Abstract
Objective
To examine the short-term effects of salpingectomy during laparoscopic hysterectomy on ovarian reserve when ovarian preservation is planned in order to determine the feasibility of conducting this study on a large scale.
Design
Pilot Randomized controlled trial.
Setting
Tertiary care, academic medical center.
Patients
Thirty premenopausal women aged 18 to 45 years underwent laparoscopic hysterectomy with ovarian preservation for benign indications from April 2012 to September 2012.
Intervention
Bilateral salpingectomy (n=15) versus no salpingectomy (n=15) at the time of laparoscopic hysterectomy with ovarian preservation.
Main Outcome Measures
Antimüllerian hormone (AMH) was measured preoperatively, 4–6 weeks postoperatively, and 3 months postoperatively. Operative time and estimated blood loss were abstracted from the medical record.
Results
Mean AMH levels were not significantly different at baseline (2.26 vs. 2.25ng/ml), 4–6 weeks postoperatively (1.03 vs. 1.25ng/ml), or 3 months postoperatively (1.86 vs. 1.82ng/ml) among women with salpingectomy versus no salpingectomy, respectively. There was also no significant temporal change in mean AMH level from baseline to 3 months postoperatively (−.07 vs. −.08ng/ml) between groups. No difference in operative time (116 vs. 115min) or estimated blood loss (70 vs. 91ml) was observed.
Conclusion
Salpingectomy at the time of laparoscopic hysterectomy with ovarian preservation is a safe procedure that does not appear to have any short-term deleterious effects on ovarian reserve, as measured by AMH level. Conducting a trial of this nature that is adequately powered with long-term follow-up would be feasible and is required to definitively confirm these results.
Keywords: Antimüllerian hormone, salpingectomy, hysterectomy, ovarian reserve
Introduction
Ovarian cancer is the fifth most common cause of cancer death in women, and is the leading cause of death due to gynecologic malignancy(1). Most ovarian cancer deaths are caused by high-grade serous carcinomas. While it has been shown that oral contraceptive use, hysterectomy, bilateral salpingo-oophorectomy, tubal ligation, multiparity, and breastfeeding can reduce ovarian cancer risk, the exact mechanism of this decreased risk remains unknown. Recent evidence suggests that the majority of high-grade pelvic serous carcinomas may actually originate in the distal fallopian tube(2–5). As a result of these findings, performing salpingectomy at the time of hysterectomy has been proposed as a strategy to reduce ovarian cancer risk(6).
Over 600,000 hysterectomies are performed on an annual basis for women in the United States, with less than half receiving concurrent bilateral salpingo-oophorectomy(7, 8). When performing a hysterectomy with planned ovarian preservation, most gynecologists do not currently perform salpingectomy out of theoretical concern that removing the shared blood supply between the fallopian tube and ovary will lead to decreased ovarian function and the premature onset of menopause(5). However, the potential deleterious effects on ovarian reserve have not been well studied. As more women decline ovarian extirpation at the time of hysterectomy, salpingectomy stands to benefit a substantial number of women as a risk-reduction strategy to prevent the development of high-grade pelvic serous cancers. Demonstrating no additional risk with salpingectomy at the time of hysterectomy may broadly change practice patterns in a meaningful way, possibly leading to a decreased incidence of a high-mortality malignancy for which there is no effective screening test.
The objective of this study was to examine the effects of salpingectomy during laparoscopic hysterectomy on ovarian reserve, when preservation of the ovaries was planned, by measuring antimüllerian hormone (AMH). We also evaluated for differences in operative time, blood loss, and complications to determine if salpingectomy poses any additional risk beyond hysterectomy itself. This was a pilot study designed to assess the feasibility of enrollment and follow-up and to estimate the effect size of our intervention in order to conduct this research on a larger scale with long-term follow-up.
Materials and Methods
This study was a single-center randomized controlled trial at the University of North Carolina Hospitals in Chapel Hill, NC. Participants were premenopausal women aged 18 to 45 years undergoing elective laparoscopic hysterectomy for benign indications with planned preservation of the ovaries. The upper age limit was chosen to prevent selecting patients in close proximity to menopause. Patients with a personal history of gynecologic malignancy, known BRCA 1/BRCA 2 carriers, and non-English speaking patients were excluded from the study. As there was no prior data on AMH assessment evaluating ovarian reserve in this setting at the initiation of this study, we set a goal of enrolling 30 women over a 6-month time period. The trial protocol was approved by the Institutional Review Board of the University of North Carolina School of Medicine and registered with ClinicalTrials.gov (NCT01578759).
Participants were recruited after the individual physician and the patient had established the final surgical plan. Eligible women were introduced to the study via an informational pamphlet provided in clinic, followed by a telephone call 1–2 weeks prior to their operation to explain the risks and benefits of participating in the study. Informed, written consent was obtained on the day of surgery.
After consent was obtained, participants were randomly allocated to the two treatment groups (salpingectomy vs. no salpingectomy) in an allocation ratio of 1:1. A computerized random number generator was used to develop the allocation sequence of random, permuted blocks of 4 and 6. Allocation concealment was maintained by having procedure indicator cards inside sequentially numbered, opaque, sealed envelopes. An unmasked research assistant not associated with the trial developed the randomization scheme and stuffed and sealed the envelopes. Participants were masked to the treatment allocation until after their operation. The physicians performing the procedures were masked to the treatment allocation until the time of surgical incision, at which time the circulating nurse in the operating room opened the envelope and revealed the treatment group.
All procedures were performed by surgeons in the Advanced Laparoscopy and Pelvic Pain division, which consists of attendings and fellows with independent surgical privileges. For patients assigned to the salpingectomy group, removal of the fallopian tubes was performed with either monopolar or bipolar electrosurgery based on the surgeon’s preference and the patient’s anatomy. Care was taken to avoid injury to the ovarian vessels and to divide the mesosalpinx as close to the fallopian tube as possible. For patients assigned to the no salpingectomy group, the fallopian tubes were divided in the proximal tubal isthmus.
Blood samples were obtained on the day of surgery prior to surgical incision in the operating room, at the patient’s postoperative visit 4–6 weeks after surgery, and again 3 months after surgery. After collection, whole blood specimens were fractionated on a centrifuge for 10 minutes at 3400rpm. The separated serum was collected and placed in 1ml vials and stored at minus 30 degrees Celsius until all specimens were obtained. The frozen serum was then shipped to the University of Southern California Reproductive Endocrine Research Lab in Los Angeles, California for analysis. AMH was measured by the use of the Ultrasensitive AMH/MIS ELISA kit obtained commercially from Ansh Labs (Webster, TX). The lowest amount of AMH that can be detected with a 95% probability is 0.023ng/ml. The assay precision (%CV) is 5.1%, 6.0% and 4.5% at 0.346ng/ml, 0.715ng/ml and 1.853ng/ml, respectively.
Demographic variables and secondary outcomes were abstracted from each participant’s medical record, including age, body mass index (BMI), uterine weight, history of prior tubal sterilization, operative time, and estimated blood loss. Comparisons between continuous variables were analyzed using the Student’s t test or Wilcoxon rank-sum test where appropriate. Statistical significance was defined as p<.05. All analyses were carried out according to intent-to-treat categorization. The conduct and analysis of the trial adhered to the 2010 CONSORT guidelines(9). Statistical analyses were conducted using Stata 12.1 (StataCorp, College Station, TX).
Results
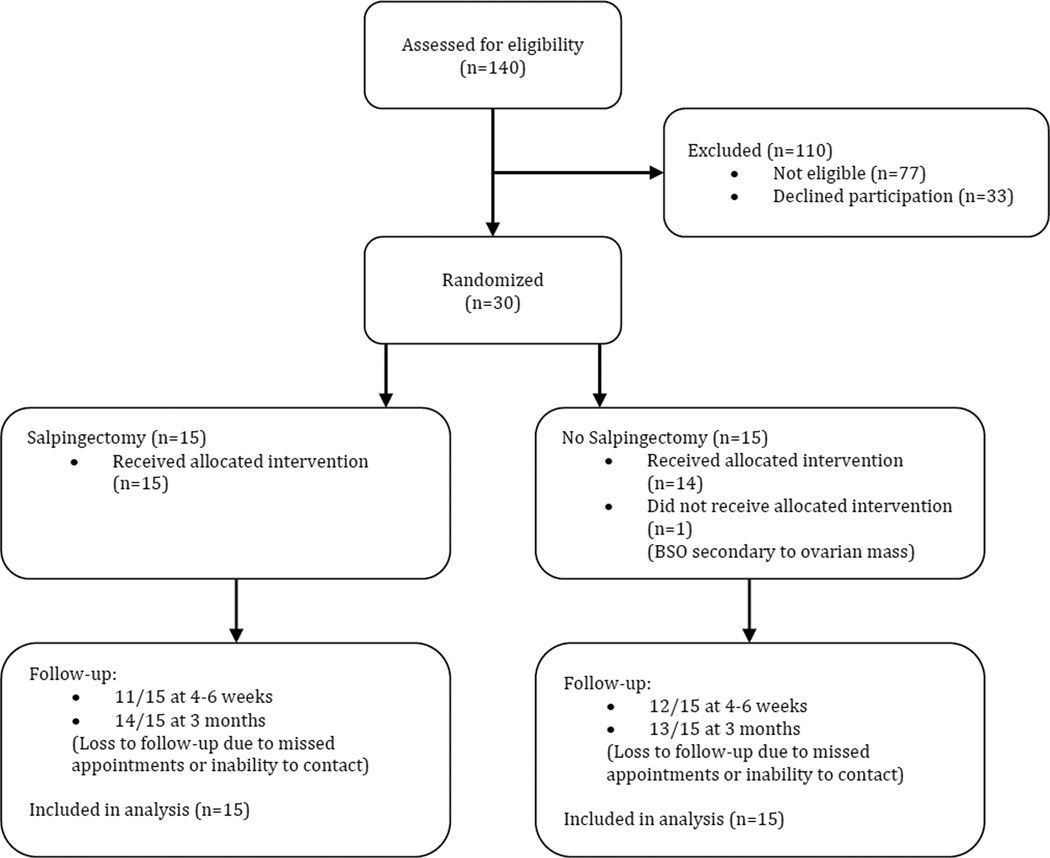
From April 2012 through September 2012, a total of 140 patients were screened for eligibility to participate in the trial. Seventy-seven patients did not meet criteria for participation, most of who were excluded for planned removal of one or both ovaries. Of the 63 eligible patients, 33 declined enrollment. Thirty patients agreed to participate and were randomized: 15 to salpingectomy and 15 to no salpingectomy. One patient assigned to the no salpingectomy group underwent bilateral salpingo-oophorectomy because of a large ovarian mass that had been diagnosed as a fibroid preoperatively. All other patients received treatment according to allocation. One intraoperative complication (inferior epigastric vessel injury) occurred, and no patients had postoperative complications. There were no complications directly attributable to performing salpingectomy. One patient in the salpingectomy group had a congenitally absent left ovary and fallopian tube that had not been diagnosed prior to her operation and one patient in the no salpingectomy group was diagnosed with well-differentiated endometrioid adenocarcinoma (FIGO Grade 1) of the endometrium on final pathology. In the salpingectomy group, 11 (73%) subjects completed the 4–6 week postoperative blood draw and 14 (93%) completed the 3-month postoperative blood draw. In the no salpingectomy group, 12 (80%) completed the 4–6 week postoperative blood draw and 13 (87%) completed the 3-month postoperative blood draw (Figure 1).
Figure 1.
Flow of participants through the trial.
Among all study participants, the mean age was 37.2 (±4.7) years, with a mean BMI of 36.3 (±9.0) kg/m2 and mean uterine weight of 211.7 (±235.2) grams. Approximately half (53%) had undergone prior tubal sterilization. There were no substantive differences in the baseline characteristics of the randomized cohort (Table 1).
Table 1.
Demographic characteristics of participants, by treatment group.
| Characteristic | Salpingectomy (n=15) | No Salpingectomy (n=15) | P |
|---|---|---|---|
| Age (y) | 36.6 ± 4.5 | 37.8 ± 5.0 | .50a |
| BMI (kg/m2) | 34.4 ± 6.8 | 38.1 ± 10.7 | .27a |
| Uterine Weight (gms) | 194.9 ± 154.6 | 228.4 ± 300.2 | .70a |
| Tubal Sterilization | 47 | 60 | .46b |
Data are means ± SD, or percent.
Student’s t-test.
Chi-Square test.
Mean AMH levels were not significantly different at baseline (2.26 vs. 2.25ng/ml, p=.99), 4–6 weeks postoperatively (1.03 vs. 1.25ng/ml, p=.76), and 3 months postoperatively (1.86 vs. 1.82ng/ml, p=.97) among women with salpingectomy versus no salpingectomy, respectively (Table 2). There were also no significant temporal changes in the mean AMH level from baseline to 3 months postoperatively (−.07 vs. −.08ng/ml, p=.98) between groups. There was no difference in mean operative time (116 vs. 115min, p=.97) or mean blood loss (70 vs. 91ml, p=.54) between both groups. Non-parametric testing of the data yielded analogous results.
Table 2.
Antimüllerian hormone, operative time, and estimated blood loss, by treatment group.a
| Outcome | Salpingectomy | No Salpingectomy | P |
|---|---|---|---|
| AMH (ng/ml) | |||
| Baseline | 2.26 ± 2.72 | 2.25 ± 2.57 | .99b |
| 4–6 weeks postoperative | 1.03 ± 1.04 | 1.25 ± 2.09 | .76b |
| 3 months postoperative | 1.86 ± 1.99 | 1.82 ± 3.12 | .97b |
| Δ AMH (Baseline-3 months) | −.07 ± .90 | −.08 ± 1.45 | .98b |
| Operative Time (min) | 115.7 ± 33 | 115.2 ± 44 | .97b |
| Estimated Blood Loss (ml) | 70.3 ± 50 | 91.3 ± 121 | .54b |
Data are means ± SD.
Student’s t-test.
Discussion
This study did not detect a significant difference in ovarian reserve at 4–6 weeks and 3 months after hysterectomy among women who underwent salpingectomy compared with women who did not receive salpingectomy, as measured by AMH level. We also did not find a difference in the change of AMH levels between groups from baseline to 3 months postoperatively. There was no difference in operative time or blood loss, and there were no complications related to performing salpingectomy. We did observe a decrease in AMH levels at the 4–6 week point that recovered to some degree by 3 months after surgery, similar to findings in previous studies examining AMH levels after ovarian surgery(10). As a pilot study, an a priori power calculation was not performed so the results of this study have to be interpreted with that in mind. We found that it was feasible to enroll participants in such a study, with nearly 50% of eligible patients consenting to participation in the study and 90% completing follow-up at 3 months postoperatively.
Our findings are similar to the only other published studies that have addressed whether or not salpingectomy performed concurrently with hysterectomy can lead to impaired ovarian function(11, 12). In 2007, Sezik et al. examined the effect of salpingectomy on ovarian reserve and stromal blood flow after abdominal hysterectomy. This study was similarly small (24 subjects), but they also did not find a difference in ovarian reserve among women who underwent salpingectomy versus those that did not. In this study, the authors used surrogate markers (FSH, LH, and estradiol) of ovarian function, which required frequent ultrasound assessment to determine early follicular phase for blood draws. In 2013, Morelli et al. performed a retrospective comparison of women who underwent hysterectomy without salpingectomy to women who underwent hysterectomy with salpingectomy when ovarian preservation was maintained. This study had a larger number of patients (79 in each group), and found no significant difference in AMH, FSH, antral follicle count, mean ovarian diameters, or peak systolic velocity between groups. These findings are also consistent with studies examining ovarian response to IVF after salpingectomy for hydrosalpinges, most of which have shown no deleterious effects on IVF outcomes(13).
The strengths of this study include the prospective and randomized design, and the use of AMH to measure ovarian reserve after hysterectomy. AMH has emerged as a validated, well-studied serum biomarker produced in the ovary that provides a direct measure of ovarian reserve. Age specific norms have been established, and AMH is now used to assess fertility, ovarian function, and predict age at menopause(14–18). AMH levels have minimal variation throughout the menstrual cycle, and remain relatively unaffected by conditions that alter endogenous gonadotropins(19), eliminating the need to determine when patients are in the follicular or luteal phase after hysterectomy. All of these factors make AMH an ideal serum marker for assessment of ovarian reserve in this setting. This study also includes patients undergoing laparoscopic, rather than abdominal hysterectomy, which is particularly relevant given the increased adoption of a minimally invasive approach to hysterectomy in the United States(20).
Limitations of this study are its small sample size and lack of long-term follow up. As a pilot study, a small cohort was appropriate and the data included here could inform a future study powered in non-inferiority design. We had to screen 140 patients undergoing laparoscopic hysterectomy to recruit 30 eligible participants to the study, and our follow up rate 3 months postoperatively was 90%, giving us a sense for how long it might take to recruit an appropriately powered study. The ideal study would follow patients through menopause and beyond to examine potential hormonal differences and, more importantly, differences in cancer rates. Such a study would incur substantial cost and time, and may not be clinically feasible. We do not know if differences would begin to appear as the participants get further out from surgery. We plan to obtain 1-year follow up data on ovarian reserve for this cohort of patients.
While removal of the ovaries and fallopian tubes clearly results in reduced risk of pelvic serous cancers(21), we do not yet know if the same applies to salpingectomy alone with ovarian retention. If indeed salpingectomy resulted in decreased ovarian function, then the decision to remove the fallopian tubes at the time of hysterectomy would involve a risk-benefit calculation similar to the discussion of adnexectomy. But if removing the fallopian tubes has no significant impact on ovarian function and does not complicate the hysterectomy, it would leave little reason not to perform salpingectomy routinely. An additional benefit would include avoidance of future operations for benign conditions of the fallopian tube such as hydrosalpinx, paratubal cyst, or tubal prolapse(22). If current research regarding the role of the fallopian tube in the pathogenesis of pelvic serous cancers continues to expand, the argument to consider prophylactic salpingectomy at the time of hysterectomy, tubal sterilization, or other elective abdominal and pelvic surgery grows stronger. Because pelvic serous cancers affect only about 1 in 70 women in the United States, the number needed to treat to prevent one cancer might be large. However, when one considers the serious morbidity of the disease and the simplicity of the prevention technique, little downside to prophylactic salpingectomy remains.
Salpingectomy at the time of laparoscopic hysterectomy with ovarian preservation did not have any short-term deleterious effects on ovarian reserve or increase surgical risk in this small cohort of women. Our results suggest obtaining AMH levels after hysterectomy is a reasonable strategy for measuring ovarian reserve, and that patients are willing to participate in enrollment and follow-up in such a study. Investigating the question more completely would likely require a large, multi-centered trial. A randomized controlled trial of this nature that is adequately powered and includes long-term follow-up is needed to definitively conclude that salpingectomy has no deleterious effects on ovarian reserve.
Acknowledgments
Support: This study was supported by a training grant from the NIH at the University of North Carolina at Chapel Hill (5 T32 HD040672-12).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Steiner is a consultant for Roche Diagnostics.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10:803–808. doi: 10.1038/nrc2946. [DOI] [PubMed] [Google Scholar]
- 3.Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42:918–931. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine DA, Argenta PA, Yee CJ, Marshall DS, Olvera N, Bogomolniy F, et al. Fallopian tube and primary peritoneal carcinomas associated with BRCA mutations. J Clin Oncol. 2003;21:4222–4227. doi: 10.1200/JCO.2003.04.131. [DOI] [PubMed] [Google Scholar]
- 5.Tone AA, Salvador S, Finlayson SJ, Tinker AV, Kwon JS, Lee CH, et al. The role of the fallopian tube in ovarian cancer. Clin Adv Hematol Oncol. 2012;10:296–306. [PubMed] [Google Scholar]
- 6.Anderson CK, Wallace S, Guiahi M, Sheeder J, Behbakht K, Spillman MA. Risk-reducing salpingectomy as preventative strategy for pelvic serous cancer. Int J Gynecol Cancer. 2013;23:417–421. doi: 10.1097/IGC.0b013e3182849dba. [DOI] [PubMed] [Google Scholar]
- 7.Asante A, Whiteman MK, Kulkarni A, Cox S, Marchbanks PA, Jamieson DJ. Elective oophorectomy in the United States: trends and in-hospital complications, 1998–2006. Obstet Gynecol. 2010;116:1088–1095. doi: 10.1097/AOG.0b013e3181f5ec9d. [DOI] [PubMed] [Google Scholar]
- 8.Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110:1091–1095. doi: 10.1097/01.AOG.0000285997.38553.4b. [DOI] [PubMed] [Google Scholar]
- 9.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstet Gynecol. 2010;115:1063–1070. doi: 10.1097/AOG.0b013e3181d9d421. [DOI] [PubMed] [Google Scholar]
- 10.Chang HJ, Han SH, Lee JR, Jee BC, Lee BI, Suh CS, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Mullerian hormone levels. Fertil Steril. 2010;94:343–349. doi: 10.1016/j.fertnstert.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Sezik M, Ozkaya O, Demir F, Sezik HT, Kaya H. Total salpingectomy during abdominal hysterectomy: effects on ovarian reserve and ovarian stromal blood flow. J Obstet Gynaecol Res. 2007;33:863–869. doi: 10.1111/j.1447-0756.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 12.Morelli M, Venturella R, Mocciaro R, Di Cello A, Rania E, Lico D, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: Primum non nocere. Gynecol Oncol. 2013;129:448–451. doi: 10.1016/j.ygyno.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Almog B, Wagman I, Bibi G, Raz Y, Azem F, Groutz A, et al. Effects of salpingectomy on ovarian response in controlled ovarian hyperstimulation for in vitro fertilization: a reappraisal. Fertil Steril. 2011;95:2474–2476. doi: 10.1016/j.fertnstert.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 14.de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–362. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 15.Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum antimullerian hormone/mullerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril. 2004;82:1323–1329. doi: 10.1016/j.fertnstert.2004.03.061. [DOI] [PubMed] [Google Scholar]
- 16.Tehrani FR, Shakeri N, Solaymani-Dodaran M, Azizi F. Predicting age at menopause from serum antimullerian hormone concentration. Menopause. 2011;18:766–770. doi: 10.1097/gme.0b013e318205e2ac. [DOI] [PubMed] [Google Scholar]
- 17.Tehrani FR, Solaymani-Dodaran M, Azizi F. A single test of antimullerian hormone in late reproductive-aged women is a good predictor of menopause. Menopause. 2009;16:797–802. doi: 10.1097/gme.0b013e318193e95d. [DOI] [PubMed] [Google Scholar]
- 18.Tehrani FR, Solaymani-Dodaran M, Tohidi M, Gohari MR, Azizi F. Modeling age at menopause using serum concentration of anti-mullerian hormone. J Clin Endocrinol Metab. 2013;98:729–735. doi: 10.1210/jc.2012-3176. [DOI] [PubMed] [Google Scholar]
- 19.Shaw CM, Stanczyk FZ, Egleston BL, Kahle LL, Spittle CS, Godwin AK, et al. Serum antimullerian hormone in healthy premenopausal women. Fertil Steril. 2011;95:2718–2721. doi: 10.1016/j.fertnstert.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright JD, Ananth CV, Lewin SN, Burke WM, Lu YS, Neugut AI, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. Jama. 2013;309:689–698. doi: 10.1001/jama.2013.186. [DOI] [PubMed] [Google Scholar]
- 21.Finch A, Beiner M, Lubinski J, Lynch HT, Moller P, Rosen B, et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. Jama. 2006;296:185–192. doi: 10.1001/jama.296.2.185. [DOI] [PubMed] [Google Scholar]
- 22.Dietl J, Wischhusen J, Hausler SF. The post-reproductive Fallopian tube: better removed? Hum Reprod. 2011;26:2918–2924. doi: 10.1093/humrep/der274. [DOI] [PubMed] [Google Scholar]