Abstract
Objective
Locomotor adaptation enables safe, efficient navigation among changing environments. We investigated how healthy young (HYA) and older (HOA) adults and persons with Parkinson’s disease (PD) adapt to walking on a split-belt treadmill, retain adapted gait parameters during re-adaptation, and store aftereffects to conventional treadmill walking.
Methods
Thirteen PD, fifteen HYA, and fifteen HOA walked on a split-belt treadmill for ten minutes with one leg twice as fast as the other. Participants later re-adapted to the same conditions to assess retention of the split-belt gait pattern. After re-adaptation, we assessed aftereffects of this pattern during conventional treadmill walking.
Results
Persons with PD exhibited step length asymmetry throughout many adaptation and adaptive learning conditions. Early adaptation was similar across groups, though HYA and HOA continued to adapt into late adaptation while PD did not. Despite pervasive step length asymmetry among conditions which were symmetric in HYA and HOA, persons with PD demonstrated significant step length aftereffects during conventional treadmill walking after split-belt walking.
Conclusions
Though they may exhibit a default asymmetry under various walking conditions, persons with PD can adapt and store new walking patterns.
Significance
Locomotor adaptation therapy may be effective in ameliorating asymmetric gait deficits in persons with PD.
Keywords: Parkinson’s disease, Aging, Split-belt treadmill, Adaptation, Gait, Motor learning
INTRODUCTION
Humans precisely coordinate the activities of many muscles to control everyday movements such as walking. This is done in the face of an environment that is constantly changing in complexity. Locomotor adaptation is a process during which changes in locomotor output are stabilized over time by the central nervous system’s incorporation of feed-forward predictive motor actions and sensorimotor feedback (Purzner et al., 2007; Cavaco et al., 2011; Bédard and Sanes, 2011; Bares et al., 2007; Bares et al., 2010; Jayaram et al., 2011; Morton and Bastian, 2006). Adaptation of human walking patterns in response to changes in internal and external environments is essential for safe, efficient community ambulation.
Split-belt treadmills (SBT) allow for creation of complex walking patterns and have recently been used to facilitate understanding of locomotor adaptation in various populations (Morton and Bastian, 2006; Dietz et al., 1995; Reisman et al., 2009). The two SBT belts can be decoupled such that one leg walks faster than the other. In healthy adults, stride length and stance time adapt to SBT walking, producing immediate asymmetric parameters between limbs (fast limb stride length is longer while stance time is shorter). These intralimb parameters remain asymmetric throughout the duration of the task (Reisman et al., 2005). Conversely, step length is an interlimb gait parameter which is initially asymmetric as the leg walking on the slow belt takes a longer step than that on the fast belt. Over time, the step lengths gradually adapt toward symmetry as the nervous system obtains the information necessary to efficiently coordinate interlimb movements (Reisman et al., 2005). Upon exposure to conventional walking immediately following SBT walking, a large aftereffect is observed in interlimb parameters such that, for instance, step length asymmetry is opposite that which was observed during initial adaptation (i.e. step length is longer for the leg that previously walked on the fast belt as compared to the leg that previously walked on the slow belt) even while both belts move at the same speeds. Little aftereffect in the intralimb parameters is typically observed during tied-belt walking immediately following a bout of SBT walking.
In this manuscript, we use the terms adaptation and adaptive learning to address two different phases of the locomotor response to an altered environment. Adaptation refers to the short-term, error-based process during which information is obtained about the altered environment and motor responses are adjusted accordingly. In contrast, adaptive learning is a long-term process representing the stored ability to predict a locomotor perturbation and adapt gait parameters, which is necessary to retain an adapted gait pattern once the environment is either altered again or returned to its original state (Jayaram et al., 2011). Prior evidence also suggests that locomotor adaptation and adaptive learning are controlled through different neurological processes. During adaptation to the SBT, it is thought that intralimb parameters (e.g. stride length and stance time) are reactively altered by feedback mechanisms likely derived from the spinal cord to accommodate an immediate response to the SBT perturbation (Rossignol et al., 1999; Pearson, 1995) while interlimb parameters (e.g. step length) require cerebellar-modulated predictive, feed-forward control to more gradually adapt (Morton and Bastian, 2006). Further, research has suggested that depression of cerebellar inhibition over the motor cortex is necessary to facilitate locomotor adaptive learning (Jayaram et al., 2011).
Individuals with Parkinson’s disease (PD) present a multitude of locomotor signs and disease manifestations including bradykinesia, akinesia, tremor, and rigidity (Knutsson, 1972; Fahn, 1995). While persons with PD demonstrate difficulty altering the locomotor system during transitional periods such as turning (Bloem et al., 2011), obstacle clearing (Stegemöller et al., 2012), and gait initiation (Hass et al., 2005), abilities to adapt steady-state gait and store adapted gait patterns have not been well-studied. Only one study has examined SBT walking in PD, demonstrating that persons with PD reactively adapted over a similar number of strides when compared to controls (Dietz et al., 1995). However, immediate reactive changes are required to mechanically maintain gait when responding to the SBT perturbation and thus the locomotor control insight provided by these findings is limited. To our knowledge, no study has investigated a longer locomotor adaptation period during which adaptation could be modulated by feed-forward mechanisms nor have assessments of retention or aftereffects been included to examine adaptive learning in persons with PD. Investigation of the ability to asymmetrically adapt gait patterns may have important clinical implications for gait rehabilitation in persons with PD as this population is often characterized by asymmetry in a multitude of gait parameters (Knutsson, 1972; Johnsen et al., 2009; Lewek et al., 2010). However, in keeping with the growing body of research suggesting that retention and aftereffect storage of learned upper extremity and visuomotor tasks are impaired in persons with PD (Bédard et al., 2011; Fernandez-Ruiz et al., 2003; Leow et al., 2012; Isaias et al., 2011; Smiley-Oyen et al., 2006; Mochizuki-Kawai et al., 2004), we postulate that persons with PD may also exhibit restricted locomotor adaptation and diminished facilitation of adaptive learning.
In addition to PD, little is known about how normal aging affects the abilities to adapt and store new gait patterns. A recent study suggested that older adults demonstrate somewhat restricted adaptation and diminished aftereffects from SBT walking; however, locomotor adaptive learning could not be fully assessed as no retention task was included (Bruijn et al., 2012). Regarding generalized motor adaptation and motor learning, the literature on effects of aging has provided largely conflicting results. A few recent studies on dynamic stability in older adults have suggested impairments in initial reaction to gait perturbations but preserved ability to predictively adapt to perturbations over time (Bierbaum et al., 2010; Pai et al., 2010). Moreover, the capacity to make visuomotor adaptations appears to remain intact with aging (Cressman et al., 2010). As the abilities to facilitate and retain adaptations in gait may be important in preventing falling in older adults (Pai et al., 2010), this study will provide knowledge as to how normal aging and PD may affect the ability to adapt and store gait patterns.
The purpose of this study was to investigate locomotor adaptation and locomotor adaptive learning in persons with PD and in normal aging. We hypothesized that during SBT walking, persons with PD and HOA would demonstrate restricted locomotor adaptation compared to healthy young adults (HYA). We also postulated that HOA and persons with PD would demonstrate diminished retention of adapted spatiotemporal gait parameters during re-adaptation and diminished aftereffects into conventional treadmill walking, with these locomotor adaptive learning deficits being more pronounced in persons with PD. However, if gait asymmetry can be altered in persons with PD, SBT training may have potential as a rehabilitative tool to restore gait symmetry in persons with PD affected by asymmetric gait deficits.
METHODS
Participants
Thirteen persons with PD (mean±SD: 64.1±8.8 yr, 172.7±3.8 cm, 81.4±9.4 kg, Unified Parkinson’s Disease Rating Scale (UPDRS) motor score: 24.6±6.9, UPDRS nonmotor score: 11.3±4.0), 15 age-matched HOA (age 65.2±8.1 yr, 169.2±7.8 cm, 72.1±15.4 kg), and 15 HYA (age 22.3±3.3 yr, 169.0±8.6 cm, 65.8±11.3 kg) participated. Our protocol was structured similarly to that used in previous studies of SBT walking (Reisman et al., 2005). No participant had walked on a SBT prior to participation nor experienced a lower limb orthopedic injury for at least one year. The HOA and HYA were free of any history of neurological impairment. Participants with PD included in this study either self-reported asymmetric gait difficulty or were referred to the study by their neurologist for demonstrating asymmetric gait characteristics. All participants with PD performed the sessions in their self-reported best-medicated state in order to best observe their daily functional ability. All participants provided written informed consent before participating in the study as approved by the University Institutional Review Board.
Locomotor data
Sixteen passive reflective markers were attached to the lower body in accordance with the Vicon Plug-in-Gait lower body marker system. Kinematic data were collected using a 7-camera motion capture system (120 Hz; Vicon, Oxford, UK) while participants walked on an instrumented SBT (Bertec Corporation, Columbus, OH). Participants first walked on the SBT while both belts moved together at a self-selected comfortable speed for five minutes to accommodate to walking on the treadmill. All participants held onto the handrails for the duration of all treadmill sessions. The speed of both belts was then increased until the participants reported being at the “fastest speed they felt comfortable walking for 15 minutes”. This speed was set as the “fast” walking speed. The “slow” walking speed was designated as 50% of the “fast” walking speed.
Participants first became acclimated with the slow and fast walking speeds. They walked for two minutes with both belts at the slow speed (BASELINE-TIED), two minutes with both belts at the fast speed, and then two-minutes at the slow speed to wash out the fast walking pattern. Gait parameters were averaged over the last 30 seconds of the BASELINE-TIED condition. Then, without stopping the treadmill after the baseline trials, the belt under the nondominant leg in HYA and HOA and the belt under the more-affected leg in PD was sped up to the fast speed while the belt under the contralateral leg remained at the slow speed. Participants walked under these conditions for 10 minutes (SPLIT). Gait parameters were averaged over the first five strides of the SPLIT condition (EARLY), five strides at the five-minute mark of the SPLIT condition (MID), and finally over last five strides of the SPLIT condition (LATE). Following the SPLIT condition, participants walked overground across a 10-m walkway ten times in order to wash out the effects of the SPLIT condition. Overground walking trials were selected as a washout rather than treadmill walking in order to remove the external stimulus and rhythmicity provided by the treadmill. Motion capture was unavailable during these overground walking trials. The participants then walked for three minutes with the treadmill again set to the same SPLIT condition (READAPT). Immediately following READAPT, the fast belt was slowed to the slow speed for five minutes (POST-TIED). Gait parameters were also averaged over the first five strides of the READAPT and POST-TIED conditions (Figure 1). Leg dominance was defined by response to the question “which leg would you use to kick a soccer ball?” Herein, the nondominant leg in HYA and HOA (or most-affected in PD) will be referred to as the “fast” leg while the dominant leg in HYA and HOA (or least-affected in PD) will be referred to as the “slow” leg. In PD, the more-affected leg was self-reported by the participants and confirmed through UPDRS scores.
Figure 1.
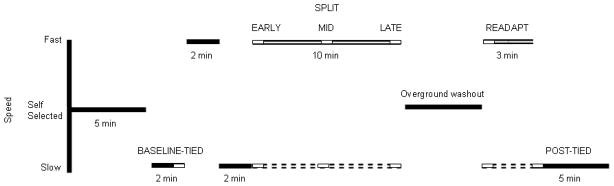
An outline of the protocol used to investigate locomotor adaptation and adaptive learning. Solid black horizontal lines indicate that the belts were moving at the same speed. The solid white lines represent the nondominant limb in HYA and HOA and the more-affected limb in PD while the dashed white lines represent the contralateral limb. “Fast” indicates self-selected fastest comfortable walking speed and “Slow” indicates slow (50% of fast) walking speed.
Data analysis
Heel-strikes and toe-offs were manually labeled in Vicon software based on marker velocity profiles. Stride length was defined as the distance traveled by the ankle marker along the walking axis from heel-strike to toe-off (Reisman et al., 2005). Stance time was defined as the percent of the gait cycle between heel-strike and subsequent toe-off of the same limb. Step length was defined as the distance between the ankle markers along the walking axis at heel-strike. We defined symmetry in each gait parameter using the following asymmetry index:
Asymmetry = (fast leg parameter – slow leg parameter)/(fast leg parameter + slow leg parameter)
An a priori sample size of n>11 per group was determined based on pilot data using F-test power analysis within G*Power 3.1 assuming β error probability of .80 and α error probability of .05. A series of 3×6 (group x condition) repeated measures ANOVAs were performed to analyze differences in stride length, stance time, and step length asymmetry among groups (HYA, HOA, and PD) and conditions (BASELINE-TIED, EARLY adaptation, MID adaptation, LATE adaptation, READAPT, POST-TIED). One-way ANOVAs were performed to analyze differences in age, height, and body mass between groups. Bonferroni post-hoc adjustments were applied to pairwise comparisons when appropriate. Levels of significance for the ANOVAs prior to Bonferroni post-hoc adjustments were set at α=.05. The ANOVAs were performed using SPSS Statistics 21 (IBM, Armonk, New York). We have also provided eta-squared (η2) effect sizes to supplement the interpretation of the results. Traditional interpretation of η2 suggests that effect sizes greater than .01 but less than .06 be considered small, greater than .06 but less than .14 be considered medium, and greater than .14 be considered large (Cohen, 1988).
RESULTS
Persons with PD had significantly more body mass than HYA (p=.005). There were no significant differences in fast treadmill walking speed or height among groups. There was no significant difference in age between PD and HOA.
Interlimb adaptation and adaptive learning
Step length asymmetry
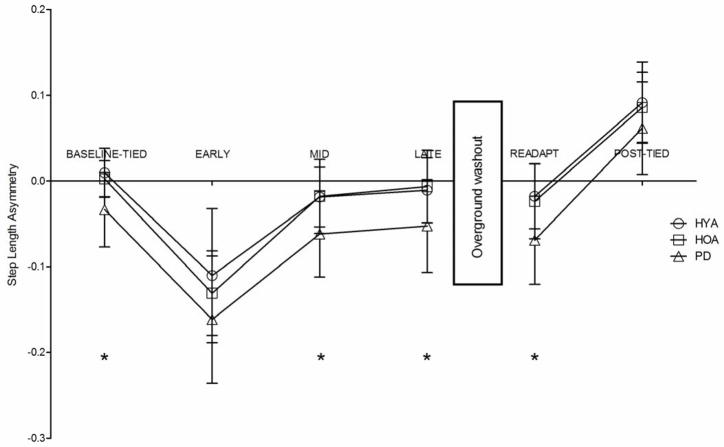
We observed main effects of both condition and group on step length asymmetry (p<.001 and p=.003, η2=.78 and η2=.25, respectively). Persons with PD exhibited significantly greater step length asymmetry (p<.05) than HYA and HOA throughout many of the locomotor adaptation and adaptive learning conditions with the exceptions of EARLY adaptation (p=.162 and p=.725, respectively) and POST-TIED (p=.564 and p=.319, respectively) (Figure 2). All groups exhibited significantly greater step length asymmetry during EARLY adaptation relative to BASELINE-TIED (all p<.001). By LATE adaptation, all groups adapted step length asymmetry such that it was not different from BASELINE-TIED (all p>.90). Thus, persons with PD demonstrated the same degree of step length asymmetry during both baseline tied-belt walking (BASELINE-TIED) and during the late stages of SBT walking (LATE adaptation).
Figure 2.
Comparisons of step length asymmetry among groups and across all walking conditions. HYA – healthy young adults, HOA- healthy older adults, PD – persons with Parkinson’s disease. Error bars indicate standard deviation. * indicates that step length asymmetry in HOA and HYA was significantly different from PD with p<.05.
All groups displayed retention of the learned gait parameters such that step length asymmetry during READAPT was not significantly different from LATE adaptation (all p>.90). Further, all groups demonstrated a significant step length aftereffect as step length asymmetry during POST-TIED was significantly greater than during BASELINE-TIED in all groups (all p<.001).
Intralimb adaptation and adaptive learning
Stride length asymmetry
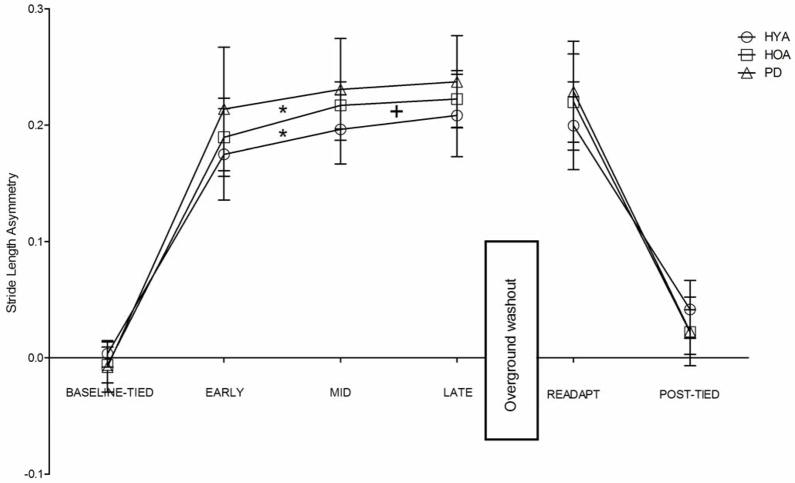
We observed a main effect of condition (p<.001, η2=.95) and a significant group x condition interaction (p=.004, η2=.01) on stride length asymmetry. All groups exhibited significant increases in stride length asymmetry during EARLY adaptation, MID adaptation, LATE adaptation, READAPT, and POST-TIED as compared to BASELINE-TIED (all p<.001) (Figure 3). We did not observe significant differences in stride length asymmetry among groups during any of the conditions. However, both HYA and HOA demonstrated a significant increase in stride length asymmetry from EARLY to LATE adaptation (both p=.001) while PD did not (p=.077). Only HYA increased stride length asymmetry from EARLY to MID adaptation and from MID to LATE adaptation (p=.019).
Figure 3.
Comparisons of stride length asymmetry among groups and across all walking conditions. HYA – healthy young adults, HOA- healthy older adults, PD – persons with Parkinson’s disease. Error bars indicate standard deviation. *indicates that stride length asymmetry during MID adaptation was significantly higher than during EARLY adaptation with p<.05. + indicates that stride length asymmetry during LATE adaptation was significantly higher than during MID adaptation with p<.05.
Stance time asymmetry
We observed a main effect of condition (p<.001, η2=.89) and a significant group x condition interaction (p=.001, η2=.02) on stance time asymmetry. All groups exhibited significantly increased stance time asymmetry during EARLY adaptation, MID adaptation, LATE adaptation, and READAPT (all p<.001) (Figure 4). We observed significantly higher stance time asymmetry during POST-TIED in the HYA and HOA relative to PD (p=.001 and p=.023, respectively) and significantly higher stance time asymmetry during POST-TIED as compared to BASELINE-TIED in HYA (p=.009) but not in HOA or PD (both p>.90).
Figure 4.
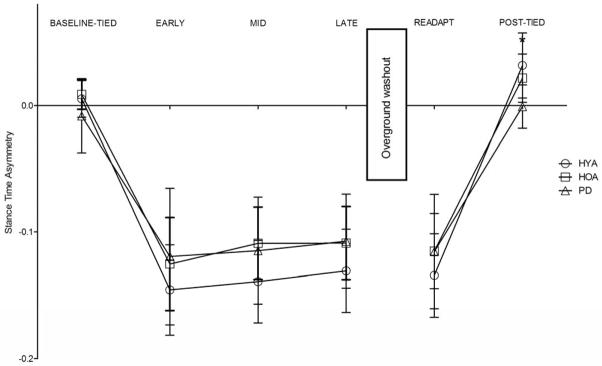
Comparisons of stance time asymmetry among groups and across all walking conditions. HYA – healthy young adults, HOA- healthy older adults, PD – persons with Parkinson’s disease. Error bars indicate standard deviation. * indicates that stance time asymmetry in HOA and HYA was significantly different from PD with p<.05.
DISCUSSION
We investigated the effects of PD and normal aging on the abilities to adapt a locomotor pattern and to retain and store aftereffects of the newly-learned pattern. Persons with PD walked with significant step length asymmetry during baseline which, interestingly, was pervasive among all walking conditions during which step length is typically symmetric in healthy adults. For example, during SBT walking in healthy adults, step length is initially asymmetric during early adaptation (Reisman et al., 2005). In the present study, the initial perturbation in step length symmetry during EARLY adaptation was not significantly different among groups. Then, over time, the step lengths gradually adapt toward symmetry in healthy adults, even while the belts continue to move at asymmetric speeds. However, persons with PD restored step length symmetry from EARLY to LATE adaptation such that the step length asymmetry observed during LATE adaptation was similar to that observed during BASELINE-TIED. Thus, step lengths were asymmetric in PD even during LATE adaptation. Likewise, a similar degree of step length asymmetry pattern was observed upon the initial re-exposure to the SBT gait perturbation during READAPT. Our findings may suggest that persons with PD who experience gait asymmetry may “default” to an asymmetric gait pattern during various walking tasks, even after the gait pattern is asymmetrically perturbed and the nervous system is forced to reorganize gait symmetry. These findings appear similar to those previously observed during SBT walking in post-stroke populations also characterized by gait asymmetry (Reisman et al., 2007).
Our results also demonstrate that persons with PD are able to at least temporarily alter this default asymmetry with training requiring locomotor adaptation. During POST-TIED, the persons with PD exhibited a significant step length aftereffect such that the more-affected limb actually took a significantly longer step than the less-affected limb (while the opposite was true during BASELINE-TIED). Thus, our findings suggest that SBT walking may have important implications for the restoration of gait symmetry in persons with PD, specifically amongst those affected by step length asymmetry. Indeed, as HOA and HYA demonstrated similar abilities to adapt and store gait patterns, the findings of the current study appear to be primarily disease- related as opposed to age-related.
Stride length and stance time have been described as rapidly-adjusting, reactively-controlled parameters, hypothesized to be obligatory to the mechanical perturbation of the belts and primarily spinally-controlled during initial locomotor adaptation (Morton and Bastian, 2006; Rossignol et al., 1999; Pearson, 1995). All groups adapted similarly during the first five strides of exposure to the SBT gait pattern, suggesting that the spinal mechanisms governing reactive locomotor adaptation remain intact/effective in PD and HOA. This finding is consistent with previous research demonstrating that persons with PD were able to adapt to SBT walking similarly to HOA over a short time period (Dietz et al., 1995) and further literature exhibiting that persons with PD are able to make mild alterations to gait during treadmill walking (Bello et al., 2008; Hong et al., 2007). Previous research has indicated that stride length adapts immediately in a rather drastic fashion upon exposure to a SBT walking task, then continues to adapt gradually throughout the task (Bruijn et al., 2012). Contrary to HOA and HYA, we observed that participants with PD did not further alter stride length from EARLY to LATE adaptation. This is in support of previous research, which suggests that consolidation of the adapted motor task over time appears to be abnormal in PD (Marinelli et al., 2009). As persons with PD demonstrated the highest stride length asymmetry during EARLY (though not significantly higher than HOA or HYA) and the smallest change from EARLY to LATE, we suggest that the modestly-higher initial adaptation may serve as a compensatory strategy for the diminished ability to consolidate task demands. This postulation is supported by the absence of significant stride length adaptation from EARLY to LATE and the similarity in stride length adaptation during EARLY and READAPT in participants with PD.
Step length is thought to be more slowly-adapting and predictively-controlled by the cerebellum (Morton and Bastian, 2006). Further, interlimb gait parameters have previously been shown to demonstrate the largest aftereffects during conventional gait following SBT walking (Reisman et al., 2005). As previous studies on upper extremity and visuomotor adaptation have shown that retention and transfer of novel skill acquisition are diminished in persons with PD (Bédard et al., 2011; Fernandez-Ruiz et al., 2003; Leow et al., 2012; Isaias et al., 2011; Smiley-Oyen et al., 2006; Mochizuki-Kawai et al., 2004), we hypothesized that retention and storage of aftereffects may also be diminished in PD. We did observe significantly greater step length asymmetry during READAPT in PD as compared to HYA and HOA; however, persons with PD were able to reproduce the LATE adaptation values of step length asymmetry upon re-exposure to the SBT walking task during READAPT. This likely indicates that persons with PD retained the LATE adaptation pattern in similar fashion to HYA and HOA. Thus, the differences among groups appear to be largely attributable to the significant step length asymmetry exhibited by persons with PD throughout the phases of the locomotor adaptation and adaptive learning conditions during which step length asymmetry is relatively symmetrical in healthy adults (BASELINE-TIED, MID and LATE adaptation, READAPT). Therefore, contrary to our hypotheses, retention of newly-learned gait patterns seems to be relatively intact in persons with PD. Moreover, the storage of aftereffects following SBT walking also appears to remain intact in PD, as there were no differences among groups in step length asymmetry during POST-TIED. Our findings suggest that the ability to store adapted gait parameters into conventional walking is preserved in PD.
As persons with PD exhibited a significant step length aftereffect during POST-TIED, SBT training may be a useful rehabilitation tool to restore gait symmetry in persons with PD affected by step length asymmetry, such as those included in this study. However, as persons with PD adapted gait parameters back to baseline asymmetric patterns given sufficient exposure to a novel SBT walking bout, whether or not gait symmetry could be more permanently restored with chronic training remains unknown. If persons with PD can learn new gait patterns over time through repetitive locomotor adaptation training, SBT walking could prove to be an effective exercise intervention in restoring gait function and improving locomotor adaptability. Data from our lab which is currently in review suggests that persons with PD can indeed learn SBT walking patterns and alter gait symmetry over time with chronic training.
Limitations to our findings are that all PD participants in this study demonstrated significant step length asymmetry during conventional treadmill walking, but not all persons with PD experience gait asymmetry. Therefore, the scope of this paper is limited to gait rehabilitation of persons with PD exhibiting asymmetric gait patterns. Also, the participants with PD performed all trials while optimally medicated. Thus, we cannot precisely ascertain how dopamine therapy and/or dopaminergic dysfunction of the PD brain affects locomotor adaptation and locomotor adaptive learning. We tested all PD participants with the more-affected limb on the faster belt, regardless of leg dominance. We do not suspect that leg dominance had any effect on our results, as previous work observed similar patterns of locomotor adaptation and retention of aftereffects regardless of which leg walked on the faster belt (Reisman et al., 2005). Persons with PD walked on the SBT at slower self-selected speeds than HYA and HOA. However, all groups attained the same degree of adaptation across all gait parameters by LATE adaptation.
CONCLUSION
Persons with PD demonstrated significant step length asymmetry which was pervasive throughout phases of locomotor adaptation and adaptive learning processes during which step lengths are relatively symmetrical in healthy adults. This may indicate that persons with PD who experience step length asymmetry default to this pattern even after gait patterns are perturbed and require reorganization. However, persons with PD demonstrated a significant aftereffect during conventional treadmill walking immediately after SBT walking. This aftereffect was similar in magnitude to the healthy groups and may have important implications for restoration of gait symmetry within this population. Further investigation is needed into the potential of split-belt treadmills as rehabilitative tools to restore gait function in PD.
Highlights.
Persons with Parkinson’s disease exhibit pervasive step length asymmetry throughout locomotor adaptation and adaptive learning conditions.
Persons with Parkinson’s disease did not adapt stride length in similar fashion to healthy adults.
Split-belt walking may have potential for gait symmetry restoration in persons with Parkinson’s disease.
Acknowledgments
This work was supported in part by NIH grants R03HD054594, 1R21AG033284-01A2 and UF National Parkinson’s Foundation Center of Excellence.
Footnotes
Conflict of Interest
The authors declare that there are no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bares M, Lungu O, Liu T, Waechter T, Gomez CM, Ashe J. Impaired predictive motor timing in patients with cerebellar disorders. Exp Brain Res. 2007;180:355–65. doi: 10.1007/s00221-007-0857-8. [DOI] [PubMed] [Google Scholar]
- Bares M, Lungu OV, Husárová I, Gescheidt T. Predictive motor timing performance dissociates between early diseases of the cerebellum and Parkinson’s disease. Cerebellum. 2010;9:124–35. doi: 10.1007/s12311-009-0133-5. [DOI] [PubMed] [Google Scholar]
- Bédard P, Sanes JN. Basal ganglia-dependent processes in recalling learned visual-motor adaptations. Exp Brain Res. 2011;209:385–93. doi: 10.1007/s00221-011-2561-y. [DOI] [PubMed] [Google Scholar]
- Bello O, Sanchez JA, Fernandez-del-Olmo M. Treadmill walking in Parkinson’s disease patients: adaptation and generalization effect. Mov Disord. 2008;23:1243–9. doi: 10.1002/mds.22069. [DOI] [PubMed] [Google Scholar]
- Bierbaum S, Peper A, Karamanidis K, Arampatzis A. Adaptational responses in dynamic stability during disturbed walking in the elderly. J Biomech. 2010;43:2362–8. doi: 10.1016/j.jbiomech.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson’s disease. J Neurol. 2001;248:950–8. doi: 10.1007/s004150170047. [DOI] [PubMed] [Google Scholar]
- Bruijn SM, Van Impe A, Duysens J, Swinnen SP. Split-belt walking: adaptation differences between young and older adults. J Neurophysiol. 2012;108:1149–1157. doi: 10.1152/jn.00018.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaco S, Anderson SW, Correia M, Magalhaes M, Pereira C, Tuna A, et al. Task-specific contribution of the human striatum to perceptual-motor skill learning. J Clin Exp Neuropsychol. 2011;33:51–62. doi: 10.1080/13803395.2010.493144. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. New York: Oxford University Press; 1988. [Google Scholar]
- Cressman EK, Salomonczyk D, Henriques DY. Visuomotor adaptation and proprioceptive recalibration in older adults. Exp Brain Res. 2010;205:533–44. doi: 10.1007/s00221-010-2392-2. [DOI] [PubMed] [Google Scholar]
- Dietz V, Zijlstra W, Prokop T, Berger W. Leg muscle activation during gait in Parkinson’s disease: adaptation and interlimb coordination. Electroencephalogr Clin Neurophysiol. 1995;97:408–15. doi: 10.1016/0924-980x(95)00109-x. [DOI] [PubMed] [Google Scholar]
- Fahn S. The freezing phenomenon in parkinsonism. Adv Neurol. 1995;67:53–63. [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Diaz R, Hall-Haro C, Vergara P, Mischner J, Nuñez L, et al. Normal prism adaptation but reduced after-effect in basal ganglia disorders using a throwing task. Eur J Neurosci. 2003;18:689–94. doi: 10.1046/j.1460-9568.2003.02785.x. [DOI] [PubMed] [Google Scholar]
- Hass CJ, Waddell DE, Fleming RP, Juncos JL, Gregor RJ. Gait initiation and dynamic balance control in Parkinson’s disease. Arch Phys Med Rehabil. 2005;86:2172–6. doi: 10.1016/j.apmr.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Hong M, Perlmutter JS, Earhart GM. Podokinetic after-rotation in Parkinson disease. Brain Res. 2007;1128:99–106. doi: 10.1016/j.brainres.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaias IU, Moisello C, Marotta G, Schiavella M, Canesi M, Perfetti B, et al. Dopaminergic striatal innervation predicts interlimb transfer of a visuomotor skill. J Neurosci. 2011;31:14458–62. doi: 10.1523/JNEUROSCI.3583-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram G, Galea JM, Bastian AJ, Celnik P. Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb Cortex. 2011;21:1901–9. doi: 10.1093/cercor/bhq263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen EL, Mogensen PH, Sunde NA, Østergaard K. Improved asymmetry of gait in Parkinson’s disease with DBS: gait and postural instability in Parkinson’s disease treated with bilateral deep brain stimulation in the subthalamic nucleus. Mov Disord. 2009;24:590–7. doi: 10.1002/mds.22419. [DOI] [PubMed] [Google Scholar]
- Knutsson E. An analysis of Parkinsonian gait. Brain. 1972;95:475–86. doi: 10.1093/brain/95.3.475. [DOI] [PubMed] [Google Scholar]
- Leow LA, Loftus AM, Hammond GR. Impaired savings despite intact initial learning of motor adaptation in Parkinson’s disease. Exp Brain Res. 2012;218:295–304. doi: 10.1007/s00221-012-3060-5. [DOI] [PubMed] [Google Scholar]
- Lewek MD, Poole R, Johnson J, Halawa O, Huang X. Arm swing magnitude and asymmetry during gait in the early stages of Parkinson’s disease. Gait Posture. 2010;31:256–60. doi: 10.1016/j.gaitpost.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli L, Crupi D, Di Rocco A, Bove M, Eidelberg D, Abbruzzese G, et al. Learning and consolidation of visuo-motor adaptation in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:6–11. doi: 10.1016/j.parkreldis.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki-Kawai H, Kawamura M, Hasegawa Y, Mochizuki S, Oeda R, Yamanaka K, et al. Deficits in long-term retention of learned motor skills in patients with cortical or subcortical degeneration. Neuropsychologia. 2004;42:1858–63. doi: 10.1016/j.neuropsychologia.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26:9107–16. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai YC, Bhatt T, Wang E, Espy D, Pavol MJ. Inoculation against falls: rapid adaptation by young and older adults to slips during daily activities. Arch Phys Med Rehabil. 2010;91:452–9. doi: 10.1016/j.apmr.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG. Proprioceptive regulation of locomotion. Curr Opin Neurobiol. 1995;5:786–91. doi: 10.1016/0959-4388(95)80107-3. [DOI] [PubMed] [Google Scholar]
- Purzner J, Paradiso GO, Cunic D, Saint-Cyr JA, Hogue T, Lozano AM, et al. Involvement of the basal ganglia and cerebellar motor pathways in the preparation of self-initiated and externally triggered movements in humans. J Neurosci. 2007;27:6029–36. doi: 10.1523/JNEUROSCI.5441-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol. 2005;94:2403–15. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130:1861–1872. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair. 2009;23:735–44. doi: 10.1177/1545968309332880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Drew T, Brustein E, Jiang W. Locomotor performance and adaptation after partial or complete spinal cord lesions in the cat. Prog Brain Res. 1999;123:349–65. doi: 10.1016/s0079-6123(08)62870-8. [DOI] [PubMed] [Google Scholar]
- Smiley-Oyen AL, Lowry KA, Emerson QR. Learning and retention of movement sequences in Parkinson’s disease. Mov Disord. 2006;21:1078–87. doi: 10.1002/mds.20906. [DOI] [PubMed] [Google Scholar]
- Stegemöller EL, Buckley TA, Pitsikoulis C, Barthelemy E, Roemmich R, Hass CJ. Postural instability and gait impairment during obstacle crossing in Parkinson’s disease. Arch Phys Med Rehabil. 2012;93:703–9. doi: 10.1016/j.apmr.2011.11.004. [DOI] [PubMed] [Google Scholar]