Abstract
Objective
Acute respiratory distress syndrome (ARDS) is a common complication of critical illness, with high mortality and limited treatment options. Preliminary studies suggest that potentially preventable hospital exposures contribute to ARDS development. We aimed to determine the association between specific hospital exposures and the rate of ARDS development among at-risk patients.
Method
In a population-based, nested, case-control study, consecutive adult patients who developed ARDS from January 2001 through December 2010 during their hospital stay (cases) were matched to similar-risk patients without ARDS (controls). They were matched for 6 baseline characteristics.
Main Outcome Measure(s)
Trained investigators blinded to outcome of interest reviewed medical records for evidence of specific exposures, including medical and surgical adverse events, inadequate empirical antimicrobial treatment, hospital-acquired aspiration, injurious mechanical ventilation, transfusion, and fluid and medication administration. Conditional logistic regression was used to calculate the risk associated with individual exposures.
Results
During the 10-year period, 414 patients with hospital-acquired ARDS were identified and matched to 414 at-risk, ARDS-free controls. Adverse events were highly associated with ARDS development (odds ratio, 6.2; 95% CI, 4.0-9.7), as were inadequate antimicrobial therapy, mechanical ventilation with injurious tidal volumes, hospital-acquired aspiration, and volume of blood products transfused and fluids administered. Exposure to antiplatelet agents during the at-risk period was associated with a decreased risk of ARDS. Rate of adverse hospital exposures and incidence of ARDS decreased during the study period.
Conclusions
Prevention of certain adverse hospital exposures in at-risk patients may limit the development of ARDS.
Keywords: acute lung injury, adverse events, case-control, health care delivery
Background
Acute respiratory distress syndrome (ARDS) is a common and often fatal condition afflicting critically ill and injured patients and imposing a substantial worldwide burden of disease (1-3). In the United States alone, estimates indicate that each year ARDS develops in approximately 190,000 patients, 74,500 of whom will die of ARDS (3). A recent systematic review described the central clinical frustration: despite decades of substantial research effort exploring various molecular, translational, and clinical aspects of ARDS, the prognosis for patients with the syndrome has remained essentially unchanged except in selected clinical trials (2). State-of-the-art treatment is limited to lung-protective ventilation and meticulous supportive care (3).
Observational studies have suggested that ARDS is rarely present during the initial emergency department evaluation or at hospital admission but rather develops in a subset of patients over a period of hours to days (4). The suggestion that ARDS could in part be viewed as a potentially preventable hospital-acquired phenomenon (5, 6) has prompted a search for tools to more accurately identify at-risk patients, along with preventive strategies to be deployed before the syndrome is fully manifested. Potentially important hospital exposures include injurious tidal volumes (5, 7), blood product transfusions (5, 8), aspiration (9), inappropriate antimicrobial therapy (9), medical and surgical adverse events (10), and fluid overload (11, 12).
Preliminary studies inadequately addressed potentially confounding baseline differences between patients at risk who did and did not develop ARDS, limiting the inferences about the causal role of hospital-acquired exposures (5, 6, 8-10). The recently developed and validated lung injury prediction score (LIPS) (4) predicts the probability of ARDS development based on the presence or absence of specific predisposing conditions (sepsis, aspiration, pneumonia, shock, trauma, high risk surgery), and risk modifiers (alcohol use, chemotherapy, acidosis, hypoxemia, tachypnea, hypoalbuminemia, diabetes) at hospital admission. Using LIPS to stratify patients at risk, we sought to test the hypothesis that hospital exposures can influence the development of ARDS in patients with similar risk at hospital admission.
Method
The study was conducted at a tertiary care teaching institution comprising 2 hospitals with 1,900 inpatient beds and 164 adult intensive care unit (ICU) beds. Detailed methods have been previously reported (13). The Mayo Clinic Institutional Review Board approved the study protocol.
Study Population
Eligible patients included consecutive adult residents of Olmsted County, Minnesota, with ARDS or at risk for ARDS, who were admitted to the hospital from January 2001 through December 2010. Screening was conducted retrospectively from January 2001 through October 2008 and prospectively from November 2008 through December 2010. We excluded patients who had ARDS or pulmonary edema at hospital admission or more than 60 days after admission, those admitted for comfort care only, and patients who died within 24 hours of admission. We also excluded patients who were readmitted to the hospital during the study period, who had recurrent episodes of ARDS, and who declined the use of their medical records for research (about 5% of hospitalized Olmsted County residents).
Ascertainment of Cases
A previously validated ARDS electronic surveillance tool (14) identified all mechanically ventilated patients who with possiblehad potential cases of ARDS; medical records of those patients were subsequently reviewed by 2 trained study investigators for accuracy and timing of ARDS development according to the American European Consensus Conference criteria (15). This process has previously been shown to have very good interobserver agreement (κ=0.83) (16). An independent third investigator resolved all disagreements.
Ascertainment of Controls
Matched controls were identified from the remaining cohort of consecutive adult Olmsted County residents admitted to the hospital from 2003 through 2010 (very limited electronic records were available for 2001-2002) who did not have ARDS, despite having at least 1 risk factor for it. Cases and controls were matched 1:1 for 6 characteristics: age, sex, high-risk surgery (elective or emergency), sepsis, ratio of peripheral oxygen saturation (SpO2) to fraction of inspired oxygen (FIO2) (17), and ARDS risk according to the LIPS (4).
Ascertainment of Hospital Exposures
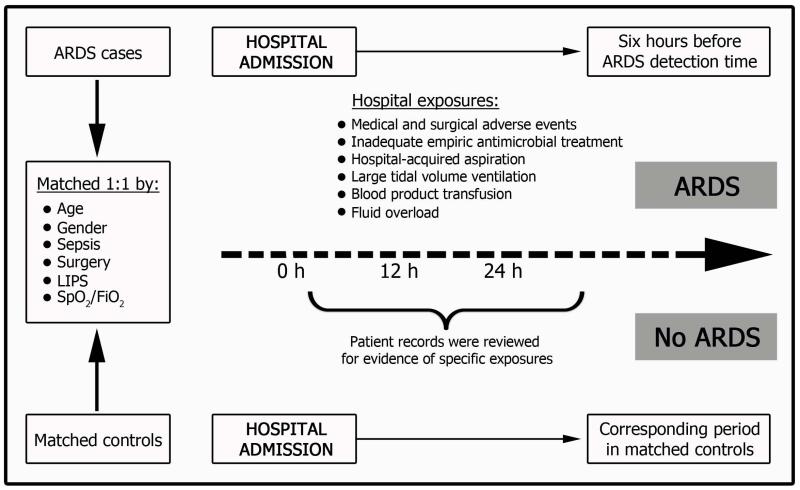
Trained study coordinators, masked to ARDS status and hospital mortality, determined the timing and intensity of hospital exposures through detailed review of the electronic health record and paper charts. Only hospital exposures occurring in a specific time range were counted (Figure 1). This range extended from hospital admission to 6 hours before meeting ARDS criteria in cases and a corresponding time period in controls (Figure 1). Standardized electronic data collection forms with embedded value range checks were used for all collected variables. After de-identification of case or control status, the statistician responsible for matching calculated the time range for data collection.
Figure 1.
Schematic of Data Collection. The investigators collected exposures occurring only from the time of hospital admission up until 6 hours before acute respiratory distress syndrome (ARDS) detection in cases and during the corresponding time period in controls. FIO2 indicates fraction of inspired oxygen; LIPS, lung injury prediction score; SpO2, peripheral oxygen saturation.
Definitions of Exposures
Adverse events were defined according to standard definitions for medical and surgical misadventures (10) (Supplemental Table E1) and were identified with the Institute for Healthcare Improvement Global Trigger Tool (18). To validate our abstracting procedure for adverse events, a blinded independent investigator reviewed a random sample of 62 subjects (about 7% of the total subjects). The interrater agreement on presence or absence of adverse events was 94%, with a κ value of 0.88 (95% CI, 0.75-1.0).Preventability was determined by clinical experts in surgical and medical critical care blinded to case-control status using de-identified clinical scenarios copied from the records without omission or addition.
Inappropriate empirical antimicrobial therapy was defined according to Kumar et al (19) by both time of administration and adherence to broadly accepted norms for empirical management of presenting syndromes. Infection and shock were defined according to standard definitions (4).
Hospital-acquired aspiration was defined by documentation of directly witnessed aspiration or by the presence of gastric contents suctioned from the endotracheal tube (20). Additional variables included inadequate head-of-bed elevation (<30° for >6 hours for ventilated patients). Delirium was defined as a positive Confusion Assessment Method for the ICU (CAM-ICU) score (21) documented by the bedside nurse. For patients hospitalized before the introduction of the CAM-ICU score (2001-2003), delirium was defined by documentation of the word agitation more than once in 12 hours. Nasogastric tube placement and intubation difficulty were determined from emergency department or ICU clinician procedure documentation.
Blood transfusion was defined as infusion of red blood cells, fresh frozen plasma, platelets, or cryoprecipitate. Fluid administration was defined as administration of any intravenous crystalloid or colloid. We also tracked the administration of selected medications previously identified as potential modifiers of ARDS development (Supplemental Table E2).
Matching Process and Statistical Analysis
Between January 1, 2001, and December 31, 2010, a total of 17,352 patients were admitted to the medical or surgical ICUs at Mayo Clinic in Rochester, Minnesota. Because of a decrease in the incidence of ARDS during the study period, we were able to enroll only 508 patients with ARDS, rather than the planned 600 (13). With a sample size of 414 matched cases and controls, and assuming the risk of ARDS increased 2-fold in patients with the risk factor, and with a 10% prevalence of a risk factor, the study had a 93% chance of finding a statistically significant effect. The presence or absence of 9 ARDS risk factors at admission was determined for all these patients and was used to form the pool of matching variables. From these baseline patient variables, controls were matched to cases on the basis of age, sex, surgery type, sepsis, SpO2/FIO2 ratio, and LIPS score. Baseline characteristics were compared using paired parametric and nonparametric testing as appropriate. Matching was carried out with a custom-programmed Mayo Clinic SAS (SAS Institute Inc, Cary, North Carolina) macro (% match, available at www.mayoclinic.org).
We used univariate and multivariate conditional logistic regression to evaluate the association between hospital exposures and the development of ARDS. Each case-control pair formed strata, and only the hospital-acquired risk factors were used in each model. Sensitivity analyses were performed to adjust for any remaining baseline differences and after exclusion of the years without controls (2001-2002). We calculated odds ratios and 95% CIs and performed hypothesis testing using PROC LOGISTIC (SAS version 9.1). Statistical significance was set at P<.05.
Results
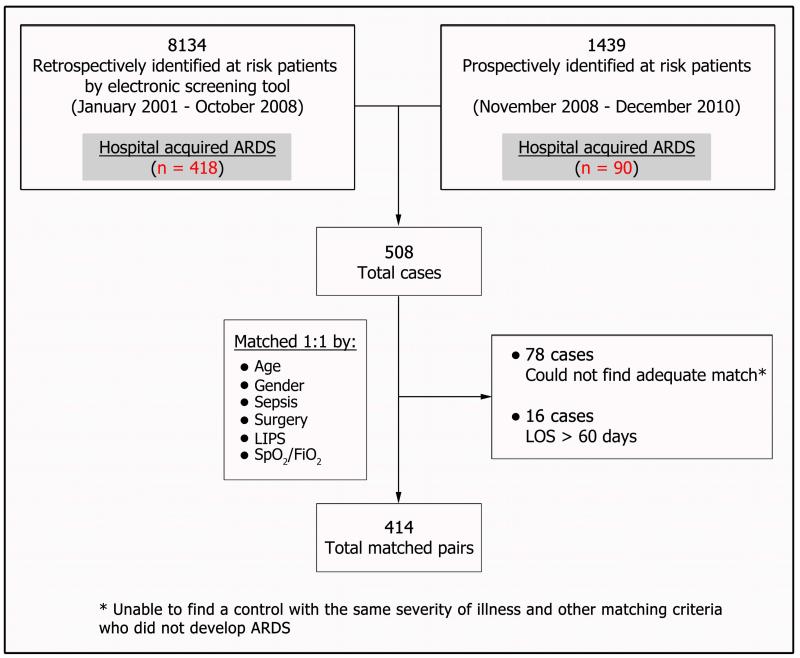
Of 508 incident cases of hospital-acquired ARDS, 414 were successfully matched (Figure 2). Seventy-eight cases could not be matched and 16 patients were excluded because they were hospitalized for more than 60 days. The median (interquartile range) time to ARDS development in cases was 58 (27-111) hours after hospital admission. Baseline characteristics collected at hospital admission were similar between cases and controls except alcohol use, hypoalbuminemia, and shock, which were more common among cases (Table 1).
Figure 2.
Study Flow Chart. AECC indicates American European Consensus Conference; ARDS, acute respiratory distress syndrome; FIO2, fraction of inspired oxygen; LIPS, lung injury prediction score; LOS, length of stay; SpO2, peripheral oxygen saturation.
Table 1.
Baseline Characteristics
| Group | |||
|---|---|---|---|
| Variable | ARDS Cases (n=414) |
Non-ARDS Controls (n=414) |
P Value |
| Age n (%) | 66 (17) | 66 (17) | … b |
| Men n (%) | 245 (59) | 245 (59) | … b |
| White n (%) | 373 (90%) | 381 (92%) | .33 |
| Risk factors for ARDS c | |||
| Sepsis b n (%) | 121 (29%) | 121 (29%) | … b |
| LIPS median(IQR) | 2 (1-3) | 2 (1-3) | … b |
| Pneumonia n (%) | 83 (20%) | 103 (25%) | .08 |
| Shock n (%) | 75 (18%) | 49 (12%) | .01 |
| Pancreatitis n (%) | 10 (2%) | 14 (3%) | .50 |
| Trauma n (%) | 24 (6%) | 16 (4%) | .24 |
| High-risk surgery n (%) | … b | ||
| Elective | 48 (12%) | 47 (11%) | |
| Emergency | 29 (7%) | 30 (7%) | |
| Pre-hospital aspiration n (%) | 14 (3%) | 14 (3%) | … b |
| Smoking n (%) | 102 (25%) | 107 (26%) | .36 |
| BMI, kg/m2 n (%) | 28 (14-83) | 28 (15-57) | .54d |
| Diabetes mellitus n (%) | 97 (23%) | 89 (22%) | .48 |
| Alcohol use disorder n (%) | 70 (17%) | 42 (10%) | .007 |
| Hypoalbuminemia (albumin <3.5 g/dL) n (%) |
175 (42%) | 108 (26%) | .001 |
| SpO2/FIO2 median (IQR) | 334.5 (194-448) | 336 (190-448) | … b |
Abbreviations: ARDS, acute respiratory distress syndrome; BMI, body mass index; FIO2, fraction of inspired oxygen; LIPS, lung injury prediction score; SpO2, peripheral oxygen saturation.
Matched variables.
At hospital admission.
Signed rank test.
Several hospital exposures were markedly more common among cases than controls (Table 2). In particular, inadequate antimicrobial therapy, medical and surgical adverse events, hospital-acquired aspiration, ventilation with potentially injurious tidal volumes, and greater volumes of blood product transfusion and fluid administration were more common in patients with ARDS (Table 2). The results were similar after adjustment for baseline differences (Table 3).
Table 2.
Comparison of Hospital Exposures and Adverse Events in ARDS Cases and Controls
| Group | ||||
|---|---|---|---|---|
| Exposure | ARDS Cases (n=414) |
Non-ARDS Controls (n=414) |
OR (95% CI) | P Value |
| Adverse eventsb | ||||
| Accidental cut, puncture, perforation, or hemorrhage n (%) |
27 (6.5) | 4 (1.0) | 12.5 (3.0-52.8) | <01 |
| Failure of sterile precautions n (%) | 4 (1.0) | 0 (0.0) | … | … |
| Retained foreign object n (%) | 0 (0.0) | 0 (0.0) | … | … |
| Instrument or apparatus failure n (%) | 8 (1.9) | 3 (0.7) | 5.3 (3.2-8.8) | <01 |
| Dosage failure n (%) | 113 (27.3) | 35 (8.5) | 3.5 (0.7-16.8) | .12 |
| Contaminated or infected blood, fluid, drug, or biological substance n (%) |
0 (0.0) | 1 (0.2) | … | … |
| Other and unspecified n (%) | 57 (13.8) | 8 (1.9) | 8.0 (3.6-17.6) | <01 |
| Any adverse event n (%) | 131 (31.6) | 47 (11.4) | 6.2 (4.0-9.7) | <01 |
| Infection control c (n=151) | ||||
| Inadequate empirical antimicrobials n (%) | 61 (40.4) | 30 (19.9) | 2.9 (1.7-5.2) | <01 |
| Time to adequate empirical antimicrobials, median (IQR) h |
7.6 (3.0-25.4) | 3.9 (2.4-8.4) | 1.3 (1.1-1.5) | <01 |
| Hospital-acquired aspiration and aspiration surrogates | ||||
| Hospital-acquired aspiration n (%) | 51 (12.3) | 1 (0.2) | 51 (7.1-369) | <01 |
| Nasogastric tube n (%) | 190 (45.9) | 60 (14.5) | 6.4 (4.2-9.9) | <01 |
| Head of bed elevation ≥30° (n=26 matched pairs)e n (%) |
16 (61.5) | 17 (65.4) | 0.8 (0.4-2.4) | .81 |
| Documented intubation difficulty n (%) | 32 (7.7) | 10 (2.4) | 3.2 (1.5-6.5) | <01 |
| Delirium n (%) | 92 (22.2) | 43 (10.4) | 2.4 (1.6-3.6) | <01 |
| Fluids for each liter infused, median (IQR) | ||||
| Crystalloid | ||||
| First 24 h | 1 (0.2,3.0) | 1.4 (0.4,3.4) | 0.9 (0.94-1.04) | 0.26 |
| Cumulative | 7.3 (3.8,11.6) | 3.6 (1.6,7.2) | 1.1 (1.09-1.17) | <01 |
| Colloid | ||||
| First 24 h | 0 (0.0,0.0) | 0 (0.0,0.0) | 1.5 (1.35-1.75) | 0.02 |
| Cumulative | 0.5 (0.0,2.4) | 0.0 (0.0,0.4) | 1.2 (1.02-1.37) | <01 |
| Blood product transfusion d | ||||
| Red blood cells n (%) | 163 (39.4) | 49 (11.8) | 1.4 (1.3-1.6) | <01 |
| Platelets n (%) | 51 (12.3) | 11 (2.7) | 2.0 (1.4-2.9) | <01 |
| Fresh frozen plasma n (%) | 85 (20.5) | 15 (3.6) | 1.4 (1.2-1.6) | <01 |
| Cryoprecipitate n (%) | 22 (5.3) | 2 (0.5) | 4.0 (1.5-11.2) | .007 |
| Mechanical ventilation parameters (n=29 pairs) | ||||
| TV by PBW, median (IQR) | 9 (7.3-10.8) | 7.7 (6.9-8.5) | 1.7 (1.1-2.6)H | .025 |
| TV>10 mL/kg PBW n (%) | 14 (48.3) | 6/26 (23.1)g | 7 (0.8-56.8) | .068 |
| FIO2 %, median (IQR) | 54 (43-62) | 55 (50-63) | 0.9 (0.9-1.0) | .92 |
| FIO2 >50 n (%) | 21 (72.4) | 15 (51.7) | 0.4 (0.1-1.2) | .12 |
Abbreviations: ARDS, acute respiratory distress syndrome; FIO2, fraction of inspired oxygen; OR, odds ratio; PBW, predicted body weight; TV, tidal volume.
Unadjusted OR
For more detailed definitions, refer to Supplemental Table E1.
Among those with infection, 151 pairs.
Any transfusion vs. none
Among those who were mechanically ventilated, 29 pairs.
Three controls were missing data.
per each mL of PBW above 6ml/kg PBW
Table 3.
Selected Exposures in ARDS Cases and Controls a
| Exposures | Cases and controls after removing 2001 -2002 (N=308 pair) |
Cases and controls after adjusting for baseline characteristics |
|---|---|---|
| OR (95% CI) P valueb | OR (95% CI) P valueb | |
| Any adverse events | 4.7 (3.0-7.6) P<0.001 | 6.5 ( 4.1-10.4) P<0.001 |
| Inadequate empiric antimicrobial | 2.5 (1.3-4.7) P = 0.006 | 3.6 (2.0-6.7) P<0.001 |
| Aspiration | 34.0 (4.7-248.4) P <0.001 | 52.0 (7.1-383.2) P<0.001 |
| Red blood cellsc | 1.4 (1.2-1.6) P <0.001 | 1.4 (1.2-1.5) P<0.001 |
| Fresh frozen plasmac | 1.4 (1.2-1.6) P <0.001 | 1.4 (1.2-1.6) P<0.001 |
| Plateletsc | 2.9 (1.6-5.5) P <0.001 | 2.0 (1.4-2.9) P<0.001 |
| TV -PBWd | 1.3 (0.82-2.2) P 0.25 | 2.1 (1.1-4.1) P = 0.025 |
Abbreviations: ARDS, acute respiratory distress syndrome; OR, odds ratio; PBW, predicted body weight; TV, tidal volume
For these exposures, differences between cases and controls remained statistically significant after adjustment for differences in baseline characteristics (shock, alcohol, and hypoalbuminemia) and after exclusion of 2001 and 2002 cases (except for the TV-PBW).
Multivariate logistic regression
Any transfusion vs. none
Per each mL of PBW above 6ml/kg PBW
Exposure to antiplatelet agents during the at-risk period was associated with a decreased risk of ARDS (odds ratio, 0.9; 95% CI, 0.8-0.9). Conversely, exposures to opioids, benzodiazepines, antacids, and furosemide were more common among cases than controls (Supplemental Table E3).
In subgroup analysis, we assessed the preventability of the adverse events as determined by blinded clinician review. Of the overall 40 identified surgical adverse events, 28 (70%) were deemed preventable and 11 (28%) non-preventable. Of a random sample of 25 medical adverse events (18% of the 138 total medical adverse events), 17 (68%) were deemed preventable and 5 (20%) non-preventable. In 1 of the surgical and 3 of the medical adverse events, there was insufficient detail to draw this distinction.
The incidence of hospital exposures and hospital-acquired ARDS decreased during the study period (Supplemental eFigure1). Both hospital mortality (36% vs 6%; P<.001) and length of stay (median [interquartile range], 17 [10-28] vs. 5 [2-8] days; P<.001) were higher among cases than controls.
Discussion
In this population-based study, we observed a strong association between certain hospital exposures and the development of ARDS among at-risk patients. Preventable medical and surgical adverse events, inadequate antimicrobial therapy, larger volumes of blood product and intravenous fluid administration, and documented pulmonary aspiration were all substantially associated with ARDS development. These findings suggest that ARDS results from a combination of non-modifiable primary risk factors (e.g. illness or injury type) and potentially modifiable secondary insults (22). Guided by this “2-hit” paradigm, quality improvement efforts to prevent modifiable second-hit exposures may decrease the risk of hospital-acquired ARDS and the overall incidence of this syndrome (6). A preliminary multicenter study proved the feasibility of introducing a checklist for lung injury prevention, intended to standardize the early application of certain critical care best practices (23). When applied to patients at high risk, this approach may decrease the incidence of ARDS, for which no treatment options exist.
As in prior studies, our cases were more likely than controls to have been exposed to certain adverse events (5, 9, 10). Most of these were deemed preventable. Patients admitted to an ICU during their hospital stay are generally more likely to experience adverse events (24). The overall incidence in our cohort (about 22%) aligns with previous estimates (25, 26), which have ranged from 20.2% (27) to 26.8% (physician observers) (28). Unlike previous investigators (29), we did not distinguish between diagnostic and management errors, although most events in our study were caused by disrupted treatment plans, particularly for time-sensitive measures (eg, antimicrobial administration). Previous work identified a correlation between the introduction of ICU quality improvement initiatives and a decrease in hospital-acquired ARDS (6, 30). Taken together, these findings suggest that attention to health care delivery factors may be protective, particularly for high-risk patients.
The association between ARDS and delayed antimicrobial administration in our data has been described by Iscimen and colleagues (9); delays in early goal-directed resuscitation also predicted ARDS development. Additionally, Kumar and colleagues (19) found that delayed or inappropriate empirical antimicrobial therapy among patients with septic shock was both common and strongly associated with mortality.
Multiple groups have described the development of ARDS after blood product administration (8). Recent studies support the effectiveness of transfusion decision support (5), and a change in blood product procurement to avoid alloimmunization was associated with the decrease of transfusion-associated ARDS (31). The association between fluid balance and ARDS development is intriguing but the study design did not allow us to adequately evaluate the specifics of timing and composition of the fluid or to exclude confounding and reverse causation.
The association between aspiration of gastric contents and ARDS is well documented for out-of-hospital aspiration (20) and can probably be extrapolated to in-hospital aspiration (9). Our data identified a higher frequency of aspiration among patients who developed ARDS; only 1 control patient had a documented aspiration event. Previous work has suggested a role for care delivery factors in limiting aspiration among ICU patients. A randomized trial of different positions for intubated patients was stopped early when interim analysis revealed a significantly lower incidence of pneumonia among semirecumbent patients (32). In our population, head elevation for ventilated patients was used almost uniformly for both cases and controls and was not significantly associated with ARDS. The association between exposure to antiplatelet agents and decreased risk of ARDS has been previously described (33) and is currently being tested in a phase II clinical trial by the US Critical Illness and Injury Trials Group (NCT01504867)
Case-control studies are prone to biases in selection, recall, exposure ascertainment, and prevalence, among others. Our study is limited by the use of single-center data and a predominantly white population, although the targets for optimized critical care delivery suggested by our data are likely race-neutral. We used 1:1 matching to minimize confounding by balancing on the basis of ARDS risk factors present at admission. This also focused the study on hospital-acquired ARDS risk factors. Moreover, we consolidated the list of exposures by eliminating those lacking sufficient accuracy and precision and those that were redundant or of little potential relevance to the outcome of interest. We did not match for all previously described ARDS risk modifiers, some of which were statistically different at baseline: alcohol use disorder (34), hypoalbuminemia (35), and shock (4). However, the results remained the same after adjustment for these baseline differences (Table 3).
More cases than controls were identified in the early years of the study. Finding a perfect control for each case was challenging and matching by ARDS risk factors and the exact year of enrollment was not feasible. Increasing numbers of critically ill patients, decreased incidence of ARDS and availability of electronic records favored larger numbers of eligible controls in the later years. Controls were available from all years other than 2001-2002, and when those years were excluded in a sensitivity analysis, the results remained unchanged. The study design did not allow us to assess the independent effect of each specific exposure. These are likely to be highly correlated as the patient exposed to accidental puncture of the bowel is likely to receive more fluid and transfusion. The parallel decrease in both exposures of interest and hospital-acquired ARDS strengthens the observed associations. However, the results are considered hypothesis generating and the more robust interventional studies are being planned under the auspice of US Critical Illness and Injury Trials Group (evaluation of CLIP – Checklist for Lung Injury Prevention)(36).
The extraction of potential errors and adverse events from the medical record is limited by the lack of reporting and nomenclature standards and substantial perceived disincentives to documentation (37). In a 2005 retrospective chart review, Lehmann and colleagues (38) reported that the associated clinical documentation contained the word iatrogenic for only 2% of patients admitted to the ICU as a result of an iatrogenic event. A substantial fraction of adverse events are likely undocumented, and identification of surrogate markers can be challenging. For example, aspiration may be more likely to be documented in patient who subsequently developed ARDS than in those who did not, potentially biasing the results. Efforts to identify adverse events using International Classification of Diseases, Ninth Revision (ICD-9) codes have been complicated by their limited meaning and the variable accuracy of coding personnel. For example, multiple investigators (including our group) have found that ICD-9 codes are more reliable for surgical than medical misadventures and that administrative databases lack standardized clinical definitions for associated diagnoses (39). To minimize these limitations in our study, the Institute for Healthcare Improvement Global Trigger Tool was applied by trained investigators with a medical background and confirmed by expert physicians, blinded to both ARDS development and hospital mortality.
Conclusions
This population-based study suggests that potentially preventable hospital exposures significantly contribute to development of ARDS in at-risk patients. Disciplined attention to health care delivery factors and the avoidance of “second hits” is advised to limit the development of ARDS and to improve safety and outcome for critically ill patients. The effectiveness of this approach needs to be prospectively evaluated. Such an approach would present very little risk and, given the lack of treatment options after ARDS has developed, critically ill patients receiving this standardized care would have much to gain.
Supplementary Material
Acknowledgments
Andrew C. Hanson helped analyze the data.
Tami Krpata helped in data collection
Shonie Buenvenida, R.N. helped in Redcap designing
Gregory Wilson, R.R.T research coordinator
A Ahmed, R Kashyap, O Gajic has access to the data
Funded in part by Mayo Foundation and National Library of Medicine RC1 LM10468Z-01
Dr. Gajic has received funding from the National Institutes of Health.
Abbreviations
- ARDS
acute respiratory distress syndrome
- CAM
Confusion Assessment Method
- FIO2
fraction of inspired oxygen
- ICD-9
International Classification of Diseases, Ninth Revision
- ICU
intensive care unit
- LIPS
lung injury prediction score
- SpO2
peripheral oxygen saturation
Footnotes
Conflict of interest: None.
The remaining authors have disclosed that they do not have any potential conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Phua J, Badia JR, Adhikari NK, et al. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. American Journal of Respiratory and Critical Care Medicine. 2009;179(3):220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Herridge MS. Epidemiology and Outcomes of Acute Lung Injury 10.1378/chest.06-1976. Chest. 2007;131(2):554–562. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 4.Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. American Journal of Respiratory and Critical Care Medicine. 2011;183(4):462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yilmaz M, Keegan MT, Iscimen R, et al. Toward the prevention of acute lung injury: protocol-guided limitation of large tidal volume ventilation and inappropriate transfusion. Crit Care Med. 2007;35(7):1660–1666. doi: 10.1097/01.CCM.0000269037.66955.F0. quiz 1667. [DOI] [PubMed] [Google Scholar]
- 6.Li G, Malinchoc M, Cartin-Ceba R, et al. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. American Journal of Respiratory and Critical Care Medicine. 2011;183(1):59–66. doi: 10.1164/rccm.201003-0436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisner MD, Thompson T, Hudson LD, et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine. 2001;164(2):231–236. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- 8.Gong MN, Thompson BT, Williams P, et al. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33(6):1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 9.Iscimen R, Cartin-Ceba R, Yilmaz M, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36(5):1518–1522. doi: 10.1097/CCM.0b013e31816fc2c0. [DOI] [PubMed] [Google Scholar]
- 10.TenHoor T, Mannino DM, Moss M. Risk Factors for ARDS in the United States: Analysis of the 1993 National Mortality Followback Study. Chest. 2001;119(4):1179–1184. doi: 10.1378/chest.119.4.1179. [DOI] [PubMed] [Google Scholar]
- 11.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. In: New England Journal of Medicine. 2006:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 12.Jia M.Eng X, Malhotra A, Saeed M, et al. Risk Factors for Acute Respiratory Distress Syndrome in Patients Mechanically Ventilated for Greater Than 48 Hours. Chest. 2008 chest.07-1121. [Google Scholar]
- 13.Thakur SJ, Trillo-Alvarez CA, Malinchoc MM, et al. Towards the prevention of acute lung injury: a population based cohort study protocol. BMC Emerg Med. 2010;10:8. doi: 10.1186/1471-227X-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herasevich V, Pickering BW, Dong Y, et al. Informatics infrastructure for syndrome surveillance, decision support, reporting, and modeling of critical illness. Mayo Clin Proc. 2010;85(3):247–254. doi: 10.4065/mcp.2009.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American Journal of Respiratory and Critical Care Medicine. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 16.Shari G, Kojicic M, Li G, et al. Timing of the onset of acute respiratory distress syndrome: a population-based study. Respir Care. 2011;56(5):576–582. doi: 10.4187/respcare.00901. [DOI] [PubMed] [Google Scholar]
- 17.Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 18.Resar RK, Rozich JD, Simmonds T, et al. A trigger tool to identify adverse events in the intensive care unit. Jt Comm J Qual Patient Saf. 2006;32(10):585–590. doi: 10.1016/s1553-7250(06)32076-4. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Ellis P, Arabi Y, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136(5):1237–1248. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 20.Pepe PE, Potkin RT, Reus DH, et al. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144(1):124–130. doi: 10.1016/0002-9610(82)90612-2. [DOI] [PubMed] [Google Scholar]
- 21.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) Jama. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 22.Villar J, Slutsky AS. Is acute respiratory distress syndrome an iatrogenic disease? Crit Care. 2010;14(1):120. doi: 10.1186/cc8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HBG MJ, Dinkels M, Hou P, Talmor DS, Gajic O, Gong MN. Checklist For Lung Injury Prevention (CLIP): A Pilot Study On Implementation Across Multiple Hospitals And Multiple Clinical Areas. Am J Resp Crit Care. 2012:A6567. [Google Scholar]
- 24.Andrews LB, Stocking C, Krizek T, et al. An alternative strategy for studying adverse events in medical care. Lancet. 1997;349(9048):309–313. doi: 10.1016/S0140-6736(96)08268-2. [DOI] [PubMed] [Google Scholar]
- 25.Bracco D, Favre JB, Bissonnette B, et al. Human errors in a multidisciplinary intensive care unit: a 1-year prospective study. Intensive Care Med. 2001;27(1):137–145. doi: 10.1007/s001340000751. [DOI] [PubMed] [Google Scholar]
- 26.Forster AJ, Kyeremanteng K, Hooper J, et al. The impact of adverse events in the intensive care unit on hospital mortality and length of stay. BMC Health Serv Res. 2008;8:259. doi: 10.1186/1472-6963-8-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothschild JM, Landrigan CP, Cronin JW, et al. The Critical Care Safety Study: The incidence and nature of adverse events and serious medical errors in intensive care. Crit Care Med. 2005;33(8):1694–1700. doi: 10.1097/01.ccm.0000171609.91035.bd. [DOI] [PubMed] [Google Scholar]
- 28.Garrouste-Orgeas M, Timsit JF, Vesin A, et al. Selected medical errors in the intensive care unit: results of the IATROREF study: parts I and II. American Journal of Respiratory and Critical Care Medicine. 2010;181(2):134–142. doi: 10.1164/rccm.200812-1820OC. [DOI] [PubMed] [Google Scholar]
- 29.Dubois RW, Brook RH. Preventable deaths: who, how often, and why? Ann Intern Med. 1988;109(7):582–589. doi: 10.7326/0003-4819-109-7-582. [DOI] [PubMed] [Google Scholar]
- 30.Ciesla DJ, Moore EE, Johnson JL, et al. Decreased progression of postinjury lung dysfunction to the acute respiratory distress syndrome and multiple organ failure. Surgery. 2006;140(4):640–647. doi: 10.1016/j.surg.2006.06.015. discussion 647-648. [DOI] [PubMed] [Google Scholar]
- 31.Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119(7):1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354(9193):1851–1858. doi: 10.1016/S0140-6736(98)12251-1. [DOI] [PubMed] [Google Scholar]
- 33.Kor DJ, Erlich J, Gong MN, et al. Association of prehospitalization aspirin therapy and acute lung injury: results of a multicenter international observational study of at-risk patients. Crit Care Med. 2011;39(11):2393–2400. doi: 10.1097/CCM.0b013e318225757f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss M, Bucher B, Moore FA, et al. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. In: Journal of the American Medical Association. 1996:50–54. [PubMed] [Google Scholar]
- 35.Mangialardi RJ, Martin GS, Bernard GR, et al. Ibuprofen in Sepsis Study Group Hypoproteinemia predicts acute respiratory distress syndrome development, weight gain, and death in patients with sepsis. Crit Care Med. 2000;28(9):3137–3145. doi: 10.1097/00003246-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 36.USCIITG [accessed Jan 14 2013];The US Critical Illness and Injury Trials Group. 2013 http://usciitg.org/index.php/usciitg-proof/ongoing-projects.
- 37.Stockwell DC, Kane-Gill SL. Developing a patient safety surveillance system to identify adverse events in the intensive care unit. Crit Care Med. 2010;38:S117–S125. doi: 10.1097/CCM.0b013e3181dde2d9. [DOI] [PubMed] [Google Scholar]
- 38.Lehmann LS, Puopolo AL, Shaykevich S, et al. Iatrogenic events resulting in intensive care admission: frequency, cause, and disclosure to patients and institutions. Am J Med. 2005;118(4):409–413. doi: 10.1016/j.amjmed.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Hougland P, Nebeker J, Pickard S, et al. Using ICD-9-CM Codes in Hospital Claims Data to Detect Adverse Events in Patient Safety Surveillance. In: Henriksen K, Battles JB, Keyes MA, Grady ML, editors. Advances in Patient Safety: New Directions and Alternative Approaches. Vol. 1. Rockville (MD): 2008. Assessment. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.