Abstract
Polyploid giant cancer cells (PGCCs) have been observed by pathologists for over a century. PGCCs contribute to solid tumor heterogeneity, but their functions are largely undefined. Little attention has been given to these cells, largely because PGCCs have been generally thought to originate from repeated mitosis/cytokinesis failure and have no capacity for long-term survival and cell proliferation. Here we report that we successfully purified and cultured PGCCs from human ovarian cancer cell lines and primary ovarian cancer. These cells are highly resistant to oxygen deprivation and could form through endoreduplication or cell fusion, generating regular-sized cancer cells quickly through budding or burst. They are positive for normal and cancer stem cell markers, divided asymmetrically and cycled slowly. They can differentiate into adipose, cartilage, and bone. A single PGCC formed cancer spheroids in vitro and generated tumors in immunodeficient mice. PGCC-derived tumor gained a mesenchymal phenotype with increased expression of cancer stem cell markers CD44 and CD133 and become more resistant to the treatment of cisplatin. Together, our results reveal that the PGCCs present a resistant form of human cancer generated in response to hypoxia stress and can contribute to generation of cancer stem-like cells and play a fundamental role in regulating tumor heterogeneity, stemness, and chemoresistance in human cancer.
Keywords: Polyploid giant cancer cells, Cancer stem cells, Cell fusion, Asymmetric cell division, Epithelial-mesenchymal transition
Introduction
Almost all human tumors display a certain level of heterogeneity(1, 2). Among the many histopathologic features of human solid tumors is the presence of large atypical cancer cells with multiple copies of DNAs, which is referred here to as polyploid giant cancer cells (PGCCs) (3). PGCCs vary in number and usually become prominent in high pathologic grades and late disease stages, or after chemotherapy. Nuclear features of PGCCs have been used to predict prognosis for ovarian cancer (4). The giant cells have often been thought to originate from repeated mitosis/cytokinesis failure or as intermediate products of genomic instability (5, 6). As a result there have been neither reports of their isolation in culture nor their detailed characterization, and the function, mechanisms of formation and role of PGCCs in the development of human tumors is largely undefined.
Hypoxia is known to play an important role in normal tissue and tumor development (7, 8) and is associated with the formation and maintenance of cancer stem cells (9); it promotes the stem-like phenotype and tumorigenesis (10). Cobalt chloride (CoCl2) has been used as a hypoxia mimic in cell culture and can activate hypoxia-mediated signaling pathways (11). Here we report the successful purification and stable maintenance of PGCCs from cancer cell lines and human primary ovarian tumors with the use of CoCl2. Our studies of stably passaged PGCCs revealed many unexpected biologic features. The division patterns of PGCCs are remarkably similar to that of simple organisms. These results strongly suggest that PGCCs represent a dormant form of cancer cells in a stress-induced cancer cell life cycle that can actively contribute to the tumor growth through the generation of cancer stem-like cells.
Results
PGCCs Exist in Multiple Cancer Cell Line
Scattered giant PGCCs were observed in HEY, SKOv3 and MDA-MB-231 lines grown in regular media. Addition of CoCl2 into the medium selectively killed the regular-sized cancer cells but not PGCCs. Typical PGCCs from HEY and MDA-MB-231 cells displayed morphology similar to that of neuron. PGCCs of SKOV3 were round and lack of branches (Fig. 1A). These PGCCs from CoCl2 have identical genotype with the parental cell lines, which have been confirmed by short tandem repeat (STR) DNA profiles analysis (Table S1). Furthermore, 10 ovarian cancer cell lines including two newly established lines from primary culture and breast cancer cell line MCF-7, phoenix and 293T cells (Fig. S1A) were treated with CoCl2. All of these cell lines showed enriched or purified PGCCs following CoCl2 treatment. PGCCs can also be induced by physiological hypoxia when cultured with 0.1% oxygen (Fig. S1B). The common morphological characteristics of PGCCs were the large size and giant nucleus. We define the PGCC as a cancer cell that is at least three times larger in size as compared with that of parental cancer cells. The mean size of the PGCCs was 3–10 times larger than those of the regular cancer cells.
Figure 1.
A. Morphologic characteristics of regular cells and PGCCs from HEY, SKOv3 and MDA-MB-231 before and after CoCl2 treatment. PGCCs were mixed with regular-sized cells before CoCl2 treatment (Black arrows) (10×). B. Flow cytometry analysis before and after CoCl2 treatment. C. X chromosome fluorescence in situ hybridization of PGCCs and diploid control HEY cells (a). D. β-galactosidase staining. No staining was observed in HEY (b), SKOv3 (c) and MDA-MB-231 (d) PGCCs compared with positive staining from senescent fibroblasts (a) (10×). E. Western blot analysis of CD133, ElF-2α and HIF-1α expression in HEY and SKOv3 PGCCs, and control cells.
Characterization of PGCCs
Flow cytometry analysis indicated that there were more G2/M phase cells after CoCl2 treatment (Fig. 1B). The presence of multiple DNA copies in individual PGCCs was confirmed by X chromosome fluorescence in situ hybridization (Fig. 1C). PGCCs were not senescent, as shown by negative beta-Galstaining (Fig. 1D). Since CoCl2 is a hypoxia mimic by stabilizing the hypoxia-inducible transcription factor 1 alpha (HIF1alpha) (12), we examined the expression of HIF1alpha. As shown in Figure 1E, HIF-1alpha expression was markedly elevated in PGCCs as compared with levels in regular HEY and SKOv3 cells. In addition, elongation initiation factor 2 alpha (EIF2alpha) expression is down-regulated in PGCCs (Fig. 1E), suggesting that protein translation may be turned off to keep slow cell cycle. Furthermore, CD133 expression is markedly increased, suggesting that PGCCs may gain the properties of cancer stem-like cells.
PGCCs Undergo Asymmetric Division and Cycle Slowly
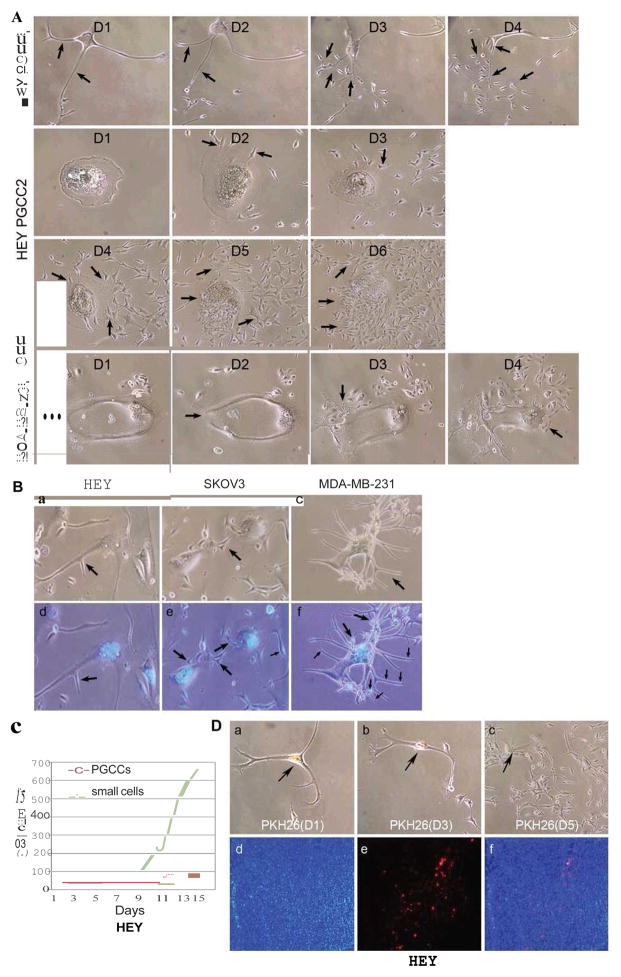
We have observed two asymmetric cell division patterns in PGCCs: budding and bursting, individually or in combination. Budding of daughter cells from PGCCs usually occurred from the branches of PGCCs (Fig. 2A, HEY PGCC1) and the body of PGCCs that contain multiple nuclei and followed by the burst release of numerous small daughter cells (Fig. 2A, HEY PGCC2, and MDA-MB-231 PGCC). To further confirm the budding division from PGCCs, we performed a time time-lapse imaging analysis on HEY PGCC and budding of small daughter cells from PGCC occurred from the body of PGCCs (Fig. S2A and S2B). These results suggest that PGCCs produced daughter cells via the mechanisms for growth and division of simple organisms such as yeasts or other unicellular organisms (13, 14).
Figure 2.
A. Patterns of cell division of HEY and MDA-MB-231 PGCCs. HEY and MDA-MB-231 PGCC generated small-sized daughter cells via budding and bursting (black arrows) over a 4-day (HEY PGCC1 and MDA-MB-231) and 6-day period (HEY PGCC2) (10×). B. Nuclear morphology of HEY, SKOv3 and MDA-MB-231 PGCCs with budding daughter cells (black arrows) stained with Hoechst 33342. (a and d) Giant Multinucleated cells of HEY PGCCs and budding daughter cells (10×). (b and e) Budding daughter cells from SKOv3 PGCCs (10×). (c and f) Giant nucleated cells of MDA-MB-231 PGCCs and budding daughter cells (10×). C. Cell counts from HEY PGCCs. The total number of small-sized cells and PGCCs were counted for 15 days in a T25 flask after treatment with 450 μM CoCl2 for 72 hours. D. Slow-cycling nature of HEY PGCCs. (a–c) Single HEY PGCCs stained with PKH26 for 5 days (10×). (d–f) PKH26 fluorescence was detected in some PGCCs but not daughters in xenografts from HEY PGCCs (10×).
Two types of PGCCs were observed after staining: giant nucleated cells or multinucleated giant cells. Figure 2B showed the cell budding from the two types of PGCCs in HEY, SKOv3 and MDA-MB-231. We performed total cell counts of the purified HEY PGCCs every 2 days for 15 days. The total number of small cells increased minimally in the first 8 days; a large number of small cells started to bud out on the 9th day, and then there was an explosive increase in the total number of cells (Fig. 2C). To further characterize the nature of the division of PGCCs, we labeled PGCCs with PKH26, which is usually lost quickly in rapidly dividing cells (15). We monitored the daughter cells from PGCCs in vitro and in vivo. PGCCs and daughter cells stained with PKH26 were observed over a period of 5 days. The PGCCs showed continued fluorescence on day 5 while the fluorescence was largely absent in the daughter cells (Fig. 2D and Fig. S3A). Nocodazole (cell cycle inhibitor) was used to inhibit PGCCs to generate the daughter cells and the fluorescent dye was maintained in PGCCs even after day 10 (Fig. S3B). In vivo, we subcutaneously injected 3000 PGCCs labeled with PKH26 into nude mice and examined frozen tumor sections for fluorescence. A few PGCCs with red fluorescence were observed in the tumor tissue, but most of tumor cells had lost fluorescence (Fig. 2D), demonstrating that the PGCCs were also slow-cycling in vivo.
PGCCs Can Generate Tumor Spheroids
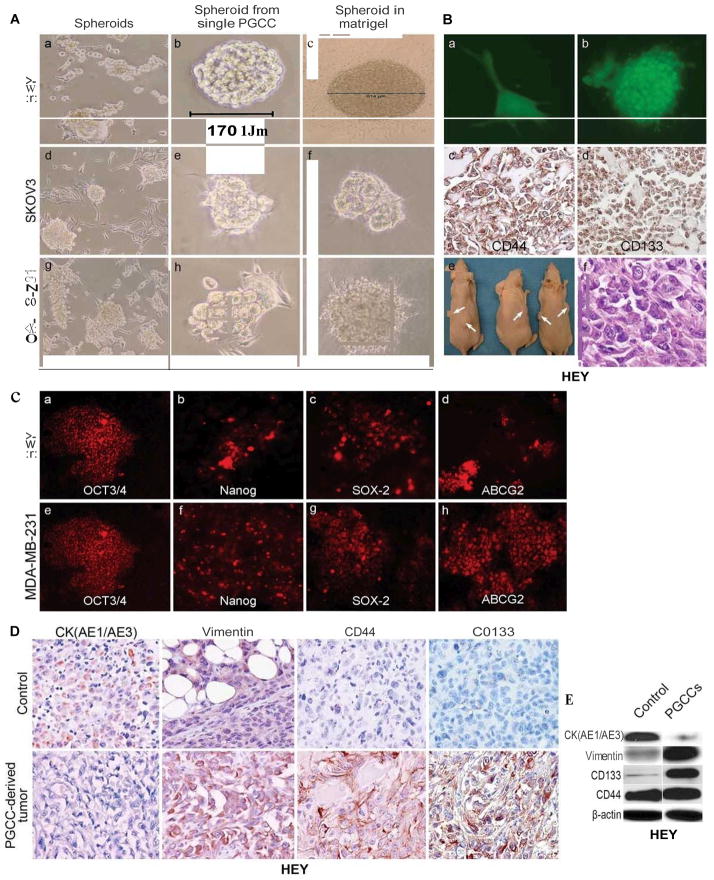
When PGCCs were cultured in stem cell medium; many spheroids of HEY, SKOv3 and MDA-MB-231 appeared in the flask (Fig. 3A). Spheroids from a single PGCC of HEY, SKOv3 and MDA-MB-231 can be observed when grown in stem cell medium and Matrigel (Fig. 3A). To further demonstrate the ability of each single PGCC to form spheroids, we serially diluted HEY PGCCs in Matrigel with 3–5 PGCCs in each well of a 24-well plate. Single PGCCs can form spheroids on the 5th day after seeding in Matrigel (Fig. 3B-a and -b). When spheroids from HEY PGCCs embedded in paraffin blocks were subjected to immunohistochemical staining, they were positive for CD44 and CD133 (Fig. 3B-c and -d). To determine the tumorigenicity of PGCCs in vivo, we subcutaneously injected a single spheroid derived from a single HEY PGCC into nude mice. Six tumors formed in all 3 nude mice with two sites injection for each mouse. Histological examination showed that the resulting tumor cells had a higher nucleus-to-cytoplasm ratio and were more mesenchymal (Fig. 3B). The spheroids from HEY and MDA-MB-231-derived PGCCs showed scattered positive cells for OCT3/4, Nanog, SOX-2, and ABCG2 (Fig. 3C), suggesting that at least part of these cells within these spheroids may have stem-like properties. The PGCC spheroid-derived tumors had lower cytokeratin expression and higher vimentin expression than the control (Fig. 3D). Results of immunohistochemical staining showed that the PGCC spheroid-derived tumors were positive for CD44 and CD133, while most of the tumor cells generated from regular HEY injection were negative (Fig. 3D). The expression of cytokeratin, vimentin, CD44 and CD133 between PGCCs and regular cancer cells is further validated by western blot (Fig. 3E).
Figure 3.
A. Formation of spheroids from PGCCs. (a, d, and g) Spheroids formed from HEY, SKOv3 and MDA-MB-231 PGCCs in stem cell media after the removal of CoCl2 (10×). Please refer Fig. 1A for control pictures of PGCCs in the presence of CoCl2. (b, e and h). Growth of spheroids from a single HEY, SKOv3 and MDA-MB-231 PGCCs in stem cell medium (10×). (c, f and i) Growth of spheroids from a single HEY, SKOv3 or MDA-MB-231 PGCC in Matrigel (10×). B. Tumor formation from single spheroid derived from a single PGCC. (a) Growth of a single eGFP-labeled HEY PGCC in Matrigel (10×). (b) Growth of spheroids from the single PGCC of (a) (10×). (c and d) Representative pictures of immunohistochemical staining against CD44 (c) and CD133 (d) on sections from the spheroids of (b) (10×). (e) A single spheroid (6/6) can form a tumor in mice. (f) H&E staining showed the morphologic characteristics of tumor from (e) (20×). C. Expression of stem cell markers in cancer spheroids. (a–h) HEY and MDA-MB-231 spheroids stained with antibodies against OCT3/4 (a and e), Nanog (b and f), SOX-2(c and g), and ABCG2 (d and h) (10×). D. Expression of cytokeratin (AE1/AE3), vimentin, CD44 and CD133 in tumors derived from control HEY cells and a single spheroid (20×). E. Western blot analysis of vimentin, CK (AE1/AE3), CD133, and CD44 in control HEY and PGCCs.
Alteration of Cell Cycle-related Protein Expression in PGCCs
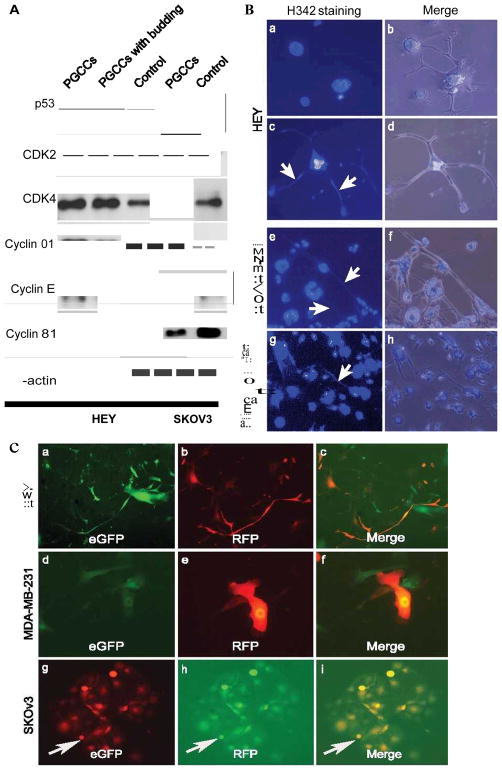
We examined the expression of a panel of proteins involved in cell cycle regulation in PGCCs from HEY and SKOv3. The expression of p53 protein is elevated in PGCCs and PGCCs with budding. Expression level of cyclin E, and cyclin D1 was markedly elevated in purified HEY PGCCs compared with regular HEY cells. HEY PGCCs with budding had the highest expression of CDK2 and cyclin B1. For SKOv3, increased expression level of CDK2 and cyclin D1 was found in purified SKOv3 PGCCs while the level of cyclin B1, cyclin E, and CDK4 expression was decreased in PGCCs as compared with control SKOv3 (Fig. 4A). These results demonstrate that PGCCs have a distinct protein expression involved in cell cycle regulation.
Figure 4.
A. Western blot analysis of protein expression related with cell cycle. The total proteins in purified PGCCs, PGCCs with 70% budding cells, control HEY and SKOv3 PGCCs, control SKOv3 cancer cells were extracted and subject for western blot analysis. B. Visualization of DNA transport via branches in PGCCs after staining with Hoechst 33342. (a and b) Lack of DNA was indicated by an absence of H342staining in the HEY PGCC branches at 8 hours after trypsin digestion (10×). (c and d) DNA was reappearance in the branches of PGCCs at 4th days after trypsin digestion (white arrow points, 10×). (e and f) DNA transportation in the branches from MDA-MB-231 PGCCs (white arrow heads) (H342staining, 10×). (g and h) Giant nucleated cells of PGCCs and DNA transportation in the branches from human ovarian cancer primary culture (white arrow heads) (H342staining, 10×). C. Generation of PGCCs via cell fusion as indicated by yellow fluorescence (10×). (a–c) Formation of HEY PGCCs fused by regular HEY cells labeled with eGFP and regular HEY cells labeled RFP (10×). (d–f). Formation of MDA-MB-231 PGCCs fused by regular MDA-MB-231 cells labeled with eGFP and regular MDA-MB-231 cells labeled RFP (10×). (g–i) Formation of SKOv3 PGCCs fused by regular SKOv3 cells labeled with eGFP and regular SKOv3 cells labeled RFP (10×).
DNA Transport of PGCC and PGCC Formation by Cell Fusion
Since PGCCs produce daughter cells via budding from the branches, we examined how DNA is transported in PGCCs. We stained these cells with Hoechst33342 to trace the branches of PGCCs for the presence or absence of DNA after trypsin digestion. DNA was not found in the branches of HEY PGCCs 8 hours after digestion (Fig. 4B-a and -b). However, DNA reappeared in the branches 4 days after trypsin digestion (Fig. 4B-c and -d). These results demonstrate that the branches of PGCCs can serve as vessels for DNA transport. DNA transportation in the branches can also be observed in MDA-MB-231 PGCCs and PGCCs from human ovarian cancer primary culture (Fig. 4B-g and -h).
It has also been reported that giant cells can be formed through cell fusion (16–19). To determine whether cell fusion is involved in PGCC formation, we mixed eGFP-labeled regular HEY cells with regular HEY cells stained with PKH26 or labeled with RFP; the mixed cells were co-cultured and then treated with CoCl2. Cell fusion was examined by fluorescence microscopy. As shown in Figures 4C, yellow fluorescence indicating cell fusion was clearly visible. High concentration of CoCl2 seclectively kill the regular-sized cells; while low concentration can induce the formation of PGCCs through cell fusion. PGCC formation via cell fusion can also been observed in MDA-MB-231, SKOv3 and labeled with eGFP and RFP (Fig. 4C). Fusion-created PGCCs were approximately 10–20% of the total number of PGCCs present in the cultures.
PGCCs are highly tumorigenic
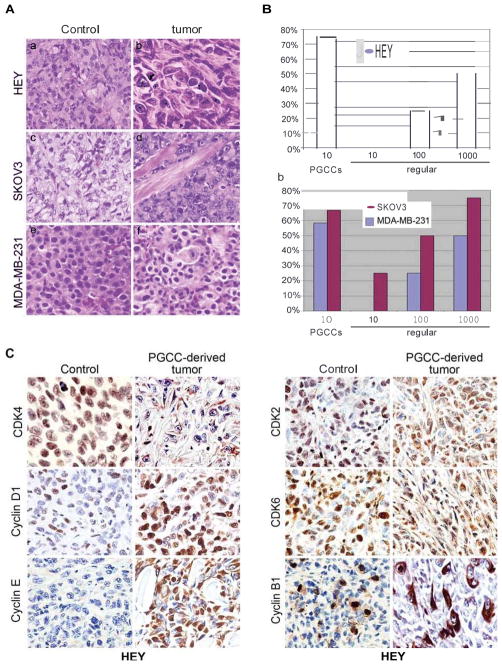
We isolated a single PGCC by means of serial dilution and injected into them into mice. The resulting tumor was excised and examined for histopathology. The tumors formed from HEY, SKOv3 and MDA-MB-231 PGCCs injection display mesenchymal changes when compared with the control (Fig. 5A). As shown in Table 1, tumors formed in 3 of 4 nude mice injected with 10 HEY PGCCs, 7 of 12 nude mice injected with a single SKOv3 PGCC and 8 of 12 nude mice injected with a single MDA-MB-231 PGCC. The rates of tumor formation from PGCCs injection are higher than their regular cancer cell controls when 10, 100 and 1000 regular cells were injected (Fig. 5B) (Table 1). Tumor formation ability was also determined with a different mouse strain; we examined the tumorigenicity in NOD.CS17-Prkdc SCID mice. By the 25th day, tumors formed in 3 of 4 mice injected with a single HEY PGCC; in contrast, a tumor formed in only 1 of 4 mice injected with 10 regular HEY cells, but in 4 of 4 mice injected with 100 and 1,000 regular HEY cells (Table 1). This result is consistent with previous report that multinucleated giant cancer cells are more tumorigenic than regular cancer cells from a sarcoma cell line model (20). Immunohistochemical staining of HEY PGCC-derived tumors also demonstrated high expression levels of cyclin D1, cyclin E, CDK2, CDK6, and cyclin B as compared with regular HEY cancer cell-derived tumors (Fig. 5C).
Figure 5.
Morphology of tumors derived from 1×106 regular HEY (a) and 10 HEY PGCCs (b), 1×106 regular SKOv3 (c) and a single SKOv3 PGCC (d), 1×106 regular MDA-MB-231(e) and a single MDA-MB-231 PGCC (f). B. Comparison of tumor formation ability (a) Comparison of tumor formation ability between 10, 100, or 1000 regular HEY and 10 HEY PGCCs; (b) Comparison of tumor formation ability between 10,100, or 1000 regular SKOv3 and MDA-MB-231 and a single SKOv3 and MDA-MB-231 PGCC. C. Expression of cell cycle-related proteins in xenograft. Immunohistochemical stainings performed on tumor tissues derived from PGCCs and regular HEY cells with antibodies against proteins involved the cell cycle regulation (20×).
TABLE 1.
Frequency of Tumor Formation of PGCCs in Mice
| Cell Lines | Number of cells inoculated per mouse | Tumor incidence/number of inoculated mice | Mouse strain | Number of days after injection that the mouse was killed |
|---|---|---|---|---|
| HEY single spheroid | 1 spheroid from 1 PGCC | 6/6 | Nude | 30 |
| HEY PGCCs from a single spheroid injection tumor | 10 PGCCs | 3/4 | Nude | 30 |
| HEY regular cells | 1 × 10 | 0/4 | n/a | |
| 1 × 102 | 1/4 | Nude | 30 | |
| 1 × 103 | 2/4 | 30 | ||
| Primary culture of 10 PGCCs | 1 PGCC | 3/4 | NOD.CS17-Prkdc SCID | 25 |
| HEY regular cells | 1 × 10 | 1/4 | 25 | |
| 1 × 102 | 4/4 | NOD.CS17- | 25 | |
| 1 × 103 | 4/4 | Prkdc SCID | 25 | |
| 1 × 103 | 4/4 | 25 | ||
| HEY PGCCs cultured with adipogenesis media | 1 × 104 | 5/5 | Nude | 30 |
| HEY chondrogenic pellets | 20–30 pellets for subcutaneous injection near the ribs | 5/5 | Nude | 30 |
| HEY chondrogenic pellets | 20–30 pellets for abdominal cavity injection | 5/5 | Nude | 40 |
| SKOv3 PGCCs | 1 PGCC | 7/12 | Nude | 49 |
| SKOv3 regular cells | 1 × 10 | 0/4 | 49 | |
| 1 × 102 | 1/4 | Nude | 49 | |
| 1 × 103 | 2/4 | 49 | ||
| MDA-MB-231 PGCCs | 1 PGCC | 8/12 | Nude | 49 |
| MDA-MB-231 regular cells | 1 × 10 | 2/4 | 49 | |
| 1 × 102 | 2/4 | Nude | 49 | |
| 1 × 103 | 3/4 | 49 | ||
| PGCCs from a human ovarian cancer sample | 50 | 2/4 | Nude | 90 |
Differentiation into Adipose, Cartilage, and Bone of PGCCs
To examine whether these PGCCs to differentiate into other cell types, PGCCs cultured with adipogenesis medium were strongly stained with oil red O (Fig. 6A-b) and strongly expressed FABP4 (Fig. 6A-d) but not in the regular HEY cells (Fig. 6A-a and -c). We subcutaneously injected 1×104 PGCCs cultured with adipogenesis medium into nude mice. Histologic examination showed a large amount of adipose tissue intermixed with the tumor cells (Fig. 6A-e). Adipose differentiation was confirmed by FABP4 immunohistochemical staining (Fig. 6A-f), and the human origin of these cells was confirmed by human-specific vimentin (Fig. 6A-g and -h). Additionally, PGCCs and regular HEY cells were cultured in chondrogenesis medium. While no morphologic changes were obvious for regular HEY cells (Fig. 6B-a), PGCCs formed chondrogenic pellets (Fig. 6B-b) which were strongly stained by Alcian blue and periodic acid-Schiff (Fig. 6B-c and -d). The chondrogenic pellets were injected both subcutaneously near the ribs and percutaneously into the abdominal cavity. The 5 animals injected subcutaneously were killed and translucent tumor nodules similar to cartilage were identified near the site of injection (Fig. 6C-a). Four of 5 abdominal cavity injections resulted in solid tumor formation. Histologic examination revealed osteoid structures within the tumor tissue (Fig. 6C-b). Osteoid differentiation was confirmed by Safranin O/fast green staining (Fig. 6C-c). The osteoid tissue was also positive for anti-eGFP (Fig. 6C-d), confirming the human origin of the bone tissue. Interestingly, 1 of 5 abdominal cavity injection tumor cells formed small, loose tumor bodies of 1–2 mm in diameter (Fig. 6C-e). Hematoxylin and eosin staining of these loose bodies showed cartilaginous tissue in the center of the small bodies (Fig. 6C-f and -g). This tissue was positive for osteopontin (Fig. 6C-h), demonstrating that PGCCs can differentiate into cartilage and bone.
Figure 6.
A. Adipose differentiation of HEY PGCCs in vitro and in vivo. Staining of regular HEY cell and PGCCs with oil red O (a and b) or FABP4 (c and d) following incubation with adipogenesis medium. (e) Histopathology of PGCC-derived xenograft tumors (H&E, 20×) and immunohistochemical staining against FABP4 (f). (g and h) immunohistochemical staining against human-specific vimentin (g:10× and h:20×). B. Cartilage differentiation of HEY PGCCs in vitro. (a) Regular HEY cells cultured in chondrogenesis medium (10×). (b) Formation of chondrogenic pellets from PGCCs cultured in chondrogenesis medium (10×). Chondrogenic pellets stained with (c) Alcian blue and (d) Alcian blue/PAS (10×). C. Cartilage and bone differentiation in vivo. (a) Cartilage-like tumor near the rib cage in nude mice. (b)Bony differentiation on sections stained with H&E (10×), (c) Safranin red and fast green (10×), and (d) anti-eGFP (10×). (e) Formation of loose tumor bodies in the abdomen following injection of chondrogenic pellets. (f) Histopathology of the loose tumor bodies (10×). (g) Cartilage differentiation (black arrow heads, 10×). (h) Bony differentiation revealed by anti-osteopontin staining (10×). D. Osteogenesis, chondrogenesis and adipose differentiation of MDA-MB-231 PGCCs. (a) Chondrogenic pellets of MDA-MB-231 PGCCs cultured with chondrogenesis medium (10×). Chondrogenic pellets stained with (b) Alcian blue and (c) Alcian blue/PAS (10×). (d) Formation of loose tumor bodies following abdominal injection of pellets. (e) Computed tomography image showing osteogenesis differentiation (white arrow heads). (f) Calcification of tumor derived from MDA-MB-231 PGCCs (black arrow heads) (10×). (g) Osteoid differentiation revealed by anti-osteopontin staining in loose bodies (10×). (h) Adipose differentiation revealed by human specific anti-vimentin staining in loose bodies (20×).
MDA-MB-231 PGCCs also formed chondrogenic pellets when cultured with chondrogenesis medium (Fig. 6D-a), which were strongly stained by Alcian blue and periodic acid-Schiff (Fig. 6D-b and -c). When the chondrogenic pellets were injected into the body cavity, small, loose tumor bodies formed (Fig. 6D-d); chondrogenic pellets injected subcutaneously into nude mice developed tumors with calcification, as confirmed with computed tomography and HE staining (Fig. 6D-e and -f). The PGCCs cultured in chondrogenesis medium differentiation into cartilage and bone was demonstrated by osteopontin immunohistochemical staining (Fig. 6D-g). Adipose tissue appeared in the center of those free, small tumor bodies, of which the human origin was confirmed by human-specific vimentin immunohistochemical staining (Fig. 6D-h).
Relationship between the number of PGCCs and resistance to CoCl2 or Cisplatin
We primarily cultured tumors derived from single PGCC injection. Compared with regular HEY cells, there was a marked increase in the total number of PGCCs, which survived the treatment of 300 μM CoCl2 (Fig. S4A). We treated regular HEY cells and PGCCs enriched by CoCl2 with 10 μg/ml cisplatin for 48 hours. Almost all the control HEY cells died, but CoCl2 enriched PGCCs survived (Fig. S4B). Furthermore, we examined 5 cases of ovarian cancer before and after chemotherapy. The number and the size variations of PGCCs were markedly increased in post-chemotherapy samples than in pre-chemotherapy samples for all five cases of human ovarian cancer (Fig. S4C).
PGCCs in Human Ovarian Cancer
To validate our findings in human cancer, we examined the existence of PGCCs in benign and malignant ovarian cancer. PGCCs were observed in serous cystadenoma, high-grade serous ovarian carcinoma, and metastatic high-grade serous ovarian carcinoma (Fig. 7A). The number of PGCCs clearly increases with increased stage and tumor grade. A case of high-grade serous ovarian carcinoma with the PGCCs indicated by arrow (Fig. 7B-a) was primarily cultured. One representative picture of PGCCs and regular cancer cells from the primary culture is shown in figure 7B-b. These PGCCs generated daughter cells via burst over an 11-day period observation (Figure S5). To determine the tumorigenic properties of PGCCs in this primary ovarian cancer, 50 PGCCs were injected into the subcutis of nude mice; tumor formation was detected 3 months after injection. When the xenograft was primarily cultured, the PGCCs (large arrows) and regular cancer cells with (small arrows) can be observed (Fig. 7B-c). After CoCl2 selectively killed the regular cancer cells, the recovered PGCCs have numerous small branches (small arrow) (Fig. 7B-d) with distinct nuclei indicated by Hoechst33342 staining (Fig. 7B -e). When cultured in stem cell medium, the PGCCs grew as tumor spheroids (Fig. 7B-f) with multiple small cancer cells budding from these spheroids (arrows).
Figure 7.
A. PGCCs in human ovarian cancers (black arrows). (a) Normal fallopian tube (20×). (b) Cystadenoma (20×). (c) PGCCs in human high-grade serous ovarian cancinoma (20×). (d) PGCCs in metastatic ovarian cancer (20×). B. Primary culture of human ovarian cancer and PGCC-derived xenograft. (a) Histology of human ovarian cancer used for primary culture. PGCCs indicated by black arrows (20×). (b) PGCCs (large arrow heads) mixed with regular cancer cells (small arrow heads) in the primary culture of (a) (10×). (c) Primary culture of mice xenografted tumor from 50 PGCCs of (b) injection showed PGCCs (large arrows) and regular cancer cells (small arrows) (10×). (d) The recovered PGCCs have numerous small branches (budding daughter cells) after CoCl2 treatment (10×). (e) Hoechst 33342 staining showed the multinucleated PGCCs and budding daughter cells (black arrows point) (10×). (f) Formation of spheroids from (d) in stem cell medium (10×). C. Cyclin B1 immunohistochemical staining in human ovarian cancers. (a) Normal human ovarian cysts negative for cyclin B1 expression (20×). (b) Nuclear expression in low-grade ovarian serous carcinoma (20×). (c) Cyclin B1 was expressed in the cytoplasm of giant nucleated cells from high-grade serous carcinoma (20×) and (d) metastatic ovarian cancer (20×).
Clinicopathologic Significances of Cyclin B1 Expression in Human Ovarian Serous Cancer
As described above, multiple cell cycle-related proteins are dysregulated in PGCCs-derived tumor. We examined the expression of cyclin B1, a cell cycle regulatory protein involved in G2/M transition of mitosis. No expression of cyclin B1 was detected in benign ovarian serous cystadenoma (Fig. 7C-a) and weak nuclear expression in low-grade serous ovarian cancer (Fig. 7C-b). However, cyclin B1 was expressed only in the cytoplasm of PGCCs from human high-grade serous carcinoma (Fig. 7C-c) and metastatic ovarian cancer (Fig. 7C-d). Statistical analysis of cyclin B1 immunohistochemical staining showed that high expression of cyclin B1 in the cytoplasm is associated with both high-grade (P = 0.028) and late-FIGO stage (P = 0.000) in 250 cases of serous ovarian cancers (Table 2). These results strongly suggest that cyclin B play an important role in regulating the formation of PGCCs and regulate the tumor aggressiveness through its re-compartmentalization.
TABLE 2.
Clinicopathologic significances of cyclin B1 different expression in the cytoplasm of ovarian serous carcinoma.
| Clinicopathologic characteristics | n | low expression | high expression | χ2 | P value |
|---|---|---|---|---|---|
| grade | 19.620 | 0.000 | |||
| high | 235 | 84 | 151 | ||
| low | 15 | 14 | 1 | ||
| stage | 7.125 | 0.028 | |||
| I–II | 11 | 7 | 4 | ||
| III | 176 | 59 | 117 | ||
| IV | 58 | 28 | 30 | ||
| unknown | 5 | 4 | 1 | ||
| Response to treatment | 1.951 | 0.377 | |||
| complete | 127 | 46 | 81 | ||
| partial | 73 | 29 | 44 | ||
| no | 30 | 15 | 15 | ||
| unknown | 20 | 8 | 12 | ||
| Ascites | 1.367 | 0.505 | |||
| yes | 163 | 52 | 101 | ||
| minimal | 18 | 8 | 10 | ||
| no | 32 | 9 | 23 | ||
| unknown | 37 | 19 | 18 | ||
| Age at diagnosis | 0.249 | 0.618 | |||
| < 60 | 115 | 47 | 68 | ||
| >=60 | 135 | 51 | 84 | ||
| family | 0.114 | 0.735 | |||
| yes | 140 | 55 | 85 | ||
| no | 97 | 36 | 61 | ||
| unknown | 13 | 7 | 6 |
Discussion
We report here the successful purification, induction and culture of PGCCs cells and characterization of their biologic properties. These PGCCs generated daughter cells via asymmetric division, formed spheroids, and were positive for multiple cancer stem cell markers. Although it remains to be determined whether PGCCs induced by CoCl2 are same as the PGCCs observed physiologic hypoxia in vivo, the PGCCs induced by CoCl2 are stable and easy to passage under the described culture condition and provide an advantage over the physiologic hypoxia in order to have “stable” PGCCs to characterize their biologic properties. PGCCs are able to generate daughter cells in vitro and were more tumorigenic than regular differentiated cells in nude mice. Furthermore, the PGCCs possessed a mesenchymal phenotype and could be induced into multiple benign lineages such as adipose tissue, bone, and cartilage, suggesting that these PGCCs gained a cancer stem cell-like properties
Mitosis is the recognized manner of cell division in eukaryotic cells that ensures the accurate distribution of duplicated genetic materials to progeny cells (21, 22). In prokaryotes and unicellular eukaryotes, cells divide by amitotic processes, including branching followed by binary fission and budding. Although mitosis prevails in complex eukaryotes, it has been well documented that variations of the mitotic cell cycle can occur and meet growth and developmental needs under stresses (13). Among these variations is the endocycle (or endoreduplication), a variation of the normal mitotic cell cycle involving multiple rounds of DNA replication without an intervening mitosis step. This process is an evolutionarily conserved means of generating multinucleated cells and is commonly employed in certain forms of growth in plants, insects, and trophoblasts (13, 23). Stress, aging (24), and an abortive cell cycle can also contribute to the generation of PGCCs. In cancer, certain anti-mitotic chemotherapy drugs increase the formation of giant cells, which are often considered to be at the stage of mitotic catastrophe and subsequent apoptosis (25), very little attention was paid to whether PGCCs can survive these treatment and become resistant cancer cells. PGCCs can generate daughter cells through budding, splitting, and bursting; these growth patterns are very different from the traditional mitotic growth of eukaryotic diploid cells. PGCCs use these evolutionarily conserved mechanisms for renewal and fast reproduction. Therefore, PGCCs may use an evolutional conserved mechanisms used in unicellular organisms to achieve the fast growth and resistance to chemotherapy.
The giant cells revert to regular-sized cancer cells through a process of reduction division called neosis or depolyploidization by previous investigators (26–28). The neosis or reductive cell division through meiosis-like depolyploidization from giant cancer cells (28) was proposed to explain this unexpected life cycle of these cells (29, 30). Overall, despite these previous reports in the literature (26–31), PGCCs have not attracted much attention in the cancer research community. Their role in tumorigenesis has not been vigorously tested. As PGCCs are present in almost all human cancers, their formation may represent an evolutionarily conserved ancient mechanism that cancer cells use in response to stress and cancer chemotherapy. First, giant cells are flexible to meet tumor developmental needs. Second, PGCCs contain multiple copies of genes, which give PGCCs ability to generate the function of different cell type via epigenetic change and DNA recombination. Thirdly, PGCCs may have different metabolic processes from different cancer cells (13). These changes allow PGCCs to be more adaptable to stress and hypoxic microenvironments. Lastly, the ability of PGCCs to release progeny cells by efficient processes such as budding or bursting is similar to the mechanisms used to release matured platelets and shedding progeny viruses in an infected host cell (32, 33). Thus, PGCCs have a distinct advantage over diploid cancer cells and can be more resistant to hypoxia and other stresses (34). Taken together, our data suggest that PGCCs represent a novel form of stress-induced cancer cell with unique cell life cycle that can actively contribute to the tumor growth, heterogeneity, epithelial to mesenchymal transitions, and chemoresistance.
Materials and Methods
Cell Lines and Culture
The human ovarian cancer cell lines HEY and SKOv3 were previously described by our laboratory (35). The breast cancer cell lines MDA-MB-231 were purchased from the American Type Culture Collection. HEY and SKOv3 were maintained in Eagle’s minimum essential medium (EMEM), and MDA-MB-231 was maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 μg/ml).
Induction and Maintenance of PGCCs
The cell lines were cultured in complete medium until the cells reached 90% confluence. Different concentrations of CoCl2 were added into the flasks and the cells were cultured for different time points. The concentration of CoCl2 and the time was dependent on the overall condition of different cell lines (Table S2). After CoCl2 treatment, almost all regular-sized cells were dead, and several purified PGCCs survived and recovered.
Flow Cytometry
The cell cycle status was detected by flow cytometry using a protocol described previously (36) and analyzed by CellQuest software (BD Biosciences).
X chromosome fluorescence in situ hybridization
A CEP X SpectrumOrange Direct Labeled Fluorescent DNA Probe Kit and the probe specific for the X chromosome were purchased from Vysis (Abbott Molecular Inc.). The fluorescence in situ hybridization was performed according to the manufacturer’s instructions.
Acidic β-Galactosidase Staining
Staining for β-galatosidase was done according to a previously published protocol (37).
PKH26 Staining and Animal Injection
The PGCCs were stained with PKH26 (Sigma-Aldrich) according to the manufacturer’s instructions. Each PGCC stained with PKH26 was monitored for its division pattern in a tissue culture dish every 12 hours. In vivo, 3000 PGCCs stained with PKH26 were injected subcutaneously into nude mice. The mice were killed on day 28 after injection, and the frozen sections of tumor were observed for fluorescence from the PKH26-stained cells.
Formation of Spheroids
Stem cell medium (GIBCO/Invitrogen) was used to grow spheroids. The PGCCs were cultured in stem cell medium for 7–10 days; the PGCCs grew as spheroids either floating in the medium or attached to the wall of the flask. For growth of spheroids in Matrigel (no. 356237; BD Biosciences), PGCCs were mixed with Matrigel and with complete EMEM at a 1:1 ratio in a total volume of 200 μl on 24-well plate. This cell-Matrigel mixture was allowed to solidify at 37°C for 10 minutes before 0.5 ml of culture medium was added.
Observation of DNA Transport in the Branches of PGCCs
The PGCCs were trypsinized and incubated with Hoechst 33342 (H342, no. B2261, Sigma) at a final concentration of 5 μg/ml at 37°C for 1 hour. After rinsed with PBS twice to remove the dye and wrapped with foil, the PGCCs were cultured with complete EMEM. Cell division patterns and DNA transportation via the PGCCs’ branches were examined and photographed 8 hours later.
Generation of PGCCs via Cell Fusion
The regular HEY and MDA-MB-231 cells were transfected with a eukaryotic expression vector for eGFP or RFP, and cells with green or red fluorescence were selected and expanded for the cell fusion experiment. Regular HEY cells were also labeled with PKH26. To generate PGCCs, regular HEY cancer cells expressing eGFP were mixed with regular HEY cells labeled with PKH26 or labeled with RFP and then treated with 300 μM CoCl2 for 72 hours. The cell fusion and the formation of PGCCs as indicated by the presence of yellow fluorescence were observed with a fluorescence microscope (Eclipse TE 2000-U; Nikon).
Immunohistochemical and immunofluorescence Staining
The detail protocol of immunofluorescence staining was described previously (35). For information on primary antibodies, see Table S3.
PGCCs Isolation from Primary Cultures of Human Ovarian Cancer
Fresh ovarian cancer tissues were collected according to a protocol approved by our institutional review board. The samples were washed with PBS and sterilized with antibiotics. The tissue was minced into 1- to 2-mm3 pieces and cultured with DMEM. The PGCCs were isolated with cloning cylinders (Sigma) and used for injection into nude mice.
Adipose, Chondrocyte and Osteoid Differentiation of PGCCs
Adipose, chondrocyte and osteoid differentiation of PGCCs were performed according to manufacturer protocols (GIBCO/Invitrogen).
Histochemical Staining
Oil Red O, Alcian Blue/PAS, and Safranin O/Fast Green Staining were performed according to the manufacturer’s instructions (Alcian blue/PAS Stain Kit, Artisan, AR169; Dako; Safranin O/fast green staining kit, no. IW-3011; NovaUltra).
Western Blotting
Western blot analyses were done as described previously (38).
Tumor Growth in Mice
Single spheroids or PGCCs together with the mixture of 0.1 ml of PBS buffer and 0.1 ml of Matrigel were drawn into the syringes and kept in ice before injection. Single PGCCs were isolated by serial dilution. Each mouse received whole-body radiation before injection. Single PGCCs were then picked out by pipette and subcutaneously injected into the flanks of nude and NOD.CS17-Prkdc SCID mice. The mice were killed and their tumors removed when the mean tumor diameter reached 0.5–1.0 cm. The care and use of the mice was approved by our Institutional Animal Care and Use Committee.
Cyclin B expression and Clinicopathologic Correlation in Human Ovarian Cancer
Tumor sample collection and tissue microarray construction have been described previously (39). 250 cases of serous ovarian carcinoma were used for clinicopathologic data analysis. All the patients were diagnosed with ovarian carcinoma and had undergone initial surgery between 30 March 1987 and 17 February 2009. Follow-up information was updated through 31 December 2011. Tumor were graded on a two-tier system (4). Disease staging was according to the International Federation of Gynecology and Obstetrics (FIGO) staging system (40). Based on the cutoff point for the score of cyclin B1 expression to reach statistical significance using X-tile software, the staining scores were defined as low expression, for those < 25% cyclin B1-positive tumor cells, and high expression, for those 25% cyclin B1-positive tumor cells (42). The use of tissue blocks and the chart reviews were approved by the MD Anderson Institutional Review Board.
Statistical Analysis
Fisher’s exact test was used to evaluate the clinicopathologic significances of cyclin B1 expression in the cytoplasm. A P-value of less than 0.05 was regarded as statistically significant. Data was analyzed using STATA 9.0 (Stats Corporation, College Station, TX, USA). All statistical tests were 2-sided.
Supplementary Material
Acknowledgments
Grant Support
J.L. was supported by an R01 grant (R01CA131183-01A2) and ovarian cancer Specialized Programs of Research Excellence (SPORE) grant (IP50CA83638) from the National Institutes of Health and multi-investigator grant from Cancer Prevention and Research Institute of Texas (CPRIT) (RP1105995). STR DNA fingerprinting was done by the Cancer Center Support Grant-funded Characterized Cell Line core, NCI # CA016672.
We thank the Department of Scientific Publications for their editorial assistance on the manuscript.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Heppner GH. Tumor heterogeneity. Cancer Res. 1984;44:2259–2265. [PubMed] [Google Scholar]
- 2.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- 3.Kumar V, Abbas AL, Fausto N, Aster JC. Robbins and Cotran Pathologic Basis of of Disease. 8. 2010. pp. 262–270. [Google Scholar]
- 4.Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Geigl JB, Obenauf AC, Schwarzbraun T, Speicher MR. Defining ‘chromosomal instability’. Trends Genet. 2008;24:64–69. doi: 10.1016/j.tig.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Holland AJ, Cleveland DW. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat Rev Mol Cell Biol. 2009;10:478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–3284. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J Clin Oncol. 2011;29:591–599. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho VT, Bunn HF. Effects of transition metals on the expression of the erythropoietin gene: further evidence that the oxygen sensor is a heme protein. Biochem Biophys Res Commun. 1996;223:175–180. doi: 10.1006/bbrc.1996.0865. [DOI] [PubMed] [Google Scholar]
- 12.Piret JP, Mottet D, Raes M, Michiels C. CoCl2, a chemical inducer of hypoxia-inducible factor-1, and hypoxia reduce apoptotic cell death in hepatoma cell line HepG2. Ann N Y Acad Sci. 2002;973:443–447. doi: 10.1111/j.1749-6632.2002.tb04680.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee HO, Davidson JM, Duronio RJ. Endoreplication: polyploidy with purpose. Genes Dev. 2009;23:2461–2477. doi: 10.1101/gad.1829209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siller KH, Doe CQ. Spindle orientation during asymmetric cell division. Nat Cell Biol. 2009;11:365–374. doi: 10.1038/ncb0409-365. [DOI] [PubMed] [Google Scholar]
- 15.Kusumbe AP, Bapat SA. Cancer stem cells and aneuploid populations within developing tumors are the major determinants of tumor dormancy. Cancer Res. 2009;69:9245–9253. doi: 10.1158/0008-5472.CAN-09-2802. [DOI] [PubMed] [Google Scholar]
- 16.Brodbeck WG, Anderson JM. Giant cell formation and function. Curr Opin Hematol. 2009;16:53–57. doi: 10.1097/MOH.0b013e32831ac52e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurdon JB, Melton DA. Nuclear reprogramming in cells. Science. 2008;322:1811–1815. doi: 10.1126/science.1160810. [DOI] [PubMed] [Google Scholar]
- 18.Lu X, Kang Y. Cell fusion as a hidden force in tumor progression. Cancer Res. 2009;69:8536–8539. doi: 10.1158/0008-5472.CAN-09-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 20.Weihua Z, Lin Q, Ramoth AJ, Fan D, Fidler IJ. Formation of solid tumors by a single multinucleated cancer cell. Cancer. 117:4092–4099. doi: 10.1002/cncr.26021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 22.Alberts BJA, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4. New York: Garland Science; 2002. < http://wwwgarlandsciencecom/textbooks/0815341059asp>. [Google Scholar]
- 23.Erenpreisa J, Cragg MS. Mitotic death: a mechanism of survival? A review Cancer Cell Int. 2001;1:1. doi: 10.1186/1475-2867-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walen KH. Human diploid fibroblast cells in senescence; cycling through polyploidy to mitotic cells. In Vitro Cell Dev Biol Anim. 2006;42:216–224. doi: 10.1290/0603019.1. [DOI] [PubMed] [Google Scholar]
- 25.Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 2008;15:1153–1162. doi: 10.1038/cdd.2008.47. [DOI] [PubMed] [Google Scholar]
- 26.Erenpreisa J, Kalejs M, Ianzini F, Kosmacek EA, Mackey MA, Emzinsh D, et al. Segregation of genomes in polyploid tumour cells following mitotic catastrophe. Cell Biol Int. 2005;29:1005–1011. doi: 10.1016/j.cellbi.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Sundaram M, Guernsey DL, Rajaraman MM, Rajaraman R. Neosis: a novel type of cell division in cancer. Cancer Biol Ther. 2004;3:207–218. doi: 10.4161/cbt.3.2.663. [DOI] [PubMed] [Google Scholar]
- 28.Erenpreisa J, Salmina K, Huna A, Kosmacek EA, Cragg MS, Ianzini F, et al. Polyploid tumour cells elicit paradiploid progeny through depolyploidizing divisions and regulated autophagic degradation. Cell Biol Int. 2011;35:687–695. doi: 10.1042/CBI20100762. [DOI] [PubMed] [Google Scholar]
- 29.Erenpreisa J, Cragg MS. Cancer: a matter of life cycle? Cell Biol Int. 2007;31:1507–1510. doi: 10.1016/j.cellbi.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Rajaraman R, Rajaraman MM, Rajaraman SR, Guernsey DL. Neosis--a paradigm of self-renewal in cancer. Cell Biol Int. 2005;29:1084–1097. doi: 10.1016/j.cellbi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Erenpreisa J, Kalejs M, Cragg MS. Mitotic catastrophe and endomitosis in tumour cells: an evolutionary key to a molecular solution. Cell Biol Int. 2005;29:1012–1018. doi: 10.1016/j.cellbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Hurley JH, Boura E, Carlson LA, Rozycki B. Membrane budding. Cell. 143:875–887. doi: 10.1016/j.cell.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316:1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- 34.Comai L. The advantages and disadvantages of being polyploid. Nat Rev Genet. 2005;6:836–846. doi: 10.1038/nrg1711. [DOI] [PubMed] [Google Scholar]
- 35.Liu G, Yang G, Chang B, Mercado-Uribe I, Huang M, Zheng J, et al. Stanniocalcin 1 and ovarian tumorigenesis. J Natl Cancer Inst. 2010;102:812–827. doi: 10.1093/jnci/djq127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang G, Cai KQ, Thompson-Lanza JA, Bast RC, Jr, Liu J. Inhibition of breast and ovarian tumor growth through multiple signaling pathways by using retrovirus-mediated small interfering RNA against Her-2/neu gene expression. J Biol Chem. 2004;279:4339–4345. doi: 10.1074/jbc.M311153200. [DOI] [PubMed] [Google Scholar]
- 37.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Yang G, Thompson-Lanza JA, Glassman A, Hayes K, Patterson A, et al. A genetically defined model for human ovarian cancer. Cancer Res. 2004;64:1655–1663. doi: 10.1158/0008-5472.can-03-3380. [DOI] [PubMed] [Google Scholar]
- 39.Yang G, Xiao X, Rosen DG, Cheng X, Wu X, Chang B, et al. The biphasic role of NF-kappaB in progression and chemoresistance of ovarian cancer. Clin Cancer Res. 2011;17:2181–2194. doi: 10.1158/1078-0432.CCR-10-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shepherd JH. Revised FIGO staging for gynaecological cancer. Br J Obstet Gynaecol. 1989;96:889–892. doi: 10.1111/j.1471-0528.1989.tb03341.x. [DOI] [PubMed] [Google Scholar]
- 41.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.