Abstract
Objective
To examine the association between use of statin and non-statin cholesterol-lowering medications and risk of nontraumatic major lower-extremity amputations (LEA) and treatment failure (LEA or death).
Design of Study
A retrospective cohort of patients with Type I and Type 2 diabetes mellitus (diabetes) was followed for five years between 2004 and 2008. The follow-up exposure duration was divided into 90-day periods. Use of cholesterol-lowering agents, diabetic medications, hemoglobin A1c, body mass index, and systolic and diastolic blood pressures were observed in each period. Demographic factors were observed at baseline. Major risk factors of LEA including peripheral neuropathy, PAD, and foot ulcers were observed at baseline and were updated for each period. LEA and deaths were assessed in each period and their hazard ratios were estimated.
Setting
US Department of Veterans Affairs Healthcare system (VA)
Subjects
Cholesterol drug-naïve patients with Type I or II diabetes who were treated in the VA in 2003 and were <65 years old at the end of follow-up.
Results
Of 83,593 patients in the study cohort, 217 (0.3%) patients experienced a major LEA and 11,716 (14.0%) patients experienced an LEA or death (treatment failure) after a mean follow-up of 4.6 years. Compared to patients who did not use cholesterol-lowering agents, statin users were 35% - 43% less likely to experience an LEA (HR = 0.65; 95% CI, 0.42–0.99) and a treatment failure (HR = 0.57; 95% CI, 0.54–0.60). Users of other cholesterol-lowering medications were not significantly different in LEA risk (HR = 0.95; 95% CI, 0.35–2.60) but had a 41% lower risk of treatment failure (HR = 0.59; 95% CI, 0.51–0.68).
Conclusions
This is the first study to report a significant association between statin use and diminished amputation risk among patients with diabetes. In this non-randomized cohort, beneficial effects of statin therapy were similar to that seen in large-scale clinical trial experience. For LEA risk, those given non-statins did not have a statistically significant benefit and its effect on LEA risk was much smaller compared to statins.. Unanswered questions to be explored in future studies include a comparison of statins of moderate versus high potency in those with high risk of coronary heart disease and an exploration of whether the effects seen in this study are simply effects of cholesterol-lowering or possibly pleiotropic effects.
Keywords: cholesterol therapy, diabetes, statins, lower-extremity amputation, amputation-free survival
INTRODUCTION
Diabetes elevates the risk of limb loss, adverse cardiovascular events, and death.1 Aggressive management of dyslipidemia is a cornerstone of risk factor modification in diabetes.2 Current Adult Treatment Panel (ATP) III guidelines define diabetes as a coronary heart disease risk equivalent and mandate a low-density lipoprotein cholesterol (LDL-c) goal of <100 mg/dL for all individuals with diabetes3 and an optional goal of LDL-c <70 mg/dL for very high-risk individuals.2
While there is trial evidence that LDL-cholesterol-lowering therapy reduces cardiovascular risk in patients with diabetes, its effect on vascular health has not been clearly evaluated.4 The landmark Heart Protection Study, which included 5,963 patients with diabetes, demonstrated a significant 22% reduction in cardiovascular events and revascularizations among subjects with diabetes randomized to simvastatin 40 mg, but failed to show a significant reduction in lower extremity-amputations (LEA).4 The Cholesterol Treatment Trialists evaluated 18,686 individuals with diabetes from 14 randomized trials of statin therapy.5 Although there was a significant reduction in vascular mortality in patients assigned to statins, the association between LEA and statin use was not reported.
Given the current lack of evidence on the effect of statins on amputation risk, our large non-trial population of individuals in the VA healthcare system with diabetes provides a unique opportunity to evaluate the association of statin and non-statin cholesterol-lowering medication use with LEA and amputation-free survival over five years of follow-up. Our objective was to examine how cholesterol-lowering medications among new users are associated with five-year amputation risk and amputation-free survival.
METHODS
Research Design
We used a retrospective cohort comprised of non-elderly (<65 years of age) diabetic patients who were not using cholesterol-lowering medications at baseline. We used inpatient and outpatient datasets for fiscal years 2002 and 2003 (October 2001 to September 2003; all years in this study are fiscal years) to identify patients who were treated for diabetes in the US Department of Veterans Affairs (VA) healthcare system. An annual inpatient data set contains patient records for all hospitalizations that occurred during each fiscal year in all VA medical centers across the country. For each hospital stay, patient conditions using up to ten ICD-9 diagnostic codes and all procedures performed are recorded using ICD-9 procedure codes. The outpatient data set contains “encounter” level data for all visits to VA hospital-based and community-based outpatient clinics each year. An encounter is a patient seen at a “clinic stop,” a concept analogous to a revenue center in the private sector. A patient can have several encounters during an outpatient care visit. Each encounter contains up to 10 patient diagnoses in ICD-9 codes and up to 20 procedures in CPT-4 codes.
We identified an individual as having diabetes if he or she had a prescription of diabetes medication in 2003 and/or two or more inpatient or outpatient care episodes with a diagnosis of diabetes (detected by an ICD-9-CM diagnostic code 250.xx) in 2002 – 2003.6
Because VA beneficiaries may also be entitled to receive health care from Medicare providers, we limited our study cohort to those aged <65 years at the end of follow-up (September 30, 2008) so that they would not be eligible for Medicare benefits on account of age before the end of follow-up. At the time of this study, we did not have access to Medicare data and diagnoses made by Medicare providers were not observable in this study.
We additionally excluded all patients who died before October 1, 2004 (the index date from when the follow-up started), who had a history of any LEA before the index date, and who were new users of, or new enrollees in, the VA healthcare system in 2003. New users and/or new enrollees were excluded because baseline comorbidities were not observable.
Identification of Amputation and Mortality
The goal of treatment for patients with diabetic complications in the lower extremities is amputation-free survival. As our main outcomes, we used LEA and treatment failure defined as an LEA or death. We identified all major (ankle or above) LEAs between 2004 and 2008 by searching both inpatient and outpatient data. The procedure codes in the ICD-9-CM or CPT used to identify LEAs are shown in Table A1 (Online Appendix). Deaths were identified by the VA Vital Status file which contains deaths for the VA beneficiaries up to April 2009. Any death before the end of follow-up was identified as a competing risk for amputation and as an event for the treatment failure. The VA Vital Status file has over 98% sensitivity and 97% specificity compared to the National Death Index.7
Cholesterol-Lowering Medications
We divided the 5-year follow-up into 90-day periods and observed cholesterol-lowering medications used during each period. A 90-day period was chosen because a large number of prescriptions for diabetic medications are filled for 90 days in the VA. The VA pharmacy prescription-level data were searched to identify all cholesterol-lowering agents dispensed to patients during each period. Medications were grouped according to their drug classes into statins (simvastatin, atorvastatin, fluvastatin, lovastatin, pravastatin, and rosuvastatin), fibrates (bezafibrate, ciprofibrate, clofibrate, gemfibrozil, and fenofibrate), nicotinic acid (niacin), bile acid sequestrants (cholestyramine, colesevelam, and colestipol), and cholesterol absorption inhibitors (ezetimibe). Days of supply filled for each class of medications were separately tallied from the dispensing date and over-supply for a period was rolled over to the next. We defined a patient as a user of a class of medications if the patient had at least 30 days’ supply of medications in the same class during each period. We likewise identified patients who had at least 30-day supply of any cholesterol-lowering medication in 2003 and excluded them from the study cohort.
Potential Confounders
Other risk factors of LEA and death were identified either at baseline or during each period from various sources that may potentially confound the association between cholesterol-lowering medication use and outcome. Demographic factors including patient age, sex, race/ethnicity, marital status, and co-existing conditions were obtained from inpatient and outpatient records in 2003. We identified some co-existing conditions in 2003 for which statins may be contraindicated and their new diagnoses during each period.
Other major risk factors of LEA or mortality include coronary artery disease (CAD), peripheral artery disease (PAD), peripheral neuropathy, foot ulcers, osteomyelitis, and history of vascular procedures. These were detected by ICD-9-CM codes in the inpatient and outpatient records for 2003 and were updated for each period. The specific codes for identifying these conditions are listed in Table A2 (Online Only Appendix).
Diabetes duration was estimated as the number of years a person had been treated for diabetes on October 1, 2003 in the VA healthcare system and was identified by searching the VA inpatient and outpatient records from 1997, the first year these data sets are available for research. Hemoglobin A1c and all cholesterol and blood pressure measures were obtained at baseline and updated for each period. The non-high-density lipoprotein cholesterol (non-HDL-c) level was computed as the total cholesterol minus high-density lipoprotein cholesterol. Non-HDL-c levels have the advantage of being calculable in the non-fasting state and include LDL-c. Moreover, in a large-scale clinical trial of patients with diabetes, non-HDL-c was shown to be a strong and independent predictor of cardiovascular endpoints.8
We also obtained all height and weight measures taken during visits to the VA hospitals or clinics in 2003 and in each period. For all period-specific measures, when a measure is not observed in a period, we used the last observation carried forward (LOCF) method to impute the missing values from the previous period, assuming that the last observed values did not change until the next measurement.9 When multiple measures were available from the same period, we used the average of all available values.
Statistical Analysis
Our primary outcomes were major LEAs and deaths identified during a five-year follow-up. Time to our primary outcomes were compared between four groups of patients according to cholesterol-lowering therapies they used: “non-users” who were not treated with any cholesterol-lowering medications, “statin users” who were treated with statins alone, “non-statin users” who were treated with cholesterol-lowering medication other than statin, and “both users” who were treated with both statins and non-statin agents. We analyzed the time to the first major LEA using a competing risk regression,10 adjusting for death as a competing risk. Time to the first LEA or death was analyzed using a Cox regression. Both models included baseline patient characteristics (age, race, marital status, diabetes duration > 7 years at baseline) and time-varying covariates such as diabetes control (A1c), diabetic medication use, BMI, and comorbidities (CAD, PAD, foot ulcers, osteomyelitis, diabetic neuropathy, and history of vascular procedures) in each person-period. Patient sex was not included in the final regression models because it was not significant in both models and a reliable estimate for LEA risk was not available due to a small number of events for females.
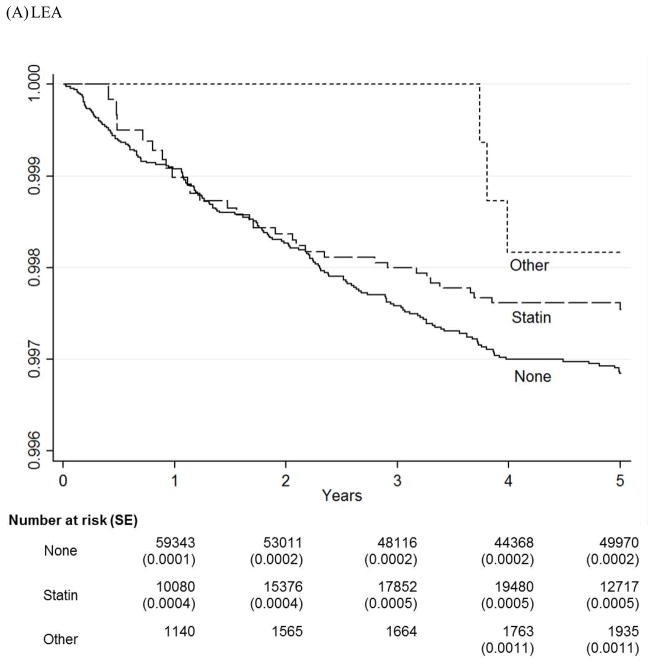
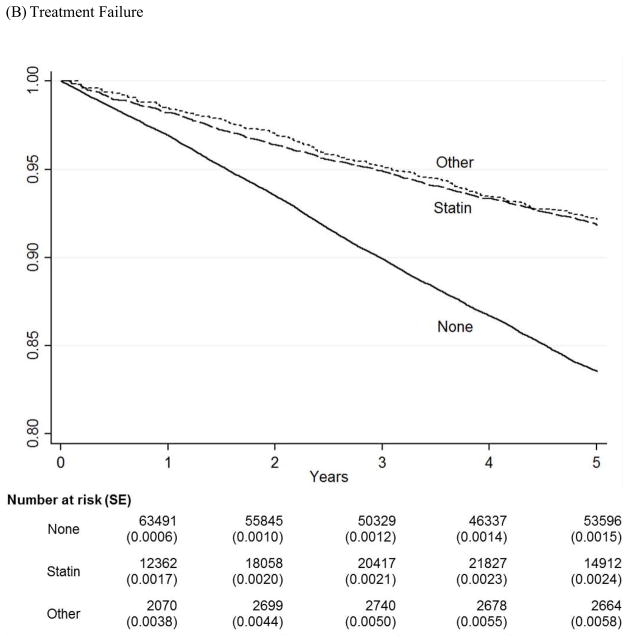
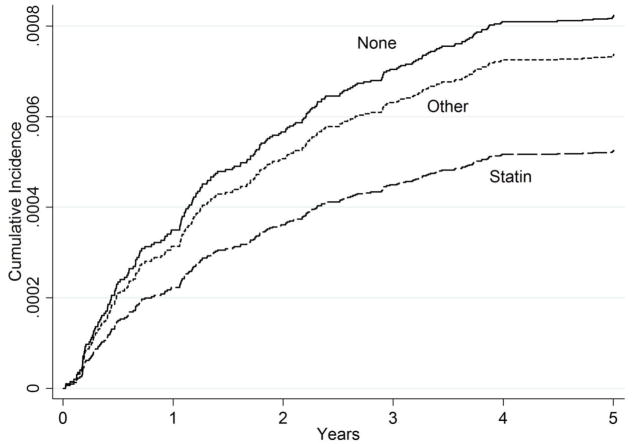
We graphically compared survival estimates by therapy groups using Kaplan-Meier Survival Curves (Figure 1). The number of patients at risk and standard errors of the estimates at each major time points were provided below the graphs. Note that none of the patients were taking any cholesterol-lowering medications at baseline and so the numbers for Year 0 are not provided. The curves for both statin and non-statin users were mainly overlapping those for statin users and were not displayed. Because Kaplan-Meier curves are not appropriate in the presence of competing risks,11 we also used the Cumulative Incidence Function (CIF) curves to compare cumulative risk of major LEAs by four therapy groups, adjusting for deaths as a competing risk (Figure 2). The sample provides power > 0.8 for a minimum detectable hazard ratio of 0.65 for LEAs and 0.95 for treatment failure between non-users and statin users and of 0.41 for LEAs and 0.85 for treatment failure between non-users and non-statin agent users with alpha < 0.05 on a two-sided test. We use Stata/SE 12.1 (StataCorp LP, College Station, TX) for statistical analysis. This study was approved by the institutional review board at the Hines VA Hospital, Hines, Illinois.
Figure 1.
Kaplan-Meier Survival Curves for LEA and Treatment Failure
* This figure shows Kaplan-Meier Survival curves for three treatment groups (None, Statin, and Other) over five years of follow-up for LEA and treatment failure.
* None = No cholesterol-lowering medications; Statin = Statins alone; Other = Cholesterol- lowering medications other than statins.
Figure 2.
Cumulative Incidence Function Curves for LEA after Adjusting for Competing Risks* This figure shows Cumulative Incidence Function curves for three treatment groups (None, Statin, and Other) over five years of follow-up for LEA, adjusting for death as a competing risk.
* LEA = Lower-extremity amputation; None = No cholesterol-lowering medications; Statin = Statins alone; Other = Cholesterol-lowering medications other than statins.
RESULTS
There were 83,593 cholesterol-drug-naïve individuals in the study cohort, of whom 217 (0.3%) experienced a major LEA and 11,716 (14.0%) experienced a treatment failure during a mean follow-up of 4.6 years (median = 5 years).
Baseline characteristics are shown in Table 1. Over half of those who had valid measures consistently did not meet ATP III goals12 recommended for persons with diabetes in cholesterol levels (LDL-c ≥ 100 mg/dL, HDL-c < 40 mg/dL), blood pressure (≥130/80 mm Hg), and body mass index (BMI ≥ 30 kg/m2).
Table 1.
Baseline Characteristics of the Study Cohort*
| Measures | N | Mean (SD) | Percentiles | ||||
|---|---|---|---|---|---|---|---|
| 5% | 25% | Median | 75% | 95% | |||
| Age, yrs | 83,593 | 52.3 (6.1) | 40.0 | 49.0 | 54.0 | 57.0 | 59.0 |
| Cholesterol Levels, mg/dL | |||||||
| LDL Cholesterol | 48,276 | 104.1 (34.1) | 48.2 | 83.3 | 104.0 | 125.0 | 159.4 |
| non-HDL Cholesterol | 55,751 | 140.6 (38.4) | 82.7 | 115.0 | 138.0 | 163.0 | 207.5 |
| Total Cholesterol | 59,329 | 182.3 (40.0) | 124.0 | 157.0 | 179.5 | 204.0 | 248.5 |
| HDL Cholesterol | 52,662 | 41.8 (14.5) | 25.0 | 33.0 | 39.0 | 47.7 | 66.5 |
| Triglycerides | 52,783 | 200.4 (132.6) | 80.0 | 114.0 | 162.0 | 241.7 | 447.0 |
| Hemoglobin A1c, % | 63,351 | 7.5 (1.8) | 5.3 | 6.2 | 7.2 | 8.6 | 11.0 |
| Blood Pressure, mmHg | |||||||
| Systolic | 53,117 | 137.0 (15.5) | 113.3 | 126.8 | 136.0 | 146.0 | 164.0 |
| Diastolic | 53,102 | 79.4 (9.0) | 65.0 | 73.5 | 79.3 | 85.0 | 94.3 |
| Body Mass Index, kg/m2 | 48,420 | 32.1 (6.8) | 22.5 | 27.4 | 31.1 | 35.8 | 44.6 |
LDL = low-density lipoprotein; HDL = high-density lipoprotein
Patient characteristics in Table 2 shows that about 75% of the cohort were aged 50 years or older and 2.9% were 60 years or older at baseline. About 44% were non-Hispanic white and 24% non-Hispanic black. Hemoglobin A1c was measured for over 75% of the cohort in 2003 and 14% of all patients (19% with A1c measures) had an average A1c >9%. Twenty-one percent had diabetes for seven years or longer at baseline. Fourteen percent were treated with insulin alone, 49% with oral medications alone, 13% with both insulin and oral medications, and the rest (23%) did not receive any pharmacological treatment for diabetes in 2003. Within this cohort, 11% had peripheral neuropathy, 10% CAD, 3% PAD, 0.12% history of vascular procedures, 2% foot ulcers, 0.3% osteomyelitis, 1.6% renal failure, and 6% liver disease at baseline.
Table 2.
Unadjusted Rates of Adverse Outcomes by Patient Characteristics at Baseline*
| LEA* | LEA or Death | No Events | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| All | 217 | 100.0% | 11,716 | 100.0% | 71,877 | 100.0% |
| Age, year | ||||||
| < 50 | 30 | 13.8% | 1,805 | 15.4% | 19,626 | 27.3% |
| 50–54 | 60 | 27.6% | 3,401 | 29.0% | 20,122 | 28.0% |
| 55–59 | 82 | 37.8% | 5,566 | 47.5% | 30,642 | 42.6% |
| 60–64 | 41 | 18.9% | 944 | 8.1% | 1,487 | 2.1% |
| Sex | ||||||
| Female | † | 243 | 2.1% | 3,412 | 4.7% | |
| Male | † | 11,473 | 97.9% | 68,465 | 95.3% | |
| Race/Ethnicity | ||||||
| Non-Hispanic White | 106 | 48.8% | 6,341 | 54.1% | 30,333 | 42.2% |
| Non-Hispanic Black | 75 | 34.6% | 3,040 | 25.9% | 17,228 | 24.0% |
| Other/Unknown | 36 | 16.6% | 2,335 | 19.9% | 24,316 | 33.8% |
| Marital Status | ||||||
| Not married | 134 | 61.8% | 7,069 | 60.3% | 35,015 | 48.7% |
| Married | 83 | 38.2% | 4,647 | 39.7% | 36,862 | 51.3% |
| Hemoglobin A1c, % | ||||||
| < 7 | 71 | 32.7% | 4,005 | 34.2% | 25,033 | 34.8% |
| 7–9 | 50 | 23.0% | 3,064 | 26.2% | 19,222 | 26.7% |
| > 9 | 58 | 26.7% | 1,870 | 16.0% | 10,157 | 14.1% |
| Unknown | 38 | 17.5% | 2,777 | 23.7% | 17,465 | 24.3% |
| Diabetes Duration, y | ||||||
| < 7 | 121 | 55.8% | 8,474 | 72.3% | 57,739 | 80.3% |
| 7 or longer | 96 | 44.2% | 3,242 | 27.7% | 14,138 | 19.7% |
| Body Mass Index, kg/m2 | ||||||
| < 25 | 33 | 15.2% | 1,564 | 13.3% | 4,835 | 6.7% |
| 25 – 29.9 | 32 | 14.7% | 1,596 | 13.6% | 10,947 | 15.2% |
| 30 or higher | 43 | 19.8% | 3,208 | 27.4% | 25,898 | 36.0% |
| Unknown | 109 | 50.2% | 5,348 | 45.6% | 30,197 | 42.0% |
| Antihyperglycemic Medications | ||||||
| None | 32 | 14.7% | 2,133 | 18.2% | 17,280 | 24.0% |
| Insulin Alone | 72 | 33.2% | 2,719 | 23.2% | 8,739 | 12.2% |
| Oral Medications Alone | 61 | 28.1% | 4,498 | 38.4% | 36,982 | 51.5% |
| Insulin & Oral Medications | 52 | 24.0% | 2,366 | 20.2% | 8,876 | 12.3% |
| Comorbidities | ||||||
| Peripheral Neuropathy | 79 | 36.4% | 1,872 | 16.0% | 7,048 | 9.8% |
| Coronary Artery Disease | 54 | 24.9% | 2,111 | 18.0% | 6,144 | 8.5% |
| Peripheral Arterial Disease | 57 | 26.3% | 705 | 6.0% | 1,477 | 2.1% |
| History of Vascular Procedure | 12 | 5.5% | 54 | 0.5% | 47 | 0.1% |
| Foot Ulcers | 98 | 45.2% | 565 | 4.8% | 1,061 | 1.5% |
| Osteomyelitis | 16 | 7.4% | 51 | 0.4% | 138 | 0.2% |
| Congestive Heart Failure | 26 | 12.0% | 945 | 8.1% | 1,090 | 1.5% |
| Hypertension | 142 | 65.4% | 6,935 | 59.2% | 37,564 | 52.3% |
| Paralysis | 10 | 4.6% | 223 | 1.9% | 465 | 0.6% |
| Chronic lung diseases | 32 | 14.7% | 1,663 | 14.2% | 5,398 | 7.5% |
| Renal Failure | 38 | 17.5% | 937 | 8.0% | 1,143 | 1.6% |
| Liver disease | 20 | 9.2% | 1,756 | 15.0% | 3,287 | 4.6% |
LEA = persons who experienced major amputations in the lower extremity; No Events = no LEA or death during follow-up
Cell with a small number.
Compared to patients who have never experienced an adverse outcome during follow-up, those who experienced a treatment failure were older (52 vs. 55 years), had slightly higher A1c (7.50 vs. 7.54 mg/dL), less likely to be obese (36.0% vs. 27.4%), but were more likely to have diabetes ≥ 7 years (19.7% vs. 27.7%), CAD (8.5% vs. 18.0%), PAD (2.1% vs. 6.0%), foot ulcers (1.5% vs. 4.8%), and osteomyelitis (0.3% vs. 0.6%) at baseline.
When we compared baseline characteristics of patients between statin users and non-users, we found that most factors significantly and positively associated with statin use were also factors that are usually associated with poor vascular health, including BMI > 30 kg/m2 (30.5% for non-users vs. 37.6% for statin users), A1c > 9% (13.4% vs. 15.3%), and PAD (2.4% vs. 2.8%). However, at baseline, more patients had foot ulcers (2.2% vs. 1.8%), osteomyelitis (0.18% vs. 0.28%), and diabetes duration >7 years (21.4% vs. 20.6%) among non-users than statin users.
Figure 1 shows Kaplan-Meier survival curves for LEA and for the treatment failure by three therapy groups over five years. For LEAs, limb survival steadily decreased for both non-user (“None”) and statin (“Statin”) groups until the fourth year and leveled off during the last year of follow-up. On the other hand, the non-statin (“Other”) group experienced most of the LEA events during the third year. While the non-statin group had the best survival of the three groups in these unadjusted Kaplan-Meier curves, the cumulative incidence function curves from the full-adjusted competing risk regression (Figure 2) shows that the statin group had the lowest cumulative incidence and the non-user group had the highest cumulative incidence of LEA with the non-statin group resembling the non-user group more than the statin group.
For the treatment failure, survival curves for the statin and non-statin groups mostly overlapped each other but they diverged from the non-user group from the very beginning of follow-up with ever widening gap in survival throughout the entire follow-up.
Table 3 characterizes cholesterol-lowering prescriptions filled over the follow-up period for individuals with LDL-c below and above 100 mg/dL. In this cholesterol-drug-naïve cohort at baseline, patients were not treated with any cholesterol-lowering medications in 72% of all periods during the five-year follow-up. Patients were treated with statins alone in 22% and by non-statin medications alone or statin and non-statin medications together in 3% and 2.7% of all periods, respectively. During periods when patients were not treated with any cholesterol-lowering medications, they had LDL-c ≥ 100 mg/dL for 38.3% of periods. In 71% of periods, patients received treatment consistent with ATP-III recommendations (LDL-c < 100 mg/dL or treated with statins). Altogether, 44.2% of all patients in the cohort have never been treated with statins during follow-up. Among patients experiencing an LEA, only 32% were treated with statins, while 55.5% were treated with statins among those who did not experience an amputation (data not shown).
Table 3.
Distribution of the cohort by cholesterol-lowering therapy and low-density lipoprotein cholesterol (LDL-c) level, all periods (N = 1,629,488)
| Therapy* | % of all periods† | LDL-c level during period, % of therapy | |
|---|---|---|---|
| < 100 mg/dL | ≥ 100 mg/dL | ||
| None | 71.9% | 61.8% | 38.3% |
| Statin | 22.3% | 59.7% | 40.3% |
| Non-statin | 3.0% | 55.1% | 44.9% |
| Both | 2.7% | 63.9% | 36.1% |
| All Patients | 100.0% | 61.1% | 36.1% |
Statin = used statins alone; Non-statin = used non-statin classes of cholesterol-lowering medications alone; Both = used both statins and non-statin classes of cholesterol-lowering medications; None = prescribed no cholesterol-lowering medications
Do not sum up to 100% due to rounding errors.
Table 4 lists hazard ratios by type of cholesterol-lowering therapy, adjusting for demographic factors, diabetes severity, and other confounders. Compared to those not receiving any cholesterol-lowering medications, users of statins alone were about 35% less likely to experience any LEA (HR = 0.65; 95% CI, 0.42–0.99; P = 0.045) and 43% less likely to experience a treatment failure (HR = 0.57; 95% CI, 0.54–0.60; P < 0.001). The LEA risk for non-statin users was not significantly different (HR = 0.95; 95% CI, 0.35–2.60; P = 0.915) from that for no users but they were 41% less likely to experience a treatment failure (HR = 0.59; 95% CI, 0.51–0.68; P < 0.001) than non-users. Individuals on both statin and non-statin agents were 50% less likely to experience a treatment failure (HR = 0.51; 95% CI, 0.43–0.59; P < 0.001).
Table 4.
Hazard Ratios and Their 95% Confidence Intervals of Any Lower-Extremity Amputation (LEA) or Any LEA or Death for Users of Different Cholesterol-Lowering Medications Compared to Non-Users*
| LEA | LEA or Death | |||
|---|---|---|---|---|
| HR (95% CI)† | P-Value | HR (95% CI) | P-Value | |
| Statin | 0.645 (0.420 – 0.991) | 0.045 | 0.569 (0.537 – 0.602) | < 0.001 |
| Non-Statin | 0.946 (0.345 – 2.596) | 0.915 | 0.586 (0.508 – 0.676) | < 0.001 |
| Both | - | - | 0.505 (0.433 – 0.589) | < 0.001 |
For LEA, a reliable estimate was not available for the “Both” group due to small number of events (n = 7); HR = hazard ratio; CI = confidence interval; Both models were adjusted for age, race/ethnicity, marital status, A1c, body mass index, insulin use and/or oral antihyperglycemic medication use, history of vascular surgery, and comorbidities (foot ulcer, coronary artery disease, peripheral arterial disease, peripheral neuropathy, foot ulcers, and osteomyelitis). The LEA model was additionally adjusted for mortality as a competing risk.
Hazard ratios for LEA were adjusted for deaths as competing risks.
DISCUSSION
Our results show that 0.3% of non-elderly patients with diabetes who did not use any cholesterol-lowering medications at baseline had a major amputation in the lower extremities and 14% died during a five-year follow-up. Within this cohort, patients who used statins had a 35% lower risk of an LEA, compared to those who were not treated with any cholesterol-lowering medications. The use of other classes of medications appears to be minimally, if at all, associated with lower risk of LEA but our sample was not adequately powered to test statistical significance of this association.. On the other hand, the use of any cholesterol-lowering medications (statins, non-statin medications, or both) was associated with increased amputation-free survival over five years. These results imply that statin use may be associated with a decrease in amputation risk but the use of non-statin agents may not share in this protection against limb loss.
Since their introduction, statins have been used not only for LDL-c reduction but for improvement in survival in patients at high-risk for cardiovascular events. Randomized controlled trials such as the Heart Protection Study,13 Collaborative Atorvastatin Diabetes Study,14 and Scandinavian Simvastatin Survival Study (4S)15 provide strong clinical evidence that statin therapy is associated with a reduction in mortality both for primary and secondary prevention, particularly in patients with diabetes.
Statins have also been shown to modify disease burden and morbidity in patients with lower-extremity occlusive disease. In the Regression Growth Evaluation Statin Study (REGRESS), pravastatin use was associated with decrease in atherosclerotic plaque burden, as assessed by ultrasound of the femoral artery.16 In small cohorts, statin therapy has been demonstrated to increase walking time and distance17 and delay functional decline.18 The 4S study was the first randomized controlled trial to demonstrate an improvement in PAD symptoms. In the 153 subjects with PAD, simvastatin was associated with a 38% reduction in new or worsening intermittent claudication.19 In patients with diabetes, peripheral neuropathy is an independent risk factor for LEA and increases the risk for foot ulcers and LEA multiplicatively in the presence of PAD.20 Previous research has shown that statins are associated with improvement in microvascular function and peripheral neuropathy, independent of LDL-c lowering.21 Despite accumulating evidence that statins have these beneficial effects on vascular health, clinical research until now has not clearly demonstrated beneficial effects of statins on amputation risk.22
Some propose that in addition to the proven benefits of LDL cholesterol-lowering on atherosclerotic cardiovascular disease (ASCVD) risk, pleiotropic effects of statins may explain other favorable results In the Justification for the Use of Statins in Primary Prevention study, a primary prevention clinical trial, investigators showed benefit of those men ≥50 and women ≥ 60 years, both with hs-CRP > 2 mg/L, randomized to rosuvastatin 20 mg/day as contrasted to placebo as regards the combined primary endpoint of ASCVD outcomes.23 While this result is explained by the statin-effects on lowering LDL-C levels, the 43% reduction in the risk of venous thromboembolism in those randomized to rosuvastatin in this trial is less convincingly assumed to be related to statin-induced LDL-C reduction. 24
The role of non-statin cholesterol-lowering medications in the maintenance of diabetic limb health is unclear. In our cohort, non-statin cholesterol-lowering medications were associated with a non-significant decrease in amputation and a less robust, but significant, reduction in treatment failure. In comparison, the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial evaluated the use of fenofibrate as monotherapy in 9,795 subjects with type 2 diabetes.25 Fenofibrate did not significantly reduce the risk of the primary outcome of coronary events or total mortality. It did reduce total cardiovascular events, mainly due to fewer non-fatal myocardial infarctions and revascularizations. Over the 5 years of follow-up, there was a significantly lower rate of minor amputations in the fenofibrate treated group but no significant difference in major amputations.26 The benefit of fenofibrate to prevent minor amputation was strongest in subjects with known microvascular disease such as retinopathy and microalbuminuria. There was no strong association between amputation and known large vessel arterial occlusive disease. Patients in our cohort were treated with non-statin agents for only 6% of all periods (3% with non-statin agents alone and 2.7% with statins and non-statins together) and, as such, our ability to draw inferences about users of non-statin agents may be limited. Both our study and the FIELD study highlighted the unique pathology of the microvasculature in diabetes.
One must recognize the limitations inherent in the inclusion/exclusion criteria for this cohort study that may limit its broad generalizability. The study population is entirely persons with diabetes, disproportionately male, and limited to those who are non-elderly. It remains unclear if a similar benefit of statins is attributable to individuals with different characteristics. Given that this is a retrospective study, we were required to assume that statin prescriptions filled during each period implied actual use. We also could not entirely exclude the possibility that some statins were filled outside the VA and were not accounted for in our analysis. Finally, as in other retrospective studies, it was impossible for us to exclude all sources of confounding, especially one due to patient selection. When we compared baseline characteristics of patients between non-users and statin users, we found that statin users did not necessarily have better vascular health at baseline than non-users to the extent that patient selection alone could have explained the differences in event rates between our comparison groups. It is noteworthy that the rate of statin use in our cohort is surprisingly low. Even though we did not have information on contraindications to safe statin use, such as allergies and adverse side effects, it is worrisome that patients in our study cohort did not meet the LDL-c target (<100 mg/dL) for 36.1% of all periods during follow-up but were not treated with any cholesterol-lowering medication in 70.8% of these periods (Table 3). Patients in general were treated with statins for 25% of all periods (22.3% with statins alone and 2.7% with both statins and other agents). Limited statin use in our cohort is not an exception, however. Under-utilization of statins and sub-optimal LDL-c levels have been documented in other diabetic populations as well.27 While this study supports a positive relationship between statin use and limb survival, our ability to draw an inference about the positive effect of statins on the micro- and macrovasculature is limited. Unfortunately, our clinical cohort remains incompletely characterized. In the literature, one third of patients with diabetes have concomitant PAD.28 Our cohort has a low reported rate of PAD (3% at baseline), which likely reflects broad underdiagnosis. Because ankle-brachial index (ABI) measures were not available for use in this study, we could not verify accuracy of PAD based on the ICD-9-CM diagnostic coding in inpatient and outpatient administrative records.
In conclusion, this is the first study that demonstrated protective effects of statins in LEA. Even though we limited our sample to non-elderly diabetic patients, our large sample from observational data allowed us to observe a striking effect of statin therapy on LEA risk and amputation-free survival. Finally, we showed that there were a large number of patients with diabetes who were not treated for cholesterol-lowering. These findings indicate an area where clinical practice may need improvement and at the same time offer an opportunity for a future study. While prospective clinical trial data support widespread use of statins in those with diabetes and ASCVD,5 it is not known if statin therapy that titrates to ATP-III goal is superior to just initiating fixed dose statin therapy in this high-risk population. A clinical trial that randomized those with diabetes and ASCVD to either an intensive treatment arm (using a maximally tolerated statin dosage to achieve an LDL-C <70 mg/dl) or merely a fixed moderate dose arm (such as simvastatin 40 mg/day or atorvastatin 10 mg/day) has the promise to provide useful information for the care of these patients. Given that moderate dose statins in the CARDS trial had a beneficial effect on CVD outcomes, it is important to know if using high-dose potent statins with their attendant increase in statin-related side effects and possibly decreased adherence is worth the increased cost and effort.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the financial support from the Center for Management of Complex Chronic Care, Hines VA Hospital, Hines, IL (LIP 42-512), the National Institutes of Health (5T32HL094293), and the Agency for Healthcare Research and Quality(1R01HS018542- 01A2). The paper presents the findings and conclusions of the authors; it does not necessarily represent the Department of Veterans Affairs, Health Services Research and Development Service, or the National Institutes of Health. The corresponding author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
DISCLOSURE
The authors have no conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
MWS researched data, wrote manuscript. NJS, EHO, WBP, and JLM wrote/edited manuscript. EBM, TAL, and WBP contributed to discussion, reviewed/edited manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) Circulation. 2006;113(11):e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 3.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 4.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005–16. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 5.Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371(9607):117–25. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 6.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27 (Suppl 2):B10–B21. doi: 10.2337/diacare.27.suppl_2.b10. [DOI] [PubMed] [Google Scholar]
- 7.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. PopulHealth Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bittner V, Hardison R, Kelsey SF, Weiner BH, Jacobs AK, Sopko G. Non-high-density lipoprotein cholesterol levels predict five-year outcome in the Bypass Angioplasty Revascularization Investigation (BARI) Circulation. 2002;106(20):2537–42. doi: 10.1161/01.cir.0000038496.57570.06. [DOI] [PubMed] [Google Scholar]
- 9.National Research Council of the National Academies. The Prevention and Treatment of Missing Data in Clinical Trials. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 10.Zhang X, Zhang MJ, Fine J. A proportional hazards regression model for the subdistribution with right-censored and left-truncated competing risks data. Stat Med. 2011;30(16):1933–51. doi: 10.1002/sim.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer. 2004;91(7):1229–35. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adult Treatment Panel III, National Institute of Health. Third Report of the National Cholesterol Education Program Expert Panel and Detection, Evaluation, and Treatment of High Blood Cholesterol in Adult. 2006. [Google Scholar]
- 13.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 14.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 15.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–9. [PubMed] [Google Scholar]
- 16.de Groot E, Jukema JW, Montauban van Swijndregt AD, Zwinderman AH, Ackerstaff RG, van der Steen AF, et al. B-mode ultrasound assessment of pravastatin treatment effect on carotid and femoral artery walls and its correlations with coronary arteriographic findings: a report of the Regression Growth Evaluation Statin Study (REGRESS) J Am Coll Cardiol. 1998;31(7):1561–7. doi: 10.1016/s0735-1097(98)00170-3. [DOI] [PubMed] [Google Scholar]
- 17.McDermott MM, Guralnik JM, Greenland P, Pearce WH, Criqui MH, Liu K, et al. Statin use and leg functioning in patients with and without lower-extremity peripheral arterial disease. Circulation. 2003;107(5):757–61. doi: 10.1161/01.cir.0000050380.64025.07. [DOI] [PubMed] [Google Scholar]
- 18.Giri J, McDermott MM, Greenland P, Guralnik JM, Criqui MH, Liu K, et al. Statin use and functional decline in patients with and without peripheral arterial disease. J Am Coll Cardiol. 2006;47(5):998–1004. doi: 10.1016/j.jacc.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen TR, Kjekshus J, Pyorala K, Olsson AG, Cook TJ, Musliner TA, et al. Effect of simvastatin on ischemic signs and symptoms in the Scandinavian simvastatin survival study (4S) Am J Cardiol. 1998;81(3):333–5. doi: 10.1016/s0002-9149(97)00904-1. [DOI] [PubMed] [Google Scholar]
- 20.Sohn MW, Stuck RM, Pinzur M, Lee TA, Budiman-Mak E. Lower-extremity amputation risk after charcot arthropathy and diabetic foot ulcer. Diabetes Care. 2010;33(1):98–100. doi: 10.2337/dc09-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leiter LA. The prevention of diabetic microvascular complications of diabetes: is there a role for lipid lowering? Diabetes Res Clin Pract. 2005;68 (Suppl 2):S3–14. doi: 10.1016/j.diabres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Aikawa M. Effects of statin therapy on vascular dysfunction. Coronary artery disease. 2004;15(5):227–33. doi: 10.1097/01.mca.0000132583.04711.47. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 24.Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360(18):1851–61. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849–61. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 26.Rajamani K, Colman PG, Li LP, Best JD, Voysey M, D’Emden MC, et al. Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a prespecified analysis of a randomised controlled trial. Lancet. 2009;373(9677):1780–8. doi: 10.1016/S0140-6736(09)60698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M, Ong KL, Tse HF, Cheung BM. Utilization of lipid lowering medications among adults in the United States 1999–2006. Atherosclerosis. 2010;208(2):456–60. doi: 10.1016/j.atherosclerosis.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.