Abstract
We describe the aetiology of community-acquired pneumonia in children before and after the introduction of the pneumococcal conjugate vaccination (PCV) programme in 2006.
Prospective studies were conducted in 2001–2002 (pre-vaccine) and 2009–2011 (post-vaccine) of children aged 0–16 years with radiologically confirmed pneumonia seen in hospital. Investigations included culture, serology, immunofluorescence antibody and urine antigen testing, with an increased use of PCR assays and expanded panels of pathogens in the post-vaccine study.
241 and 160 children were enrolled in the pre- and post-vaccine studies, respectively (73% aged <5 years). Identification of a causative pathogen was higher post-vaccination (61%) than pre-vaccination (48.5%) (p=0.019). Rates of bacterial infections were not different between post- and pre-vaccine studies (17.5% versus 24%, p=0.258). Viral (31%) and mixed (12.5%) infections were found more often post-vaccination (19.5%, p=0.021) than pre-vaccination (5%, p=0.015). Rates of identified pneumococcal infections were comparable between pre- and post-vaccine studies (14.7% versus 17.4%, p=0.557). Diagnosis of pneumococcal infection post-vaccination improved when PCR was used compared to culture (21.6% versus 6%, p=0.0004). Serotypes included in PCV13 but not PCV7 were identified in 75% (18 out of 24) post-vaccination.
Infection with nonvaccine pneumococcal serotypes continues to be a significant cause of pneumonia in children in the UK.
Short abstract
Aetiology of community-acquired pneumonia in children following a pneumococcal conjugate vaccination programme http://ow.ly/p9Wub
Introduction
The range of implicated pathogens in paediatric community-acquired pneumonia (CAP) is wide and includes viruses, bacteria and co-infection with both [1, 2]. Studies of pneumonia frequently report low levels of pathogen identification, although improved knowledge of pneumonia aetiology is essential for the development of targeted management and effective public health strategies such as vaccination [3, 4]. In the UK, the 7-valent pneumococcal conjugate vaccine (PCV) was introduced routinely in September 2006 and replaced by PCV13 from April 2010. The vaccine schedule is three doses administered at 2, 4 and 13 months of age. When first introduced, those over and under 1 year of age received one and two doses, respectively, as part of a catch-up programme for children aged <2 years.
Identifying the aetiology of paediatric CAP is challenging, with a large number of potential pathogens, some of which may also be carried as commensal organisms, which can complicate the interpretation of the results of testing nasopharyngeal samples [5]. Conventional methods such as blood culture and serology often have limited sensitivity due to inadequate sample volume or lack of convalescent sera [3]. Molecular diagnostics are now routinely used in the assessment of viral respiratory infections and similar techniques have been developed for the detection of bacterial respiratory infections [6, 7]. Resti et al. [8] demonstrated a significant improvement in the identification of pneumococcal pneumonia in children by PCR on blood samples (15.4%) when applied simultaneously with blood culture (3.8%). In a recent study of Italian children aged <5 years, overall bacteraemic pneumococcal pneumonia was identified in 14.3%, which was established by PCR in 92%, blood culture in 1% and both in 7% of subjects [9].
The introduction of PCV was expected to decrease the incidence of pneumonia in children, and this was supported by a region-wide epidemiological prospective survey [10]. We present data from studies conducted over two periods, before (2001–2002) and after (2009–2011) the addition of PCV. These were designed to describe the proportion of causative pathogens in paediatric CAP and describe how the identification of causative pathogens could be improved with the application of more PCR-based assays. As disease incidence declined, a longer recruitment period in the post-vaccine study was therefore planned, in order to have a larger cohort with representative aetiological data.
Methods
Participants
Two prospective studies were undertaken from August 2001 to July 2002 and October 2009 to March 2011. Enrolled children were from the North East of England (excluding Cumbria) who were aged 0–16 years and who presented with clinical and radiological features suggestive of pneumonia. They were admitted to the paediatric services at the Great North Children's Hospital, Newcastle upon Tyne (formerly Newcastle General and Royal Victoria Infirmary), the regional cardiothoracic centre at the Freeman Hospital (Newcastle upon Tyne) or the James Cook Hospital (Middlesbrough). The cohort of 2001–2002 study was a proportion of children with pneumonia seen at these recruitment sites as part of a previously published regional epidemiological survey [11]. They consented and were enrolled in the aetiological study with an extended panel of investigations. Written informed consent was obtained from parents and assent obtained from older children. Ethical approval was granted by the Newcastle and North Tyneside research ethics committee and the research approval board at South Tees Hospitals NHS Trust, Middlesbrough. Caldicott approval was also obtained.
Research teams of doctors and nurses led and ascertained the standardised diagnosis of pneumonia and the recruitment procedures. Recruitment methodology and enrolment criteria were consistent across the two studies and included children with any history, signs or symptoms suggestive of lower respiratory tract infection, including any of fever, tachypnoea (defined age-specific respiratory rates), dyspnoea, cough, respiratory distress, chest wall retractions and auscultatory findings such as crackles, bronchial breathing or reduced breath sounds, together with chest radiographic findings consistent with pneumonia as determined initially by the local paediatrician. Data on C-reactive protein and full blood count indices were used when clinically indicated by the admitting teams to inform the diagnosis of pneumonia. Exclusion criteria included residing outside North East England, clinical diagnosis of bronchiolitis, hospitalisation in the preceding 3 weeks or normal chest radiograph after formal reporting by a radiologist.
All chest radiographs were reviewed by second consultant radiologists (one for each study) at the regional centre in Newcastle upon Tyne who were blinded to both the clinical data and the first reports. Radiological findings were categorised into lobar, patchy or perihilar, according to the World Health Organization criteria [12]. Pneumococcal conjugate immunisation history including the valency of PCV was obtained from parents and cross-checked with the child's parent-held health records. General practice surgeries were contacted to clarify the doses given if there was uncertainty. Immunisation history was not collected in the pre-vaccine study.
Laboratory procedures
Blood samples were collected for serum, blood culture (BacT/ALERT; bioMérieux, Craponne, France) and pneumococcal PCR testing. Approximately 4 weeks later blood was collected for convalescent serology. Parents often declined returning for these convalescent samples (all in the post-vaccine study), contributing to the variability in the number of investigations performed. Nasopharyngeal secretions included aspirates from infants, and/or swabs from older children. Nasopharyngeal aspirate samples were placed in 0.9% sodium chloride transport solution or swabs (Medical Wire and Equipment Co. Ltd, Corsham, UK). Tracheobronchial secretions (collected via endotracheal tube or bronchoalveolar lavage) and pleural fluid were tested when obtained. The nature of the collected samples were standardised across all ages and in both studies. Recovery of bacterial pathogens from nasopharyngeal secretions, sputum or by urinary pneumococcal antigen was not considered evidence of definite infection, due to the risk of physiological colonisation [3, 13]. Therefore, only positive results that were classified as likely causative pathogens of pneumonia according to defined diagnostic criteria are presented and discussed (table 1). Any positive results from the potentially colonised above-mentioned sites were added together separately and rates were grouped among “unknown causes” in figure 1 for the reader’s information.
Table 1– Laboratory investigations and diagnostic criteria.
| Sample | Pathogen/antigen | Tests | Diagnosis of causative pathogens# | |
| 2001–2002 study | 2009–2011 study | |||
| Serum | Respiratory viruses | Complement fixation | Complement fixation | Acute titre ≥1/128 or four-fold rise between paired sera |
| Atypical bacteria | ||||
| Mycoplasma | IgM antibody | IgM antibody | Positive | |
| Group A Streptococcus | ASOT (IU·mL−1) | ASOT (IU·mL−1) | Acute two-fold rise or four-fold rise between paired sera | |
| Blood | Bacteria | Culture | Culture | Growth |
| Streptococcus pneumoniae | Real-time PCR | Real-time PCR | Positive | |
| Nasopharyngeal secretions/sputum | Respiratory viruses | IFAT | Real-time PCR | Positive |
| Bacteria | Culture | Culture/real-time PCR | Not applicable | |
| Tracheobronchial secretions (bronchoalveolar lavage/endotracheal) | Respiratory viruses | IFAT | Real-time PCR | Positive |
| Bacteria | Culture | Culture/real-time PCR | Growth/positive | |
| Pleural fluids | Bacteria | Culture | Culture | Growth |
| Pneumococcal antigen | ELISA | ELISA | Positive | |
| Streptococcus pneumoniae | Not tested | Real-time PCR | Positive | |
ASOT: antistreptolysin O titre; IFAT: immunofluorescence antibody testing. #: definite/probable.
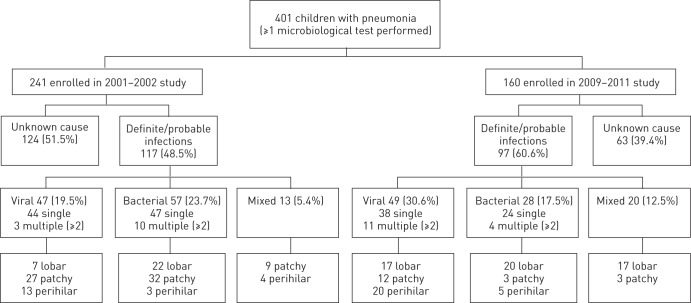
Figure 1–
Summary of the aetiological and radiological classifications.
Where tests were not part of routine clinical care, samples were stored at -20°C for subsequent analysis. Investigations were performed in the microbiology laboratory, Newcastle upon Tyne Hospitals NHS Trust and the Health Protection Agency (HPA) Public Health Laboratory, Newcastle upon Tyne. Apart from locally performed routine diagnostic tests, samples from Middlesbrough were transported to Newcastle upon Tyne via daily transport services. Pneumococcal isolates from blood and respiratory secretions including pleural fluids were serotyped by multiplexed immunoassay using xMAP beads (Bio-Plex, Bio-Rad, Hercules, CA, USA) for detection of serotype-specific Streptococcus pneumoniae antigens at the national HPA Respiratory and Systemic Infection Laboratory (London, UK) [14].
Viral laboratory diagnostic tests
Pre-vaccination, immunofluorescence antibody testing (IFAT) was applied to respiratory secretions using Chemicon (Temecula, CA, USA) SimuFluor fluorescein isothiocyanate for respiratory syncytial virus (RSV), influenza A and B, parainfluenza 1–3 and adenovirus. Human metapneumovirus (hMPV) was tested for using IFAT utilising an in-house pool of anti-hMPV monoclonal antibodies [15]. Viral screening was performed in the post-vaccine study using an in-house multiplex real-time PCR assay. The target panel was expanded to include pandemic influenza A subtype H1N1, parainfluenza virus 4, rhinovirus, coronavirus (229E, OC43 and NL63) and bocavirus, plus the viruses previously tested for by IFAT. Viral serological tests included RSV, influenza A and B and adenovirus.
Bacterial laboratory diagnostic tests
Total nucleic acid was extracted from blood samples from both studies and tested using a S. pneumoniae-specific PCR assay targeting the pneumolysin gene [16]. An acute complement fixation test antibody screen for “atypical” bacteria and respiratory viruses was also performed, which included Mycoplasma IgM antibody, M. pneumoniae, Chlamydia pneumoniae and C. psittaci, as well as Coxiella burnetii and Legionella pneumophila serogroup 1–6 in the post-vaccine study. Antistreptolysin O titre was assayed in both studies using a Rheumajet ASO kit (Launch Diagnostics, Longfield, UK).
Statistical analysis
Data were analysed using Epi InfoTM 7 (Centers for Disease Control and Prevention, Atlanta, GA, USA). A summary of the isolated pathogens was presented as frequencies and categorised as viral, bacterial or mixed viral–bacterial infections. Detection rates of pathogens are expressed as proportions of those tested, and results were compared in relation to age group. The age group classification was under or above 5 years, as by the start of post-vaccine study; children in the <5 years age group should have been vaccinated with PCV. This would allow the investigation of the relative contribution of pneumococcal infection in causing pneumonia, as well as the role of other pathogens. Where there was a common methodology for diagnosis between studies, identification rates of infections were compared using Fisher's exact test with odds ratios and 95% confidence intervals.
A subgroup analysis within the post-vaccine study was performed on children enrolled before the PCV13 was introduced in April 2010. This was to compare the rates of infection groups in relation to the data from the pre-vaccine study and all from the post-vaccine study (October 2009 to March 2011).
Results
A total of 401 children were enrolled; 241 and 160 in the pre- and post-vaccine studies, respectively. All had at least one microbiological investigation performed. There were similar demographic characteristics between the pre- and post-vaccine studies, including median age (2.5 versus 2.6 years), proportions of males (57% versus 56%) and aged <5 years (75.5% versus 69%). figure 1 summarises the aetiological and radiological classifications and table 2 lists the results of the diagnostic tests performed. Lobar consolidation was more often present post-vaccination (61%) compared to 23% pre-vaccination (p<0.001). A likely causative pathogen was established in 61% of children post-vaccination, compared to 48.5% pre-vaccination (OR 0.6, 95% CI 0.41–0.92; p=0.019) when the results of all tests were combined.
Table 2– Results of the diagnostic tests performed.
| 2001–2002 study# | 2009–2011 study¶ | |||
| Tests performed | Positive results | Tests performed | Positive results | |
| Blood and serology | 238 | 75 (31.5) | 138 | 32 (23.2) |
| Blood overall | 236 | 36 (15.3) | 136 | 13 (9.6) |
| Bacterial culture | 185 | 6 (3.2) | 126 | 7 (5.6) |
| Streptococcus pneumoniae PCR | 228 | 30 (13.2) | 86 | 7 (8.1) |
| Serology overall | 181 | 49 (27.0) | 105 | 22 (21.0) |
| Acute serology | ||||
| Mycoplasma IgM antibody | 34 | 11 (32.4) | 77 | 8 (10.4) |
| ASOT | 158 | 12 (7.6) | 80 | 9 (11.3) |
| Mycoplasma/Chlamydia | 128 | 8 (6.3)/0 | 51/39 | 0 |
| Legionella/Q-fever | 0 | 0 | 50/42 | 0 |
| Influenza A/B | 158 | 1 (0.6)/2 (1.2) | 68/62 | 7 (10.3)/0 |
| RSV/adenovirus | 158 | 2 (1.2)/2 (1.2) | 52/46 | 0 |
| Convalescent serology | ||||
| ASOT | 52 | 2 (3.8) | 0 | 0 |
| Mycoplasma/Chlamydia | 14 | 3 (21.4)/0 | 0 | 0 |
| Influenza A/B | 101 | 1 (1.0)/0 | 0 | 0 |
| RSV/adenovirus | 101 | 6 (6.0)/6 (6.0) | 0 | 0 |
| Respiratory secretions overall | 175 | 59 (33.7) | 151 | 121 (80.1) |
| Viral screen | 158 | 44 (27.9) | 141 | 63 (44.7) |
| Bacterial culture (TBS) | 14 | 5 (35.7) | 12 | 7 (58.3) |
| Pleural fluids overall | 17 | 4 (23.5) | 40 | 27 (67.5) |
| Bacterial culture | 17 | 2 (11.8) | 40 | 10 (25.0) |
| Pneumococcal antigen | 17 | 2 (11.8) | 30 | 7 (23.3) |
| Pneumococcal real-time PCR | 0 | 0 | 30 | 18 (60.0) |
Data are presented as n or n (%). ASOT: antistreptolysin O titre; RSV: respiratory syncytial virus; TBS: tracheobronchial secretions. #: n=241; ¶: n=160.
41 children were not eligible for the PCV due to age criteria, while its uptake was 94% (112 out of 119) among eligible children (89 PCV7, 10 PCV13 and 13 received combined doses of each with age-appropriate schedule). Of those vaccinated with PCV7 either routinely or according to the catch-up programmes, 83 received age-appropriate doses (57 had full schedule) whereas six children had a partial schedule with one dose less for their age. Among those who had PCV13, one received the full schedule, one child had one dose less for age, and eight had not completed but had appropriate doses for their age.
Viral infections
table 3 shows the number of pathogens identified with age-group distribution. Viral (31%) and mixed infections (12.5%) were significantly higher post-vaccination than pre-vaccination, at 19.5% (p=0.021) and 5% (p=0.015), respectively. The detection of viruses using a combination of PCR and serological assays post-vaccination (85 (57%) out of 149) was higher than that achieved testing with immunofluorescence and serology pre-vaccination (65 (30.5%) out of 213). This improvement in viral detection was thought to be due to the application of PCR assays (44.7%) replacing immunofluorescence testing (27.9%) on respiratory secretions (p=0.003). Post-vaccination, acute viral serological assays were only positive in seven patients with influenza A virus infection, whereas pre-vaccination, combined acute and convalescent serology identified eight patients with RSV infection, eight patients with adenovirus, four with influenza A/B viruses, and one with Epstein–Barr virus.
Table 3– Detected likely causative pathogens by age group.
| 2001–2002 study | 2009–2011 study | |||||
| <5 years | 5–16 years | n/N (%) | <5 years | 5–16 years | n/N (%) | |
| Bacterial | ||||||
| Streptococcus pneumoniae | 28 | 7 | 35/238 (14.7) | 14 | 10 | 24/138 (17.4) |
| Mycoplasma pneumoniae | 9 | 13 | 22/176 (12.5) | 2 | 6 | 8/81 (9.9) |
| Group A Streptococcus | 5 | 9 | 14/202 (7.0) | 6 | 8 | 14/133 (10.5) |
| Staphylococcus aureus | 3 | 2 | 5/189 (2.6) | 1 | 2 | 3/130 (2.3) |
| Haemophilus influenzae | 0 | 2 | 2/189 (1.0) | 3 | 0 | 3/130 (2.3) |
| Bordetella pertussis | 1 | 0 | 1/189 (0.5) | 0 | 0 | 0/121 |
| Moraxella catarrhalis | 1 | 0 | 1/189 (0.5) | 2 | 1 | 3/130 (2.3) |
| Streptococcus intermedius | 1 | 0 | 1/189 (0.5) | 0 | 1 | 1/130 (0.8) |
| α-haemolytic Streptococcus | 1 | 0 | 1/189 (0.5) | 0 | 0 | 0/130 |
| Klebsiella pneumoniae | 0 | 0 | 0/189 | 1 | 0 | 1/130 (0.8) |
| Viral | ||||||
| RSV (not typed) | 29 | 3 | 32/213 (15.0) | 0 | 0 | 0 |
| RSV type A | 0 | 0 | 0 | 19 | 0 | 19/147 (13.0) |
| RSV type B | 0 | 0 | 0 | 11 | 1 | 12/147 (8.2) |
| Influenza A and B viruses | 9 | 4 | 13/213 (6.0) | 7 | 4 | 11/149 (7.4) |
| Adenovirus | 11 | 2 | 13/213 (6.0) | 10 | 0 | 10/145 (6.9) |
| Parainfluenza 1–4 | 5 | 0 | 5/158 (3.2) | 5 | 1 | 6/141 (4.3) |
| Human metapneumovirus | 0 | 0 | 0/48 | 1 | 0 | 1/141 (0.7) |
| Epstein–Barr virus | 0 | 1 | 1/1 (100) | Not tested | Not tested | |
| Varicella zoster virus | 0 | 1 | 1/1 (100) | Not tested | Not tested | |
| Rhinovirus | Not tested | Not tested | 10 | 2 | 12/141 (8.5) | |
| Pandemic influenza A H1N1 | Not tested | Not tested | 4 | 3 | 7/141 (5.0) | |
| Bocavirus | Not tested | Not tested | 2 | 2 | 4/121 (3.3) | |
| Coronavirus (type OC43) | Not tested | Not tested | 2 | 1 | 3/121 (2.5) | |
| Total | 103 | 44 | 147 | 100 | 42 | 142 |
Data are presented as n, unless otherwise stated. N represents the total number of performed tests with positive results classified as definite/probable infections. RSV: respiratory syncytial virus.
Post-vaccination, RSV was detected in 21% (31 out of 147) of samples, of which 19 were type A, with rhinovirus (8.5%), influenza (7%) and adenoviruses (7%). These figures were comparable with those pre-vaccination for adenovirus and influenza A/B (6% each); but higher than that for RSV (15%). Of the 142 identified causative pathogens post-vaccination, 71 (50%) viruses were detected among those aged <5 years, compared to the finding in the pre-vaccine study (54 (36%) out of 149). hMPV was not detected in any of the 48 pre-vaccination respiratory samples tested, but was identified in one child in the post-vaccine study.
Bacterial infections
There was no difference in the rates of bacterial infections post-vaccination, (17.5% of the total) compared to 24% pre-vaccination (p=0.258). Overall, identified pneumococcal infections were not different between the studies (p=0.557). They represent 17.4% among children tested post-vaccination (14 (15%) out of 93 and 10 (22.2%) out of 45 in those aged <5 years and >5 years, respectively). This was compared to 14.7% pre-vaccination (28 (15.6%) out of 180 and seven (12%) out of 58 among those aged <5 years and >5 years, respectively). In the post-vaccine study, diagnosis of pneumococcal infection improved when PCR was used (21 (21.6%) out of 97) compared to culture (eight (6%) out of 132) (p=0.0004). A serotype was identified in 75% (18 out of 24) in the post-vaccine study. These were serotypes 1 (44.4%), 3 (27.8%), 19A (22.2%) and 7A/F (5.6%). The rate of positive blood culture post-vaccination was almost double (5.6%) that pre-vaccination (3.2%).
Group A streptococcal infections were confirmed in a greater proportion of children (10.5%) post-vaccination than in the pre-vaccination group (7%). These infections were associated with severe disease, and in two-thirds of cases with empyema. M. pneumoniae was identified from acute serology in 9.9% of children post-vaccination, with 4% (two out of 51) in those aged <5 years and 20% (six out of 30) in patients aged >5 years. The rate of detected mycoplasma infection was higher pre-vaccination (12.5%) when paired acute and convalescent samples were available, with 7% (nine out of 128) in those aged <5 years and 27% (13 out of 48) in those aged >5 years.
Subgroup analysis: October 2009 to March 2010
Among 67 children enrolled during this period, the causative pathogen was identified in 37 (55%). S. pneumoniae was identified in 18.3% (11 out of 60) compared to 16.7% (13 out of 78) during the first year of PCV13 vaccination (p=0.824). Rates of infections were 22.4% bacterial, 22.4% viral and 10.5% mixed. The rate of bacterial infection is similar to the figures from the pre- and entire post-vaccine studies. There was no difference between the rates of viral infection before and after the introduction of PCV13 during the post-vaccine study (p=0.079).
Discussion
This is the first published study to describe the aetiology of CAP in UK children prior to and following the introduction of the PCV7. The timing of this comprehensive study, 3 years after the introduction of PCV7 and during the first year of PCV13, provides a baseline for future comparative studies of the pneumonia aetiology in the same setting. The causative pathogens identified were predominately viruses in both studies, with the detection of pneumococcal infections increasing from pre-to post-vaccine studies, presumably as a consequence of the application of molecular diagnostic methods.
Previous UK studies investigating the aetiology of pneumonia prior to the introduction of the PCV7 were able to identify the causative pathogens in up to 54% of children [17–19]. In addition to blood cultures, blood was tested for S. pneumoniae, Mycoplasma and Chlamydia using PCR and identified 8% of children with pneumococcal pneumonia [18]. Another study identified 6% pneumococcal infection using pneumolysin ELISA on blood [19]. The identification rate of 61% for the likely causative pathogens in the post-vaccine study is similar to that reported by Don et al. [20], who used serological assays. However, this is lower than the detection rates of ∼80% from studies which used serological and/or molecular approaches [21–23], but higher than the rates previously found prior to the introduction of the conjugate vaccine [24, 25]. The improved detection rate between our two studies appears to be related to the different laboratory approaches used.
While molecular diagnostic methods have improved respiratory virus detection and bacterial detection from normally sterile sites, the interpretation of results can be more problematic when it is applied to nasopharyngeal secretions and other respiratory samples [3, 13]. Additionally, pneumococcal pneumolysin DNA can be detected in the blood of healthy children colonised with pneumococcus [26]. The pneumolysin gene can also be detected in non-pneumococcal Viridans-group streptococci, particularly S. pseudopneumoniae and S. mitis [5]. Potential confounders resulting from using a pneumolysin PCR in this study therefore include false positives associated with pneumococcal carriage or cross-reactivity with other Viridans-group streptococci. Given the very low pneumococcal carriage rate in this population (7%), the former is unlikely. Although not well explored, viral carriage is also a clinical possibility, leading to positive PCR results that do not necessarily correlate to the observed pneumonia [27]. Hence the results of PCR-based approaches can be limited in making a definite diagnosis of causative pathogens in pneumonia.
The rates of pneumococcal infection from the two datasets are lower than in studies in other countries [3], but are higher than those previously described in the UK [18, 19]. Improvement of pneumococcal identification with the application of PCR when compared to culture alone is consistent with previous studies [9, 28–30]. This is similar to the reported increase in a recent Italian study (from 3.8% to 15.4%) [8]. It is interesting that despite the overall decrease in the incidence and hospitalisation of pneumonia since the introduction of PCV7 [31, 32], the rates of pneumococcal infection were comparable between the two studies. Replacement with non-PCV7 serotypes causing invasive pneumococcal disease is well recognised [33], and this may explain our findings in the face of reduction in disease incidence. Where pneumococcal serotyping was possible, with the majority being identified from pleural fluids, all serotypes recovered were non-PCV7 but included in PCV13. This is similar to data from the USA on children with empyema, where 98% were non-PCV7 serotypes and primarily similar to the serotypes in our study [34]. Despite the lack of comprehensive serotype data, this suggests that PCV13 could substantially reduce invasive pneumococcal disease [35, 36].
Serological evidence of Mycoplasma infection was detected in 12.5% and 9.9% of children in the pre- and post-vaccination studies, respectively, rates that are similar to the published literature [19, 25]. M. pneumoniae is traditionally considered a pathogen of older children, and in these studies was identified more frequently in those >5 years of age. No other serological evidence of other “atypical” organisms was identified, although this may have been as a consequence of the lack of convalescent sera. S. aureus and group A streptococcal infections were often associated with severe pneumonia and empyema [37, 38]. In keeping with previous findings, group A Streptococcus can be found in up to 7% of children with pneumonia, compared to 7% and 10.5%, respectively, in the pre- and post-vaccination datasets [19, 39]. With the introduction of PCV and decrease in pneumococcal pneumonia it is possible that the relative proportion of bacteria such as group A Streptococcus and S. aureus, as well as M. pneumoniae causing severe pneumonia will increase.
Viruses, either alone or as co-pathogens, were detected in 25% and 43% of children in the pre- and post-vaccine studies, respectively, with RSV being the most commonly detected pathogen, as previously reported [3, 40]. This was followed by rhinovirus, influenza and adenovirus at ∼7% each, similar to data previously described for the same region [19]. Diagnosis of viral infection was achieved mainly through the testing of respiratory secretions rather than by serology, which was only positive in seven children with influenza A virus. The improvement in the detection of viruses in the post-vaccine study was mainly achieved by the application of PCR assays for respiratory viral screening. Most of the viruses detected were identified in those aged <5 years, consistent with other studies [2, 23]. In the post-vaccine study, viral screening was expanded to include eight viruses with their subgroups, including pandemic H1N1, and to delineate their contribution in causing CAP in children in the UK. Considering the timing of the second recruitment period, pandemic influenza A H1N1 as a single pathogen was not implicated in many cases of pneumonia. The low isolation rates of bocavirus, coronavirus and hMPV highlight the minimal contribution of these viruses to the aetiology of pneumonia in children in the UK. The rates of mixed viral–bacterial infection were variable between the two studies and likely to be dependent on the screening methods used to identify the causative pathogens [3].
There are several limitations to our data, such as potential seasonal bias to the data of the post-vaccine study in which recruitment was carried out over 18 months which included two winter seasons (48% of enrolled children). Although the post-vaccine study covered two winter seasons, the numbers of children enrolled were fewer than in the pre-vaccine study, which could be a true reflection of decreased disease incidence and hospitalisation. The findings from the post-vaccine study may have been hampered by the lack of convalescent sera, which may have led to the underestimation of the role of atypical bacteria in paediatric pneumonia, but this effect is probably minimal as Mycoplasma infection was only detected in three children by paired serum samples in the pre-vaccine study. Lack of serotype data of the identified pneumococci from the pre-vaccine study limits the true comparison with the serotype profile after the implementation of the conjugate vaccine. Another limitation is the variation between the two studies in the diagnostic methods used and the pathogens investigated, which makes the interpretation of comparative findings guarded. The significant improvement in pathogen identification by the application of more PCR assays adds further evidence to the importance of using these techniques to monitor changes in the epidemiology of paediatric CAP and pneumococcal serotype replacement.
In conclusion, although viruses are the most common cause of pneumonia, around a fifth of children had bacterial infections. The combined use of culture, serology and PCR-based diagnostic tests significantly improved the identification of causative pathogens in paediatric CAP. Replacement of PCV7 with PCV13 was likely to be associated with as significant a reduction in pneumococcal disease as non-PCV7, but PCV13 serotypes predominated. This requires continued surveillance to monitor for the emergence of serotype replacement.
Acknowledgments
We are indebted to the paediatric staff for their facilitation of the recruitment. We thank the research nurses J. Kelly, K. Pollard and C. Simmister (Dept of Paediatric Infectious Disease and Immunology, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK) and C. Barwick and P. Singleton (Dept of Paediatrics, James Cook University Hospital, Middlesbrough, UK) for their assistance with data collection. Thanks are also due to R. Lee and M. Muller at the Dept of Radiology (Newcastle upon Tyne Hospitals NHS Foundation Trust) for reviewing the chest radiographs of the 2001 and 2009 studies, respectively, and A. Nicholson (Dept of Microbiology, Freeman Hospital, Newcastle upon Tyne, UK) for laboratory support. Our thanks also go to R. George and C. Sheppard (Respiratory and Systemic Infection Laboratory, Health Protection Agency, London, UK) for running the xMAP immunoassay for serotyping the pneumococcal isolates.
Footnotes
Support statement: The work was supported by Pfizer Vaccines UK (0887X1-4479).
Conflict of interest: Disclosures can be found alongside the online version of this article at www.erj.ersjournals.com
References
- 1.McIntosh K. Community-acquired pneumonia in children. N Engl J Med 2002; 346: 429–437 [DOI] [PubMed] [Google Scholar]
- 2.Cilla G, Oñate E, Perez-Yarza EG, et al. Viruses in community-acquired pneumonia in children aged less than 3 years old: high rate of viral coinfection. J Med Virol 2008; 80: 1843–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 2011; 66: Suppl. 2, ii1–ii23 [DOI] [PubMed] [Google Scholar]
- 4.Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010; 375: 1969–1987 [DOI] [PubMed] [Google Scholar]
- 5.Carvalho Mda G, Tondella ML, McCaustland K, et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J Clin Microbiol 2007; 45: 2460–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michelow IC, Lozano J, Olsen K, et al. Diagnosis of Streptococcus pneumoniae lower respiratory infection in hospitalized children by culture, polymerase chain reaction, serological testing, and urinary antigen detection. Clin Infect Dis 2002; 34: E1–11 [DOI] [PubMed] [Google Scholar]
- 7.Hamano-Hasegawa K, Morozumi M, Nakayama E, et al. Comprehensive detection of causative pathogens using real-time PCR to diagnose pediatric community-acquired pneumonia. J Infect Chemother 2008; 14: 424–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Resti M, Moriondo M, Cortimiglia M, et al. Community-acquired bacteremic pneumococcal pneumonia in children: diagnosis and serotyping by real-time polymerase chain reaction using blood samples. Clin Infect Dis 2010; 51: 1042–1049 [DOI] [PubMed] [Google Scholar]
- 9.Esposito S, Marchese A, Tozzi AE, et al. Bacteremic pneumococcal community-acquired pneumonia in children less than 5 years of age in Italy. Pediatr Infect Dis J 2012; 31: 705–710 [DOI] [PubMed] [Google Scholar]
- 10.Elemraid MA, Rushton SP, Shirley MD, et al. Impact of the 7-valent pneumococcal conjugate vaccine on the incidence of childhood pneumonia. Epidemiol Infect 2013; 141: 1697–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark JE, Hammal D, Hampton F, et al. Epidemiology of community-acquired pneumonia in children seen in hospital. Epidemiol Infect 2007; 135: 262–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherian T, Mulholland EK, Carlin JB, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ 2005; 83: 353–359 [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011; 53: e25–e76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheppard CL, Harrison TG, Smith MD, et al. Development of a sensitive, multiplexed immunoassay using xMAP beads for detection of serotype-specific Streptococcus pneumoniae antigen in urine samples. J Med Microbiol 2011; 60: 49–55 [DOI] [PubMed] [Google Scholar]
- 15.Fenwick F, Young B, McGuckin R, et al. Diagnosis of human metapneumovirus by immunofluorescence staining with monoclonal antibodies in the North-East of England. J Clin Virol 2007; 40: 193–196 [DOI] [PubMed] [Google Scholar]
- 16.Corless CE, Guiver M, Borrow R, et al. Simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J Clin Microbiol 2001; 39: 1553–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaacs D. Problems in determining the etiology of community-acquired childhood pneumonia. Pediatr Infect Dis J 1989; 8: 143–148 [PubMed] [Google Scholar]
- 18.Clements H, Stephenson T, Gabriel V, et al. Rationalised prescribing for community acquired pneumonia: a closed loop audit. Arch Dis Child 2000; 83: 320–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drummond P, Clark J, Wheeler J, et al. Community acquired pneumonia – a prospective UK study. Arch Dis Child 2000; 83: 408–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Don M, Fasoli L, Paldanius M, et al. Aetiology of community-acquired pneumonia: serological results of a paediatric survey. Scand J Infect Dis 2005; 37: 806–812 [DOI] [PubMed] [Google Scholar]
- 21.Michelow IC, Olsen K, Lozano J, et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics 2004; 113: 701–707 [DOI] [PubMed] [Google Scholar]
- 22.Tsolia MN, Psarras S, Bossios A, et al. Etiology of community-acquired pneumonia in hospitalized school-age children: evidence for high prevalence of viral infections. Clin Infect Dis 2004; 39: 681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juvén T, Mertsola J, Waris M, et al. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J 2000; 19: 293–298 [DOI] [PubMed] [Google Scholar]
- 24.Laundy M, Ajayi-Obe E, Hawrami K, et al. Influenza A community-acquired pneumonia in East London infants and young children. Pediatr Infect Dis J 2003; 22: Suppl. 10, S223–S227 [DOI] [PubMed] [Google Scholar]
- 25.Wubbel L, Muniz L, Ahmed A, et al. Etiology and treatment of community-acquired pneumonia in ambulatory children. Pediatr Infect Dis J 1999; 18: 98–104 [DOI] [PubMed] [Google Scholar]
- 26.Dagan R, Shriker O, Hazan I, et al. Prospective study to determine clinical relevance of detection of pneumococcal DNA in sera of children by PCR. J Clin Microbiol 1998; 36: 669–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Bergh MR, Biesbroek G, Rossen JW, et al. Associations between pathogens in the upper respiratory tract of young children: interplay between viruses and bacteria. PLoS One 2012; 7: e47711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toikka P, Nikkari S, Ruuskanen O, et al. Pneumolysin PCR-based diagnosis of invasive pneumococcal infection in children. J Clin Microbiol 1999; 37: 633–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menéndez R, Córdoba J, de La Cuadra P, et al. Value of the polymerase chain reaction assay in noninvasive respiratory samples for diagnosis of community-acquired pneumonia. Am J Respir Crit Care Med 1999; 159: 1868–1873 [DOI] [PubMed] [Google Scholar]
- 30.Strachan RE, Cornelius A, Gilbert GL, et al. Pleural fluid nucleic acid testing enhances pneumococcal surveillance in children. Respirology 2012; 17: 114–119 [DOI] [PubMed] [Google Scholar]
- 31.Koshy E, Murray J, Bottle A, et al. Impact of the seven-valent pneumococcal conjugate vaccination (PCV7) programme on childhood hospital admissions for bacterial pneumonia and empyema in England: national time-trends study, 1997–2008. Thorax 2010; 65: 770–774 [DOI] [PubMed] [Google Scholar]
- 32.Black SB, Shinefield HR, Ling S, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J 2002; 21: 810–815 [DOI] [PubMed] [Google Scholar]
- 33.Miller E, Andrews NJ, Waight PA, et al. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011; 11: 760–768 [DOI] [PubMed] [Google Scholar]
- 34.Byington CL, Hulten KG, Ampofo K, et al. Molecular epidemiology of pediatric pneumococcal empyema from 2001 to 2007 in Utah. J Clin Microbiol 2010; 48: 520–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jefferies JM, Macdonald E, Faust SN, et al. 13-valent pneumococcal conjugate vaccine (PCV13). Hum Vaccin 2011; 7: 1012–1018 [DOI] [PubMed] [Google Scholar]
- 36.Miller E, Andrews NJ, Waight PA, et al. Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine 2011; 29: 9127–9131 [DOI] [PubMed] [Google Scholar]
- 37.Grijalva CG, Nuorti JP, Zhu Y, et al. Increasing incidence of empyema complicating childhood community-acquired pneumonia in the United States. Clin Infect Dis 2010; 50: 805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Kaabi N, Solh Z, Pacheco S, et al. A comparison of group A Streptococcus versus Streptococcus pneumoniae pneumonia. Pediatr Infect Dis J 2006; 25: 1008–1012 [DOI] [PubMed] [Google Scholar]
- 39.Dodman T, Clark J, Cant AJ. Community acquired pneumonia: review of investigations, aetiology, treatment and outcome for inpatients from a UK centre. Eur J Pediatr 1999; 158: 1005. [DOI] [PubMed] [Google Scholar]
- 40.García-García ML, Calvo C, Pozo F, et al. Spectrum of respiratory viruses in children with community-acquired pneumonia. Pediatr Infect Dis J 2012; 31: 808–813 [DOI] [PubMed] [Google Scholar]