Abstract
Ageing is the main risk factor for the development of dementing neurodegenerative diseases (NDs) and it is accompanied by the accumulation of variations in mitochondrial DNA. The resulting tissue-specific alterations in ATP production and availability cause deteriorations of cerebral clearance mechanisms that are important for the removal of toxic peptides and its aggregates. ABC transporters were shown to be the most important exporter superfamily for toxic peptides, e.g. β-amyloid and α-synuclein. Their activity is highly dependent on the availability of ATP and forms a directed energy-exporter network, linking decreased mitochondrial function with highly impaired ABC transporter activity and disease progression. In this paper, we describe a network based on interactions between ageing, energy metabolism, regeneration, accumulation of toxic peptides and the development of proteopathies of the brain with a focus on Alzheimer’s disease (AD). Additionally, we provide new experimental evidence for interactions within this network in regenerative processes in AD.
Keywords: ageing, ABC transporters, mitochondria, Alzheimer’s disease, Parkinson’s disease, energy metabolism, dementia, neurodegeneration, ABCB1, ABCC1, ABCA7, ABCA1, Aqp4
Introduction
Neurodegenerative diseases (NDs) and dementias in particular have become a serious and global challenge. In 2010, an estimated number of 35 million people suffered from dementia. According to the most recent dementia report from the World Health Organization, the world population aged 60 years and older will reach 2 billion by the year 2050 (WHO, 2012). Because of the ageing Western and Asian societies, the prevalence of NDs is rising continuously, because age is still the main and only risk factor thus far widely acknowledged. In addition to the physiological and psychological burden for the affected and their relatives, a high economic burden, with worldwide expenses of US$604 billion, was estimated in 2010 (WHO, 2012). Therefore, the rapid development of novel strategies is critical to match the increasing prevalence of these disorders. The pathogenesis of most NDs is commonly hallmarked by the accumulation of insoluble neurotoxic peptides, e.g. β-amyloid (Aβ) in Alzheimer’s disease (AD) or α-synuclein (αSyn) in Parkinson’s disease (PD) and Dementia with Lewy-bodies (DLB), and a consequent decline of neuronal and synaptic function (Johnson, 2000). Although these peptides are physiologically produced during the whole life and have distinct functions in the brain, their concentration gradually reaches toxic levels in specific brain regions of the elderly, leading to specific clinical disease hallmarks. In sporadic cases, the distinct localizations do not indicate localized overproduction, but rather insufficiencies in clearance processes such as degradation by proteases, proteasomes and/or autophagy or active export across the blood-brain barrier and the perivascular drainage channels (Mawuenyega et al., 2010; Pahnke et al., 2009b). Since the precise mechanisms underlying the accumulation of harmful levels of soluble peptides/oligomers and non-soluble aggregates in the brain remain are still unknown, current treatment strategies focus on proposed pathogenesis-related markers, such as peptide production by cleavage enzymes from precursor proteins, peptide deposits and neuronal functional deficiencies (Selkoe, 2001). However, neither of these strategies (e.g. active or passive immunization, and secretase inhibition in AD) could accomplish sufficient clinical effects to be introduced in routine treatment. Most of the studies were halted in early phases (e.g. phase 2 trial of bapineuzumab), or even late in phase 3 (e.g. phase 3 trials of solanezumab, a mixture of two humanized anti-Aβ-monoclonal antibodies) due to the lack of observed benefit for the patients (Imbimbo et al., 2012; Salloway et al., 2009). Hence, a desperate need has driven the search for new approaches apart from the current ‘overproduction and aggregation’ hypotheses.
New strategies may aim toward i) normalization of the soluble peptide equilibrium/flow between brain tissue and vasculature/serum and simultaneously, and ii) the replacement of the lost or impaired neurons. The removal of insoluble aggregates from the brain was thought to be a promising strategy, especially because Aβ plaques have been recognized as one of the first histological applicable structures during neurodegeneration in AD (Alzheimer, 1907). However currently, the opinions have changed tremendously: the most toxic effect on neurons is assumed to be facilitated by small peptide-oligomers, rather than by large fibrillar species (reviewed in (Pahnke et al., 2009b)). Mechanisms influencing their clearance from the brain are widely accepted as new targets for fighting NDs. In this context, two major brain barriers have recently been in the focus of research. Aβ accumulation in the epithelial cells of the choroid plexus and in cerebrovascular walls has been correlated with disruption and dysfunction in the blood-cerebrospinal fluid barrier (BCSFB) as well as in the blood-brain barrier (BBB) (Liu, 1991). Both barriers regulate the exchange of toxic peptides, compounds and metabolites by the specificity of their cellular structures. Beside structural specificity of cells, drainage of small molecules to the blood, e.g. Aβ, is age-dependent and correlates with changes in capillary density (Hawkes et al., 2011).
The BCSFB is defined by having a fenestrated basal membrane and highly a specialized choroid plexus epithelial layer, which are located in the ventricles. The capillary BBB is a structure composed of a monolayer of brain endothelial cells and encompasses pericytes and astrocytes’ endfeet separated from the capillary endothelium by a tight basal lamina. It is unique in that it lacks fenestration and the presence of tight junctions, which support the maintenance of the brain’s microenvironment (reviewed in (Pahnke et al., 2009b; Zlokovic, 2008)). Tight junctions are located between the endothelia and allow only small, nonpolar molecules to passively diffuse through the barrier (Pardridge, 1999). The active transport of nutrients to the brain and the efflux of toxic compounds via the BCSFB and BBB are facilitated by specified membrane receptors and transporters (Weiss et al., 2009). Many of these membrane transporter proteins are encoded by a superfamily of genes that can be found in both prokaryotes and eukaryotes: the ATP-binding-cassette (ABC) transporters (Saurin et al., 1999).
Together, 49 human ABC transporters are known and they are divided into seven subfamilies (ABCA to ABCG). They are characterized by having broad substrate specificity and two conserved ATP-binding sites that generate the energy needed for transport processes. Most human ABC transporters are expressed in a strict pattern of polarization, which leads to an asymmetrical arrangement, not only at barriers of the brain, and ensures a very restricted influx of substrates through the blood-brain barrier (Pahnke et al., 2009b; Pahnke et al., 2008). Consequently, ABC transporters have been identified as a crucial protein superfamily representing potential targets in the prevention and treatment of various diseases. They share responsibility for development of multidrug resistance in cancer and of cerebral proteopathies (reviewed in (Bartels et al., 2009; Kanwar et al., 2012)).
Neurodegeneration is defined by the reduction or even total loss of distinct groups of neurons in specified locations that lead to specific clinical symptoms, e.g. short-term memory deficits in mild cognitive impairment (MCI)/AD, motor and cognitive deficits in DLB and PD. Hence, the continuous energy-dependent renewal and reconstruction of the neuronal network is crucial to maintain normal function. It is widely acknowledged that alterations in the mitochondrial genome occur during the ageing process, which is referred to as ‘the free radical theory of aging’, and which have also been found in NDs (reviewed in (Coskun et al., 2012; Harman, 2003; Lin and Beal, 2006; Wallace, 1992). Regenerative mechanisms are insufficient or become insufficient during age progression and disease development. We hypothesize that the dysfunction of mitochondria that leads to alterations in ATP production has an important impact on active transport at the BBB and BCSFB. This article summarizes recent literature describing various energy-dependent mechanisms involving ABC transporters and highlights how these findings support the hypothesis that each of these contributes to the pathogenesis of age-related NDs with a focus on AD as the most common cause of neurodegeneration and dementia.
ABC transporter function in ageing-related diseases
Barriers of the brain provide effective protection against the influx of harmful and dangerous substances from the blood. However, substances and metabolites produced in the brain must also cross these barriers to reach the circulatory system. Both directions of active transport are tightly regulated and facilitated by specific transport molecules. Unfortunately, the brain lacks a lymphatic circulation that could support gross clearance processes. Thus, the metabolites/substances have to fulfill specific requirements related to their size and charge to be excreted or imported by the transporters. If these requirements are not met, bioavailability in the brain is low or accumulation in the brain tissue may occur. This not only accounts for small molecules, but also for peptides that are produced at physiologic levels in the brain throughout the whole life, e.g. Aβ.
Several studies have described an association between decreased ABC transporter activity and increased incidence of neurodegenerative disease, showing that these transporters are important for the export of toxic peptide species from the brain. One of the first in vivo pieces of evidence was provided by Bartels and colleagues in an investigation of ABC transporter function in patients with neurodegenerative syndromes: PD, progressive supranuclear palsy (PSP), and multi-system atrophy (MSA), which are characterized by accumulation of αSyn or Tau (Bartels et al., 2008b). These disorders were examined by positron-emission tomography (PET) using an ABC transporter-specific probe, [11C]-verapamil, that is exported exclusively by ABCB1 (Bartels et al., 2008b). They demonstrated a specific accumulation of the radio-probe in brain regions where these diseases primarily start, e.g. Substantia nigra in PD, and described a decrease of ABCB1 function precisely in these locations. Moreover, using Parkinson’s patients with frontal basal brain involvement, mimicking DLB, these regions were also noted to accumulate [11C]-verapamil. This method was applied to the third disease with αSyn accumulation, MSA, that primarily affects central basal ganglia to detect this primary pathogenic locus. Assema and colleagues gathered similar results for AD. They showed decreased ABCB1 function in AD patients with mild to moderate disease status using [11C]-verapamil PET (Assema et al., 2012). Reduced binding potential values were also found in brain regions affected late in AD, such as the occipital cortex, but not in unrelated areas.
Does an impairment of ABC transporters in distinct brain regions lead to a disturbed detoxification of these areas, which results in the development of toxic peptide aggregates (Bartels et al., 2008a; Pahnke et al., 2009a)?
Mawuenyega and colleagues proved an impairment of the detoxification mechanisms/or processes by measuring both the production of Aβ40/Aβ42 and the clearance rates from the CNS of elderly patients suffering from AD and age-matched controls. They showed that the clearance rates of Aβ40 and Aβ42 were impaired in the affected group, as compared to rates of the control group. In fact, Aβ clearance rates decreased by approximately 30% in the AD group and, thus, the authors considered inhibition/reduction of clearance rates to be essential in the development of AD (Mawuenyega et al., 2010). Recent investigations by our group have revealed a mechanism that may underlie the effects found by Mawuenyega et al.(Krohn et al., 2011). We observed that decreased ABC transporter function of ABCB1 and ABCC1 leads to a highly increased accumulation and aggregation of Aβ in vivo. Investigations of ABCC1-deficient mice illustrated that the accumulation of Aβ is 12- to 14-fold higher than in non-deficient control animals (Krohn et al., 2011). Using a mathematical model, we were also able to show that a decline of export function by only 11% accounts for a 4-fold increase of Aβ in the brain of mice. In keeping with these observations, we hypothesized that a chronic decline of export activity of ABCC1 and ABCB1 caused by nutrition and drugs can lead to a reduction of continuous removal of toxic peptide species (Krohn et al., 2011; Pahnke et al., 2009b).
Furthermore, Cirrito et al. showed a decreased clearance of [125I]Aβ40 and [125I]Aβ42 microinjected into the CNS of ABCB1-deficient mice, and increased Aβ levels within the brain interstitial fluid after APP-transgenic mice were dosed with an ABCB1 inhibitor (Cirrito et al., 2005). Consistently, Hartz et al. demonstrated a reduced ABCB1 expression and transport activity ex vivo in brain capillaries as compared with wild-type controls. Additionally, they showed that compared to control mice, brain Aβ levels were significantly reduced after the restoration of ABCB1 expression and transport activity in brain capillaries (Hartz et al., 2010). This data further supports our hypothesis that reduced ABC transporter function in AD is a cause rather than a consequence of Aβ accumulation. Very recently, one important piece was added to the puzzle: how Aβ arrives at the vessels and perivascular drainage channels (PVDC) to be excreted by ABC transporters. Iliff and colleagues revealed that a substantial amount of Aβ is transported by an intercellular ‘water flow’ that is driven by aquaporin-4 water channels (Iliff et al., 2012). Aquaporin-4 is expressed highly polarized in perivascular endfeet of astrocytes and is proposed to facilitate various functions in maintaining convective currents that drive the clearance of interstitial solutes from the brain parenchyma to the CSF (Iliff et al., 2012; Noell et al., 2012). Because ABC transporters (esp. ABCC1) are also highly expressed at the choroid plexus (CP), both mechanisms may contribute to the establishment a constant physiological route for Aβ drainage. Other ABC transporters that interact with Apolipoprotein E variants (ABCA1) (Fitz et al., 2012) and membrane receptors such as LRP1/RAGE (low-density lipoprotein receptor-related protein/ receptor for advanced glycation end products) (Deane et al., 2003; Shibata et al., 2000) may also contribute to the ‘Aβ excretion highway’.
ABC transporters in general play a key role in the pathology and treatment of several diseases, and therefore, are denoted as ‘multidrug-resistance transporters’. More importantly, drugs and their metabolites are able to modulate the binding affinity for substrates that often results in a change in their transport kinetics. Several drugs that are prescribed to reduce blood pressure (β-blockers, calcium-antagonists) are known to decrease ABC transporter function (reviewed in (Schinkel and Jonker, 2003) and (Lee, 2010)). In this context, it would be interesting to evaluate the risk for ND in patients being treated with such drugs. To our knowledge, this has not yet been done. With advancing age, such drugs and age-related, structural changes in the vasculature (media thickening, lipid storage, and calcifications) could lead to an impaired clearance of toxic peptides from the brain. Lipid metabolism seems to play an important role in the development of AD. ABCA1, ABCA2, and ABCA7 are representatives of the A-subfamily of ABC transporters that mediate the transport of a broad range of physiologic lipid compounds across membrane barriers, including the BBB (reviewed in (Piehler et al., 2012)). Their effects on the development of AD, however, as well as their modes of interaction differ. ABCA1 is highly expressed in neurons, microglia, astrocytes, and oligodendrocytes and also found in brain capillary endothelial cells (Kim et al., 2006; Koldamova et al., 2003; Panzenboeck et al., 2002). Studies have shown that ABCA1 enhances amyloid formation indirectly via facilitation of apoE lipidation; specifically, the binding of apoE-lipid particles with Aβ facilitates the cellular uptake and clearance of Aβ via the ApoE-receptor LRP1 (Bell et al., 2007; Hirsch-Reinshagen et al., 2009; Hirsch-Reinshagen et al., 2007; Wahrle et al., 2008). Fitz and colleagues recently showed that the human ApoE4 gene Aβ deposition and, consequently, cognitive impairment are exacerbated in ABCA1-deficient mice (Fitz et al., 2012). In animals, the loss of one ABCA1 allele seems to be compensated with the ApoE3 gene. With regard to this knowledge, further research is necessary to examine interactions between the ABCA1 and ApoE isoforms. Current opinions suggest that ABCA1 acts as a suppressor of Aβ production and deposition. Recent experiments involving ABCA1 and liver X receptor agonists have shown promising, anti-amyloidogenic effects and, thus, highlight the importance of ABCA1 as a drug target (Koldamova et al., 2003).
Other members of the A-family, e.g. ABCA2 and ABCA7, also affect Aβ production per se. ABCA2 depletion leads to a shift from β- to α-secretase dependent cleavage of APP, resulting in increased levels of APPsα and a decrease in APPsβ production (Davis, 2010; Michaki et al., 2012). ABCA7 prevents Aβ accumulation by inhibiting Aβ production and promoting Fcγ-receptor-independent phagocytosis (Bacskai et al., 2002; Chan et al., 2008). ABCA7 transporters are highly expressed in microglia and play an active role in the phagocytic uptake of debris and plaques generated in NDs (Bacskai et al., 2002; Das et al., 2003; Kim et al., 2006; Stolzing and Grune, 2004).
Several genome-wide association studies (GWASs) found associations between the A-subfamily of ABC transporters and AD in large cohorts (Hollingworth et al., 2011; Naj et al., 2011). For example, variants of ABCA7 were identified in several joint- and meta-analyses positively correlated with an increased risk for AD. Allen and colleagues revealed a correspondence between small nucleotide polymorphisms (SNPs) and an increased expression of ABCA7 and Apolipoprotein J, which is associated with cell debris and apoptosis (Allen et al., 2012). Both proteins, ABCA7 and Apolipoprotein J, are proposed to have protective effects. Further analyses of SNPs in ABCA2, with regard to the development of AD, were ethnicity-dependent and thus not conclusive (Wollmer et al., 2006). Reviewing the results of the previous investigations, the use of ABC transporters as diagnostic markers seems promising, but has to be confirmed in further studies. The previous GWASs examined only specific haplotypes. As a consequence of the promising results from molecular and GWASs, whole-exon-sequencing, including the promoter and regulatory regions, will be of high interest in the near future.
Impact of mitochondrial alterations on ageing and neurodegeneration
The fact that the central nervous system already consumes 20% of the bodies’ energy indicates not only that mitochondria play an essential role in the maintenance of neuronal and synaptic function, but also in important cell signaling pathways (Di Filippo et al., 2010). In addition to their role in ATP production, these organelles are highly important for Ca2+ homeostasis, apoptosis, synthesis of iron-sulfur clusters, and steroids (reviewed in (Martin, 2012)). They have also been investigated for their contribution to ageing. A widely acknowledged hypothesis implies a causative role of accumulated mitochondrial DNA (mtDNA) mutations acquired during life and the resulting excessive production of reactive oxygen species (ROS) in the pathogenesis of NDs, like AD and PD (reviewed in (Harman, 2003; Lin and Beal, 2006; Wallace, 2005, 2011). ROS production occurs endogenously as a natural process during oxidative phosphorylation and is normally compensated for by the presence of antioxidants, including glutathione, carotenoids, and antioxidant enzymes (reviewed in (Naik and Dixit, 2011)). The hypothesis further states that the produced ROS mainly targets mitochondria and cause further damage to the cells and mitochondria itself. Since mitochondrial DNA is not protected by histones, ROS leads to mutations in the mitochondrial genome and can promote mitochondrial dysfunction even more (Keil et al., 2004). The vicious cycle thus created leads to cellular dysfunction and altered ATP production. In PD, it has been recognized for many years that mitochondrial changes are correlated with pathogenesis. In cells of the affected regions (e.g. Substantia nigra pars compacta), complex I activity of the mitochondrial respiratory chain were reduced and inhibitors of this complex, like the toxin 1,2,3,6-methyl-phenyl-tetrahydropyridine (MPTP), caused damage to disease-related (dopaminergic) neurons in an animal model (reviewed in (Martin, 2012; Olanow and Tatton, 1999)).
The first experimental evidence supporting the hypothesis that mutated mtDNA contributes to deleterious neurodegenerative effects during the process of ageing was presented by Trifunovic and colleagues in 2004 using a knock-in mouse model with a mutated mtDNA-polymerase A (Trifunovic et al., 2004). Because of a deficiency in the proof-reading function, the number of mtDNA mutations increased, resulting in a decreased life span for these mice due to the early onset of various ageing-related phenotypes. Several subsequent studies revealed that mitochondrial dysfunction is, at least in part, also a consequence of pathological changes occurring during neurodegeneration. With regard to AD, Aβ was shown to influence key enzymes, inhibit respiration, and cause imbalances in mitochondrial function, e.g. cytochrome c oxidase (COX), α-ketoglutarate dehydrogenase and pyruvate dehydrogenase (Casley et al., 2002; Crouch et al., 2005; Devi et al., 2006; Hernandez-Zimbron et al., 2012; Manczak et al., 2006; Rui et al., 2006). Until now, knowledge of this interaction has permitted only a few distinct statements to be made, because of the complexity of the relationships between energy-producing enzymes. For example, on one hand Aβ was recognized to induce inhibition of cytochrome c oxidase by binding the amino-terminal region of subunit one of the enzyme (Hernandez-Zimbron et al., 2012) or directly induce cytochrome c oxidase release and mitochondrial swelling in neurons followed by activation of caspase-3, finally leading to apoptotic death (Kim et al., 2002). On the other hand, cytochrome c oxidase-deficiency itself resulted in decreased ROS production and increased amyloid burden in an animal model, while the expression of the amyloid precursor protein (APP) remained unchanged. These conflicting data points prevented researchers from drawing the conclusion that COX impairment plays an initiative role in the disease development and is an indicator for a potential disturbance in APP processing (Fukui et al., 2007). Thus, the role of cytochrome c oxidase in the pathology of NDs remains elusive.
Several other examples regarding the influence of Aβ on mitochondrial key enzymes have been identified. The Aβ-binding alcohol dehydrogenase (ABAD) is located in neuronal mitochondria and is involved in mitochondrial functional alterations during neurodegeneration. ABAD binds Aβ and, thereby, causes mitochondrial dysfunction (Lustbader et al., 2004; Takuma et al., 2005). Inhibition of this interaction leads to reduced Aβ accumulation and improved mitochondrial function (Yao et al., 2011). Other groups reported that Aβ directly participated in mitochondrial processes like NO and ATP production. Aβ mediates increased NO levels that lead to a reversible inhibition of cytochrome c oxidase and, in turn, to decreased ATP levels. If NO persists at high levels, RNS (reactive nitrogen species) are generated that may irreversibly inactivate respiratory chain complexes and ATP synthase (Keil et al., 2004; Radi et al., 2002). Further evidence for the mitochondrial link in α-synucleinopathies was recently presented by Rhinn and colleagues, who described a specific transcript variant of αSyn, referred to as αSynL, which showed a shift in the preferred accumulation site from synaptic terminals towards mitochondria. These observations, consistent with PD pathology, were supported by the increase of αSynL transcript expression in the presence of PD risk factors like age, toxin exposure and genetic variants of αSyn, indicating a possible role in the initiation of the disease pathology (Rhinn et al., 2012).
Even though direct toxic effects of neurodegeneration-related peptides on mitochondria that lead to dysfunction in complexes of the respiratory chain have been demonstrated several times, indicating that mtDNA mutations may not play an initial role in the development of NDs, this important issue remains highly controversial. Recent data acquired using AD mice show that higher ATP levels caused by polymorphic mtDNA is accompanied by lower Aβ plaque number and size, as well as lower soluble Aβ levels (Scheffler et al., 2012). Additionally, evidence exists that these alterations increase the phagocytic activity of microglia. An ageing-related switch of microglial function from neuroprotective to neurotoxic was observed in a mouse model of AD, demonstrating an age-dependent release of cytotoxic factors (e.g. TNFα) that is followed by an increase in neuronal death (Jimenez et al., 2008).
According to the observed alterations in mitochondrial function, we hypothesize that variations in ATP levels have a great impact on the progression of NDs. Reasons may include the revealed connection between mtDNA mutations and alterations in microglial phenotype, but more likely involve the critical role of ATP in various vital mechanisms for peptide degradation/efflux, e.g. the ubiquitin proteasome complex. The main function of the ubiquitin proteasome complex is the degradation of aggregated or misfolded macromolecules. Consequences of its dysfunction were shown in the context of PD. Parkin (part of the ubiquitin proteasome complex)-knock-out mice showed a loss of function in dopaminergic neurons in the striatum and a decrease in expression levels of proteins involved in the mitochondrial oxidative phosphorylation and, thereby, respiration. These accumulated changes lead to a further decrease in ATP levels (reviewed in (Martin, 2012)).
Furthermore, proteases for Aβ degradation, e.g. the insulin-degrading enzyme (IDE) or Neprilysin (NEP), are influenced by fluctuations in the ATP levels. Although findings using IDE could thus far only confirm an increase in small peptide degradation upon an increase of ATP production (Song et al., 2004), an evaluation of the effects on Aβ monomers degradation remains elusive.
Yet another important mechanism for peptide removal, which highly depends on the availability of ATP, is active transport across the blood-brain barrier by ABC transporters. As described above, these transporters facilitate peptide efflux, and this activity has a strong therapeutic relevance. While decreased ATP levels reduced transporter activity and accumulation of toxic Aβ (Scheffler et al., 2012), the pharmaceutical increase of transporter activity was accompanied by lower plaque burden, plaque number and size in a mouse model of AD (Krohn et al., 2011). Neuroregeneration provided by neuronal stem/progenitor cells (NSPCs) was decreased in mice due to the loss of specific ABC transporter function (Schumacher et al., 2012) and, thereby, could be determined to be highly dependent on ATP availability, too.
ABC transporter function in neuroregeneration
While the involvement of ABC transporter dysfunction in AD has been recently demonstrated (Krohn et al., 2011), previous studies had previously indicated that ABC transporters might also participate in neuroregenerative processes because they were found to be abundantly and transiently expressed in neurosphere-forming stem and progenitor cells (NSPCs). In those studies, robust expression rates of ABCB1 and ABCG2 were reported and the high abundance of these transporters was noted to decrease during lineage commitment (Islam et al., 2005a; Islam et al., 2005b). Both ABCB1 and ABCG2 were proposed to play a functional role in maintaining the stem cell status (Lin et al., 2006). Accordingly, our most recent study on the role of ABC transporters in neuroregeneration demonstrated how ABC transporter dysfunction contributed to adult neuroregeneration in mice (Schumacher et al., 2012). Interestingly, the impairments in neuroregeneration seem not to be related to cell-intrinsic dysfunctions of NSPCs, since in vitro experiments on proliferation and differentiation capacity did not reveal significant impairments as a result of ABC transporter-deficiency. Instead, the data suggested that the loss of ABC transporter function alters the export kinetics at both the blood-brain and cerebrospinal fluid- brain barriers and, thereby, might significantly compromise the homeostasis of the brain’s stem cell niche.
The concept that a so-called stem cell niche is a specific extracellular compartment that enables stem cells to maintain their undifferentiated and proliferating state was introduced in the late 1970’s (Schofield, 1978). Since then, researchers identified this niche in various tissue types in various organisms (Li and Xie, 2005). All stem cell niches thus far studied share a variety of anatomical features, such as diffusible signaling molecules and cell-to-cell and cell-to-extracellular matrix interactions, which allow the maintenance of the specific microenvironment needed for maintaining the stem cell pool. A common feature of these niches is that they always have a close interaction with the vasculature. The vasculature not only provides a scaffold for stem cell migration (Palmer et al., 2000), but also facilitates the local and long distance transport of signaling molecules (Jones and Wagers, 2008). Even signaling pathways like Notch 1, which is mostly responsible for local signaling between neighboring cells using the ligands Delta and Jagged, are to a certain degree interacting via diffusible ligands. It has been shown that diffusible components of the extracellular matrix can also activate the Notch pathway via β-integrin receptors (Campos et al., 2006).
Keeping in mind that ABC transporters convey many biological and metabolic substrates, (Ferreira and Sa-Nogueira, 2010; Gisin et al., 2010; Linton, 2007) while distinct cell types (e.g. endothelial cells) are able to release soluble factors that directly affect NSPC functions (Shen et al., 2004), we postulate that loss of ABC transporter function could severely change the brain’s homeostasis, leading to the observed results. Data from further regeneration-inducing experiments support this hypothesis and show strong implications for the importance of ABC transporters in maintaining the homeostasis of the stem cell niche. The location of the stem cell niche between the vascular and the ventricular system indicates that impairments in ABC transporter-mediated export of metabolites could directly affect NSPC fate. NSPCs may even interact with both systems (Shen et al., 2008), and the assumption that NSPC fate depends on ABC transporter functions is theoretically valid. In the absence of specific ABC transporters, distinct metabolites could reach functional or even toxic concentrations within the stem cell niche and, thereby, affect NSPC maturation. Additionally, many natural and pharmacological ABC transporter ligands are known to alter ABC transport kinetics (Lee, 2010; Shukla et al., 2008). Thus, these ligands could easily contribute to altered neuroregeneration, which is relevant at advanced ages or during chronic medication.
Neuroregeneration in AD mouse models depends on ABC transporter expression
ABC transporters have been recently acknowledged to play a prominent role in Aβ clearance across barriers of the brain (Cirrito et al., 2005; Koldamova et al., 2005; Krohn et al., 2011; Rodriguez-Rodriguez et al., 2007; Vogelgesang et al., 2004) and in regular stem cell differentiation and neuroregeneration (Islam et al., 2005a; Schumacher et al., 2012). It is of great importance to understand how alterations in ABC transporter function contribute to peptide clearance and also to altered neuroregeneration during the pathogenesis of AD.
Aβ peptides are mostly deemed to be neurotoxic in low molecular weight oligomeric states (Cleary et al., 2005; Lacor et al., 2004), but evidence also exists that they can have a neuroprotective role. Whereas the ability of Aβ1-28 monomers to enhance survival of hippocampal neurons in vitro was first observed more than a decade ago (Whitson et al., 1989), other Aβ species have recently been demonstrated to have some neuroprotective traits when present in their monomeric form. Aβ1-42 monomers were shown to rescue developing neurons, even under trophic-factor deprivation. The IGF-1 pathway is seemingly required for the Aβ’s neuroprotective effect, because Aβ was able to phosphorylate IRS-1 (insulin receptor substrate 1) (Giuffrida et al., 2009) and locally induce insulin production (Townsend et al., 2007) or bind the insulin receptor directly (Xie et al., 2002). Evidence for a direct function in neural stem cell differentiation has also been presented. The two amyloidogenic Aβ species, Aβ1-40 and Aβ1-42, thus contrarily affect the differentiation fate of NSPCs. While Aβ1-40 promotes pro-neuronal NSPC differentiation, Aβ1-42 has the opposite effect, leading towards promoting astroglial differentiation. In contrast to Aβ1-42, which seemed to act directly on the NSPCs, Aβ1-40 appeared to indirectly support the pro-glial differentiation by inducing cytokine release in active astrocytes, which are toxic to developing neurons and inhibit axonal growth (Chen and Dong, 2009).
Nonetheless, the toxic effects of Aβ deposition during AD pathogenesis still outweigh its possible minor positive effects toward neuroregeneration.
Studies on neuroregeneration during AD pathogenesis are mostly based on animal models, examining Aβ deposition by the use of mutated human APP, with or without additional presenilin transgenes, as well as the activity of different promoters driving their expression. Therefore, the observed effects on neurogenic functions differ substantially. While some studies suggest that in general neurogenesis increased (Jin et al., 2004), at least at distinct time points (Ermini et al., 2008), most studies reported impairing effects on neuroregeneration (Feng et al., 2001; Haughey et al., 2002a; Haughey et al., 2002b).
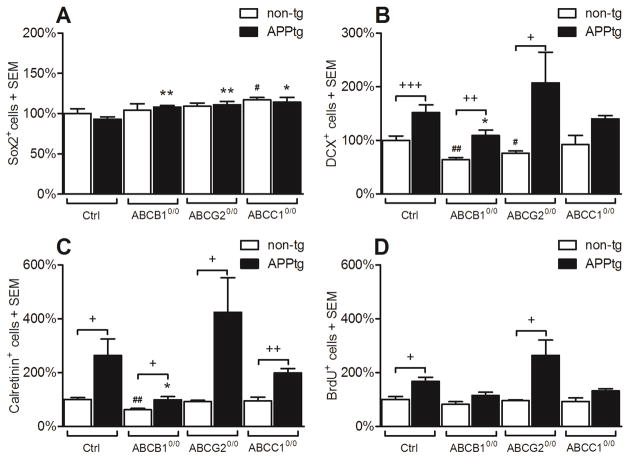
In the next section, we highlight as-yet unpublished information using the combined Thy1-APPKM670/671NL - Thy1-PS1L166P (APP/PPS1) amyloidosis mouse model (Radde et al., 2006). While Aβ deposition leads to a robust increase in neuroregenerative function in transgenic mice in comparison to non-transgenic littermates, the pro-neurogenic effect is strongly influenced by ABC transporter expression (Figure 1), which fits with the knowledge that this expression strongly influences the equilibrium of soluble and aggregated forms of Aβ (Krohn et al., 2011). Comparative data on the neuroregenerative capacity of ABC transporter-deficient and control mice were recently published (Schumacher et al., 2012). The data imply that dysfunctions in ABC transporters may affect neuroregeneration at least at two different levels: i) impairment of the microenvironment in the stem cell niche, and ii) shift in the concentration and composition of different Aβ species/aggregates that specifically influence NSPC maturation. The latter is particularly supported by the presented datasets (Figure 1), which show that loss of ABC transporter function not only increases the concentration of intracerebral Aβ species, but also triggers adult neuronal differentiation. The effect of Aβ on neurogenesis does not seem to be altered simply in an Aβ concentration-depended manner; compared to their non-transgenic counterparts, neurogenesis was most significantly increased in APP/PS1xABCG20/0 mice. Aβ levels in those mice where comparable to APP/PS1 controls, but APP/PS1 mice showed much significantly lower Aβ-deposition levels as compared to ABCC0/0 transgenic mice. Both showed comparable levels of neurogenesis induction compared to their respective non-transgenic controls (see also (Krohn et al., 2011)). At the age analyzed for neurogenesis, quantification of soluble and insoluble Aβ revealed decreased concentrations of soluble Aβ42 in ABCG20/0 transgenic mice as compared to ABCC10/0 and control transgenic mice, while the insoluble Aβ42 concentration was between that of the ABCC10/0 and the control AD mice (see also (Krohn et al., 2011)). These subtle ratio differences of soluble and insoluble Aβ suggest that Aβ in distinct states of oligomerization displays different binding affinities to the analyzed ABC transporters and NSPCs. Where distinct transporters are lacking, alterations in the overall concentration of intracortical Aβ as well as the homeostasis of oligomeric states of the β-amyloids may occur, leading to the observed effects on adult neurogenesis.
Figure 1. Aβ deposition triggers neurogenesis dependent on ABC transporter expression. ABC transporter dysfunction and Aβ proteopathy impair the neuroregeneration.
Graphs were established from quantification of immunofluorescence staining. Procedures were the same as in (Schumacher et al., 2012).
(A) Aβ deposition does not significantly alter the quantity of Sox2+ (transcription factor, active only in stem and progenitor cells) stem cells in comparison to non-trangenic littermates. However, ABC transporter-deficiency increases the relative number in APPtg mice (black bars, ABCB10/0 +16%, ABCG20/0 +19%, ABCC10/0 +12%).
(B) The pool of DCX+ (doublecortin, a microtubule associated protein which is transiently expressed only in neuronal progenitor cells) neuroblasts is increased in APPtg animals (ctrls +52%, black bars), ABCB10/0 (+71%), ABCG20/0 (+172%), and ABCC10/0 (+52%, not significant) mice versus non-transgenic littermates. A significant difference between APPtg and APPtg/ABC0/0 strains is only observable in the ABCB10/0 strain (−28%). ABC transporter-deficiency leads to a loss of DCX+ cells even in non-transgenic (non-tg) mice (ABCB10/0 −36%; ABCG20/0 −24%)
(C) In case of newly generated Calretinin+ neurons, the increase in cell numbers reaches statistical significance in all APPtg mice as compared to non-transgenic littermates (ctrls +164%, ABCB10/0 +59%, ABCG20/0 +357%, ABCC10/0 +109%). In the APPtg lines a significant effect is only observable for the ABCB10/0 strain which is decreased by −62%. In non-tg mice only the deficiency of ABCB10/0 leads to a significant decline in Calretinin+ cell number (−38%).
(D) The incorporation rate of BrdU (bromodeoxyuridine a halogenated thymidine analogue) during proliferation showed significant effects only in APPtg controls (+68%) and APPtg/ABCG20/0 (+175%) mice compared to non-tg littermates.
Summarizing, ABC transporter dysfunction and Aβ proteopathy impair the neuroregeneration. Numbers of cells are presented relatively to the previously published data on non-tg littermates (white bars, (Schumacher et al., 2012)).
Diagrams indicate means +SEM;
t-Test: APPtg ctrls vs. APPtg/ABC0/0 strains: * ≤ 0.05; ** ≤ 0.01; n≥4 (males)
t-Test: APPtg vs. non-transgenic littermates: + ≤ 0.05; ++ ≤ 0.01; +++ ≤ 0.001; n≥4; (males)
t-Test: non-transgenic ctrls vs non-transgenic ABC0/0 strains: # ≤ 0.05; ## ≤ 0.01; n≥4 (males)
Interestingly, precisely the same ABC transporters that are known to clear Aβ peptides across cellular membranes are also expressed in NSPCs. How do dysfunctions of these transporters contribute to the cytotoxic effects of oligomeric Aβ species on NSPCs in vitro? Various protocols have been established to generate solutions containing distinct Aβ oligomer species (Dahlgren et al., 2002). Studies with these species have revealed different traits, mostly concerning their respective level of cytotoxicity, but also their potential influence on stem cell functions (Dahlgren et al., 2002; Shruster et al., 2011). Most researchers have used the original Aβ preparation protocol of Dahlgren et al., but their results are not easily comparable. Oligomeric Aβ42 was shown to impair neuronal differentiation in neural stem cells at low micromolar concentrations by one study (Chen and Dong, 2009), but the opposite effect was demonstrated by others (Heo et al., 2007; Shruster et al., 2011). With regard to the different effects of distinct oligomeric Aβ species (Shruster et al., 2011), minor and involuntary changes in the Aβ preparation protocol could lead to unpredictable changes in the configuration of the comprising Aβ oligomer species and, thereby, lead to the conflicting results. Similarily, our experiments using control and ABC transporter-deficient NSPCs (ABCB10/0, ABCC10/0, and ABCG20/0) showed that NSPC proliferation was not affected by Aβ administration per se (data not shown). The increase of pro-neuronal cell differentiation (Tuj1+ cells) was statistically significance only in ABCG20/0 cells after treatment with oligomeric Aβ42 in vitro, indicating that the expression of ABC transporters in NSPCs may be of importance for protection against Aβ. ABC transporter dysfunction, however, affects brain homeostasis directly and, thereby, only indirectly affects NSPC function. Studies have shown that changes in the Aβ deposition characteristics during AD pathogenesis influence adult neuroregeneration significantly (Heo et al., 2007, 2007). This data taken together indicate that the observed inconsistency in results from in situ analyses of neuroregeneration in mice can be explained by the combined effects of the disturbed stem cell microenvironment and the specific concentrations and compositions of Aβ species in different mouse strains (i.e. variability in numerous experimental parameters)
The ‘Ageing-Energy-Export’ network – linking physiologic ageing and pathologic protein accumulation
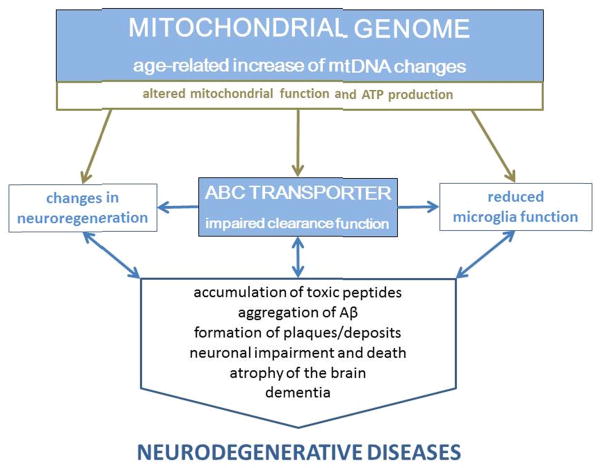
Ageing itself is thus far the only consistent risk factor for most sporadic, neurodegenerative and dementing disorders, and therefore, the mechanisms involved in and altered by this exceptional biological condition are vitally important. Recent data have provided numerous insights into a network of age-related processes, in which rates of accumulation of mitochondrial mutations and efflux/export of toxic peptides play a central role (Figure 2). Strong, albeit debatable, evidence exists that randomly occurring, age-dependent mitochondrial mtDNA changes result in subtle functional changes, with substantial, long-term metabolic effects (reviewed in (Lin and Beal, 2006; Wallace, 2005, 2011)). Although the precise location of specific changes varies, the biochemical effect unexceptionally leads to dysfunction or alterations in mitochondrial respiratory chain complexes and, consequently, to changes in the availability of ATP (Casley et al., 2002; Crouch et al., 2005; Devi et al., 2006; Hernandez-Zimbron et al., 2012; Manczak et al., 2006; Rui et al., 2006). Deficiency in chemical energy production induces a wide range of negative effects on the cellular or tissue levels. Concomitant decrease in the export function of the BBB and BCSFB, respectively, due to the reduced activity of transporter molecules, gradually leads to accumulation of peptides near vessels, in cells, or in organelles, causing long-term toxic effects that result in dysfunction. Aquaporin-4, a highly expressed water channel at the endfeet of astrocytes, however, can limit the accumulation of peptides to a non-toxic level (Iliff et al., 2012). This channel transfers Aβ peptides by a paravascular ‘water flow’ that reaches CSF spaces and the choroid plexus and finally results in a clearance of the brain. Besides AQP4, the ATP-dependent ABC transporters play an important role in the maintenance of the brain’s microenvironment. The deregulation of metabolic exchange via the brain’s barriers drives the pathogenesis of diseases, resulting in the aggregation of toxic peptides (Graf et al., 2004; Krishnamurthy et al., 2006).
Figure 2.
A hypothetical outline of interactions within a complex ageing network involving ABC transporters and mitochondria: i) continuous accumulation of mutations in the mitochondrial DNA and ii) decreased ATP-binding-cassette (ABC) transporter function in all cell types (neurons, microglia, astrocytes, neurosphere-forming stem and progenitor cells) closely interact.
Increasing numbers of mitochondrial DNA mutations result in mitochondrial dysfunction that lead to increased ROS production and decreased ATP production and availability. Due to the loss of ABC transporter function, neuroregenerative processes are disturbed and the replacement of lost neurons is reduced. Increasing amounts of highly aggregated Aβ shift the microglia phenotype from neuroprotective to neurotoxic, further promoting neurodegeneration and cognitive decline.
ABC transporters are also expressed in different cell types and organelles, e.g. microglia, astrocytes and mitochondria (reviewed in (Kim et al., 2006; Zutz et al., 2009)). The consequences of disturbances in energy production and ABC transporter function affect these cells and organelles. Defects in the phagocytotic function of microglia, decreased protease activity, and reduced export rates from mitochondria lead to further toxic effects and enhanced ROS production, processes that are widely acknowledged in ND pathology (Gibson et al., 2012; Graf et al., 2004; Krishnamurthy et al., 2006; Song et al., 2004). The resulting accumulation of toxic peptides (e.g. Aβ, αSyn) within the brain culminates in the formation of poorly soluble deposits (e.g. senile plaques and Lewy bodies). The presence of these deposits and their peptide predecessors induces a decline in neuronal and synaptic function and leads to the clinical symptoms seen in patients and animal models (Johnson, 2000). The progressing atrophy of the parenchyma, however, may be suspended by the replacement of dead neurons through neuroregeneration. With decreased ABC transporter activity, this process is also impaired, because the homeostasis of the stem cell niche and the differentiation of NSPC into neurons highly depends on the number and function of ABC transporters and is, thereby, also linked to the energy level (ATP) provided by mitochondria (Schumacher et al., 2012).
This ‘Ageing-Energy-Export’ network is not only endogenously influenced by metabolites, but more importantly is influenced by a genetic predisposition, such as we know from AD. Studies support that the risk of developing AD is increased when the patient’s mother and grandmother were also sufferers, an observation that directly correlates with the maternal inheritance of mitochondria (Honea et al., 2010; Mosconi et al., 2010).
The described complex coherencies between ageing, energy production and export function offer new opportunities for the development of new diagnostic tools and treatment strategies. The risk of being affected may be determined by screening the mitochondrial genome and assessing the mitochondrial function of the elderly. Whether skin fibroblasts or white blood cells will be most appropriate for such a screening approach has yet to be determined.
Additionally, the improvement of the ABCC1 and ABCB1 transporter function using specific compounds may be a novel treatment strategy, since both transporters contribute to Aβ clearance via the BBB and BCSFB (Krohn et al., 2011). The results of improvement of the maintenance of the stem cell niche, seems to be promising, but are still highly speculative and the contribution of ABC transporters herein has to be determined in more detail.
Highlights.
The paper describes the ‘ageing-energy-export’ network.
This network is impaired in neurodegenerative diseases.
Energy production is facilitated by mitochondria, and fails due to mtDNA mutations.
ABC transporters need ATP and facilitate Abeta export from brain.
Acknowledgments
We sincerely thank Sara Crockett (Karl-Franzens-University, Graz, Austria) for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen M, Zou F, Chai HS, Younkin CS, Crook J, Pankratz VS, Carrasquillo MM, Rowley CN, Nair AA, Middha S, Maharjan S, Nguyen T, Ma L, Malphrus KG, Palusak R, Lincoln S, Bisceglio G, Georgescu C, Schultz D, Rakhshan F, Kolbert CP, Jen J, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD, Petersen RC, Graff-Radford NR, Dickson DW, Younkin SG, Ertekin-Taner N. Novel late-onset Alzheimer disease loci variants associate with brain gene expression. Neurology. 2012;79:221–228. doi: 10.1212/WNL.0b013e3182605801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiat Psych-Gerichtl Med. 1907;64:3. [Google Scholar]
- Assema DM, Lubberink M, Bauer M, van der Flier WM, Schuit RC, Windhorst AD, Comans EF, Hoetjes NJ, Tolboom N, Langer O, Muller M, Scheltens P, Lammertsma AA, van Berckel BN. Blood-brain barrier P-glycoprotein function in Alzheimer’s disease. Brain. 2012;135:181–189. doi: 10.1093/brain/awr298. [DOI] [PubMed] [Google Scholar]
- Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels AL, Kortekaas R, Bart J, Willemsen AT, de Klerk OL, de Vries JJ, van Oostrom JC, Leenders KL. Blood-brain barrier P-glycoprotein function decreases in specific brain regions with aging: a possible role in progressive neurodegeneration. Neurobiol Aging. 2009;30:1818–1824. doi: 10.1016/j.neurobiolaging.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Bartels AL, van Berckel BN, Lubberink M, Luurtsema G, Lammertsma AA, Leenders KL. Blood-brain barrier P-glycoprotein function is not impaired in early Parkinson’s disease. Parkinsonism Relat Disord. 2008a;14:505–508. doi: 10.1016/j.parkreldis.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Bartels AL, Willemsen AT, Kortekaas R, de Jong BM, de Vries R, de Klerk O, van Oostrom JC, Portman A, Leenders KL. Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J Neural Transm. 2008b;115:1001–1009. doi: 10.1007/s00702-008-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos LS, Decker L, Taylor V, Skarnes W. Notch, epidermal growth factor receptor, and beta1-integrin pathways are coordinated in neural stem cells. J Biol Chem. 2006;281:5300–5309. doi: 10.1074/jbc.M511886200. [DOI] [PubMed] [Google Scholar]
- Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem. 2002;80:91–100. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- Chan SL, Kim WS, Kwok JB, Hill AF, Cappai R, Rye KA, Garner B. ATP-binding cassette transporter A7 regulates processing of amyloid precursor protein in vitro. J Neurochem. 2008;106:793–804. doi: 10.1111/j.1471-4159.2008.05433.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Dong C. Abeta40 promotes neuronal cell fate in neural progenitor cells. Cell death and differentiation. 2009;16:386–394. doi: 10.1038/cdd.2008.94. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Coskun P, Wyrembak J, Schriner SE, Chen HW, Marciniack C, Laferla F, Wallace DC. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim Biophys Acta. 2012;1820:553–564. doi: 10.1016/j.bbagen.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, Masters CL, Trounce IA. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1-42. J Neurosci. 2005;25:672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- Das P, Howard V, Loosbrock N, Dickson D, Murphy MP, Golde TE. Amyloid-beta immunization effectively reduces amyloid deposition in FcRgamma-/- knock-out mice. J Neurosci. 2003;23:8532–8538. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W., Jr The ATP-binding cassette transporter-2 (ABCA2) increases endogenous amyloid precursor protein expression and Abeta fragment generation. Curr Alzheimer Res. 2010;7:566–577. doi: 10.2174/156720510793499002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo M, Chiasserini D, Tozzi A, Picconi B, Calabresi P. Mitochondria and the link between neuroinflammation and neurodegeneration. J Alzheimers Dis. 2010;20(Suppl 2):S369–379. doi: 10.3233/JAD-2010-100543. [DOI] [PubMed] [Google Scholar]
- Ermini FV, Grathwohl S, Radde R, Yamaguchi M, Staufenbiel M, Palmer TD, Jucker M. Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis. Am J Pathol. 2008;172:1520–1528. doi: 10.2353/ajpath.2008.060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CB, Martin GM, Kim SH, Langdon RB, Sisodia SS, Tsien JZ. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32:911–926. doi: 10.1016/s0896-6273(01)00523-2. [DOI] [PubMed] [Google Scholar]
- Ferreira MJ, Sa-Nogueira I. A multitask ATPase serving different ABC-type sugar importers in Bacillus subtilis. Journal of bacteriology. 2010;192:5312–5318. doi: 10.1128/JB.00832-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz NF, Cronican AA, Saleem M, Fauq AH, Chapman R, Lefterov I, Koldamova R. Abca1 deficiency affects Alzheimer’s disease-like phenotype in human ApoE4 but not in ApoE3-targeted replacement mice. J Neurosci. 2012;32:13125–13136. doi: 10.1523/JNEUROSCI.1937-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui H, Diaz F, Garcia S, Moraes CT. Cytochrome c oxidase deficiency in neurons decreases both oxidative stress and amyloid formation in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:14163–14168. doi: 10.1073/pnas.0705738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CJ, Hossain M, Richardson JR, Aleksunes LM. Inflammatory Regulation of ABC Efflux Transporter Expression and Function in Microglia. J Pharmacol Exp Ther. 2012 doi: 10.1124/jpet.112.196543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisin J, Muller A, Pfander Y, Leimkuhler S, Narberhaus F, Masepohl B. A Rhodobacter capsulatus member of a universal permease family imports molybdate and other oxyanions. Journal of bacteriology. 2010;192:5943–5952. doi: 10.1128/JB.00742-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A, Garozzo D, Nicoletti F, Rizzarelli E, Copani A. Beta-amyloid monomers are neuroprotective. J Neurosci. 2009;29:10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf SA, Haigh SE, Corson ED, Shirihai OS. Targeting, import, and dimerization of a mammalian mitochondrial ATP binding cassette (ABC) transporter, ABCB10 (ABC-me) J Biol Chem. 2004;279:42954–42963. doi: 10.1074/jbc.M405040200. [DOI] [PubMed] [Google Scholar]
- Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Miller DS, Bauer B. Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer’s disease. Mol Pharmacol. 2010;77:715–723. doi: 10.1124/mol.109.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Liu D, Nath A, Borchard AC, Mattson MP. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer’s disease. Neuromolecular Med. 2002a;1:125–135. doi: 10.1385/NMM:1:2:125. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J Neurochem. 2002b;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- Hawkes CA, Hartig W, Kacza J, Schliebs R, Weller RO, Nicoll JA, Carare RO. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 2011;121:431–443. doi: 10.1007/s00401-011-0801-7. [DOI] [PubMed] [Google Scholar]
- Heo C, Chang KA, Choi HS, Kim HS, Kim S, Liew H, Kim JA, Yu E, Ma J, Suh YH. Effects of the monomeric, oligomeric, and fibrillar Abeta42 peptides on the proliferation and differentiation of adult neural stem cells from subventricular zone. J Neurochem. 2007;102:493–500. doi: 10.1111/j.1471-4159.2007.04499.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Zimbron LF, Luna-Munoz J, Mena R, Vazquez-Ramirez R, Kubli-Garfias C, Cribbs DH, Manoutcharian K, Gevorkian G. Amyloid-beta Peptide Binds to Cytochrome C Oxidase Subunit 1. PloS one. 2012;7:e42344. doi: 10.1371/journal.pone.0042344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Burgess BL, Wellington CL. Why lipids are important for Alzheimer disease? Mol Cell Biochem. 2009;326:121–129. doi: 10.1007/s11010-008-0012-2. [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Chan JY, Wilkinson A, Tanaka T, Fan J, Ou G, Maia LF, Singaraja RR, Hayden MR, Wellington CL. Physiologically regulated transgenic ABCA1 does not reduce amyloid burden or amyloid-beta peptide levels in vivo. J Lipid Res. 2007;48:914–923. doi: 10.1194/jlr.M600543-JLR200. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Jones N, Stretton A, Thomas C, Richards A, Ivanov D, Widdowson C, Chapman J, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Beaumont H, Warden D, Wilcock G, Love S, Kehoe PG, Hooper NM, Vardy ER, Hardy J, Mead S, Fox NC, Rossor M, Collinge J, Maier W, Jessen F, Ruther E, Schurmann B, Heun R, Kolsch H, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Gallacher J, Hull M, Rujescu D, Giegling I, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Pankratz VS, Sando SB, Aasly JO, Barcikowska M, Wszolek ZK, Dickson DW, Graff-Radford NR, Petersen RC, van Duijn CM, Breteler MM, Ikram MA, DeStefano AL, Fitzpatrick AL, Lopez O, Launer LJ, Seshadri S, Berr C, Campion D, Epelbaum J, Dartigues JF, Tzourio C, Alperovitch A, Lathrop M, Feulner TM, Friedrich P, Riehle C, Krawczak M, Schreiber S, Mayhaus M, Nicolhaus S, Wagenpfeil S, Steinberg S, Stefansson H, Stefansson K, Snaedal J, Bjornsson S, Jonsson PV, Chouraki V, Genier-Boley B, Hiltunen M, Soininen H, Combarros O, Zelenika D, Delepine M, Bullido MJ, Pasquier F, Mateo I, Frank-Garcia A, Porcellini E, Hanon O, Coto E, Alvarez V, Bosco P, Siciliano G, Mancuso M, Panza F, Solfrizzi V, Nacmias B, Sorbi S, Bossu P, Piccardi P, Arosio B, Annoni G, Seripa D, Pilotto A, Scarpini E, Galimberti D, Brice A, Hannequin D, Licastro F, Jones L, Holmans PA, Jonsson T, Riemenschneider M, Morgan K, Younkin SG, Owen MJ, O’Donovan M, Amouyel P, Williams J. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Swerdlow RH, Vidoni ED, Goodwin J, Burns JM. Reduced gray matter volume in normal adults with a maternal family history of Alzheimer disease. Neurology. 2010;74:113–120. doi: 10.1212/WNL.0b013e3181c918cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid beta. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbimbo BP, Ottonello S, Frisardi V, Solfrizzi V, Greco A, Seripa D, Pilotto A, Panza F. Solanezumab for the treatment of mild-to-moderate Alzheimer’s disease. Expert Rev Clin Immunol. 2012;8:135–149. doi: 10.1586/eci.11.93. [DOI] [PubMed] [Google Scholar]
- Islam MO, Kanemura Y, Tajria J, Mori H, Kobayashi S, Hara M, Yamasaki M, Okano H, Miyake J. Functional expression of ABCG2 transporter in human neural stem/progenitor cells. Neurosci Res. 2005a;52:75–82. doi: 10.1016/j.neures.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Islam MO, Kanemura Y, Tajria J, Mori H, Kobayashi S, Shofuda T, Miyake J, Hara M, Yamasaki M, Okano H. Characterization of ABC transporter ABCB1 expressed in human neural stem/progenitor cells. FEBS Lett. 2005b;579:3473–3480. doi: 10.1016/j.febslet.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Jimenez S, Baglietto-Vargas D, Caballero C, Moreno-Gonzalez I, Torres M, Sanchez-Varo R, Ruano D, Vizuete M, Gutierrez A, Vitorica J. Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer’s disease: age-dependent switch in the microglial phenotype from alternative to classic. J Neurosci. 2008;28:11650–11661. doi: 10.1523/JNEUROSCI.3024-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WG. Late-onset neurodegenerative diseases--the role of protein insolubility. J Anat. 2000;196 ( Pt 4):609–616. doi: 10.1046/j.1469-7580.2000.19640609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- Kanwar JR, Sriramoju B, Kanwar RK. Neurological disorders and therapeutics targeted to surmount the blood-brain barrier. Int J Nanomedicine. 2012;7:3259–3278. doi: 10.2147/IJN.S30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil U, Bonert A, Marques CA, Scherping I, Weyermann J, Strosznajder JB, Muller-Spahn F, Haass C, Czech C, Pradier L, Muller WE, Eckert A. Amyloid beta-induced changes in nitric oxide production and mitochondrial activity lead to apoptosis. J Biol Chem. 2004;279:50310–50320. doi: 10.1074/jbc.M405600200. [DOI] [PubMed] [Google Scholar]
- Kim HS, Lee JH, Lee JP, Kim EM, Chang KA, Park CH, Jeong SJ, Wittendorp MC, Seo JH, Choi SH, Suh YH. Amyloid beta peptide induces cytochrome C release from isolated mitochondria. Neuroreport. 2002;13:1989–1993. doi: 10.1097/00001756-200210280-00032. [DOI] [PubMed] [Google Scholar]
- Kim WS, Guillemin GJ, Glaros EN, Lim CK, Garner B. Quantitation of ATP-binding cassette subfamily-A transporter gene expression in primary human brain cells. Neuroreport. 2006;17:891–896. doi: 10.1097/01.wnr.0000221833.41340.cd. [DOI] [PubMed] [Google Scholar]
- Koldamova R, Staufenbiel M, Lefterov I. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem. 2005;280:43224–43235. doi: 10.1074/jbc.M504513200. [DOI] [PubMed] [Google Scholar]
- Koldamova RP, Lefterov IM, Ikonomovic MD, Skoko J, Lefterov PI, Isanski BA, DeKosky ST, Lazo JS. 22R-hydroxycholesterol and 9-cis-retinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid beta secretion. J Biol Chem. 2003;278:13244–13256. doi: 10.1074/jbc.M300044200. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, Wang J, Sosa-Pineda B, Murti KG, Schuetz JD. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443:586–589. doi: 10.1038/nature05125. [DOI] [PubMed] [Google Scholar]
- Krohn M, Lange C, Hofrichter J, Scheffler K, Stenzel J, Steffen J, Schumacher T, Bruning T, Plath AS, Alfen F, Schmidt A, Winter F, Rateitschak K, Wree A, Gsponer J, Walker LC, Pahnke J. Cerebral amyloid-beta proteostasis is regulated by the membrane transport protein ABCC1 in mice. J Clin Invest. 2011;121:3924–3931. doi: 10.1172/JCI57867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH. Reversing agents for ATP-binding cassette drug transporters. Methods in molecular biology (Clifton, N J. 2010;596:325–340. doi: 10.1007/978-1-60761-416-6_14. [DOI] [PubMed] [Google Scholar]
- Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lin T, Islam O, Heese K. ABC transporters, neural stem cells and neurogenesis - a different perspective. Cell Res. 2006;16:857–871. doi: 10.1038/sj.cr.7310107. [DOI] [PubMed] [Google Scholar]
- Linton KJ. Structure and function of ABC transporters. Physiology (Bethesda) 2007;22:122–130. doi: 10.1152/physiol.00046.2006. [DOI] [PubMed] [Google Scholar]
- Liu DH. A study of the relationship of total urinary hydroxyproline: creatinine ratio to body height. Zhonghua Yu Fang Yi Xue Za Zhi. 1991;25:19–22. [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Martin LJ. Biology of mitochondria in neurodegenerative diseases. Prog Mol Biol Transl Sci. 2012;107:355–415. doi: 10.1016/B978-0-12-385883-2.00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaki V, Guix FX, Vennekens K, Munck S, Dingwall C, Davis JB, Townsend DM, Tew KD, Feiguin F, De Strooper B, Dotti CG, Wahle T. Down-regulation of the ATP-binding cassette transporter 2 (Abca2) reduces amyloid-beta production by altering Nicastrin maturation and intracellular localization. J Biol Chem. 2012;287:1100–1111. doi: 10.1074/jbc.M111.288258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Berti V, Swerdlow RH, Pupi A, Duara R, de Leon M. Maternal transmission of Alzheimer’s disease: prodromal metabolic phenotype and the search for genes. Hum Genomics. 2010;4:170–193. doi: 10.1186/1479-7364-4-3-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK, Larson EB, Bird TD, Boeve BF, Graff-Radford NR, De Jager PL, Evans D, Schneider JA, Carrasquillo MM, Ertekin-Taner N, Younkin SG, Cruchaga C, Kauwe JS, Nowotny P, Kramer P, Hardy J, Huentelman MJ, Myers AJ, Barmada MM, Demirci FY, Baldwin CT, Green RC, Rogaeva E, St George-Hyslop P, Arnold SE, Barber R, Beach T, Bigio EH, Bowen JD, Boxer A, Burke JR, Cairns NJ, Carlson CS, Carney RM, Carroll SL, Chui HC, Clark DG, Corneveaux J, Cotman CW, Cummings JL, DeCarli C, DeKosky ST, Diaz-Arrastia R, Dick M, Dickson DW, Ellis WG, Faber KM, Fallon KB, Farlow MR, Ferris S, Frosch MP, Galasko DR, Ganguli M, Gearing M, Geschwind DH, Ghetti B, Gilbert JR, Gilman S, Giordani B, Glass JD, Growdon JH, Hamilton RL, Harrell LE, Head E, Honig LS, Hulette CM, Hyman BT, Jicha GA, Jin LW, Johnson N, Karlawish J, Karydas A, Kaye JA, Kim R, Koo EH, Kowall NW, Lah JJ, Levey AI, Lieberman AP, Lopez OL, Mack WJ, Marson DC, Martiniuk F, Mash DC, Masliah E, McCormick WC, McCurry SM, McDavid AN, McKee AC, Mesulam M, Miller BL, Miller CA, Miller JW, Parisi JE, Perl DP, Peskind E, Petersen RC, Poon WW, Quinn JF, Rajbhandary RA, Raskind M, Reisberg B, Ringman JM, Roberson ED, Rosenberg RN, Sano M, Schneider LS, Seeley W, Shelanski ML, Slifer MA, Smith CD, Sonnen JA, Spina S, Stern RA, Tanzi RE, Trojanowski JQ, Troncoso JC, Van Deerlin VM, Vinters HV, Vonsattel JP, Weintraub S, Welsh-Bohmer KA, Williamson J, Woltjer RL, Cantwell LB, Dombroski BA, Beekly D, Lunetta KL, Martin ER, Kamboh MI, Saykin AJ, Reiman EM, Bennett DA, Morris JC, Montine TJ, Goate AM, Blacker D, Tsuang DW, Hakonarson H, Kukull WA, Foroud TM, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noell S, Ritz R, Wolburg-Buchholz K, Wolburg H, Fallier-Becker P. An allograft glioma model reveals the dependence of aquaporin-4 expression on the brain microenvironment. PloS one. 2012;7:e36555. doi: 10.1371/journal.pone.0036555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annual review of neuroscience. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Pahnke J, Krohn M, Scheffler K. The role of blood-brain barrier in the pathogenesis of Alzheimer dementia--implications for immunological therapies for plaque dissolution. Fortschritte der Neurologie-Psychiatrie. 2009a;77(Suppl 1):S21–24. doi: 10.1055/s-0028-1109601. [DOI] [PubMed] [Google Scholar]
- Pahnke J, Walker LC, Scheffler K, Krohn M. Alzheimer’s disease and blood-brain barrier function-Why have anti-beta-amyloid therapies failed to prevent dementia progression? Neuroscience and biobehavioral reviews. 2009b;33:1099–1108. doi: 10.1016/j.neubiorev.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahnke J, Wolkenhauer O, Krohn M, Walker LC. Clinico-pathologic function of cerebral ABC transporters - implications for the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2008;5:396–405. doi: 10.2174/156720508785132262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Panzenboeck U, Balazs Z, Sovic A, Hrzenjak A, Levak-Frank S, Wintersperger A, Malle E, Sattler W. ABCA1 and scavenger receptor class B, type I, are modulators of reverse sterol transport at an in vitro blood-brain barrier constituted of porcine brain capillary endothelial cells. J Biol Chem. 2002;277:42781–42789. doi: 10.1074/jbc.M207601200. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier biology and methodology. J Neurovirol. 1999;5:556–569. doi: 10.3109/13550289909021285. [DOI] [PubMed] [Google Scholar]
- Piehler AP, Ozcurumez M, Kaminski WE. A-Subclass ATP-Binding Cassette Proteins in Brain Lipid Homeostasis and Neurodegeneration. Front Psychiatry. 2012;3:17. doi: 10.3389/fpsyt.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jaggi F, Wolburg H, Gengler S, Haass C, Ghetti B, Czech C, Holscher C, Mathews PM, Jucker M. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006;7:940–946. doi: 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- Rhinn H, Qiang L, Yamashita T, Rhee D, Zolin A, Vanti W, Abeliovich A. Alternative alpha-synuclein transcript usage as a convergent mechanism in Parkinson’s disease pathology. Nat Commun. 2012;3:1084. doi: 10.1038/ncomms2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Rodriguez E, Mateo I, Llorca J, Sanchez-Quintana C, Infante J, Garcia-Gorostiaga I, Sanchez-Juan P, Berciano J, Combarros O. Association of genetic variants of ABCA1 with Alzheimer’s disease risk. Am J Med Genet B Neuropsychiatr Genet. 2007;144:964–968. doi: 10.1002/ajmg.b.30552. [DOI] [PubMed] [Google Scholar]
- Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J Neurosci. 2006;26:10480–10487. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, Mulnard R, Barakos J, Gregg KM, Liu E, Lieberburg I, Schenk D, Black R, Grundman M. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurin W, Hofnung M, Dassa E. Getting in or out: early segregation between importers and exporters in the evolution of ATP-binding cassette (ABC) transporters. Journal of molecular evolution. 1999;48:22–41. doi: 10.1007/pl00006442. [DOI] [PubMed] [Google Scholar]
- Scheffler K, Krohn M, Dunkelmann T, Stenzel J, Miroux B, Ibrahim S, von Bohlen Und Halbach O, Heinze HJ, Walker LC, Gsponer JA, Pahnke J. Mitochondrial DNA polymorphisms specifically modify cerebral beta-amyloid proteostasis. Acta Neuropathol. 2012;124:199–208. doi: 10.1007/s00401-012-0980-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55:3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Schumacher T, Krohn M, Hofrichter J, Lange C, Stenzel J, Steffen J, Dunkelmann T, Paarmann K, Frohlich C, Uecker A, Plath AS, Sommer A, Bruning T, Heinze HJ, Pahnke J. ABC Transporters B1, C1 and G2 Differentially Regulate Neuroregeneration in Mice. PloS one. 2012;7:e35613. doi: 10.1371/journal.pone.0035613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shruster A, Eldar-Finkelman H, Melamed E, Offen D. Wnt signaling pathway overcomes the disruption of neuronal differentiation of neural progenitor cells induced by oligomeric amyloid beta-peptide. J Neurochem. 2011;116:522–529. doi: 10.1111/j.1471-4159.2010.07131.x. [DOI] [PubMed] [Google Scholar]
- Shukla S, Wu CP, Ambudkar SV. Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges. Expert opinion on drug metabolism & toxicology. 2008;4:205–223. doi: 10.1517/17425255.4.2.205. [DOI] [PubMed] [Google Scholar]
- Song ES, Juliano MA, Juliano L, Fried MG, Wagner SL, Hersh LB. ATP effects on insulin-degrading enzyme are mediated primarily through its triphosphate moiety. J Biol Chem. 2004;279:54216–54220. doi: 10.1074/jbc.M411177200. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Grune T. Neuronal apoptotic bodies: phagocytosis and degradation by primary microglial cells. Faseb J. 2004;18:743–745. doi: 10.1096/fj.03-0374fje. [DOI] [PubMed] [Google Scholar]
- Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, Trillat AC, Stern DM, Arancio O, Yan SS. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. FASEB J. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- Townsend M, Mehta T, Selkoe DJ. Soluble Abeta inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem. 2007;282:33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- Vogelgesang S, Warzok RW, Cascorbi I, Kunert-Keil C, Schroeder E, Kroemer HK, Siegmund W, Walker LC, Pahnke J. The role of P-glycoprotein in cerebral amyloid angiopathy; implications for the early pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2004;1:121–125. doi: 10.2174/1567205043332225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Kim J, Li A, Knoten A, Jain S, Hirsch-Reinshagen V, Wellington CL, Bales KR, Paul SM, Holtzman DM. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest. 2008;118:671–682. doi: 10.1172/JCI33622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science. 1992;256:628–632. doi: 10.1126/science.1533953. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Bioenergetic origins of complexity and disease. Cold Spring Harb Symp Quant Biol. 2011;76:1–16. doi: 10.1101/sqb.2011.76.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788:842–857. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Whitson JS, Selkoe DJ, Cotman CW. Amyloid beta protein enhances the survival of hippocampal neurons in vitro. Science. 1989;243:1488–1490. doi: 10.1126/science.2928783. [DOI] [PubMed] [Google Scholar]
- WHO. World Health Organization Report. World Health Organization; 2012. [Google Scholar]
- Wollmer MA, Kapaki E, Hersberger M, Muntwyler J, Brunner F, Tsolaki M, Akatsu H, Kosaka K, Michikawa M, Molyva D, Paraskevas GP, Lutjohann D, von Eckardstein A, Hock C, Nitsch RM, Papassotiropoulos A. Ethnicity-dependent genetic association of ABCA2 with sporadic Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:534–536. doi: 10.1002/ajmg.b.30345. [DOI] [PubMed] [Google Scholar]
- Xie L, Helmerhorst E, Taddei K, Plewright B, Van Bronswijk W, Martins R. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci. 2002;22:RC221. doi: 10.1523/JNEUROSCI.22-10-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Du H, Yan S, Fang F, Wang C, Lue LF, Guo L, Chen D, Stern DM, Gunn Moore FJ, Xi Chen J, Arancio O, Yan SS. Inhibition of amyloid-beta (Abeta) peptide-binding alcohol dehydrogenase-Abeta interaction reduces Abeta accumulation and improves mitochondrial function in a mouse model of Alzheimer’s disease. J Neurosci. 2011;31:2313–2320. doi: 10.1523/JNEUROSCI.4717-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zutz A, Gompf S, Schagger H, Tampe R. Mitochondrial ABC proteins in health and disease. Biochim Biophys Acta. 2009;1787:681–690. doi: 10.1016/j.bbabio.2009.02.009. [DOI] [PubMed] [Google Scholar]