Received 22 July 2012; revised 29 January 2013; accepted 4 March 2013
Aims
Aldosterone plays a crucial role in cardiovascular disease. ‘Systemic’ inhibition of its mineralocorticoid receptor (MR) decreases atherosclerosis by reducing inflammation and oxidative stress. Obesity, an important cardiovascular risk factor, is an inflammatory disease associated with increased plasma aldosterone levels. We have investigated the role of the ‘endothelial’ MR in obesity-induced endothelial dysfunction, the earliest stage in atherogenesis.
Methods and results
C57BL/6 mice were exposed to a normal chow diet (ND) or a high-fat diet (HFD) alone or in combination with the MR antagonist eplerenone (200 mg/kg/day) for 14 weeks. Diet-induced obesity impaired endothelium-dependent relaxation in response to acetylcholine, whereas eplerenone treatment of obese mice prevented this. Expression analyses in aortic endothelial cells isolated from these mice revealed that eplerenone attenuated expression of pro-oxidative NADPH oxidase (subunits p22phox, p40phox) and increased expression of antioxidative genes (glutathione peroxidase-1, superoxide dismutase-1 and -3) in obesity. Eplerenone did not affect obesity-induced upregulation of cyclooxygenase (COX)-1 or prostacyclin synthase. Endothelial-specific MR deletion prevented endothelial dysfunction in obese (exhibiting high ‘endogenous’ aldosterone) and in ‘exogenous’ aldosterone-infused lean mice. Pre-incubation of aortic rings from aldosterone-treated animals with the COX-inhibitor indomethacin restored endothelial function. Exogenous aldosterone administration induced endothelial expression of p22phox in the presence, but not in the absence of the endothelial MR.
Conclusion
Obesity-induced endothelial dysfunction depends on the ‘endothelial’ MR and is mediated by an imbalance of oxidative stress-modulating mechanisms. Therefore, MR antagonists may represent an attractive therapeutic strategy in the increasing population of obese patients to decrease vascular dysfunction and subsequent atherosclerotic complications.
Keywords: Obesity, Endothelial, Aldosterone, Mineralocorticoid receptor
Introduction
Obesity has reached pandemic dimensions.1 In association with insulin resistance, hypertension, and dyslipidaemia, obesity forms the ‘metabolic syndrome’, a hallmark of cardiovascular risk.2 The ‘white’ adipose tissue (WAT) is increased in both obese mice and humans3 and is characterized by an increase in the inflammatory cytokines and oxidative stress.4 Furthermore, adipocytes can induce aldosterone synthesis in the adrenocortical gland in an endocrine fashion.5 As a result, visceral obesity is associated with increased plasma aldosterone levels.6 Many of its effects are mediated by activation of its nuclear receptor, the mineralocorticoid receptor (MR).
Aldosterone itself plays a crucial role in cardiovascular diseases. Mineralocorticoid receptor antagonists reduce morbidity and mortality in patients with congestive heart failure.7,8 In corresponding mouse models, the beneficial effects of MR antagonism were associated with decreased expression of inflammatory mediators.9 Atherosclerosis is initiated at sites of endothelial dysfunction which may be caused by enhanced generation of COX-derived vasoconstricting prostanoids or reactive oxygen species (ROS).10,11 The increased oxidative stress is mainly due to a disturbed expression of ROS-producing enzymes such as NADPH oxidase and enzymes involved in antioxidant defense such as glucose-6-phosphate dehydrogenase (G6PDH) and glutathione peroxidase (GPx).12–14
Pharmacological MR antagonism decreases atherosclerosis in mice by diminishing oxidative stress and inflammation.15 Moreover, MR blockade attenuates endothelial dysfunction in animal models of heart failure.16 MR activation induces expression of NADPH oxidase subunits, thereby contributing to generation of oxidative stress.17 Furthermore, aldosterone alters expression of prostanoid-producing enzymes (enhanced) and G6PDH (reduced) that are associated with endothelial dysfunction.18,19
Mineralocorticoid receptor is expressed in both endothelial cells and adipocytes. While it may induce an array of mediators involved in endothelial dysfunction20 in the former, it acts in adipocytes as a pro-adipogenic transcription factor.21 Pharmacological MR blockade using eplerenone reduces insulin resistance, macrophage infiltration, expression of pro-inflammatory factors, and ROS release in WAT.22,23 Of note, the effects of MR blockade on obesity-induced endothelial dysfunction, the role of the endothelial MR in this context, and the corresponding molecular mediators remain unknown.
Thus, we assessed endothelial function of lean and diet-induced obese mice, differing in endogenous aldosterone levels, and of lean mice with and without exogenous aldosterone infusion. Mineralocorticoid receptor activity was modulated by a pharmacological and a genetic approach. As non-selective receptor binding properties of spironolactone may result in an increase in plasma cortisol24 and also thereby potentially influence endothelial function, we used in this study, the more selective MR-antagonist eplerenone. To address the role of the endothelial MR, we used a genetic loss-of-function approach targeted to endothelial cells (Tie2-driven endothelial MR deletion).
Methods
Online data supplements are available for (i) Animals, (ii) Signal transduction and inflammation, (iii) Blood glucose and components of the renin-angiotensin-aldosterone system, (iv) Analysis of vascular function, (v) Isolation of fresh aortic endothelial cells and RNA expression analyses, and (vi) Statistical analyses.
Results
Pro-inflammatory changes in obese mice are attenuated by eplerenone
To address the contribution of endogenous aldosterone in the development of obesity-induced endothelial dysfunction, we exposed 6-week-old male C57BL/6 mice to a high-fat diet without (HFD) or with eplerenone (HFD EPL). Control mice were fed a normal diet (ND) and were considered as lean mice for the purpose of these experiments.
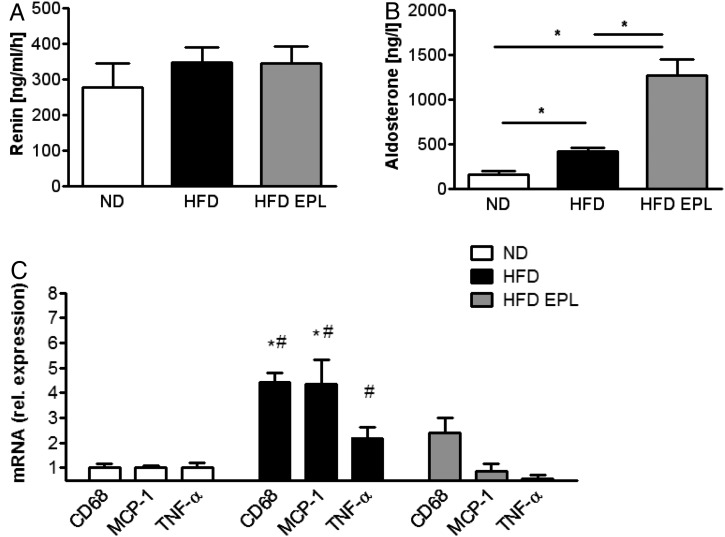
To assess a putative activation of the renin–angiotensin–aldosterone system, aldosterone and renin plasma concentration were measured. Aldosterone levels were significantly higher, whereas renin concentration remained unaltered in mice fed an HFD compared with lean mice (Figure 1A and B). Compared with HFD-fed obese mice, EPL treatment of obese mice did not change plasma renin, whereas it increased plasma aldosterone levels (Figure 1A and B). Feeding an HFD for 14 weeks resulted in a significant increase in body weight and epididymal WAT, it increased fasting plasma glucose, and reduced glucose tolerance compared with mice fed an ND. Treatment with EPL administered with the HFD did not interfere with epididymal WAT and total body weight gain. However, EPL treatment did prevent worsening of glucose metabolism in these mice (Supplementary material online, Table S2).
Figure 1.
Plasma aldosterone is increased in obesity and further elevated by mineralocorticoid receptor antagonism; obesity-induced expression of pro-inflammatory cytokines in white adipose tissue is attenuated by mineralocorticoid receptor antagonism. C57BL/6 mice on ND, HFD, and HFD EPL were analysed concerning (A) plasma-renin and (B) plasma-aldosterone levels; n = 8–10; *P < 0.05. (C) mRNA levels of epididymal fat pad in C57BL/6 mice on ND, HFD, and HFD EPL, normalized to ND levels; n = 6–8; *P < 0.05 compared with ND levels, #P < 0.05 compared with HFD EPL. ND, normal chow diet; HFD, high-fat diet; HFD EPL, high-fat diet with eplerenone.
mRNA levels of pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α) and monocyte chemoattractant protein-1 (MCP-1) tended to be increased (TNF-α) or were increased (MCP-1) in the epididymal WAT of obese mice compared with lean control mice (Figure 1C). Chronic administration of eplerenone prevented an increase in mRNA expression levels of these cytokines as well as macrophage-specific glycoprotein CD68 in the epididymal WAT. These data suggest that MR antagonism diminished the obesity-induced pro-inflammatory changes in the epididymal WAT.
Pharmacological mineralocorticoid receptor antagonism prevents obesity-induced endothelial dysfunction
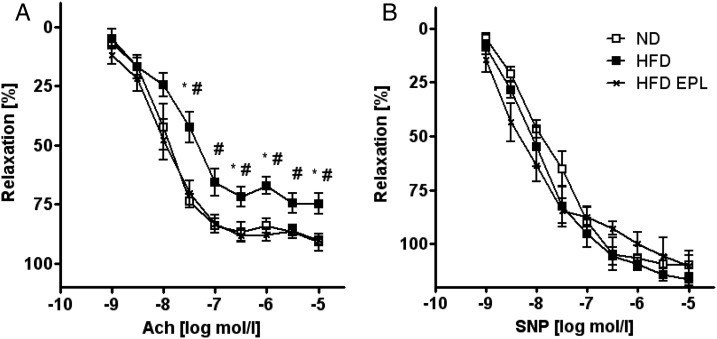
To determine the effects of increased endogenous aldosterone levels in obesity on endothelial function, we performed ex vivo aortic ring contraction/relaxation experiments with aortae from C57BL/6 mice kept on ND, HFD, or HFD EPL, respectively (Figure 2). Endothelium-dependent relaxation to acetylcholine was blunted in aortae of obese mice starting at a concentration of 3 × 10−8M of acetylcholine and reached a maximal relaxation at 10−5 M of acetylcholine. Chronic eplerenone treatment in obese mice prevented endothelial dysfunction (Figure 2A). Endothelium-independent relaxation to sodium nitroprusside (SNP) was similar in all groups (Figure 2B). Contractions to KCl and norepinephrine (NE) were unaffected in mice fed HFD or HFD EPL, respectively (data not shown). These findings show that pharmacological MR antagonism prevents obesity-induced endothelial dysfunction.
Figure 2.
Mineralocorticoid receptor antagonism prevents obesity-induced worsening of endothelial function. Endothelial function studies in C57BL/6 mice on ND, HFD, and HFD EPL. Responses of aortic rings to increasing doses of (A) acetylcholine (Ach), mediating endothelial release of endogenous nitric oxide, and (B) sodium nitroprusside (SNP), a nitric oxide donor. See Supplementary material online, Table S4 for EC50 and Emax values; n = 8–10; *P < 0.05 for ND vs. HFD; #P < 0.05 for HFD vs. HFD EPL. ND, normal chow diet; HFD, high-fat diet; HFD EPL, high-fat diet with eplerenone.
Mineralocorticoid receptor antagonism attenuates pro-inflammatory and pro-oxidative changes in aortic endothelial cells of obese mice
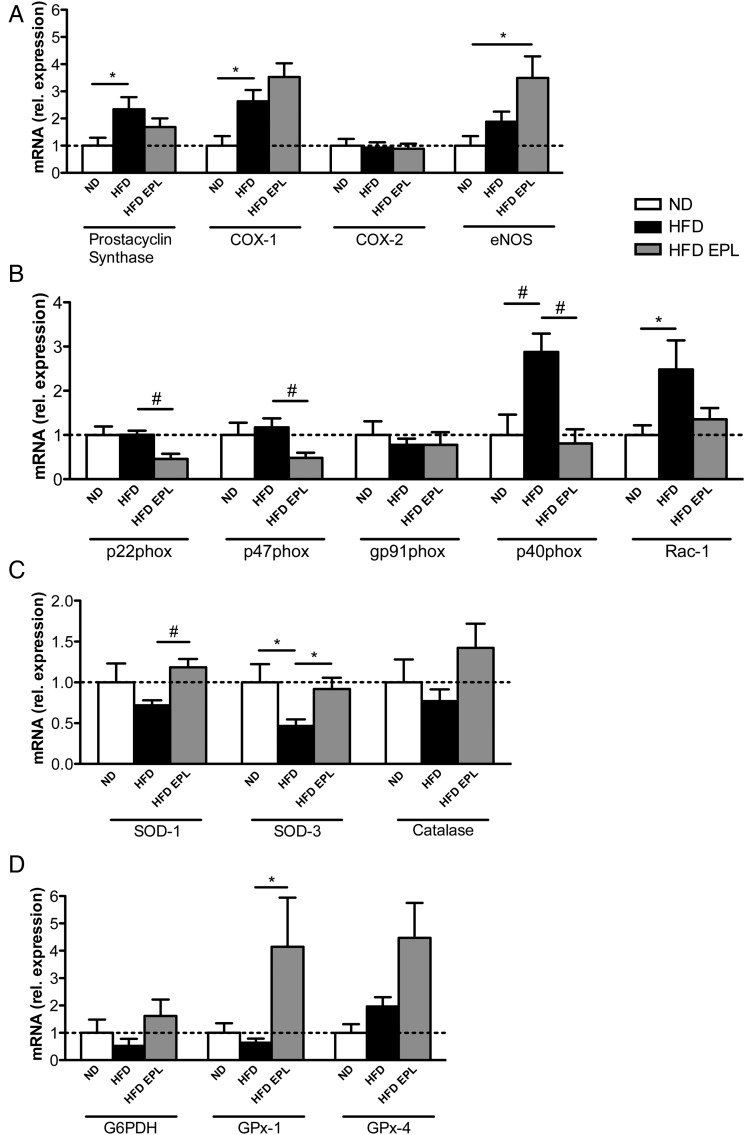
Obesity is known to promote a pro-inflammatory, pro-oxidative, and vasoconstricting state in the vascular endothelium, thereby inducing endothelial dysfunction. To investigate the direct effects of obesity and eplerenone treatment on the genes expressed in aortic endothelial cells, we developed a method to isolate fresh endothelial cells from C57BL/6 mice exposed to ND, HFD, or HFD EPL (Supplementary material online, Figure S2A and B). We observed a slight increase in the MR mRNA expression under HFD and an even higher increase upon additional chronic eplerenone treatment (Supplementary material online, Figure S3A). Mineralocorticoid receptor mRNA was reduced in endothelial cells of mice in which MR was deleted (EC MR−/−), indicating successful ablation of endothelial MR (Supplementary material online, Figure S3B). Analyses of mediators involved in endothelial dysfunction revealed that prostacyclin synthase and COX-1 expression were upregulated in obesity, whereas COX-2 remained unaltered (Figure 3A). Eplerenone treatment did neither prevent HFD-induced upregulation of prostacyclin synthase nor COX-1 expression. Furthermore, endothelial nitric oxide synthase (eNOS) expression increased in HFD EPL compared with ND mice (Figure 3A).
Figure 3.
Obesity-induced pro-inflammatory and pro-oxidative changes are modulated by mineralocorticoid receptor antagonism in aortic endothelial cells. Aortic endothelial cell mRNA levels in C57BL/6 mice on ND, HFD, HFD EPL after 14 weeks (A) prostacyclin synthase, COX-1, COX-2, eNOS, (B) p22phox, 47phox, gp91phox, p40phox, Rac-1, (C) SOD-1, SOD-3, catalase, (D) G6PDH, GPx-1, GPx-4, normalized to ND levels, n = 10, *P < 0.05, #P < 0.01. ND, normal chow diet; HFD, high-fat diet; HFD EPL, high-fat diet with eplerenone.
Given the association of aldosterone with oxidative stress, we tested mRNA expression of NADPH oxidase subunits in isolated aortic endothelial cells. mRNA levels of the p22phox, p47phox, and gp91phox were not affected by diet-induced obesity. However, additional eplerenone treatment decreased the expression of p22phox and p47phox (Figure 3B). On the other hand, obesity increased the expression levels of p40phox and Rac-1; the increase in p40phox was attenuated by eplerenone (Figure 3B).
mRNA expression levels of the antioxidant enzymes SOD-1 and SOD-3 were lower under HFD compared with ND (trend for SOD-1) and HFD EPL conditions; catalase expression remained unaltered (Figure 3C). The mRNA expression of the ROS-scavenging enzyme GPx-1 was enhanced by eplerenone in obesity, whereas GPx-4 and G6PDH remained unchanged (Figure 3D).
Taken together, we observed the generation of an obesity-induced vasoconstrictive and pro-oxidative mRNA expression profile in aortic endothelial cells that was partially prevented by pharmacological MR antagonism.
Endothelium-specific mineralocorticoid receptor ablation neither affects hyperglycaemia nor inflammation in white adipose tissue of obese or aldosterone-infused lean mice
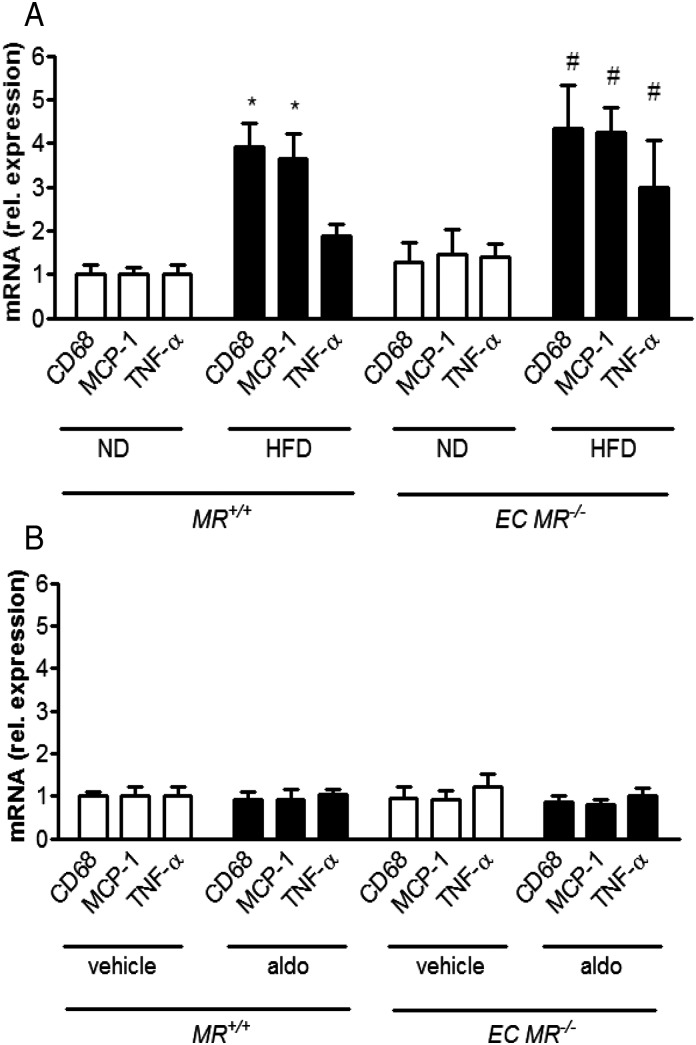
To investigate the role of the endothelial MR on obesity-induced metabolic changes, we exposed EC MR−/− mice and their corresponding MR+/+ littermates to an ND or HFD for 14 weeks. After 14 weeks on a HFD, both obese MR+/+ mice and EC MR−/− showed a similar increase in plasma aldosterone levels (Supplementary material online, Figure S4A), weight gain, glucose intolerance (Supplementary material online, Table S3), as well as expression of pro-inflammatory markers MCP-1, and CD68; TNF-α was upregulated only in EC MR−/− mice (Figure 4A).
Figure 4.
The obesity-induced increase in the expression of pro-inflammatory cytokines in white adipose tissue is independent of endothelial mineralocorticoid receptor; aldosterone infusion in lean animals does not induce inflammation. mRNA levels of the epididymal fat pad in wild-type (MR+/+) and endothelial-specific MR knockout (EC MR−/−) mice (A) on ND or HFD and (B) vehicle- or aldosterone-infused, standardized to S12 and normalized to ND levels; n = 6–8; *P < 0.05 for MR+/+ ND vs. MR+/+HFD; #P < 0.05 for EC MR−/− ND vs. EC MR−/−HFD. ND, normal chow diet; HFD, high-fat diet.
To test the effects of exogenous aldosterone on the endothelial MR, we implanted osmotic minipumps containing aldosterone or vehicle in lean MR+/+ and EC MR−/− mice. Aldosterone infusion for 2 weeks increased plasma aldosterone to the same extent in both EC MR−/− and MR+/+ mice kept on ND (Supplementary material online, Figure S4B) and did not induce the expression of TNF-α, MCP-1, or CD68 in the epididymal WAT (Figure 4B). These data indicate that endothelial MR deletion neither affects glucose tolerance nor the pro-inflammatory state of the WAT of obese or aldosterone-infused lean mice.
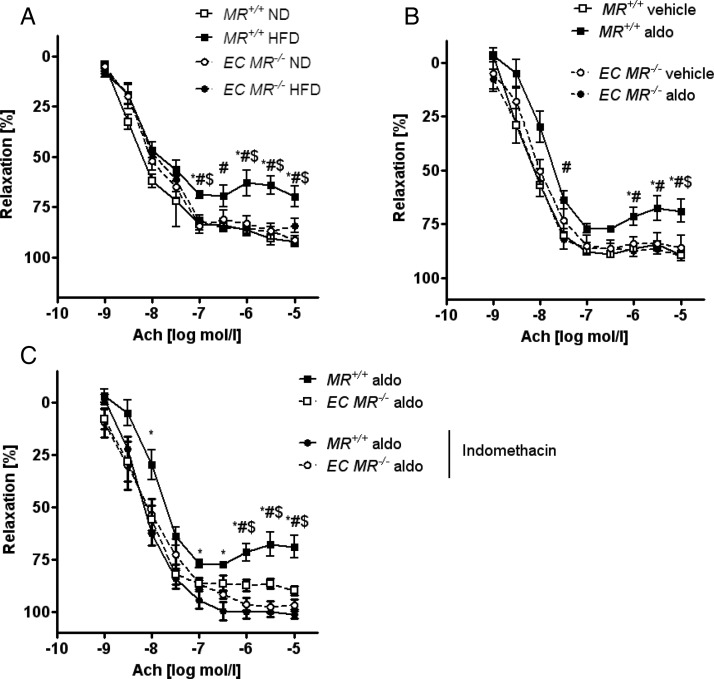
Deletion of the endothelial mineralocorticoid receptor prevents obesity- or exogenous aldosterone-induced endothelial dysfunction to a similar extent as does COX inhibition
To determine whether obesity-induced endothelial dysfunction was mediated by the endothelial MR, we exposed EC MR−/− and MR+/+ mice to ND or HFD for 14 weeks. Compared with lean controls, obese MR+/+ mice demonstrated a significant impairment of endothelium-dependent vasodilation, whereas MR deletion in endothelial cells prevented endothelial dysfunction (Figure 5A). Of note, aortic walls of obese MR+/+ mice contained no macrophages (Supplementary material online, Figure S5). Infusion of aldosterone for 2 weeks using minipumps blunted endothelial function in lean mice. Genetic deletion of the endothelial MR prevented endothelial dysfunction (Figure 5B). Thus, the endothelial MR mediates both obesity-induced endogenous as well as exogenous aldosterone-induced endothelial dysfunction.
Figure 5.
Loss of endothelial MR prevents obesity-induced endothelial dysfunction and COX inhibition restores aldosterone-induced endothelial dysfunction. The vascular response of aortic rings pre-constricted with norepinephrine to increasing doses of the endothelium-dependent vasodilator acetylcholine (Ach) was measured in wild-type (MR+/+) and endothelial-specific MR knockout (EC MR−/−) mice; n = 6–8. (A) MR+/+and EC MR−/− mice on ND and HFD; *P < 0.05 for MR+/+ ND vs. MR+/+ HFD; #P < 0.05 for MR+/+ HFD vs. EC MR−/− HFD; $P < 0.05 for MR+/+ HFD vs. EC MR−/− ND. (B) MR+/+ and EC MR−/− mice after aldosterone or vehicle infusion; *P < 0.05 for MR+/+ vehicle vs. MR+/+ aldo; #P < 0.05 for MR+/+ aldo vs. EC MR−/− aldo; $P < 0.05 for MR+/+ aldo vs. EC MR−/− vehicle. (C) MR+/+ on ND and HFD or after aldosterone or vehicle infusion; rings were pre-treated for 30 min with indomethacin. See Supplementary material online, Table S4 for EC50 and Emax values. *P < 0.05 for MR+/+ aldo vs. MR+/+ aldo (indomethacin-treated); #P < 0.05 for MR+/+ aldo vs. EC MR−/− aldo; $P < 0.05 for MR+/+ aldo vs. EC MR−/− aldo (indomethacin-treated). ND, normal chow diet; HFD, high-fat diet.
COX inhibition using indomethacin normalized endothelial function in aldosterone-infused lean mice to the same extent as endothelial MR deletion (Figure 5C). Therefore, aldosterone induces endothelial dysfunction by activating COX-dependent pathways.
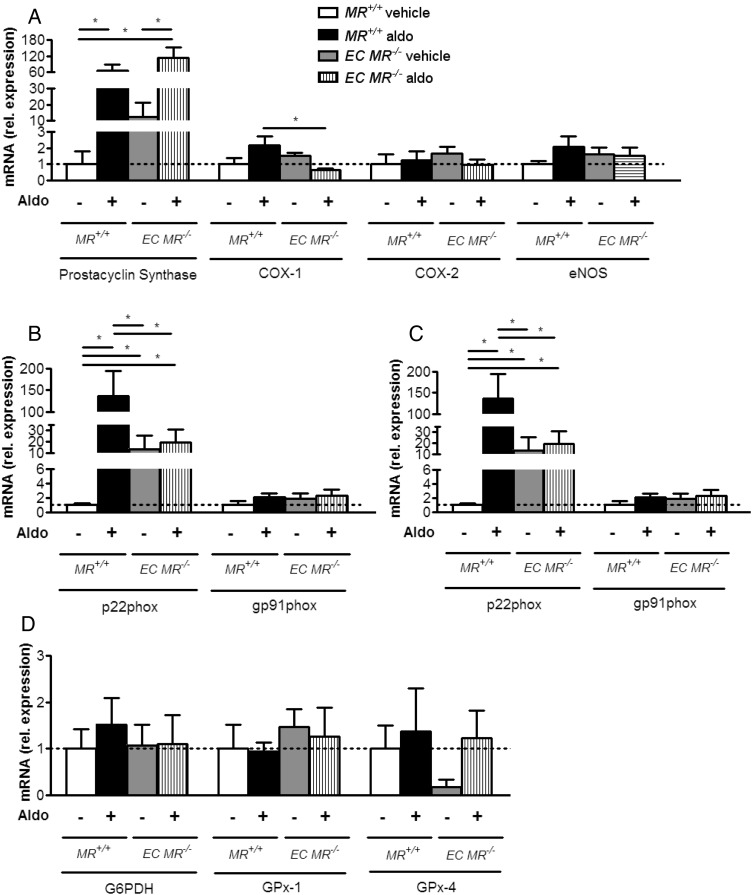
Aldosterone-infused endothelium-specific MR−/− lean mice exhibit pro-inflammatory changes in aortic endothelial cells
Next we evaluated the effects of aldosterone infusion or MR ablation on mediators of inflammation, vasoconstriction, and oxidative stress in aortic endothelial cells. For this purpose, we isolated fresh aortic endothelial cells from lean MR+/+ and EC MR−/− mice infused with aldosterone for 2 weeks and determined mRNA expression by qPCR. Our analyses showed that prostacyclin synthase expression was enhanced by exogenous aldosterone, independent of the presence of endothelial MR (Figure 6A); in contrast, aldosterone-induced COX-1 expression was decreased in EC MR−/− mice, whereas COX-2 and eNOS expression remained unchanged.
Figure 6.
Aldosterone-induced expression of COX-1 and reactive oxygen species-generating enzymes in endothelial aortic cells depend on endothelial mineralocorticoid receptor. Aortic endothelial cell mRNA levels in wild-type (MR+/+) and endothelial-specific MR knockout (EC MR−/−) mice after aldosterone or vehicle infusion (A) prostacyclin synthase, COX-1, COX-2, eNOS, (B) p22phox, gp91phox, (C) SOD-1, SOD-3, (D) G6PDH, GPx-1, GPx-4, standardized to S12 and normalized to ND levels; n = 6–8; *P < 0.05. ND, normal chow diet.
Analyses of enzymes enhancing oxidative stress revealed that mRNA expression of the NADPH oxidase subunit p22phox was upregulated by aldosterone, whereas this effect was abolished in EC MR−/− mice; gp91phox remained unaltered in all conditions (Figure 6B). Expression levels of the NADPH oxidase subunits p47phox, p40phox, and Rac-1 were below the detection limit (data not shown). Regarding antioxidant enzymes, expression of SOD-1 mRNA was decreased by aldosterone, but did not reach statistical significance (P = 0.077). SOD-3 mRNA was decreased upon aldosterone infusion and remained low in EC MR−/− mice, both in the presence or absence of aldosterone (Figure 6C). Catalase could not be detected (data not shown). ROS-scavenging enzymes GPx-1, GPx-4, and G6PDH remained unaltered in all conditions (Figure 6D). Thus, aldosterone enhances the expression of mediators involved in the generation of ROS and prostanoids in an endothelial MR-dependent manner.
Discussion
Principle findings
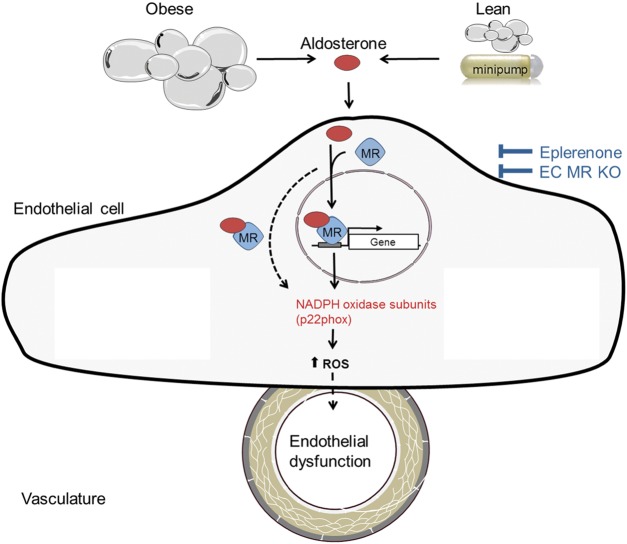
This experimental study provides three main novel findings (Figure 7): (i) diet-induced obesity generates endothelial dysfunction by enhancing expression of NADPH oxidase and through activation of COX-1-dependent pathways in freshly isolated aortic endothelial cells; (ii) genetic deletion of the endothelial MR prevents obesity- as well as aldosterone-induced endothelial dysfunction without affecting glucose tolerance or pro-inflammatory pathways in the WAT; (iii) activation of the endothelial MR is sufficient to induce endothelial dysfunction, in part via increased endothelial p22phox expression.
Figure 7.
Mechanisms of aldosterone-induced endothelial dysfunction in obesity. The expression of NADPH oxidase subunit p22phox can be blocked by both eplerenone and endothelial mineralocorticoid receptor ablation. Therefore, p22phox seems to be the crucial mediator in inducing aldosterone-induced endothelial dysfunction in aortic endothelial cells of obese mice through genomic (solid arrow) or non-genomic (dashed arrow) effects.
Added value of this study
Our study provides added value in the following contexts:
In mice fed an HFD, we observed an increase in plasma aldosterone. This effect was more pronounced if mice were pre-treated with eplerenone; renin levels remained unaltered. This is in line with publications showing a positive correlation between obesity and plasma aldosterone levels independent of plasma renin activity.25,26 Since adipocytes induce the release of aldosterone by adrenocortical cells,27 we propose a renin-independent mechanism in obesity that stimulates aldosterone secretion. We observed a further increase in plasma aldosterone levels upon eplerenone therapy corroborating previous findings.28,29 This phenomenon is likely related to a displacement of the mineralocorticoid from its receptor and/or its enhanced production during MR blockade due to a positive physiological feedback loop. We show for the first time that deletion of endothelial MR does not increase plasma aldosterone levels. Thus, we propose that the endothelial MR is not involved in the regulatory feedback loop that induces eplerenone-induced aldosterone production.
Our data reveal that pharmacological MR blockade in diet-induced obesity improved glucose tolerance. In line with our observation, MR antagonism has been reported to reduce insulin resistance in genetically obese mice.23 Obesity-associated pro-inflammatory changes in WAT can contribute to the development of insulin resistance.30 We and others22 demonstrated that eplerenone decreases mRNA of pro-inflammatory cytokines in WAT. Moreover, we extend these findings by showing for the first time that endothelial MR ablation neither prevents impaired glucose tolerance nor increases the expression of pro-inflammatory mediators in the obese WAT. Thus, endothelial MR signalling is neither involved in these obesity-induced diabetic nor pro-inflammatory changes.
Genetically obese mice develop endothelial dysfunction.31 In the current study, pharmacological MR antagonism prevented endothelial dysfunction in diet-induced obesity, suggesting that activation of MR with a concomitant release of inflammatory molecules impaired endothelium-dependent relaxation. Notably, endothelium-specific MR deletion completely prevented both diet- and aldosterone-induced endothelial dysfunction. These novel findings demonstrate that activation of the endothelial MR is sufficient to mediate endothelial dysfunction in diet-induced obesity.
Aldosterone increases expression of COX-2, prostacyclin production,32 and vascular inflammation.33 In the context of diet-induced obesity with increased endogenous aldosterone levels, we observed that COX-1 was upregulated in freshly isolated aortic endothelial cells. Mineralocorticoid receptor antagonism did not attenuate this increased COX-1 expression. Our endothelial function studies revealed that pre-treatment of aortic rings with the COX inhibitor indomethacin completely normalized endothelium-dependent relaxations in response to acetylcholine in obese mice. Similar effects were observed in spontaneously hypertensive rats.11 Our results corroborate another study reporting that obesity induces endothelial dysfunction in a COX-1-dependent manner.34 Analyses of the prostacyclin synthase revealed that both diet-induced obesity as well as exogenous aldosterone administration enhanced expression of prostacyclin synthase in aortic endothelial cells. In line with our observation, it has been shown that prostacyclin increased endothelial dysfunction in hypertensive rats.32 Furthermore, inhibition of COX that produces prostacyclin synthase substrates, restores aldosterone-induced endothelial dysfunction.35 Of note, neither pharmacological MR antagonism in diet-induced obesity, nor genetic endothelial-specific MR deletion during aldosterone infusion attenuated prostacyclin synthase expression. These findings imply that increased endogenous aldosterone in obesity induces vasoconstricting prostanoids independent of the endothelial MR. It is likely that aldosterone acts in this context by non-genomic effects.36 A more detailed mechanistic insight would be of interest. However, we consider the corresponding analyses beyond the scope of the current study.
We demonstrate that diet-induced obesity was associated with an increased expression of the pro-oxidant NADPH subunits (p40phox and rac-1) as well as a reduced expression of the antioxidant proteins SOD-1 and -3. Thus, increased ROS production may contribute to endothelial dysfunction. Indeed, the endothelial MR mediates superoxide generation via Rac-1 activation.17 This is in line with our observation that MR antagonism in obesity conferred beneficial effects on the expression of the NADPH oxidase subunits (p22phox, p47phox, p40phox, and rac-1), antioxidant enzymes SOD-1 and -3, and the ROS scavenging enzyme GPx-1. Exogenous aldosterone administration modulated exclusively the NADPH oxidase subunit p22phox mRNA expression in aortic endothelial cells in an MR-dependent manner. Since endothelial MR ablation is sufficient to abolish both obesity- and exogenous aldosterone-induced endothelial dysfunction, we postulate that the endothelial MR-mediated p22phox expression is sufficient to increase a pro-oxidative pathway leading to endothelial dysfunction.
Overexpression of endothelial MR in mice fed a normal chow induces an enhanced vasoconstrictive response in resistance arteries with a mild hypertension. However, these vessels do not exhibit signs of endothelial dysfunction nor increased oxidative stress.37 Differences in experimental settings (our study: diet-induced obesity, high cholesterol diet, and constitutive EC MR expression) are likely to account for the diverse findings.
Potential limitations
We provide evidence of increased endothelial MR expression in obese compared with lean mice and of a lack of MR expression in endothelial cells of EC MR−/− mice—all at the DNA and RNA level. Despite extensive efforts, we did not succeed in visualizing MR expression at the protein level using various lots of previously described antibodies.38 Since the knockout procedure consistently deletes the gene, we consider that our data provide solid evidence for MR deletion in endothelial cells. As the Tie2 promotor used to drive MR deletion in endothelial cells is also active in myeloid cells, we cannot conclude that endothelial protection seen in EC MR−/− mice is due exclusively to endothelial MR ablation.39 Indeed, we observed MR ablation in macrophages of EC MR−/− mice. Moreover, MR ablation in macrophages reduces MR-mediated cardiac pro-inflammatory responses in a mouse model of cardiac fibrosis.40 Since we found no macrophages in the aortae of obese mice with intact MR and we observed similar pro-inflammatory expression patterns in the WAT of obese EC MR−/− and MR+/+ mice, we propose that Tie2-driven ablation of MR in adipose tissue macrophages is unlikely to contribute to endothelial protection in diet-induced obesity in EC MR−/− mice.
Blood pressure in the different animal groups was not assessed, although a minor increase in blood pressure of obese animals and a decrease in mice lacking the endothelial MR are possible. Indeed, increased blood pressure has been observed upon exogenous aldosterone administration19 and in mice with endothelium-specific MR overexpression.37 Thus, changes in blood pressure could have taken place and may have modified aortic endothelial gene expression and participated in the observed alterations of endothelial function.
Conclusions and possible implications
We demonstrate that both endogenous aldosterone in obesity as well as exogenous aldosterone cause endothelial dysfunction by inducing expression of ROS-generating enzymes in an endothelial MR-dependent fashion and by activating the COX-1 pathway independent of the endothelial MR. Pharmacological MR blockade abolishes obesity-induced glucose intolerance, adipose tissue inflammation, and endothelial dysfunction. Genetic ablation of the MR in endothelial cells identifies the endothelial MR as a crucial mediator of obesity-induced endothelial dysfunction (Figure 7). In addition, changes in blood pressure secondary to endothelial MR ablation and/or the deletion of the MR in macrophages may have contributed to the observed prevention of obesity-induced effects by Tie2-driven MR deletion.
Our experimental findings have the following potential clinical implications: MR-antagonizing drugs appear worth testing for endothelial function in the rapidly growing population of obese patients that are particularly prone to atherosclerosis, hypertension, and insulin resistance.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by grants from the Swiss National Science Foundation 310030-130626/1 (C.M.M.), and 3100-068118 (T.F.L.) the University Research Priority Program Integrative Human Physiology at the University of Zurich (N.S., F.R, T.F.L., F.V, and C.M.M.). Further support was provided by an unrestricted grant from Pfizer (NYC, USA).
Conflict of interest: C.M.M. received grant from ZIHP, University of Zurich; MSD; AstraZeneca; EliLilly; Sirtris; Bayer; Swiss National Science Foundation; MSD; AstraZeneca; Roche; has expert testimony to MSD; has patent rights to Mabimmune, CH.
Supplementary Material
Acknowledgements
We thank the members of the cardiovascular research and epithelial transport laboratories for their help. Eplerenone was provided by Pfizer (Groton, CT, USA).
References
- 1.James PT, Rigby N, Leach R. The obesity epidemic, metabolic syndrome and future prevention strategies. Eur J Cardiovasc Prev Rehab. 2004;355:763–778. doi: 10.1097/01.hjr.0000114707.27531.48. [DOI] [PubMed] [Google Scholar]
- 2.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 3.Ahima RS. Adipose tissue as an endocrin organ. Obesity. 2006;14:242S–249S. doi: 10.1038/oby.2006.317. [DOI] [PubMed] [Google Scholar]
- 4.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erhardt-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci USA. 2003;100:14211–14216. doi: 10.1073/pnas.2336140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodfriend TL, Egan BM, Kelley DE. Aldosterone in obesity. Endocr Res. 1998;24:789–796. doi: 10.3109/07435809809032689. [DOI] [PubMed] [Google Scholar]
- 7.Pitt B, Remme W, Zannad F. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 8.Pitt B, Zannad F, Remme WJ. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 9.Garnier A, Bendall JK, Fuchs S, Escoubet B, Rochais F, Hoerter J, Nehme J, Ambroisine ML, Angelis ND, Morineau G, d`Estienne P, Fischmeister R, Heymes C, Pinet F, Declayre C. Cardiac specific increase in aldosterone production induces coronary dysfunction in aldosterone synthase-transgenic mice. Circulation. 2004;110:1819–1825. doi: 10.1161/01.CIR.0000142858.44680.27. [DOI] [PubMed] [Google Scholar]
- 10.Tang EH, Ku D, Tipoe GL, Feletou M, Man RY, Vanhoutte PM. Endothelium-dependent contractions occur in the aorta of wild-type and COX2-/- knockout but not COX1-/- knockout mice. J Cardiovasc Pharmacol. 2005;46:761–765. doi: 10.1097/01.fjc.0000187174.67661.67. [DOI] [PubMed] [Google Scholar]
- 11.Luscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986;344:344–348. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- 12.Leopold JA, Cap A, Scribner AW, Stanton RC, Loscalzo L. Glucose-6-phosphate dehydrogenase deficiency promotes endothelial oxidative stress and decreases endothelial nitric oxide bioviability. FASEB J. 2001;15:1771–1773. doi: 10.1096/fj.00-0893fje. [DOI] [PubMed] [Google Scholar]
- 13.Hoen PAC, Lans CACVd, Eck MV, Bijsterbosch MK, Berkel TJCV, Twisk J. Aorta of ApoE-deficient mice responds to atherogenic stimuli by a prelesional increase and susequent decrease in the expression of antioxidant enzymes. Circ Res. 2003;93:262–269. doi: 10.1161/01.RES.0000082978.92494.B1. [DOI] [PubMed] [Google Scholar]
- 14.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szöcs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor R, Griendling KK. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki J, Iwai M, Mogi M, Oshita A, Yoshii T, Higaki J, Horiuchi M. Eplerenone with valsartan effectively reduces atherosclerotic lesion by attenuation of oxidative stress and inflammation. Arterioscler Thromb Vasc Biol. 2006;26:917–921. doi: 10.1161/01.ATV.0000204635.75748.0f. [DOI] [PubMed] [Google Scholar]
- 16.Sartorio CL, Fraccarollo D, Galuppo P, Leutke M, Ertl G, Stefanon I, Bauersachs J. Mineralocorticoid receptor blockade improves vasomotor dysfunction and vascular oxidative stress early after myocardial infarction. Hypertension. 2007;50:919–925. doi: 10.1161/HYPERTENSIONAHA.107.093450. [DOI] [PubMed] [Google Scholar]
- 17.Iwashima F, Yoshimoto T, Minami I, Sakurada M, Hirono Y, Hirata Y. Aldosterone induces superoxide generation via rac1 activation in endothelial cells. Endocrinology. 2008;149:1009–1014. doi: 10.1210/en.2007-0864. [DOI] [PubMed] [Google Scholar]
- 18.Blanco-Rivero J, Cachofeiro V, Lahera V, Aras-Lopez R, Márquez-Rodas I, Salaices M, Xavier FE, Ferrer M, Balfagón G. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hypertension. 2005;46:107–112. doi: 10.1161/01.HYP.0000171479.36880.17. [DOI] [PubMed] [Google Scholar]
- 19.Leopold JA, Dam A, Maron BA, Scribner AW, Liao R, Handy DE, Stanton RC, Pitt B, Loscalzo J. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med. 2007;13:189–197. doi: 10.1038/nm1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caprio M, Newfell B, Sala Al, Baur W, Fabbri A, Rosano G, Mendelsohn ME, Jaffe IZ. Functional mineralocorticoid receptor in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res. 2008;102:1359–1367. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caprio M, Feve B, Claes A, Viengchareun S, Lombes M. Pivotal role of the mineralocorticoid receptor in corticoid-induced adipogenesis. FASEB J. 2007;21:1–10. doi: 10.1096/fj.06-7970com. [DOI] [PubMed] [Google Scholar]
- 22.Guo C, Ricchiuti V, Lian BQ, Yao TM, Couthino P, Romero JR, Li J, Wiliams GH, Adler G. Mineralocorticoid receptor blockade reserves obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and pro-inflammatory adipocytokines. Circulation. 2008;117:2253–2261. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirata A, Maeda N, Hiuge A, Hibuse T, Fujita K, Okada T, Kihara S, Funahashi T, Shimomura I. Blockade of mineralocorticoid receptor reverses adipocyte dysfunction and insulin resistance in obese mice. Cardiovasc Res. 2009;84:164–172. doi: 10.1093/cvr/cvp191. [DOI] [PubMed] [Google Scholar]
- 24.Yamaji M, Tsutamoto T, Kawahara C, Nishiyama K, Yamamoto T, Fujii M, Horie M. Effect of eplerenone versus spironolactone on cortisol and hemoglobin A1c levels in patients with chronic heart failure. Am Heart J. 2012;160:915–921. doi: 10.1016/j.ahj.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res. 1999;7:355–361. doi: 10.1002/j.1550-8528.1999.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 26.Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, Martin M. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med. 1989;321:580–585. doi: 10.1056/NEJM198908313210905. [DOI] [PubMed] [Google Scholar]
- 27.Goodfriend TL, Ball DL, Egan BM, Campbell WB, Nithipatikom K. Epoxy-keto derivative of linoleic acid stimulates aldosterone secretion. Hypertension. 2004;43:358–363. doi: 10.1161/01.HYP.0000113294.06704.64. [DOI] [PubMed] [Google Scholar]
- 28.Hlavacova N, Jezova D. Effects of single treatment with the antihypertensive drug eplerenone on hormone levels and anxiety-like behaviour in rats. Endocr Regul. 2008;42:147–153. [PubMed] [Google Scholar]
- 29.Krum H, Nolly H, Workman D, He W, Roniker B, Krause S, Fakouhi K. Efficacy of eplerenone added to renin-angiotensin blockade in hypertensive patients. Hypertension. 2002;40:117–123. doi: 10.1161/01.hyp.0000025146.19104.fe. [DOI] [PubMed] [Google Scholar]
- 30.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avogaro A, de Kreutzenberg SV. Mechanisms of endothelial dysfunction in obesity. Clin Chim Acta. 2005;360:9–26. doi: 10.1016/j.cccn.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 32.Xavier FE, Aras-Lopez R, Arroyo-Villa I, Campo LD, Salaices M, Rossoni LV, Ferrer M, Balfagon G. Aldosterone induces endothelial dysfunction in resistance arteries from normotensive and hypertensive rats by increasing thromboxane A2 and prostacyclin. Br J Pharmacol. 2008;154:1225–1235. doi: 10.1038/bjp.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283:H1802–H1810. doi: 10.1152/ajpheart.01096.2001. [DOI] [PubMed] [Google Scholar]
- 34.Traupe T, Lang M, Goettsch W, Münter K, Morawietz H, Vetter W, Barton M. Obesity increases prostanoid-mediated vasoconstriction and vascular thromboxan receptor gene expression. J Hypertens. 2002;20:2239–2245. doi: 10.1097/00004872-200211000-00024. [DOI] [PubMed] [Google Scholar]
- 35.Félétou M, Huang Y, Vanhoutte P. Vasoconstrictor prostanoids. Pflügers Archiv. 2010;459:941–950. doi: 10.1007/s00424-010-0812-6. [DOI] [PubMed] [Google Scholar]
- 36.Gros R, Ding Q, Sklar LA, Prossnitz EE, Arterburn JB, Chorazyczewski J, Feldman RD. GPR30 expression is required for the mineralocorticoid receptor-independent rapid vascular effects of aldosterone. Hypertension. 2011;57:442–451. doi: 10.1161/HYPERTENSIONAHA.110.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen Dinh Cat A, Griol-Charhbili V, Loufrani L, Labat C, Benjamin L, Farman N, Lacolley P, Henrion D, Jaisser F. The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J. 2010;24:2454–2463. doi: 10.1096/fj.09-147926. [DOI] [PubMed] [Google Scholar]
- 38.Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, Gomez-Sanchez EP. Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology. 2006;147:1343–1348. doi: 10.1210/en.2005-0860. [DOI] [PubMed] [Google Scholar]
- 39.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Aispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: A tool for lineage analysis and gene deletion in endothelial cells. Dev Dynam. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 40.Bienvenu LA, Morgan J, Rickard AJ, Tesch GH, Cranston GA, Fletcher EK, Delbridge LM, Young MJ. Macrophage mineralocorticoid receptor signaling plays a key role in aldosterone-independent cardiac fibrosis. Endocrinology. 2012;153:3416–3425. doi: 10.1210/en.2011-2098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.