This editorial refers to ‘Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity’†, by N. Schäfer et al., on page 3515
Obesity is a highly prevalent chronic condition and a major risk factor for several metabolic and cardiovascular (CV) diseases. Numerous human studies have demonstrated that obesity is associated with hypertension and vascular dysfunction secondary to arterial stiffness, reduced endothelium-dependent relaxation, and atherosclerosis.1 The exact mechanisms implicated in obesity-related vascular dysfunction have not been fully elucidated, but are likely to involve a complex interplay between multiple endocrine and paracrine factors, inflammation, oxidative stress, and structural modifications of the vessel.
In recent years there has been a growing interest in the simultaneous worldwide increase in the prevalence of obesity, hypertension, and hyperaldosteronism, and a possible inter-relationship between these conditions. The relevance of aldosterone to human health is supported by prospective studies demonstrating a positive correlation between plasma levels of aldosterone and CV mortality.2 Further, pivotal clinical trials have shown that mineralocorticoid receptor (MR) antagonists, such as spironolactone or eplerenone, markedly improve morbidity and mortality in heart failure.3 We and others have shown that obesity is associated with increased aldosterone production in humans and increased aldosterone and MR levels in obese diabetic mice.4,5 MR blockade improved the proinflammatory cytokine profiles of adipose tissue in the obese mice. These findings led to the hypothesis that elevated aldosterone levels contribute to CV injury, progression of atherosclerosis, and metabolic disorders in obesity (Figure 1). The specific mechanisms for CV injury may be related to aldosterone's renal effects to promote sodium retention and volume expansion as well as to MR activation in multiple extrarenal tissues, including the heart, vasculature, monocytes/macrophages, and adipose tissue. Specifically in vessels, aldosterone and/or MR activation increases vascular macrophage infiltration, enhances the proinflammatory cytokine profile, reduces endothelial progenitor cell migration and nitric oxide (NO) release, and increases superoxide radicals, leading to endothelial dysfunction, atherosclerosis, fibrosis, and remodelling.4,6 The MR is expressed in endothelial cells, vascular smooth muscle cells, adipocytes, leucocytes, and fibroblasts, and thus is found throughout the blood vessel and in the perivascular adipose tissue (PVAT). MR's actions are mediated through classic genomic effects and also through rapid (within minutes), non-genomic actions. In addition, a G protein-coupled membrane receptor GPR30 may mediate some of the effects of aldosterone.7
Figure 1.

Potential mechanisms for increased MR activation in obesity include: 1) adipocyte production of aldosterone and aldosterone secretagogues; 2) increased MR; and 3) alterations in MR-interacting proteins (e.g. Rac1, AT1R, angiotensin II receptor type 1; EGFR, epidermal growth factor receptor; LSD-1, lysine-specific demethylase 1).
Pharmacological and genetic approaches have been used to demonstrate a critical role for MR in the pathogenesis of vascular injury and the importance of MR in all layers of the vessel to this process. An interesting study by Briones et al. reported that adipocytes express aldosterone synthase and produce aldosterone, basally and when stimulated by angiotensin II.8 Further, these investigators demonstrated that paracrine secretion of aldosterone by PVAT increased vascular dysfunction in obese db/db mice and that MR blockade prevented this vascular dysfunction, suggesting that the cross-talk between adipocyte-derived factors and blood vessels is involved in the pathogenesis of obesity-related CV complications. Further, selective deletion of macrophage MR reduces cardiac fibrosis and blood pressure in an MR agonist/high sodium mouse model,9 which raises the intriguing possibility that macrophage MR may affect vascular pathology and inflammation. McCurley and colleagues recently published a study showing that aged mice with a vascular smooth muscle cell- (VSMC) specific deletion of MR have reduced vascular oxidative stress, reduced vasoconstriction, and less hypertension. Thus, MR in the media layer appears to influence CV ageing and vascular contraction.10 Finally, in aortic endothelial cells, aldosterone acting via the MR increased the surface expression of the proatherogenic leucocyte adhesion molecule [intercellular adhesion molecule 1 (ICAM-1)], leading to increased leucocyte adhesion. Both effects were inhibited by spironolactone.11 The above-mentioned studies demonstrate that MR is expressed throughout the vessel and has important effects on endothelial function, vascular smooth muscle contraction, and the interplay between PVAT and blood vessels (Figure 2).
Figure 2.
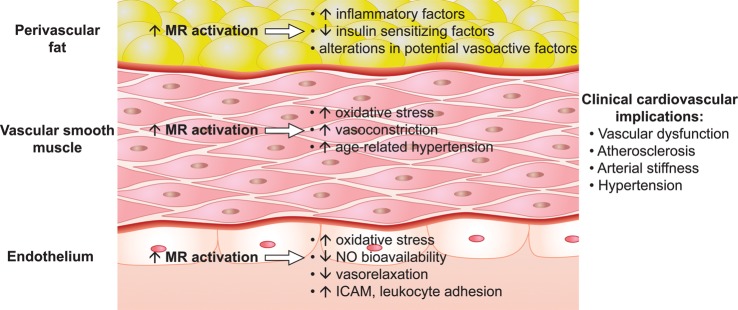
MR-mediated effects in endothelium, vascular smooth muscle and perivascular adipose tissue may contribute to vascular dysfunction and injury in obesity. NO, nitric oxide; ICAM, intercellular adhesion molecule.
Schaefer and colleagues now describe a sequence of carefully crafted in vivo studies which demonstrate a novel role for MR in the vascular endothelium.12 The investigators studied C57Bl/6 mice fed a high-fat vs. normal diet, and demonstrated that the high-fat regimen induced a plethora of obesity-associated characteristics: excessive weight gain, increased epididymal fat weight, inflammation, and aldosterone levels, impaired glucose metabolism, and vascular dysfunction. The obesity-induced vascular dysfunction was characterized by a 15–20% decrease in maximum acetylcholine-induced relaxation (an endothelium-mediated response). Furthermore, Schaefer et al. showed that pharmacological MR blockade prevented the endothelial dysfunction, improved glucose metabolism, and reduced inflammatory cytokine expression in white adipose tissue without affecting the diet-induced increases in body and epididymal fat pad weight. The diffuse nature of systemic MR blockade made it impossible to discern whether the improvements in vascular function were due to direct vascular effects or to indirect effects of MR blockade. To resolve this issue, the authors generated a mouse with endothelium-specific ablation of the MR and demonstrated that these mice were protected from obesity-induced endothelial dysfunction. Loss of MR from endothelial cells did not protect the mice from developing the usual obesity-associated impairments in systemic insulin resistance and adipose tissue inflammation, thus demonstrating that MR-mediated endothelial dysfunction can occur independently from systemic effects of MR. Whether the endothelial effects of MR involve cross-talk with insulin signalling within the endothelial cell remains to be determined.
To establish further the critical role of endothelial MR in vascular dysfunction, Schaefer and colleagues infused aldosterone into lean mice, achieving blood aldosterone levels similar to those in the obese mice. Aldosterone administration induced endothelial dysfunction in wild-type animals, but not in endothelium-specific MR-ablated mice. This vascular dysfunction was shown to be due in part to an imbalance in endothelial mechanisms of oxidative stress, including an MR-mediated increase in expression of the NADPH oxidase component p22phox. The specific mechanisms involved in MR-mediated endothelial dysfunction remain to be elucidated. Future studies are needed to determine the relevance of MR's genomic and non-genomic actions as well as the role of known aldosterone-regulated genes such as glucose-6-phosphate dehydrogenase in mediating MR's effects on endothelial function. All together, the innovative study presented by Schaefer and colleagues firmly establishes the endothelial MR as a key player in endothelium-mediated vascular dysfunction in obese mice and in lean animals with increased aldosterone.
The specific mechanisms leading to excess MR activation in obesity need further clarification. As noted above, adipocytes produce aldosterone that can act in a paracrine fashion and produce factors that increase adrenal production of aldosterone (Figure 1). Further, it is possible that obesity induces increases in endothelial MR as has been shown to occur in other tissues. Also, it is becoming increasingly clear that MR activity is influenced by many cellular proteins (Figure 1). There is cross-talk between MR and angiotensin II receptor type 1 (AT1R), between MR and epidermal growth factor receptor (EGFR), and probably between MR and striatin7. We have shown that lysine-specific demethylase 1 (LSD1; an epigenetic regulator) and caveolin 1 (cav-1; a scaffolding membrane protein) modulate vascular function and MR activity.13,14 Recently Rac1, a member of the Rho family of GTPases, was shown to increase MR activity. In humans, mononuclear Rac1 levels were associated with increased body mass index and higher levels of oxidative stress.15 Consistent with this human study, Schaefer et al. showed that Rac1 expression was increased in endothelial cells from obese mice. Since dietary sodium intake alters the levels of almost all of these MR-interacting proteins and increases aldosterone-mediated CV injury in many animal models, the effects of salt intake on obesity-related endothelial dysfunction and on MR-modulating proteins warrant further investigation. Finally, MR may be activated by glucocorticoids as well as by aldosterone. Cortisol could have a role in obesity-associated vasculopathy, especially if obesity is associated with alterations in factors that increase local cortisol production, such as 11 β-HSD1 (11 β-hydroxysteroid dehydrogenase type 1), or with alterations in 11 β-HSD2, which is associated with MR in some cell types and serves to protect MR by converting cortisol to the inactive cortisone.16.
The findings by Schaefer et al. are highly relevant to the role of aldosterone in CV injury in obesity. Another common disorder—hypertension—has long been associated with endothelial dysfunction. It would be of interest to determine the role of endothelial MR in blood pressure control and vascular dysfunction in normotensive and hypertensive humans with and without obesity. Further, given the influence of VSMC MR in the development of ageing-related hypertension, future studies are needed to assess the role of endothelial MR in CV ageing. These studies showing a prominent role for endothelial MR in the pathogenesis of vasculopathy lead one to speculate that development of a blood vessel-specific MR antagonist may avoid the adverse effects on potassium of current MR antagonists and could have important, beneficial effects on vascular health in human obesity.
Funding
The National Institutes of Health [K24 HL103845 to G.K.A. and R01 HL104032 to L.H.P.].
Conflict of interest: none declared.
References
- 1.Campia U, Tesauro M, Cardillo C. Human obesity and endothelium-dependent responsiveness. Br J Pharmacol. 2012;165:561–573. doi: 10.1111/j.1476-5381.2011.01661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivanes F, Susen S, Mouquet F, Pigny P, Cuilleret F, Sautiere K, Collet JP, Beygui F, Hennache B, Ennezat PV, Juthier F, Richard F, Dallongeville J, Hillaert MA, Doevendans PA, Jude B, Bertrand M, Montalescot G, Van Belle E. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J. 2012;33:191–202. doi: 10.1093/eurheartj/ehr176. [DOI] [PubMed] [Google Scholar]
- 3.Tirosh A, Garg R, Adler GK. Mineralocorticoid receptor antagonists and the metabolic syndrome. Curr Hypertens Rep. 2010;12:252–257. doi: 10.1007/s11906-010-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calhoun DA, Sharma K. The role of aldosteronism in causing obesity-related cardiovascular risk. Cardiol Clin. 2010;28:517–527. doi: 10.1016/j.ccl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo C, Martinez-Vasquez D, Mendez GP, Toniolo MF, Yao TM, Oestreicher EM, Kikuchi T, Lapointe N, Pojoga L, Williams GH, Ricchiuti V, Adler GK. Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology. 2006;147:5363–5373. doi: 10.1210/en.2006-0944. [DOI] [PubMed] [Google Scholar]
- 6.Briet M, Schiffrin EL. Vascular actions of aldosterone. J Vasc Res. 2013;50:89–99. doi: 10.1159/000345243. [DOI] [PubMed] [Google Scholar]
- 7.Krug AW, Pojoga LH, Williams GH, Adler GK. Cell membrane-associated mineralocorticoid receptors? New evidence. Hypertension. 2011;57:1019–1025. doi: 10.1161/HYPERTENSIONAHA.110.159459. [DOI] [PubMed] [Google Scholar]
- 8.Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Correa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD, Touyz RM. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59:1069–1078. doi: 10.1161/HYPERTENSIONAHA.111.190223. [DOI] [PubMed] [Google Scholar]
- 9.Rickard AJ, Morgan J, Tesch G, Funder JW, Fuller PJ, Young MJ. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54:537–543. doi: 10.1161/HYPERTENSIONAHA.109.131110. [DOI] [PubMed] [Google Scholar]
- 10.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caprio M, Newfell BG, la Sala A, Baur W, Fabbri A, Rosano G, Mendelsohn ME, Jaffe IZ. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res. 2008;102:1359–1367. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schäfer N, Lohmann C, Winnik S, van Tits LJ, Miranda MX, Vergopoulos A, Ruschitzka F, Nussberger J, Berger S, Lüscher TF, Verrey F, Matter CM. Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J. 2013;34:3515–3524. doi: 10.1093/eurheartj/eht095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pojoga LH, Adamova Z, Kumar A, Stennett AK, Romero JR, Adler GK, Williams GH, Khalil RA. Sensitivity of NOS-dependent vascular relaxation pathway to mineralocorticoid receptor blockade in caveolin-1-deficient mice. Am J Physiol Heart Circ Physiol. 2010;298:H1776–H1788. doi: 10.1152/ajpheart.00661.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams JS, Chamarthi B, Goodarzi MO, Pojoga LH, Sun B, Garza AE, Raby BA, Adler GK, Hopkins PN, Brown NJ, Jeunemaitre X, Ferri C, Fang R, Leonor T, Cui J, Guo X, Taylor KD, Ida Chen YD, Xiang A, Raffel LJ, Buchanan TA, Rotter JI, Williams GH, Shi Y. Lysine-specific demethylase 1: an epigenetic regulator of salt-sensitive hypertension. Am J Hypertens. 2012;25:812–817. doi: 10.1038/ajh.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun M, Huang X, Yan Y, Chen J, Wang Z, Xie M, Li J. Rac1 is a possible link between obesity and oxidative stress in Chinese overweight adolescents. Obesity. 2012;20:2233–2240. doi: 10.1038/oby.2012.63. [DOI] [PubMed] [Google Scholar]
- 16.Baudrand R, Carvajal CA, Riquelme A, Morales M, Solis N, Pizarro M, Escalona A, Boza C, Perez G, Dominguez A, Arrese M, Fardella CE. Overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in hepatic and visceral adipose tissue is associated with metabolic disorders in morbidly obese patients. Obes Surg. 2010;20:77–83. doi: 10.1007/s11695-009-9937-0. [DOI] [PubMed] [Google Scholar]


