Abstract
Aims
Aircraft noise disturbs sleep, and long-term exposure has been shown to be associated with increases in the prevalence of hypertension and an overall increased risk for myocardial infarction. The exact mechanisms responsible for these cardiovascular effects remain unclear.
Methods and results
We performed a blinded field study in 75 healthy volunteers (mean age 26 years), who were exposed at home, in random order, to one control pattern (no noise) and two different noise scenarios [30 or 60 aircraft noise events per night with an average maximum sound pressure level (SPL) of 60 dB(A)] for one night each. We performed polygraphy during each study night. Noise caused a worsening in sleep quality (P < 0.0001). Noise60, corresponding to equivalent continuous SPLs of 46.3 dB (Leq) and representing environmental noise levels associated with increased cardiovascular events, caused a blunting in FMD (P = 0.016). As well, although a direct comparison among the FMD values in the noise groups (control: 10.4 ± 3.8%; Noise30: 9.7 ± 4.1%; Noise60: 9.5 ± 4.3%, P = 0.052) did not reach significance, a monotone dose-dependent effect of noise level on FMD was shown (P = 0.020). Finally, there was a priming effect of noise, i.e. the blunting in FMD was particularly evident when subjects were exposed first to 30 and then to 60 noise events (P = 0.006). Noise-induced endothelial dysfunction (ED) was reversed by the administration of Vitamin C (P = 0.0171). Morning adrenaline concentration increased from 28.3 ± 10.9 to 33.2 ± 16.6 and 34.1 ± 19.3 ng/L (P = 0.0099). Pulse transit time, reflecting arterial stiffness, was also shorter after exposure to noise (P = 0.003).
Conclusion
In healthy adults, acute nighttime aircraft noise exposure dose-dependently impairs endothelial function and stimulates adrenaline release. Noise-induced ED may be in part due to increased production in reactive oxygen species and may thus be one mechanism contributing to the observed association of chronic noise exposure with cardiovascular disease.
Keywords: Endothelial function, Aircraft noise, Cardiovascular risk
See page 3472 for the editorial comment on this article (doi:10.1093/eurheartj/eht339)
Introduction
The WHO estimates that in high-income Western European countries (population ∼340 million) at least 1 million healthy life years are lost every year due to environmental noise.1 The negative health outcomes of noise include annoyance,2 sleep disturbance,3 cardiovascular disease,4,5 and impairment of cognitive performance in children.6
Aircraft noise has been shown to be more annoying than road- and railway noise at the same equivalent noise level.7 Epidemiologic studies have demonstrated associations between long-term exposure to aircraft noise and an increased incidence of arterial hypertension and therefore cardiovascular disease.7,8 The mechanisms underlying these adverse cardiovascular effects of aircraft noise are not fully understood. Nocturnal noise exposure seems to be more relevant for the genesis of cardiovascular disease than daytime noise exposure,9 probably due to repeated autonomic arousals that have been shown to habituate to a lesser degree to noise than, e.g. cortical arousals.10 In general, the risk increases with exposure duration, and is higher in those who decide to sleep with open windows.11,12
Undisturbed sleep of sufficient length is obligatory for the maintenance of daytime performance and health.13 The human organism recognizes, evaluates, and reacts to environmental sounds even while asleep.14 These reactions are part of an integral activation process of the organism that expresses itself, e.g. as changes in sleep structure or increases in blood pressure and heart rate.10,15 Environmental noise may decrease the restorative power of sleep by means of repeatedly occurring activations (so-called sleep fragmentation) that are associated with more awakenings/arousals, less deep sleep and rapid eye movement sleep, and early awakenings in the morning. Although healthy subjects have been shown to habituate to aircraft noise exposure to a certain degree,10 the habituation is not complete, and noise-induced awakenings and, especially, activations of the autonomic nervous system can still be observed in subjects that have been exposed to aircraft noise for several years.16 Sleep disturbance and especially sleep restriction in turn have been shown to cause hormonal and metabolic changes,17–19 which could predispose to a future development of cardiovascular disease.
Circadian changes related to altered sleep may also adversely affect the immune system20,21 and may increase the responsiveness of the heart to hypertrophic stimuli.22 Although plausible, the link between polysomnographic evidence of sleep disturbance during aircraft noise exposure and cardiovascular outcomes is not well established. It is largely unknown which changes or indices predict long-term risk.23
Furthermore, polysomnography (i.e. the simultaneous measurement of the electroencephalogram, electrooculogram, and electromyogram) is a complex and cumbersome method, which is not very well suited for larger studies in the general population.24 Therefore, other methods, like actigraphy (a non-invasive technique to monitor human rest/activity cycles) and behaviourally confirmed awakenings, have been used in this context.
In the case of aircraft noise, hypertension may be a consequence of the noise-induced release of stress hormones such as epi- and norepinephrine and/or the development of vascular (endothelial) dysfunction. Endothelial dysfunction (ED) is considered an early step in the development of atherosclerotic changes of the vasculature (for review see25) and can be assessed non-invasively. Recent studies indicate that in patients with coronary artery disease and hypertension, ED assessment in the forearm may have prognostic implications.25
Based on these considerations, the primary aim of the present study was to test whether nocturnal exposure to aircraft noise may induce ED. The morning plasma level of adrenaline was a secondary endpoint. In a subgroup of noise 60 subjects, we also tested whether acute vitamin C challenges may improve ED.
Methods
The study was approved by the ethics committee of University Medical Center Mainz. All participants were volunteers and signed informed consent. Anti-aircraft noise activists were excluded from the study as were persons with high nighttime traffic noise exposure at home as determined by noise maps available from municipal online resources (LA,eq,22-6h > 40 dB for aircraft noise and LA,eq,22-6h > 45 dB for road and rail traffic noise).
Study population
The study enrolled 75 healthy non-smokers between 20 and 60 years of age. Before the study, audiometry was performed in all participants. Persons with an age-adjusted hearing loss of 20 dB or more on one or both ears were excluded from the study. Subjects with sleep disorders [score >10 on the Pittsburgh Sleep Quality Index (PSQI)]26 or psychiatric disorders (assessed by M.I.NI. Screen interview) were also ineligible. Study participants were instructed to refrain from consumption of coffee, tea, alcohol, sleep altering medications, and nicotine on the day prior to the study night. Otherwise, they were told to continue their usual diet and daily routines. Hormonal contraception was allowed but care was taken to synchronize study nights with the hormonal status. Other hormonal therapies were excluded.
Study procedures
After inclusion, participants returned to the laboratory for three visits. During the night preceding each visit, subjects were exposed in a randomized order to one of three noise patterns. One night served as the control night, and subjects were exposed to normal background noise. During the other two nights, subjects were exposed to recording reproducing different numbers of flights: Noise30 with playback of 30 aircraft noise events, and Noise60 with playback of 60 aircraft noise events. Study visits were prescheduled with at least three non-study nights between two study nights and on the same weekday if possible. In premenopausal women, the visits were scheduled to occur in the same phase of the hormonal cycle. Supplemental vitamins, alcohol, and caffeine containing beverages were prohibited on the evening and night before the study.
Participants were randomly given one of six different sequences of noise and control nights according to the randomization plan (C-30-60, C-60-30, 30-C-60, 30-60-C, 60-C-30, 60-30-C). At study onset, subjects and investigators were both blinded to the noise pattern sequence. Participants slept in their usual home environment and were asked to maintain their usual sleep–wake rhythm. They wore portable polygraphic screening devices (SOMNOwatch™ plus, SOMNOmedics, Randersacker, Germany) during the night with continuous recording of ECG, SpO2, actimetry, light, and derived parameters as described in previous studies.27–29
In the noise exposure nights, the same aircraft noise event was played back repeatedly. It was originally recorded in the bedroom of a resident living in the vicinity of Düsseldorf airport (window tilted open), and was already used in previous studies on the effects of aircraft noise on sleep.30,31 Noise patterns were recorded as MP3 files and played back on a standard portable audio system with a fixed speaker position relative to the head of the subject. The playback volume was levelled at each measurement site to guarantee similar SPLs at all study sites. During the night, the SPL was continuously recorded in the bedroom with class-2 sound level meters (Datalogger DL-160S, Voltcraft, Germany; Model 407764A Datalogger, Extech Instruments, USA) to assure subject compliance. They were placed on the nightstand close to the participants. All sound files were coded with a study number and were of equal length and file size, making inadvertent unblinding less likely. All noise patterns started with a constant tone of 30 s duration to allow testing of equipment function. The first aircraft noise event was played back after 39.5 min to facilitate sleep onset. The last aircraft noise event was played back after 415 min. Each noise event lasted roughly 45 s. Noise events followed a short–long–short pattern with time between events roughly 6:40 min and 16:40 min for Noise30 and 4:05 min and 6:40 min for Noise60 (Figure 1).
Figure 1.
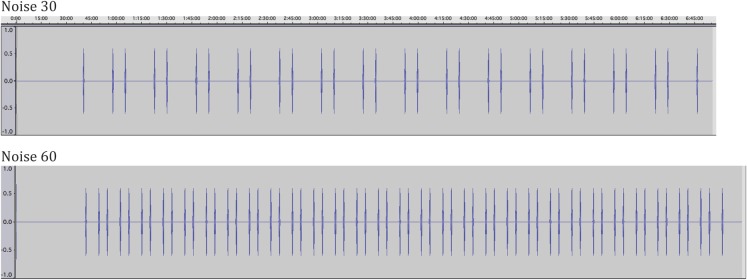
Schematic representation of the noise events.
After the study night, participants returned to the study centre in a fasting state for further testing. Flow-mediated dilatation of the brachial artery was measured at the same time in the early morning and before 10 a.m. by a technician using standardized techniques described previously.25,32,33 Briefly, brachial artery diameter is measured with a linear ultrasound probe at rest and after a 5 min occlusion period with a pressure cuff. Changes in diameter are given in percent and reflect the endothelial release of vasodilatory substances such as nitric oxide (NO). To address the role of reactive oxygen species in causing ED, FMD was also measured in a subset of five subjects exposed to Noise60 before and after administration of vitamin C (2 g, p.o.) as previously described.34 After FMD measurement, blood samples were drawn and questionnaires were filled out. Blood samples were transported directly to a clinical laboratory for evaluation. Part of the blood was centrifuged, aliquoted, and frozen at below −62°C for later testing. Global noise sensitivity was measured using the Dortmund Noise Sensitivity Questionnaire.35 The Horne-Ostberg Morningness-Eveningness Questionnaire (MEQ)36 was used to assess individual chronotype. Pulse transit time (PTT, time between the R wave in the ECG and peak oxygen saturation measured at the tip of the first finger of the right hand) and heart rate accelerations (number of accelerations >20 bpm and >2 s per h) were calculated. Interleukin-6 and cortisol were measured in serum with chemiluminescence immunoassay. Adrenaline was measured from NH4-heparine anticoagulated blood drawn 30 min after puncture and cooled during transport to the lab.
Statistical analysis
The primary endpoint of the study was the change in %FMD induced by the different levels of noise. Secondary outcomes included the changes in all variables measured (neurohormones, PTT, inflammatory markers, etc), the existence of a relationship between dose of noise and blunting of FMD (dose–effect relationship), and whether Noise30 or Noise60 had a priming effect on the blunting in FMD induced by, respectively, Noise60 or Noise30. A separate study was conducted to test the effect of Vitamin C on FMD in subjects exposed to Noise60. Data are presented as mean ± standard deviation. The Kolmogorov–Smirnov test was used to assess whether the data were normally distributed. To address the primary endpoint, we first compared the effect of Noise60, which reproduces the increase in night noise previously shown to be associated with an increased incidence of cardiovascular events and prevalence of hypertension,9 with the control visit. Further, a multi-factor ANOVA [taking into account noise exposure, night of exposure, and subject id (for subject-related differences)] was performed. A test for a monotone effect of the exposure (dose of nighttime aircraft noise: 0, 30, or 60) was performed by using exposure as a pseudo-continuous factor in the ANOVA. Further, a (post hoc) multi-factor ANOVA was performed with two additional factors: one for the comparison of FMD values after Noise60 in all subjects allocated to control–Noise30–Noise60 or Noise30–Noise60–control to FMD values of all other patients, and the other for the same comparison after Noise30 in all subjects exposed to Noise60 directly preceding Noise30. P-values <0.05 were considered significant. All tests were two-sided. P-values for secondary outcome variables are shown without adjustment for multiple testing. Based on the paper by Ghiadoni et al.,37 a difference between means of 2% could be expected (with SD of about 3%). With a sample size of 75 and a standard deviation of FMD differences between Noise60 and control of 3%, one may expect to detect a FMD difference of 0.98% with a power of 80% at the alpha-level 0.05.
Results
Study population and setting
A total of 88 subjects were enrolled. Thirteen of them were excluded from the final analysis. Reasons for dropouts (3 study subjects before and 10 after the first study night) included the diagnosis of hyperthyroidism, relocation to noise-affected areas, protocol violations, and inadequate data recording quality. The study subjects included in the final analysis were on average 26 years (range 20–54 years) old, 61% were females. FMD data could not be analysed for one visit in two subjects. The study population did not have relevant sleep disorders as assessed with the PSQI, and had a moderate trend towards evening chronotype (characteristics shown in Table 1). None reported significant diseases.
Table 1.
Baseline characteristics of the study population
| Age | (min–max) | 25.7 (20–54) |
| Gender | % female | 61.3 |
| Height | cm | 174.6 ± 10.2 |
| Weight | kg | 67.7 ± 11.9 |
| BMI | kg/m2 | 22.1 ± 2.4 |
| Baseline noise sensitivity, chronotype, sleep quality index | ||
| NoiSeQ | 0–3 | 1.22 ± 0.38 |
| Horne–Östberg | 14–86 | 49.41 ± 9.79 |
| PSQI | 0–21 | 3.73 ± 1.72 |
| Laboratory values | ||
| Total cholesterol | mg/dL | 182.9 ± 32.9 |
| LDL | mg/dL | 104.7 ± 25.6 |
| HDL | mg/dL | 60.7 ± 15.3 |
| Triglycerides | mg/dL | 87.2 ± 41.9 |
| C-reactive protein | mg/L | 1.3 ± 1.5 |
| Creatinin | mg/dL | 1.0 ± 0.5 |
| HbA1C | % | 5.3 ± 0.5 |
Data are presented as mean ± SD.
NoiSeQ, Dortmund Noise Sensitivity Questionnaire with three greatest noise sensitivity; Horne-Östberg, Morningness-Eveningness Questionnaire; PSQI, Pittsburgh Sleep Quality Index.
The average maximum SPL of aircraft noise events recorded in participants' bedrooms is presented in Table 2. Overall nighttime SPLs had average peak levels of 49.6 dB(A) (control), 59.9 dB(A) (Noise30), and 60.9 dB(A) (Noise60) (both P < 0.0001 compared with control). Corresponding equivalent continuous SPLs Leq(3) were 35.4 dB(A), 43.1 dB(A), and 46.3 dB(A), respectively. The mean time between awakening and start of image acquisition for FMD did not differ across visits (P > 0.5).
Table 2.
Effects of nighttime noise on the quality of sleep, haemodynamic parameters, cortisol levels, and inflammation parameters
| Control | Noise 30 | Noise 60 | P (ANOVA) | |
|---|---|---|---|---|
| PeakdB(A) | 48.63 ± 3.47 | 59.89 ± 3.28 | 60.87 ± 2.46 | <0.001 |
| Leq3dB(A) | 35.44 ± 8.08 | 43.12 ± 4.91 | 46.28 ± 3.89 | <0.001 |
| Sleep quality | 6.70 ± 1.92 | 5.20 ± 2.28 | 4.37 ± 2.23 | <0.001 |
| Movement index | 3.94 ± 5.40 | 3.06 ± 2.85 | 3.23 ± 3.44 | 0.639 |
| Haemodynamic parameters | ||||
| HR mean | 58.7 ± 7.6 | 59.5 ± 7.7 | 59.7 ± 7.8 | 0.345 |
| HR max | 102.6 ± 13.3 | 104.3 ± 13.2 | 106.9 ± 17.5 | 0.325 |
| BPsys mean (mmHg) | 109.8 ± 15.4 | 114.9 ± 13.9 | 115.2 ± 12.4 | 0.120 |
| BP rise Index | 2.3 ± 2.3 | 2.5 ± 2.32 | 3.8 ± 5.9 | 0.397 |
| HR_accel Index | 25.8 ± 32.4 | 22.8 ± 23.0 | 23.9 ± 26.5 | 0.215 |
| Pulse transit time (ms) | 271.8 ± 12.3 | 270.9 ± 18.7 | 264.9 ± 15.7 | 0.003 |
| Laboratory parameters | ||||
| Adrenaline (ng/L) | 28.3 ± 10.9 | 33.2 ± 16.6 | 34.1 ± 19.3 | 0.010 |
| Cortisol (μg/L) | 15.34 ± 5.47 | 16.43 ± 5.55 | 15.76 ± 5.78 | 0.197 |
| Neutrophils (%) | 51.0 ± 11.39 | 49.77 ± 9.48 | 50.04 ± 7.87 | 0.353 |
| IL-6 (pg/mL) | 2.6 ± 3.45 | 2.27 ± 1.25 | 2.57 ± 3.29 | 0.383 |
| C-reactive protein (mg/L) | 2.26 ± 6.30 | 2.27 ± 4.82 | 1.55 ± 2.16 | 0.512 |
Data are presented as mean ± SD.
Leq3 dB, long-term equivalent continuous sound level; PTT, pulse transit time; BP, blood pressure; HR accel, heart rate acceleration; IL-6, interleukin 6.
Control and noise exposure nights did not differ significantly with regard to outside and body temperatures, total time in bed or subjective well being prior to the study night (data not shown). All data were normally distributed.
Haemodynamic changes in response to night noise
As a secondary predefined endpoint, we also found a dose-dependent decrease in minimum PTT (Table 2) after the noise nights, which was mirrored by the changes in systolic blood pressure (P = 0.11 for the changes among visits, Table 2). Automated heart rate analysis detected no significant change in mean and maximum heart rate. Heart rate acceleration index as detected by the polygraphic device did not differ between noise exposure and control nights.
With increasing number of noise events, study subjects reported deteriorating sleep quality in the morning after the respective study night (P = 0.001).
Effects of nocturnal noise on endothelial function
The comparison of the FMD values measured after the control visit and the Noise60 visit demonstrated a blunting in endothelial responses after noise (P = 0.016). When all three levels of noise were compared, and noise exposure (0, 30, 60) was used as a pseudo-continuous covariate in the AN(C)OVA in order to test for a dose-dependency in the effect of noise on FMD, a linear relationship between FMD values and exposure was found (P = 0.020), confirming that the exposure to more severe noise causes more severe ED. Although a standard comparison among the three noise levels within the ANOVA, i.e. without assuming a monotone effect for dose as a pseudo-continuous covariate, did not reach statistical significance (control night: 10.4 ± 3.8%; after 30 noise events: 9.7 ± 4.1%; after 60 noise events: 9.5 ± 4.3%, P = 0.052, Figure 2A), the introduction of the two additional factors described in the Methods section evidenced a priming effect of Noise30 nights on the blunting in FMD induced by Noise60 (P = 0.006), i.e. Noise60 had the largest impact on FMD in the subjects who had already been exposed to Noise30. Finally, there was no effect of the randomization sequence (means after each visit adjusted for the effect of effect of noise: first visit: 9.8%, second visit: 10.0%, third visit: 9.4%, P = 0.757).
Figure 2.
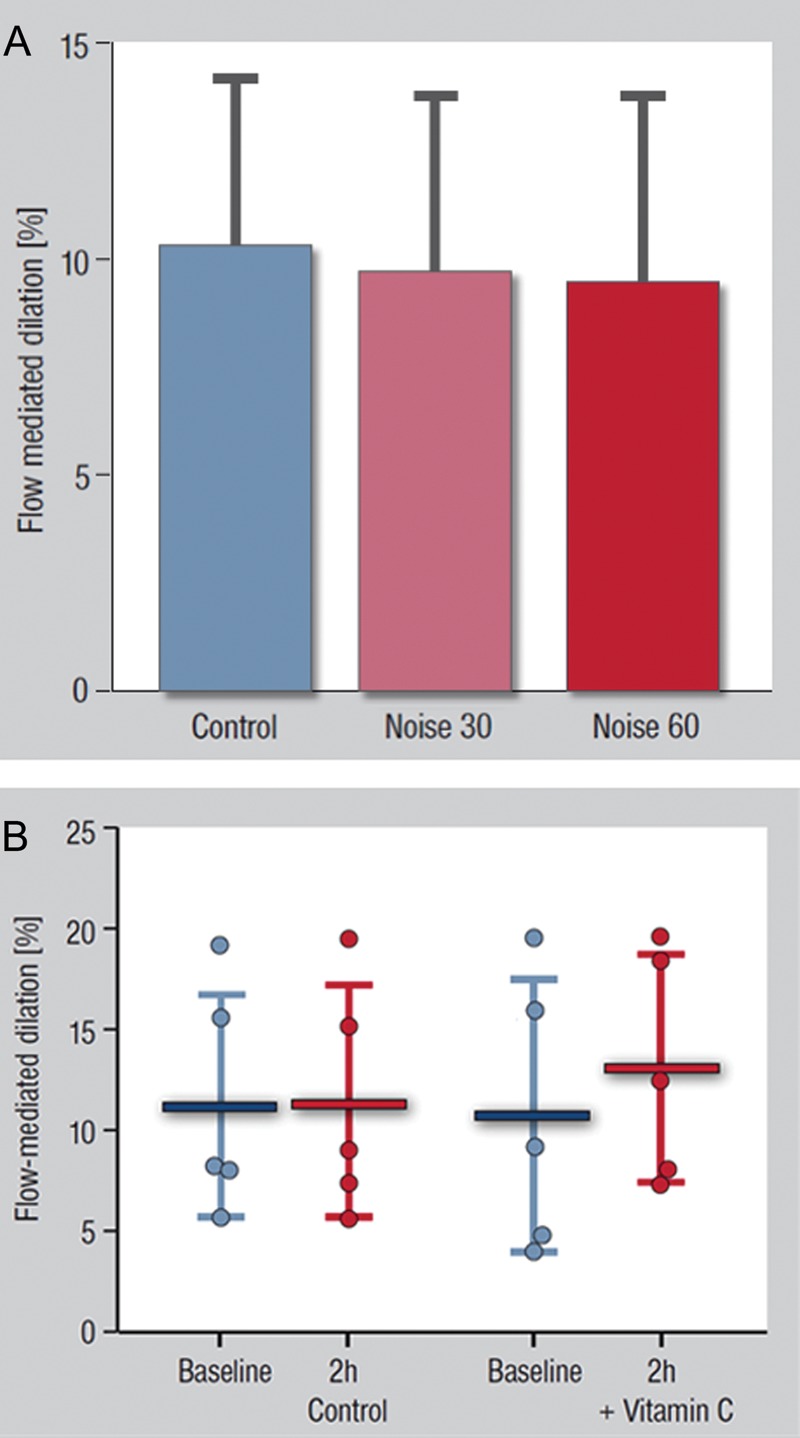
(A) Effects of Noise30 and Noise60 on flow-mediated dilation (FMD). Data are mean ± SD; P = 0.020 for a test using the level of noise a pseudo-continuous variable, demonstrating a linear relationship between FMD values and noise exposure. (B) Effects of Vitamin C (2 g, p.o.) in FMD of the brachial artery. 2 h after Vitamin C administration, the antioxidant improved significantly FMD in five control subjects exposed to Noise60. Date are presented as mean ± SD; P = 0.0171 for the effect of Vitamin C on FMD, paired t-test.
Noise had no effect on blood flow and reactive hyperaemia (control: 855 ± 357%; Noise30: 900 ± 423%; Noise60: 900 ± 389%, P = 0.55). As well, baseline arterial diameter did not significantly influence the effect of noise on FMD.
In order to study the mechanism of the blunting in FMD induced by Noise60, we tested the impact of acute challenges with vitamin C in five control subjects. In these subjects, 2 h after the administration of Vitamin C, FMD was markedly improved (Figure 2B, P = 0.0171). In contrast, in a separate control group of subjects exposed to Noise60 without Vitamin C, FMD did not change as an effect of time (11.21 ± 5.56%; FMD at 2 h: 11.47 ± 5.80%; P = 0.842).
Effects of night noise on neurohormones and markers of inflammation (Table 2)
We found a marked increase in plasma adrenaline concentrations between control and Noise30 and 60 exposure nights, respectively (control: 28.3 ± 10.9 ng/L; Noise30: 33.2 ± 16.6; Noise60: 34.1 ± 19.3 ng/L, P = 0.0099, Figure 3). In contrast, morning plasma levels of cortisol did not increase with noise exposure. Likewise, inflammatory markers IL-6 and C-reactive protein were unaffected by noise exposure.
Figure 3.
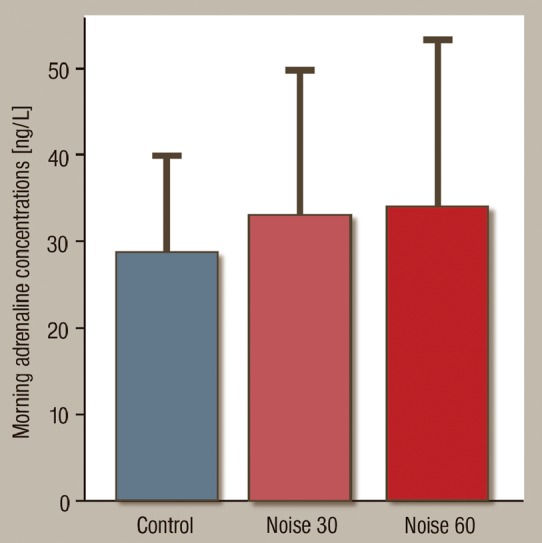
Effects of Noise30 and Noise60 on plasma adrenaline levels. Nighttime noise exposure significantly increases circulating catecholamine levels. Data are mean ± SD. P < 0.01, ANOVA.
Discussion
We demonstrate cardiovascular effects of nighttime aircraft noise in young and healthy individuals with low cardiovascular risk. Nighttime aircraft noise increased plasma epinephrine levels, worsened sleep quality, and decreased pulse transit time, a parameter of arterial stiffness, which varies inversely to arterial blood pressure. A dose-dependent decrease in endothelial function after exposure to increasing levels of noise was also observed. Acute Vitamin C challenges improved endothelial function in a separate group of subjects exposed to Noise60. We found no effect of aircraft noise exposure on nocturnal motility, heart rate or blood cortisol, neutrophils, IL-6, or C-reactive protein.
Interestingly, a priming effect of aircraft noise on ED was observed, i.e. previous exposure to Noise30 caused Noise60 to have larger effects on endothelial function. These data demonstrate that aircraft noise can affect endothelial function, and that rather than habituation, prior exposure to noise seems to amplify the negative effect of noise on endothelial function. Although the mechanisms of these observations cannot be characterized at a molecular level in vivo in humans, it has been previously shown that other forms of mental stress lead to a decrease in endothelial function.37–40 With regards to the molecular mechanisms, previous studies indicate that noise leads to an up-regulation, rather than a downregulation, of the eNOS.41 Interestingly, such an increased eNOS activity does not necessarily result in improved endothelial responses. For instance, in animal models of diabetes and/or hypertension, increased expression of an uncoupled (superoxide-producing) eNOS is associated with impaired endothelial function (reviewed in42). Since measurements of NO and/or superoxide production in the local vascular microenvironment are impossible to perform in humans, this question cannot be addressed at the present time. The improvement in FMD observed in our study 2 h after application of the antioxidant vitamin C in subjects exposed to Noise60 is compatible with this evidence, and it suggests that exposure to aircraft noise might lead to ED due to increased vascular oxidative stress.34
We also demonstrate changes in PTT, a parameter that correlates inversely with changes in blood pressure. Briefly, PTT is measured as the time it takes a pulse wave to travel between two arterial sites. Rises in blood pressure cause vascular tone to increase, leading to increased arterial stiffness and a shorter PTT. As mentioned above, these data are compatible with those of the HYENA project, in which an increase prevalence of hypertension was reported in subjects exposed to nocturnal noise in the range of 50 dB (similar to our Noise60 condition; 46.3 dB).9 Similarly, acute noise events were associated in this study with increased systolic and diastolic blood pressure by 6.2 and 7.4 mmHg, a phenomenon which, interestingly, was not necessarily associated with awakenings.
With regard to the pathophysiological mechanism behind the changes in blood pressure and vascular function, we also report elevated epinephrine levels after exposure to noise. It has been demonstrated that intermittent release of adrenaline may be implicated in the development of hypertension.43 Epinephrine is released as a response to different stressors such as noise44 and increases the release and the effects of norepinephrine.45 Interestingly, increased epinephrine levels have been found in patients with borderline hypertension,45,46 suggesting a role in the early history of hypertension.
Importantly, increased plasma catecholamines have also been shown to correlate negatively with endothelial function as measured by FMD.47 A recent study has linked autonomic sympathetic activation to the development of hypertension in elderly patients independent of the cause of activation of the autonomic nervous system.48
Our results are congruent with the growing amount of data linking short sleep duration or sleep disturbances of various kinds to the development of cardiovascular disease. For example, shift work has been shown to cause impaired endothelial function, sympathetic activation, and metabolic changes.49,50 Extensive evidence exists for the relation between obstructive sleep apnoea, hypertension, ED, and subsequently cardiovascular disease.51 Recently, the restless legs syndrome has been identified as another cause for sleep disruption, and it has been shown to increase the risk for myocardial infarction in women.52 There is ample evidence that nocturnal aircraft noise exposure disturbs and fragments sleep, leads to changes in sleep structure, increases sleepiness during the following day, and leads to impairments of cognitive performance.10,23,53,54 The results of our study suggest that these changes in sleep structure negatively affect the cardiovascular system, and that these changes, in the case of long-term exposure, may predispose to the development of hypertension and cardiovascular disease.
The study by design eliminated noise adaptation processes, which can often mask effects of environmental influences. Therefore, it is unclear whether the negative cardiovascular effects observed in this study persist after weeks or months with continued noise exposure. However, biologic adaptation is often incomplete and requires physiologic resources therefore also putting strain on the system as a whole. Effects of aircraft noise in population-based studies are likely to be mitigated by partial physiologic adaptation and avoidance of residential areas with high levels of noise exposure by highly sensitive individuals. Other environmental factors like air pollution, which has also been shown to influence endothelial function,55 may interfere with noise effects in epidemiological studies. Therefore, data from interventional studies may be helpful in judging the effect of nocturnal noise on cardiovascular health and disease.
Limitations of the study
The protocol was designed as a field study with minimal sleep disruption due to environment and equipment, thus creating ecologically valid conditions. We avoided on purpose a pure laboratory environment where ambient conditions, sound levels, and external stimuli can be controlled at the expense of creating artificial rather than familiar conditions. Sleep quality is very sensitive to changes in surroundings and study subjects usually show more pronounced alterations of sleep in the laboratory than in the field.56 There were no adaptation nights prior to study nights due to logistic constraints and because, since subjects were not required to sleep in non-familiar environments, our study design did not demand such adaptation. Reinforcing this, the analysis did not show a significant first-night effect for our primary outcome,57 which supports the validity of our study design and results. Study subjects were healthy, young, and with a female majority and are therefore not representative of the whole population. In general, younger adults usually show less sleep problems and disturbance than older persons when exposed to noise, and the fact that noise had an impact also on such a low-risk population rather emphasizes the potential clinical relevance of the present findings. Finally, endothelium-independent vasodilation was not systematically measured and the data are not presented: nitroglycerin responses were measured initially, but these measures were discontinued due to refusal by many study participants related to the side effects of the drug.
Summary and conclusions
In a group of young and healthy volunteers, we found evidence for significant impairment of endothelial function after only one night of aircraft noise exposure with 60 noise events. Pointing to a significant contribution of oxidative stress in this phenomenon, these adverse changes of the vasculature were markedly improved by acute Vitamin C challenges. Endothelial dysfunction was paralleled by significant increases in circulating adrenaline levels and a substantial, dose-dependent decrease in sleep quality and an increase in systolic blood pressure. These findings indicate that hypertension observed in response to nighttime exposure to noise might be explained by increased sympathetic activation but also by the occurrence of vascular dysfunction. Accumulating data increasingly confirms that sleep disturbance of different causes might represent a novel, important health risk. An undisturbed night's sleep is important for health and well-being and should be protected as far as possible, and reducing nocturnal aircraft noise can therefore be regarded as a preventive measure for cardiovascular disease. Since the present studies demonstrate adverse effects of endothelial function and stress hormones in healthy adults, the implications for patients with known cardiovascular disease will need to be tested in further studies.
Funding
The Study was funded by the Foundation Heart of Mainz, the Rober Müller Foundation and the Department of Cardiology of the University Medical Center Mainz. T.G. receives a Grant from the Federal Ministry of Education and Research (within project BMBF 01EO1003).
Conflict of interest: none declared.
References
- 1.Fritschi L, Brown AL, Kim R, Schwela DH, Kephalopoulos S. Burden of disease from environmental noise, World Health Organization. 2011 http://www.euro.who.int/__data/assets/pdf_file/0008/136466/e94888.pdf. [Google Scholar]
- 2.Miedema HME, Oudshoorn CGM. Annoyance from transportation noise: relationships with exposure metrics DNL and DENL and their confidence intervals. Environ Health Perspect. 2001;109:409–416. doi: 10.1289/ehp.01109409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muzet A. Environmental noise, sleep and health. Sleep Med Rev. 2007;11:135–142. doi: 10.1016/j.smrv.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 4.van Kempen E, Babisch W. The quantitative relationship between road traffic noise and hypertension: a meta-analysis. J Hypertens. 2012;30:1075–1086. doi: 10.1097/HJH.0b013e328352ac54. [DOI] [PubMed] [Google Scholar]
- 5.Sörensen M, Andersen ZJ, Norsborg RB, Jensen SS, Lillelund KG, Beelen R, Schmidt EB, Tjönneland A, Overvad K, Raaschou-Nielsen O. Road traffic noise and incident myocardial infarction: a prospective cohort study. PLoS ONE. 2012;7:1–7. doi: 10.1371/journal.pone.0039283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stansfeld SA, Matheson MP. Noise pollution: non-auditory effects on health. Br Med Bull. 2003;68:243–257. doi: 10.1093/bmb/ldg033. [DOI] [PubMed] [Google Scholar]
- 7.Miedema HM, Oudshoorn CG. Annoyance from transportation noise: relationships with exposure metrics DNL and DENL and their confidence intervals. Environ Health Perspect. 2001;109:409–416. doi: 10.1289/ehp.01109409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenlund M, Berglind N, Pershagen G, Jarup L, Bluhm G. Increased prevalence of hypertension in a population exposed to aircraft noise. Occup Environ Med. 2001;58:769–773. doi: 10.1136/oem.58.12.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarup L, Babisch W, Houthuijs D, Pershagen G, Katsouyanni K, Cadum E, Dudley ML, Savigny P, Seiffert I, Swart W, Breugelmans O, Bluhm G, Selander J, Haralabidis A, Dimakopoulou K, Sourtzi P, Velonakis M, Vigna-Taglianti F. Hypertension and Exposure to Noise near Airports: the HYENA study. Environ Health Perspect. 2008;116:329–333. doi: 10.1289/ehp.10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basner M, Müller U, Elmenhorst E-M. Single and combined effects of air, road, and rail traffic noise on sleep and recuperation. Sleep. 2011;34:11–23. doi: 10.1093/sleep/34.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lercher P, Widmann U, Kofler W. Transportation noise and blood pressure: the importance of modifying factors. In Cassereau D, ed. Proceedings of the 29th International Congress and Exhibition on Noise Control Engineering. 2000:2071–2075. Nice, France: Sociéte Francaise d, Acoustique. [Google Scholar]
- 12.Huss A, Spoerri A, Egger M, Roosli M. Aircraft noise, air pollution, and mortality from myocardial infarction. Epidemiology. 2010;21:829–836. doi: 10.1097/EDE.0b013e3181f4e634. [DOI] [PubMed] [Google Scholar]
- 13.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- 14.Oswald I, Taylor AM, Treisman M. Discriminative responses to stimulation during human sleep. Brain. 1960;83:440–453. doi: 10.1093/brain/83.3.440. [DOI] [PubMed] [Google Scholar]
- 15.Basner M, Müller U, Griefahn B. Practical guidance for risk assessment of traffic noise effects on sleep. Appl Acoustics. 2010;71:518–522. [Google Scholar]
- 16.Basner M, Isermann U, Samel A. Aircraft noise effects on sleep: application of the results of a large polysomnographic field study. J Acoust Soc Am. 2006;119:2772–2784. doi: 10.1121/1.2184247. [DOI] [PubMed] [Google Scholar]
- 17.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Lehnert H, Born J, Schultes B. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep. 2009;34:371–377. doi: 10.1093/sleep/34.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wehrens SM, Hampton SM, Finn RE, Skene DJ. Effect of total sleep deprivation on postprandial metabolic and insulin responses in shift workers and non-shift workers. J Endocrinol. 2010;206:205–215. doi: 10.1677/JOE-10-0077. [DOI] [PubMed] [Google Scholar]
- 19.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157:549–557. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange T, Dimitrov S, Fehm HL, Westermann J, Born J. Shift of monocyte function toward cellular immunity during sleep. Arch Intern Med. 2006;166:1695–1700. doi: 10.1001/archinte.166.16.1695. [DOI] [PubMed] [Google Scholar]
- 21.Dimitrov S, Lange T, Nohroudi K, Born J. Number and function of circulating human antigen presenting cells regulated by sleep. Sleep. 2007;30:401–411. doi: 10.1093/sleep/30.4.401. [DOI] [PubMed] [Google Scholar]
- 22.Durgan DJ, Tsai JY, Grenett MH, Pat BM, Ratcliffe WF, Villegas-Montoya C, Garvey ME, Nagendran J, Dyck JR, Bray MS, Gamble KL, Gimble JM, Young ME. Evidence suggesting that the cardiomyocyte circadian clock modulates responsiveness of the heart to hypertrophic stimuli in mice. Chronobiol Int. 2011;28:187–203. doi: 10.3109/07420528.2010.550406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basner M, Van den Berg M, Griefahn B. Aircraft noise effects on sleep: mechanisms, mitigation and research needs. Noise Health. 2010;12:95–109. doi: 10.4103/1463-1741.63210. [DOI] [PubMed] [Google Scholar]
- 24.Basner M, Brink M, Elmenhorst EM. Critical appraisal of methods for the assessment of noise effects on sleep. Noise Health. 2012;14:321–329. doi: 10.4103/1463-1741.104902. [DOI] [PubMed] [Google Scholar]
- 25.Munzel T, Sinning C, Post F, Warnholtz A, Schulz E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med. 2008;40:180–196. doi: 10.1080/07853890701854702. [DOI] [PubMed] [Google Scholar]
- 26.Zisberg A, Gur-Yaish N, Shochat T. Contribution of routine to sleep quality in community elderly. Sleep. 2010;33:509–514. doi: 10.1093/sleep/33.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gesche H, Grosskurth D, Kuchler G, Patzak A. Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method. Eur J Appl Physiol. 2012;112:309–315. doi: 10.1007/s00421-011-1983-3. [DOI] [PubMed] [Google Scholar]
- 28.Bartsch S, Ostojic D, Schmalgemeier H, Bitter T, Westerheide N, Eckert S, Horstkotte D, Oldenburg O. Validation of continuous blood pressure measurements by pulse transit time: a comparison with invasive measurements in a cardiac intensive care unit. Dtsch Med Wochenschr. 2010;135:2406–2412. doi: 10.1055/s-0030-1269408. [DOI] [PubMed] [Google Scholar]
- 29.Pepin JL, Delavie N, Pin I, Deschaux C, Argod J, Bost M, Levy P. Pulse transit time improves detection of sleep respiratory events and microarousals in children. Chest. 2005;127:722–730. doi: 10.1378/chest.127.3.722. [DOI] [PubMed] [Google Scholar]
- 30.Basner M, Samel A. Nocturnal aircraft noise effects. Noise Health. 2004;6:83–93. [PubMed] [Google Scholar]
- 31.Basner M, Samel A, Isermann U. Aircraft noise effects on sleep: application of the results of a large polysomnographic field study. J Acoust Soc Am. 2006;119:2772–2784. doi: 10.1121/1.2184247. [DOI] [PubMed] [Google Scholar]
- 32.Ostad MA, Eggeling S, Tschentscher P, Schwedhelm E, Boger R, Wenzel P, Meinertz T, Munzel T, Warnholtz A. Flow-mediated dilation in patients with coronary artery disease is enhanced by high dose atorvastatin compared to combined low dose atorvastatin and ezetimibe: results of the CEZAR study. Atherosclerosis. 2009;205:227–232. doi: 10.1016/j.atherosclerosis.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 33.Warnholtz A, Wild P, Ostad MA, Elsner V, Stieber F, Schinzel R, Walter U, Peetz D, Lackner K, Blankenberg S, Munzel T. Effects of oral niacin on endothelial dysfunction in patients with coronary artery disease: results of the randomized, double-blind, placebo-controlled INEF study. Atherosclerosis. 2009;204:216–221. doi: 10.1016/j.atherosclerosis.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Gokce N, Keaney JF, Jr, Frei B, Holbrook M, Olesiak M, Zachariah BJ, Leeuwenburgh C, Heinecke JW, Vita JA. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1999;99:3234–3240. doi: 10.1161/01.cir.99.25.3234. [DOI] [PubMed] [Google Scholar]
- 35.Schutte M, Marks A, Wenning E, Griefahn B. The development of the noise sensitivity questionnaire. Noise Health. 2007;9:15–24. doi: 10.4103/1463-1741.34700. [DOI] [PubMed] [Google Scholar]
- 36.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 37.Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, O'Connor G, Betteridge J, Klein N, Steptoe A, Deanfield JE. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102:2473–2478. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- 38.Harris CW, Edwards JL, Baruch A, Riley WA, Pusser BE, Rejeski WJ, Herrington DM. Effects of mental stress on brachial artery flow-mediated vasodilation in healthy normal individuals. Am Heart J. 2000;139:405–411. doi: 10.1016/s0002-8703(00)90083-8. [DOI] [PubMed] [Google Scholar]
- 39.Spieker LE, Hurlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, Hayoz D, Deanfield JE, Luscher TF, Noll G. Mental stress induces prolonged endothelial dysfunction via endothelin-a receptors. Circulation. 2002;105:2817–2820. doi: 10.1161/01.cir.0000021598.15895.34. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Dangardt F, Osika W, Berggren K, Gronowitz E, Friberg P. Age- and sex-related differences in vascular function and vascular response to mental stress. Longitudinal and cross-sectional studies in a cohort of healthy children and adolescents. Atherosclerosis. 2012;220:269–274. doi: 10.1016/j.atherosclerosis.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 41.Heinrich UR, Selivanova O, Feltens R, Brieger J, Mann W. Endothelial nitric oxide synthase upregulation in the guinea pig organ of corti after acute noise trauma. Brain Res. 2005;1047:85–96. doi: 10.1016/j.brainres.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 42.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 43.Brown MJ, Macquin I. Is adrenaline the cause of essential hypertension? Lancet. 1981;2:1079–1082. doi: 10.1016/s0140-6736(81)91279-4. [DOI] [PubMed] [Google Scholar]
- 44.Andren L, Hansson L, Bjorkman M, Jonsson A. Noise as a contributory factor in the development of elevated arterial pressure. A study of the mechanisms by which noise may raise blood pressure in man. Acta Med Scand. 1980;207:493–498. doi: 10.1111/j.0954-6820.1980.tb09760.x. [DOI] [PubMed] [Google Scholar]
- 45.Nezu M, Miura Y, Adachi M, Kimura S, Toriyabe S, Ishizuka Y, Ohashi H, Sugawara T, Takahashi M. The effects of epinephrine on norepinephrine release in essential hypertension. Hypertension. 1985;7:187–195. doi: 10.1161/01.hyp.7.2.187. [DOI] [PubMed] [Google Scholar]
- 46.Nezu M, Miura Y, Adachi M, Kimura S, Toriyabe S, Ishizuka Y, Ohashi H, Sugawara T, Takahashi M. The role of epinephrine in essential hypertension. Jpn Circ J. 1983;47:1242–1246. doi: 10.1253/jcj.47.1242. [DOI] [PubMed] [Google Scholar]
- 47.Kaplon RE, Walker AE, Seals DR. Plasma norepinephrine is an independent predictor of vascular endothelial function with aging in healthy women. J Appl Physiol. 2011;111:1416–1421. doi: 10.1152/japplphysiol.00721.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dauphinot V, Barthelemy JC, Pichot V, Celle S, Sforza E, Achour-Crawford E, Gosse P, Roche F. Autonomic activation during sleep and new-onset ambulatory hypertension in the elderly. Int J Cardiol. 2011;155:155–159. doi: 10.1016/j.ijcard.2011.10.097. [DOI] [PubMed] [Google Scholar]
- 49.Amir O, Alroy S, Schliamser JE, Asmir I, Shiran A, Flugelman MY, Halon DA, Lewis BS. Brachial artery endothelial function in residents and fellows working night shifts. Am J Cardiol. 2004;93:947–949. doi: 10.1016/j.amjcard.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 50.Wehrens SM, Hampton SM, Skene DJ. Heart rate variability and endothelial function after sleep deprivation and recovery sleep among male shift and non-shift workers. Scand J Work Environ Health. 2011;38:171–181. doi: 10.5271/sjweh.3197. [DOI] [PubMed] [Google Scholar]
- 51.Levy P, Tamisier R, Arnaud C, Monneret D, Baguet JP, Stanke-Labesque F, Dematteis M, Godin-Ribuot D, Ribuot C, Pepin JL. Sleep deprivation, sleep apnea and cardiovascular diseases. Front Biosci (Elite Ed) 2012;4:2007–2021. doi: 10.2741/521. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Walters AS, Chiuve SE, Rimm EB, Winkelman JW, Gao X. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation. 2012;126:1689–1694. doi: 10.1161/CIRCULATIONAHA.112.112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elmenhorst EM, Elmenhorst D, Wenzel J, Quehl J, Mueller U, Maass H, Vejvoda M, Basner M. Effects of nocturnal aircraft noise on cognitive performance in the following morning: dose-response relationships in laboratory and field. Int Arch Occup Environ Health. 2010;83:743–751. doi: 10.1007/s00420-010-0515-5. [DOI] [PubMed] [Google Scholar]
- 54.Basner M. Nocturnal aircraft noise increases objectively assessed daytime sleepiness. Somnologie. 2008;12:110–117. [Google Scholar]
- 55.Krishnan RM, Adar SD, Szpiro AA, Jorgensen NW, Van Hee VC, Barr RG, O'Neill MS, Herrington DM, Polak JF, Kaufman JD. Vascular responses to long- and short-term exposure to fine particulate matter: MESA Air (Multi-Ethnic Study of Atherosclerosis and Air Pollution) J Am Coll Cardiol. 2012;60:2158–2166. doi: 10.1016/j.jacc.2012.08.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pearsons K, Barber D, Tabachnick BG, Fidell S. Predicting noise-induced sleep disturbance. J Acoust Soc Am. 1995;97:331–338. [Google Scholar]
- 57.Agnew HW, Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]


