Abstract
Degraded lands are defined by soils that have lost primary productivity due to abiotic or biotic stresses. Among the abiotic stresses, drought, salinity, and heavy metals are the main threats in tropical areas. These stresses affect plant growth and reduce their productivity. Nitrogen-fixing plants such as actinorhizal species that are able to grow in poor and disturbed soils are widely planted for the reclamation of such degraded lands. It has been reported that association of soil microbes especially the nitrogen-fixing bacteria Frankia with these actinorhizal plants can mitigate the adverse effects of abiotic and biotic stresses. Inoculation of actinorhizal plants with Frankia significantly improves plant growth, biomass, shoot and root N content, and survival rate after transplanting in fields. However, the success of establishment of actinorhizal plantation in degraded sites depends upon the choice of effective strains of Frankia. Studies related to the beneficial role of Frankia on the establishment of actinorhizal plants in degraded soils are scarce. In this review, we describe some examples of the use of Frankia inoculation to improve actinorhizal plant performances in harsh conditions for reclamation of degraded lands.
1. Introduction
In recent years, land degradation has increased considerably due to climatic factors as well as human intervention resulting in a reduction in fertility, biodiversity, and productivity. Since tropical countries are characterized by rapid demographic growth, the population dependency on ecosystems is high resulting in ecosystem degradation. Thus, the rehabilitation of degraded lands is critical. To overcome the problem of the lack of fertility in degraded soils, fast growing nitrogen fixing trees such as actinorhizal plants in combination with biofertilizers are used [1, 2]. Actinorhizal plants are nitrogen-fixing plants from 8 families including Betulaceae, Casuarinaceae, Coriariaceae, Datiscaceae, Elaeagnaceae, Myricaceae, Rhamnaceae, and Rosaceae, [3, 4] distributed in 25 genera and approximately 200 angiosperms species. Generally, these nitrogen-fixing plants are able to grow in poor and disturbed soils and are well adapted to abiotic stresses such as drought, salinity, and flooding [5]. Among them, plants of Casuarinaceae and Betulaceae families are the most widely planted around the world for the rehabilitation of degraded lands [6]. In addition to the positive aspects of actinorhizal plants on the remediation of degraded ecosystem, some actinorhizal plants such as Hippophae rhamnoides (sea buckthorn), Hippophae L., and Rubus ellipticus (Yellow Himalayan raspberry) are used as food ingredients or for medicinal purposes [7–9]. Actinorhizal plants are also pioneer species and have a potential role to enhance plant establishment on disturbed sites, to improve soil fertility and stability.
In degraded soils, one of the scarcest nutrients is nitrogen. The symbiotic relationship with nitrogen-fixing bacteria Frankia increases soil fertility and enhances the performance of trees during their plantation in degraded lands. Upon establishment, actinorhizal tree seedlings must be inoculated in nursery or in the field [10]. The success of establishment of Casuarina plantation in degraded sites depends on the effective nodulation by appropriate Frankia strains [2, 11]. However, studies related to the beneficial role of the nitrogen fixing actinorhizal bacteria on actinorhizal plants for the rehabilitation of degraded soils are very scarce. In this paper, we review these efforts and highlight the positive role of Frankia in improving actinorhizal plant performances in harsh conditions.
2. Frankia
Frankia is a gram-positive nitrogen-fixing actinobacterium that forms a symbiotic association with actinorhizal plants. It is a filamentous free-living bacterium [12] found in root nodules or in soil [13]. The genus Frankia has been classified in the order of Actinomycetales on the basis of morphology, cell chemistry, and 16S rRNA sequences [14]. Genomic studies have been undertaken to characterize Frankia [15–17] and to better understand the functioning of actinorhizal symbiosis. Among species of Frankeniaceae, the capacity to fix nitrogen is restricted to Frankia [3, 18]. This microaerophilic bacterium is characterized by a high GC% content and a slow growth rate [19]. In liquid culture and depending on the condition of culture, Frankia forms hyphae and multilocular sporangia which are located on hyphae either terminal or intercalary [20] (Figure 1). Ultrastructure showed that the hyphae, free living structures are septate, and sporangia are multilocular and contain the spores, the effective propagules of the bacteria. Vesicles are the site of nitrogenous activity and are generally formed when nitrogen is very limited in the medium [21] (Figure 1). Due to the presence of resistant structure in culture, Frankia inoculum is easier to conserve than Rhizobium inoculum [22]. However, Frankia morphology in the nodule varies according to host plant. The size of hyphae and the presence or the absence of vesicles depends on the bacterial partner [23, 24].
Figure 1.
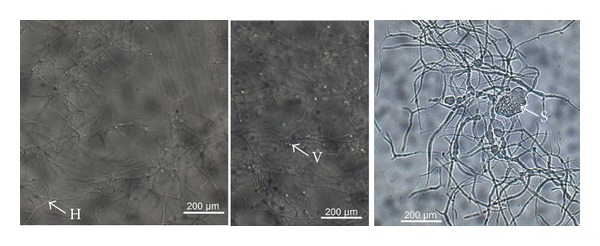
Structures of Frankia bacteria H: hyphae, V: vesicle, and S: sporangia.
The symbiosis between actinorhizal plants and Frankia induces the formation of a perennial root organ called nodule, wherein bacteria is hosted and nitrogen is fixed [25, 26]. In the field, actinorhizal nodule can have variable forms and colours [27]. Comparison of actinorhizal and leguminous nodules shows that morphology, anatomy, origin, and functioning of nodules are different for these two nitrogen-fixing plants [28]. Two types of nodule formation occur in actinorhizal symbiosis: the intercellular and the extracellular infection [29].
3. Actinorhizal Plants
Actinorhizal plants are grouped in the clade of Rosid I [30]. With the exception of two species belonging to Datiscaceae family, actinorhizal plants are mostly trees or woody shrubs [4]. They are found on all continents with the exception of Antarctica [31]. Casuarina and Alnus are the most important and widely spread actinorhizal plants due to their uses in soil reclamation, agroforestry systems, dune stabilization, and windbreaks [32]. They are generally pioneer species that colonize disturbed environments with low soil fertility and facilitate the establishment and development of subsequent plant communities [33].
Among the actinorhizal plants, Casuarinaceae trees are widely distributed in tropical areas [34]. This family is composed of 4 genera with 96 species. These plants are originated from Australia, South-East Asia, Malaysia, Melanesian, and Polynesian regions of the Pacific, New Guinea [35, 36]. Casuarina species are able to grow well under a range of stresses such as drought, flooding, salinity, and sites polluted by heavy metal [27, 37]. Casuarina plantations improve physical and microbiological quality of degraded soils [38]. These plants have an important ecological and economical role in tropical countries. They contribute to the improvement of soil fertility by fixing nitrogen and producing thick leaf litter from needles that can be used as compost by farmers [2, 39–41].
Casuarina trees are widely used for the rehabilitation of degraded lands in South Africa, Kenya [42]. They are used as windbreaks to protect adjacent crops and fix sand dunes in Senegal (Figure 2(a)), Tunisia, Egypt, China, and India [2, 36, 43]. In agroforestry system, these trees are used to improve soil fertility and increase crop yield (e.g, intercropping with legumes) in China and India [44]. Casuarina trees are also used in the production of smokeless fuelwood with a high calorific value, and hardwood in the construction of houses in Benin [45] and in the production of paper pulp wood in India [46]. In Asia, shelter belts formed by Casuarina trees have played a major protective role during typhoons and tsunami [44]. Association with Frankia increased Casuarina growth and biomass [2, 47, 48]. Furthermore, in this symbiotic relationship, bacteria confer to plants a high resistance to abiotic and biotic stresses [27, 49]. By using C. equisetifolia inoculated with Frankia Ceq1, Tani and Sasakawa [37] showed that actinorhizal symbiosis plays an important role in the reclamation of degraded lands. In India, about 5,000,000 ha are planted with C. equisetifolia (Figure 2(b)) and produce 10 million tonnes of pulpwood [50]. Recognising the importance of symbiotic association between Casuarina-Frankia, farmers have begun to use these biofertilizers through inoculation with crushed nodules [50]. The positive role of Casuarina and its ability to be propagated by seed, cutting, and tissue culture [44] makes it an ideal species for the reclamation of degraded lands. However, C. equisetifolia clones have been reported to show marked variation in their ability to tolerate salt stress [51–54]. Thus, identifying stress tolerant clones that can grow in degraded lands are crucial for the rehabilitation of these lands.
Figure 2.

(a) Casuarina equisetifolia plantation in Niayes region for fixation of dunes, Senegal; (b) C. equisetifolia plantation in Magnesite mined out lands, India.
Like Casuarina, Betulaceae contains members that play an important role in improving soil fertility [55]. They are used in the production of firewood, pulp, and timber. These species are also used in land reclamation, agroforestry, and as windbreak to avoid erosion [56, 57] and promote the establishment of the more nutrient demanding plants [58]. Inoculation with Frankia increases the growth and biomass of Alnus [59, 60]. Furthermore, alder growth is higher when plants are inoculated with more Frankia strains [57]. Inoculation with Frankia, increases the leaf N content of Alnus glutinosa [61]. Alder plants inoculated with Frankia have a higher productivity, a higher shoot length, root length, dry matter production, and chlorophyll content [62]. Under nursery conditions, Frankia provides good alder plants to be used in reforestation programs [60]. Inoculation with Frankia increases the survival rate of transplanted seedlings in the field [63]. A plantation of Alnus acumita increases soil N content by about 279 kg h−1 [64]. Some other species of actinorhizal plants are also used as pioneer plants such as Coriaria and Datisca [65].
When compared to legume-rhizobia symbiosis, actinorhizal symbiosis fixes at similar high rate of N2 estimated at around 240–350 kg ha−1 y−1 [3].
4. The Nitrogen Fixing Bacteria Frankia Improves Actinorhizal Plant Performance in Reforestation Programs
4.1. Actinorhizal Symbiosis and Plant Growth
Inoculation with the nitrogen-fixing bacteria Frankia improves the nutrient status and enhances actinorhizal plant development [2, 59, 66, 67]. However, the successful establishment and growth of actinorhizal plants in nutrient deficient and/or marginal soils depends on the formation of the symbiotic relationship between plant and the nitrogen-fixing bacteria. Thus, to optimize association between the actinorhizal/Frankia, an efficient combination in nitrogen fixation well adapted to environmental conditions is recommended [2].
In fields, inoculation with Frankia is commonly carried out with crushed nodule, Frankia suspension, Frankia enrobed in alginate bead, soil containing Frankia, or leaf litter from around nodulated plants [10, 39]. The response to Frankia inoculation is strongly linked to factors such as provenance source, Frankia strain, and nutrients status of the site such as nitrogen [68]. Frankia inoculation in nursery and field conditions is beneficial to Casuarina given that it reduces transplantation shock [22, 68]. Another study [69] showed that Casuarinas generally do not form nodule outside their zone of origin; however, nodulation occurs when they are inoculated with Frankia from the plants' zone of origin. When Casuarina trees are planted in sites where they have not previously been planted, inoculation with Frankia is recommended for the successful establishment of the plantation [44] given that Casuarina strains are generally absent in zone without the host plant [69]. However, for some actinorhizal species infective Frankia strains can be found in zone without the host plant [70, 71]. Generally, arid soils are not infected with Frankia [71] but plant bioassays have demonstrated that members of the Frankia survive and remain infective in soils that are devoid of host plants [72].
Therefore, it becomes important to transfer stress tolerant actinorhizal plants along with effective Frankia strains during reforestation programs. Attention also needs to be given to match Frankia strains, plant, and environment, as the response of different clones to nodulation by Frankia may differ in different environments [2].
Casuarina plantations are spread all over Senegal and used for multiple purposes (Figure 2). In the coastal sandy dunes of the Niayes region of Senegal, C. equisetifolia was established in 1925 to fix dunes (Figure 2(a)) and to protect adjacent crops. Presently, about 10,000 ha are planted with C. equisetifolia along this coastal zone [74]. Inoculation with Frankia was achieved in the Niayes region later in 1976 during a reforestation program with support from FAO [75]. Based on Maggia's studies [76], nodules have been found in C. equisetifolia trees planted before 1976. This nodulation may be due to the natural presence of Frankia strains in Senegal or to the presence of Frankia associated with Casuarina plant from Australia and established in Senegal in 1925. To inoculate Casuarina plants in the Niayes region, the nodules from the Casuarina plantation established before were crushed and soaked for 4-5 days in water and used for irrigating plants [43]. About two weeks after inoculation, nodulation was observed in root system of inoculated plants [77]. In the Niayes region, association of Frankia with Casuarina plants enhanced plant growth and development [78], improved soil fertility, enhanced vegetable production largely used by the local population, and increased population incomes [43].
Pure cultures of Frankia were used to inoculate Casuarina plantations in 1984 in the Notto region of Senegal [39]. Plants were inoculated in a nursery condition before transplantation with Frankia strains ORS 021001. A few years after transplantation on fields, results obtained showed that Frankia strains were very effectively associated with C. equisetifolia [39]. Actinorhizal plants inoculated with Frankia were taller (Table 1) and had better photosynthesis activity, a higher shoot biomass, and a higher N2 content in roots and needles (Table 2). Casuarina plantations are replenished in Senegal by planting young seedlings inside the old Casuarina plantations (Figure 3(a)) to improve soil fertility and increase agricultural yield and wood production.
Table 1.
Casuarinaceae field trials: effect of inoculation with Frankia on plant height.
| Species | Treatments | Plant height (cm) | References |
|---|---|---|---|
|
Casuarina equisetifolia
(2-year-old field plantation) |
Control | 64.80 | Karthikeyan et al., [50] |
| Frankia | 94.60 | ||
|
C. cunninghamiana
(6–8-year-old field plantation ) |
Control | 580 | Bulloch, [73] |
| Frankia | 680 | ||
|
C. glauca
(6–8-year-old field plantation ) |
Control | 780 | |
| Frankia | 890 | ||
|
C. obesa
(6–8-year-old field plantation ) |
Control | 500 | |
| Frankia | 550 | ||
| Allocasuarina verticillata | Control | 580 | |
| Frankia | 700 |
Table 2.
Casuarinaceae fields trials: effect of inoculation with Frankia on plants biomass and N content.
| Species | Treatments | Dry weight (g) | Total N (g)/plant | References |
|---|---|---|---|---|
|
Casuarina equisetifolia
(3-year-old field plantation ) |
Control | 1000 | 15.57 | Sougoufara et al., [39] |
| Frankia | 1356 | 23. 35 | ||
|
Casuarina equisetifolia
(2-year-old field plantation ) |
Control | 300 | 1.02 | Karthikeyan et al., [50] |
| Frankia | 680 | 3.8 | ||
|
Alnus crispa
(1.5-year-old field plantation ) |
Control | 111.8 | 3.5 | Lefrançois et al., [79] |
| Frankia | 154.1 | 4.8 |
Figure 3.

C. equisetifolia plantation in Mboro: (a) old trees are replaced by young seedlings; (b) and (c) uses of C. equisetifolia wood as firewood (b) or timber (c).
Plants inoculated with Frankia are also used in China for the rehabilitation of degraded lands. In 2001, more than 6 species of Casuarina were planted in degraded lands and inoculated with Frankia. Results from these experiments showed that Frankia application improves survival, biomass productivity, and plant growth [44].
For other actinorhizal species, such as members of Betulaceae, inoculation with Frankia has shown similar positive effects by improving plant growth and development in marginal soils. Alnus plants inoculated with Frankia have a higher productivity, higher shoot length, root length, dry matter production, chlorophyll content, and leaf N content [59, 61, 62]. Studies carried out by Lefrançois et al. [79, 80], showed that Frankia-alder symbiotic relationship improves soil quality. Taken together, inoculation of actinorhizal plants with Frankia is a promising tool for the rehabilitation of degraded lands.
4.2. Actinorhizal Symbiosis and Environment Stresses
Actinorhizal plants are generally tolerant of abiotic stresses. This tolerance can be improved when plants are associated with Frankia [81]. The actinorhizal plant-Frankia system is widely used for reclaiming lands affected by abiotic stresses [82]. Most of the Frankia strains were resistant to an elevated level of several heavy metals [83] and also to salinity [84]. In association with actinorhizal plants, Frankia strains resistant to abiotic stresses confer and/or increase capacity of the plant partner to tolerate abiotic stresses. Generally Frankia strains are absent or rare in stressed soils, which indicate the requirement of inoculation of the host plant before transplantation for rehabilitation of saline soil [85].
Some Frankia strains are very tolerant to salinity and can be used as biofertilizers in land affected by salt [84]. It has been demonstrated that nodulation occurred under saline conditions until 300 mM, approximately 28 dSm−1 [37]. Given that Frankia improves plant performance in stressed conditions [37], the symbiotic association between C. equisetifolia and Frankia can be widely used for the recovery of saline soils. However, selection of compatible salt tolerant Frankia and host plant is a key for obtaining the right actinorhizal plant-Frankia for rehabilitation of salt affected soils [37, 86] since differences in salt tolerance between individual Frankia strains have been shown by Ngom et al. (unpublished).
Actinorhizal symbioses are a biological tool generally used for the remediation and revegetation of soils affected by salt, heavy metal, oil, and so forth [62, 87]. It has been demonstrated that alder-Frankia symbiosis improves remediation capabilities and enhances soil quality by improving soil nutrients, pH, and cation exchange capacity and enhancing plant performance in these harsh conditions [80]. To restore the landscape of the Bamburi Cement Factory in Mombasa, Kenya, R. D. Haller initiated some plantation by testing 26 species in 1971. After six months, only three species, C. equisetifolia sp., Conocarpus lancifolius sp., and coconut palm survived. Among them, C. equisetifolia J.R et G. Forst was the most adapted to this environment where it acts as pioneer plant. In this reclamation program, Casuarina plants inoculated with Frankia showed a higher survival rate after transplantation [88].
Inoculation with Frankia has a beneficial effect on restoration and reforestation of bauxite mine spoils [50]. Their studies showed that in bauxite mine spoils the growth and nutrients uptake (N, P, K) of plants inoculated with Frankia was higher than those of noninoculated plants (Table 2). Beside, the important role of Casuarina on lands reclamation, the symbiotic relationship between Frankia-actinorhizal plant can also be used as a biocontrol tool against diseases such as bacterial wilt (Ralstonia), cataplexy (Rhizoctonia sp.), powdery mildew (Oidium sp.), Hexenbesen (mycoplasma-like organism; bacteria-like organism), and canker (Phomopsis sp.) [89]. Given that actinobacterium is a group of bacteria that generally produces antagonistic compounds against pathogens [90], the establishment of actinorhizal symbiosis could mitigate the development of plant disease [91]. In nursery experiments, results obtained by kang [92] showed that Casuarina plants inoculated with Frankia are more resistant to bacterial wilt disease than noninoculated plants. Furthermore, it has been demonstrated by [49] that Frankia strains could counterbalance root rot of C. equisetifolia caused by Rhizoctonia sp. There is a positive correlation between the dose of Frankia, the efficiency of disease control, and also nodule formation. Taken together, these results highlight the positive role of Frankia as an eco-friendly tool for the control of plant disease and the improvement of agricultural productivity [1].
5. Conclusion
Ecosystem degradation leads to soil infertility and crop losses therefore leading to decreased food security. Plantation of pioneer trees such as actinorhizal plants that are well adapted to such harsh conditions is a promising tool for restoring these degraded lands. However, for the successful establishment of the plantation, care should be taken to select appropriate tree species for a specific environment. In the context of the significant intraspecies variation in their tolerance to salinity, it becomes important to select tolerant clones for plantation programmes. To reduce the transplantation shock during reforestation programs and to increase the productivity of these plantations, Frankia inoculation must be carried out during the nursery stage. Association between actinorhizal plant and Frankia must be optimized by selecting a more efficient symbiosis. Thus, appropriate combinations of actinorhizal plants as well as compatible Frankia strains well adapted to environmental conditions are recommended. Given that the symbiotic microorganism Frankia significantly improves performance of actinorhizal plantations, it becomes very important to enhance Frankia production particularly in arid and semiarid areas for large-scale adoption by farmers.
Acknowledgments
The authors, work on interaction between Casuarina and soil symbiotic microorganisms is supported by the International Foundation for Sciences (IFS, no. AD/22680), The Academy of Sciences for the Developing World (TWAS, no. 11-214 RG/BIO/AF/AC_I), and the Institut de Recherche pour le Développement (IRD). Nathalie Diagne was the recipient of a CV Raman International Fellowship for African Researchers. The authors thank Dr. Laura M. Boykin (Australian Research Council Centre of Excellence in Plant Energy Biology) for critical reading of their paper.
References
- 1.Mahdi SS, Hassan GI, Samoon SA, Rather HA, Dar SA, Zehra B. Bio-fertilizers in organic agriculture. Journal of Phytology. 2010;2:42–54. [Google Scholar]
- 2.Sayed WF. Improving Casuarina growth and symbiosis with Frankia under different soil and environmental conditions-review. Folia Microbiologica. 2011;56(1):1–9. doi: 10.1007/s12223-011-0002-8. [DOI] [PubMed] [Google Scholar]
- 3.Wall LG. The actinorhizal symbiosis. Journal of Plant Growth Regulation. 2000;19(2):167–182. doi: 10.1007/s003440000027. [DOI] [PubMed] [Google Scholar]
- 4.Dawson JO. The ecology of actinorhizal plants, in Nitrogen-fixing actinorhizal symbioses. In: Pawlowski K, Newton WE, editors. Nitrogen Fixation: Applications and Research Progress. Vol. 6. Dordrecht, The Netherlands: Springer; pp. 199–234. [Google Scholar]
- 5.Echbab H, Arahou M, Ducousso M, et al. Successful nodulation of Casuarina by Frankia in axenic conditions. Journal of Applied Microbiology. 2007;103(5):1728–1737. doi: 10.1111/j.1365-2672.2007.03425.x. [DOI] [PubMed] [Google Scholar]
- 6.Schwencke J, Carú M. Advances in actinorhizal symbiosis: host plant-Frankia interactions, biology, and applications in arid land reclamation. A review. Arid Land Research and Management. 2001;15(4):285–327. [Google Scholar]
- 7.Abid H, Ali J, Hussain A, Afridi SR. Production and quality evaluation of sea buckthorn (Hippophae rhamnoides L.) vinegar using Accetobacter acceti. Pakistan Journal of Biochemestry and Molecular Biology. 2010;43:185–188. [Google Scholar]
- 8.Becking JH. N2-fixing tropical non-legumes. In: Dommergues YR, Diem HG, editors. Microbiology of Tropical Soil and Plant Productivity. The Hague, The Netherlands: Martinus Nijhoff; 1982. pp. 109–146. [Google Scholar]
- 9.Kalia RK, Singh R, Rai MK, Mishra GP, Singh SR, Dhawan AK. Biotechnological interventions in sea buckthorn (Hippophae L.): current status and future prospects. Trees: Structure and Function. 2011;25(4):559–575. [Google Scholar]
- 10.Mansour SR, Dewedar A, Torrey JG. Isolation, culture, and behaviour of Frankia strain HFPCgI4 from root nodules of Casuarina glauca . Botanical Gazatte. 1990;151(4):490–496. [Google Scholar]
- 11.Sayed WF, Zahran HH, Salem WM. Dominant rhizospheric microorganisms under some casuarinas and its effect on Frankia growth and nodulation capacity. Egypt Journal of Biotechnology. 2007;2:201–218. [Google Scholar]
- 12.Normand P, Orso S, Cournoyer B, et al. Molecular phylogeny of the genus Frankia and related genera and emendation of the family Frankiaceae. International Journal of Systematic Bacteriology. 1996;46(1):1–9. doi: 10.1099/00207713-46-1-1. [DOI] [PubMed] [Google Scholar]
- 13.Chaia EE, Wall LG, Huss-Danell K. Life in soil by the actinorhizal root nodule endophyte Frankia. A review. Symbiosis. 2010;51(3):201–226. [Google Scholar]
- 14.Lechevalier MP. Taxonomy of the genus Frankia (Actinomycetales) International Journal of Systematic Bacteriology. 1994;44(1):1–8. [Google Scholar]
- 15.Normand P, Lapierre P, Tisa LS, et al. Genome characteristics of facultatively symbiotic Frankia sp. strains reflect host range and host plant biogeography. Genome Research. 2007;17(1):7–15. doi: 10.1101/gr.5798407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persson T, Benson DR, Normand P, et al. Genome sequence of “candidatus Frankia datiscae” Dg1, the uncultured microsymbiont from nitrogen-fixing root nodules of the dicot Datisca glomerata . Journal of Bacteriology. 2011;193(24):7017–7018. doi: 10.1128/JB.06208-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Udwary DW, Gontang EA, Jones AC, et al. Significant natural product biosynthetic potential of actinorhizal symbionts of the genus Frankia, as revealed by comparative genomic and proteomic analyses. Applied and Environmental Microbiology. 2011;77(11):3617–3625. doi: 10.1128/AEM.00038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franche C, Lindström K, Elmerich C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant and Soil. 2009;321(1-2):35–59. [Google Scholar]
- 19.Benson DR, Silvester WB. Biology of Frankia strains, actinomycete symbionts of actinorhizal plants. Microbiological Reviews. 1993;57(2):293–319. doi: 10.1128/mr.57.2.293-319.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obertello M, Sy MO, Laplaze L, et al. Actinorhizal nitrogen fixing nodules: infection process, molecular biology and genomics. African Journal of Biotechnology. 2003;2(12):528–538. [Google Scholar]
- 21.Zhang X, Benson DR. Utilization of amino acids by Frankia sp. strain CpI1. Archives of Microbiology. 1992;158(4):256–261. [Google Scholar]
- 22.Brunck F, Colonna JP, Dommergues M. La maîtrise de l’inoculation des arbres avec leurs symbioses racinaires. Synthèse d’une sélection d’essais au champ en zone tropicale. Bois et Forêts des Topiques. 1990;223:24–42. [Google Scholar]
- 23.Diem HG, Dommergues YR. In vitro production of specialized reproductive torulose hyphae by Frankia strain ORS 021001 isolated from Casuarina junghuhniana root nodules. Plant and Soil. 1985;87(1):17–29. [Google Scholar]
- 24.Gauthier D. La Symbiose Frankia Casuarina Equisetifolia. Université Paris VII; 1984. [Google Scholar]
- 25.Perrine-Walker F, Gherbi H, Imanishi L, et al. Symbiotic signaling in actinorhizal symbioses. Current Protein and Peptide Science. 2011;12(2):156–164. doi: 10.2174/138920311795684896. [DOI] [PubMed] [Google Scholar]
- 26.Tromas A, Parizot B, Diagne N, et al. Heart of endosymbioses: transcriptome analysis reveals conserved genetic programme between arbuscular mycorrhizal, actinorhizal and legume-rhizobial symbioses. PloS ONE. 2012;7(9) doi: 10.1371/journal.pone.0044742.44742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bargali K. Actinorhizal plants of Kumaun Himalaya and their ecological significance. African Journal of Plant Science. 2011;5:401–406. [Google Scholar]
- 28.Hocher V, Auguy F, Bogusz D, et al. Actinorhizal nitrogen fixing symbiosis: an example of adaptation against soil abiotic stresses. Cahiers Agricultures. 2009;18(6):498–505. [Google Scholar]
- 29.Pawlowski K, Demchenko KN. The diversity of actinorhizal symbiosis. Protoplasma. 2012;249(4):967–979. doi: 10.1007/s00709-012-0388-4. [DOI] [PubMed] [Google Scholar]
- 30.Soltis ED, Soltis PS. Contributions of plant molecular systematics to studies of molecular evolution. Plant Molecular Biology. 2000;42(1):45–75. [PubMed] [Google Scholar]
- 31.Baker DD, Schwintzer CR. Introduction. In: Schwintzer CR, Tjepkema JD, editors. The Biology of Frankia and Actinorhizal Plants. New York, NY, USA: Academic Press; 1990. pp. 3–13. [Google Scholar]
- 32.Dommergues YR. Contribution of actinorhizal plants to tropical soil productivity and rehabilitation. Soil Biology and Biochemistry. 1997;29(5-6):931–941. [Google Scholar]
- 33.Gtari M, Dawson JO. An overview of actinorhizal plants in Africa. Functional Plant Biology. 2011;38(8-9):653–661. doi: 10.1071/FP11009. [DOI] [PubMed] [Google Scholar]
- 34.Diagne N, Diouf D, Svistoonoff S, et al. Casuarina in Africa: distribution, role and importance of arbuscular mycorrhizal, ectomycorrhizal fungi and Frankia on plant development. Journal of Environmental Management. 2013;128:204–209. doi: 10.1016/j.jenvman.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 35.He XH, Critchley C. Frankia nodulation, mycorrhization and interactions between Frankia and mycorrhizal fungi in Casuarina plants. In: Varma A, editor. Mycorrhiza. 3rd edition. chapter 6. Berlin, Germany: Springer; 2008. pp. 767–781. (Biomedical and life Sciences). [Google Scholar]
- 36.Zhong C, Zhang Y. Introduction and management of Casuarina tree species in China. China Forestry Science and Technology. 2003;17:3–5. [Google Scholar]
- 37.Tani C, Sasakawa H. Salt tolerance of Casuarina equisetifolia and Frankia Ceq1 strain isolated from the root nodules of C. equisetifolia . Soil Science and Plant Nutrition. 2003;49(2):215–222. [Google Scholar]
- 38.Izquierdo I, Caravaca F, Alguacil MM, Hernández G, Roldán A. Use of microbiological indicators for evaluating success in soil restoration after revegetation of a mining area under subtropical conditions. Applied Soil Ecology. 2005;30(1):3–10. [Google Scholar]
- 39.Sougoufara B, Diem HG, Dommergues YR. Response of field-grown Casuarina equisetifolia to inoculation with Frankia strain ORS 021001 entrapped in alginate beads. Plant and Soil. 1989;118(1-2):133–137. [Google Scholar]
- 40.Cisse M, Gournière F. Décomposition de la litière de Filao (Casuarina equisetifolia forst) au senegal. Activité de la microfaune détritivore. Dakar, Senegal: ORSTOM; 1993. [Google Scholar]
- 41.Forrester DI, Bauhus J, Cowie AL, Vanclay JK. Mixed-species plantations of Eucalyptus with nitrogen-fixing trees: a review. Forest Ecology and Management. 2006;233(2-3):211–230. [Google Scholar]
- 42.Andéké-Lingui MA, Dommergues . Coastal sand dune stabilization in Senegal. In: Midgley SJ, Turnbull JW, Johnston RD, editors. Casuarina Ecology, Management and Utilization. Melbourne, Australia: Common wealth Scientific and Industrial Research Organization (CSIRO); 1983. pp. 158–166. [Google Scholar]
- 43.Mailly D, Ndiaye P, Margolis HA, Pineau M. Fixation des dunes et reboisement avec le filao (Casuarina equisetifolia) dans la zone du littoral nord du Senegal. Forestry Chronicle. 1994;70(3):282–290. [Google Scholar]
- 44.Zhong C, Zhang Y, Chen Y, et al. Casuarina research and applications in China. Symbiosis. 2010;50(1-2):107–114. [Google Scholar]
- 45.Buffe J. Les plantations de Casuarina equisetifolia (Filao) dans le Sud-Dahomey. Bois; 1962. [Google Scholar]
- 46.Jain JK, Mohan M. Improving Smallholder Livelihoods through Improved Casuarina Productivity. 2011. Casuarina in farm forestry for sustainable livelihoods: the andhra pradesh paper mills experience; pp. 232–238. (Proceedings of the 4th International Casuarina Workshop). [Google Scholar]
- 47.Shah SK, Shah RP, Xu HL, Aryal UK. Biofertilizers: an alternative source of nutrients for sustainable production of tree crops. Journal of Sustainable Agriculture. 2007;29(2):85–95. [Google Scholar]
- 48.Rajendran K, Devaraj P. Biomass and nutrient distribution and their return of Casuarina equisetifolia inoculated with biofertilizers in farm land. Biomass and Bioenergy. 2004;26(3):235–249. [Google Scholar]
- 49.Gopinathan S. Biological control of Rhizoctonia sp. root rot of Casuarina equisetifolia seedlings by Frankia spp. strains. Biology and Fertility of Soils. 1995;20(4):221–225. [Google Scholar]
- 50.Karthikeyan A, Deeparaj B, Nepolean P. Reforestation in bauxite mine spoils with Casuarina equisetifolia frost. and beneficial microbes. Forests Trees and Livelihoods. 2009;19(2):153–165. [Google Scholar]
- 51.Balasubramanian A. Screening for salinity resistance in clones of Casuarina equisetifolia forst [Ph.D. thesis] Jorhat, India: Forest Research Institute (Deemed University); 2001. [Google Scholar]
- 52.Tripathi SB, Mathish NV, Gurumurthi K. Changes in protein profiles in Casuarina equisetifolia during salt stress. In: Gurumurthi K, editor. Proceedings of the 5th Annual Workshop on Casuarina; October 2001; Rajahmundry, India. Regional Forest Research Center; pp. 126–134. [Google Scholar]
- 53.Warrier KCS, Venkataramanan KS. Suitable clones of Casuarina equisetifolia for sodic soils in India. Proceedings of the 4th International Casuarina Meeting on Improving Smallholder Livelihoods through Improved Casuarina Productivity; 2011; pp. 194–198. [Google Scholar]
- 54.Mathish NV, Johnson Prabhu S, Roopesh M, et al. Development of an In silico gene bank for plant abiotic stresses: towards its utilization for molecular analysis of salt tolerant and susceptible Casuarina equisetifolia clones. Proceedings of the 4th International Casuarina Meeting on Improving Smallholder Livelihoods through Improved Casuarina Productivity; 2010; pp. 144–151. [Google Scholar]
- 55.Kohls SJ, Baker DD, Kremer DA, Dawson JO. Water-retentive polymers increase nodulation of actinorhizal plants inoculated with Frankia . Plant and Soil. 1999;214(1-2):105–115. [Google Scholar]
- 56.National Research Council. Casuarinas: Nitrogen-Fixing Trees for Adverse Sites. Washington, DC, USA: Office of International Affairs; 1984. [Google Scholar]
- 57.Kendall JM, Tanaka Y, Myrold DD. Dual inoculation increases plant growth with Frankia on red alder (Alnus rubra Bong.) in fumigated nursery beds. Symbiosis. 2003;34(3):253–260. [Google Scholar]
- 58.Roy S, Khasa DP, Greer CW. Combining alders, frankiae, and mycorrhizae for the revegetation and remediation of contaminated ecosystems. Canadian Journal of Botany. 2007;85(3):237–251. [Google Scholar]
- 59.Yamanaka T, Akama A, Li CY, Okabe H. Growth, nitrogen fixation and mineral acquisition of Alnus sieboldiana after inoculation of Frankia together with Gigaspora margarita and Pseudomonas putida . Journal of Forest Research. 2005;10(1):21–26. [Google Scholar]
- 60.Quoreshi AM, Roy S, Greer CW, Beaudin J, McCurdy D, Khasa DP. Inoculation of green alder (Alnus crispa) with Frankia-ectomycorrhizal fungal inoculant under commercial nursery production conditions. Native Plants Journal. 2007;8:271–281. [Google Scholar]
- 61.Oliveira RS, Castro PML, Dodd JC, Vosátka M. Synergistic effect of Glomus intraradices and Frankia spp. on the growth and stress recovery of Alnus glutinosa in an alkaline anthropogenic sediment. Chemosphere. 2005;60(10):1462–1470. doi: 10.1016/j.chemosphere.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 62.Vendan RT, Rajeshwari T, Thamizh R, Narayanan R. Studies on strain specificity of Frankia in Alnus nepalensis . Tropical Agriculture Research and Extension. 1999;2:124–125. [Google Scholar]
- 63.Steele DB, Ramirez K, Stowers MD. Host plant growth response to inoculation with Frankia . Plant and Soil. 1989;118(1-2):139–143. [Google Scholar]
- 64.Carlson PT, Dawson JO. Soil nitrogen changes, early growth, and response to soil internal drainage of a plantation of Alnus jorullerzsis in the Colombian highlands. Turrialba. 1985;35:141–150. [Google Scholar]
- 65.Chaudhary AH, Hafeez ADL, Akkermans MF, Chaudhary MF, Mirza MS. Morphology, physiology and potential in agroforestry of Datisca, Coriaria and Alnus species from Pakistan. In: Malik KA, Naqvi SHM, Aleem MIH, editors. Nitrogen and the Environment. Faisalabed, Pakistan: NTAB; 1985. pp. 213–224. [Google Scholar]
- 66.El-Lakany MH. Contribution of Casuarina to soil fertility in Egypt. Nitrogen Fixing Trees Workshop: Biological Improvement of Soil; March 1986; Dakar, Senegal. [Google Scholar]
- 67.Kang LH, Li SC, Peng YQ, Liu YL, Chen HC, Luo CJ. Study on the inoculation effects of field-grown Casuarina equisetifolia with Frankia alginate beads. Forest Research. 2000;13(1):39–43. [Google Scholar]
- 68.Landis TD, Dumroese RK. Applying the target plant concept to nursery stock quality. In: MacLennan L, Fennessy J, editors. Proceedings of the COFORD Conference on Plant Quality: A Key to Success in Forest Establishment; 2006; Dublin, Ireland. National Council for Forest Research and Development; pp. 1–10. [Google Scholar]
- 69.Simonet P, Navarro E, Rouvier C, et al. Co-evolution between Frankia populations and host plants in the family Casuarinaceae and consequent patterns of global dispersal. Environmental Microbiology. 1999;1(6):525–533. doi: 10.1046/j.1462-2920.1999.00068.x. [DOI] [PubMed] [Google Scholar]
- 70.Smolander A. Frankia populations in soils under different tree species-with special emphasis on soils under Betula pendula . Plant and Soil. 1990;121(1):1–10. [Google Scholar]
- 71.Benson DR, Vanden Heuvel BD, Potter D. Actinorhizal symbioses: diversity and biogeography. Bios Scientific Publisher. 2004;15:99–130. [Google Scholar]
- 72.Maunuksela L. Molecular and Physiological Characterization of Rhizosphere Bacteria and Frankia in Forest Soils Devoid of Actinorhizal Plants. Faculty of Science Department of Biosciences, Division of General Microbiology, and Graduate School in Microbiology and Viikki Graduate School in Biosciences University of Helsinki; 2001. [Google Scholar]
- 73.Bulloch BT. Nodulation by Frankia increases growth of Casuarinaceae in a New Zealand horticultural soil. New Zealand Journal of Crop and Horticultural Science. 1994;22(1):39–44. [Google Scholar]
- 74.Bâ AM, Diédhiou AG, Prin Y, Galiana A, Duponnois R. Management of ectomycorrhizal symbionts associated to useful exotic tree species to improve reforestation performances in tropical Africa. Annals of Forest Science. 2010;67(3):p. 301. [Google Scholar]
- 75.FAO. Rapport Technique. 1. Rome, Italy: PNUD; 1981. Fixation des dunes, protection des niayes et des sols diors de la grande côte Sénégal. Manuel de reboisement FO: DPFAFfl41308, FO: DPlSENl731012. [Google Scholar]
- 76.Maggia L. Diversité génétique de Frankia, symbiote de Casuarina equisetifolia L. Johnson en Afrique de l’Ouest (Sénégal et Gambie) Université Paris VII; 1991. [Google Scholar]
- 77.Andeke-Lengui MA, Dommergues YR. Coastal sand dune stabilization in Senegal. In: Midgley SJ, Tumbull JW, Johnston RD, editors. Casuarina Ecology Management and Utilization. Melbourne, Australia: Common Wealth Scientific and Industrial Research Organization (CSIRO); 1981. pp. 158–166. (Proceedings of an International Workshop). [Google Scholar]
- 78.Dommergues YR, Duhoux E, Diem HG. Les arbres fixateurs d’azote : caractéristiques fondamentales et rôle dans l’aménagement des écosystèmes méditerranéens et tropicaux, avec référence particulière aux zones subhumides et arides. St. Etienne, France: Impression Dumas; 1999. [Google Scholar]
- 79.Lefrançois E, Quoreshi A, Khasa D, et al. Alder-Frankia symbionts enhance the remediation and revegetation of oil sands tailings. 2007, http://esaa-events.org/
- 80.Lefrançois E, Quoreshi A, Khasa D, et al. Field performance of alder-Frankia symbionts for the reclamation of oil sands sites. Applied Soil Ecology. 2010;46(2):183–191. [Google Scholar]
- 81.Bélanger PA, Beaudin J, Roy S. High-throughput screening of microbial adaptation to environmental stress. Journal of Microbiological Methods. 2011;85(2):92–97. doi: 10.1016/j.mimet.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 82.Salt DE, Blaylock M, Kumar NPBA, et al. Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology. 1995;13(5):468–474. doi: 10.1038/nbt0595-468. [DOI] [PubMed] [Google Scholar]
- 83.Richards JW, Krumholz GD, Chval MS, Tisa LS. Heavy metal resistance patterns of Frankia strains. Applied and Environmental Microbiology. 2002;68(2):923–927. doi: 10.1128/AEM.68.2.923-927.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Srivastava A, Singh SS, Mishra AK. Sodium transport and mechanism(s) of sodium tolerance in Frankia strains. Journal of Basic Microbiology. 2012;52(1):1–2. doi: 10.1002/jobm.201100586. [DOI] [PubMed] [Google Scholar]
- 85.Hafeez FY, Sohail Hameed A, Malik KA. Frankia and Rhizobium strains as inoculum for fast growing trees in saline environment. Pakistan Journal of Botany. 1999;31(1):173–182. [Google Scholar]
- 86.Ng BH. The effects of salinity on growth, nodulation and nitrogen fixation of Casuarina equisetifolia . Plant and Soil. 1987;103(1):123–125. [Google Scholar]
- 87.Mehda P. Evaluating the Potential of Alder-Frankia Symbionts for the Remediation and Revegetation of Oil Sands Tailings. McGil University; 2006. [Google Scholar]
- 88.Baumer M, Darnhofer I, Guandalino S. Baobab farm Itd ou que faire d'une carrière après exploitation? Bois et Forêts des Tropiques. 1990;226:48–60. [Google Scholar]
- 89.Huang J, Hueyou H, Shouping C, yuzhu K, Duanqin C, Chensheng T. Current research on main Casuarina diseases and insects in China. In: Zhong C, Pinyopusarerk K, Kalinganire A, Franche C, editors. Improving Smallholder Livelihoods through Improved Casuarina Productivity. 2011. pp. 232–238. (Proceedings of the 4th International Casuarina Workshop). [Google Scholar]
- 90.Beaulieu C. Actinomycetes, promising tools to control plant diseases and to promote plant growth. Phytoprotection. 2001;82(3):85–102. [Google Scholar]
- 91.Zheng HC. Studies on the relation among the Casuarina equisetifolia clones resistant or sensitive to Pseudomonas solanacearum nodule nitrogen fixation activity and biomass. Journal of Fujian Forestry Science and Technology. 1996;23:44–47. [Google Scholar]
- 92.Kang L. Inhibition of bacterial wilt growth by Frankia isolates from Casuarinaceae. Forest Research. 1999;12(1):42–46. [Google Scholar]


