Abstract
Objective
To evaluate whether higher circulating levels of complement proteins C3 and C4 are associated with menopausal status and with hemostatic/thrombus formation markers (circulating factor VII (factor VIIc), fibrinogen, plasminogen activator inhibitor-1 (PAI-1) and tissue plasminogen activator antigen (tPA-ag)) in a sample of midlife women.
Methods and Results
A total of 100 women (50 late peri-/postmenopausal and 50 pre-/early peri menopausal women) from the Study of Women’s Health Across the Nation (SWAN) Pittsburgh site were included in the present analysis. Factor VIIc and PAI-1 were log transformed. Linear regression was used for analysis. The mean age of the study participants was 50.5±2.6 years with 73% were Caucasian and 27% were African American. C3 but not C4 was significantly higher in postmenopausal women compared to premenopausal women (P value=0.03), adjusting for age, race and BMI. In final model (adjusting for age, race, BMI and menopausal status), C3 was associated with higher levels of log PAI-1 (P value=0.0009) and tPA-ag (P value=0.0003), while C4 was associated with higher levels of log factor VIIc (P value=0.04) and fibrinogen (P value=0.005).
Conclusions
These data suggest that C3 and C4 may be related to blood clots via their associations with hemostatic markers and that C3 is related to menopausal status. Complement proteins C3 and C4 could be possible pathways by which postmenopausal women are at higher risk of atherosclerosis and cardiovascular related events. It is important to replicate these findings in a larger sample size.
Keywords: Epidemiology, Risk Factors; Coagulation, Fibrinolysis; Menopause
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the United States and the risk of CVD increases in women after the age of 50,1,2 a time period that coincident with the menopausal transition. Increasing evidence suggests that the complement system is associated with atherosclerosis and CVD.3–10 However, the underlying mechanism is not completely understood. The complement system includes a series of proteins that have a critical role in innate immunity and inflammatory reactions. The activation of the complement system produces pro-inflammatory mediators that can attack the endothelium and enhance leukocyte recruitment to inflammatory sites.11 Among the several complement proteins that mediate activation of the complement system are complement proteins C3 and C4, which play critical roles in the complement system activation.11,12 Depositions of both C3 and C4 have been found in arterial lesions.13, 14 Recent data suggest a potential role of complement protein C3 in clot stability with hypofibrinolytic and prothrombotic features.15
Previous studies showed that serum C3 is significantly higher in women compared to men,8 with women over 50 years of age having significantly higher C3 levels compared with younger women.16 This pattern raises the possibility that reproductive hormones play a role in the complement proteins. Whether higher levels of complement proteins are related to the menopausal transition, independent of aging, or play any role in explaining the increasing risk of CVD in women after the menopause is not clear. For the present study, we evaluated whether higher levels of complement proteins are associated with postmenopausal status, independent of age, race, and BMI, and sought to determine their relationship with hemostatic/thrombus formation markers (factor VIIc, fibrinogen, plasminogen activator inhibitor-1 (PAI-1) antigen and tissue plasminogen activator antigen (tPA-ag)) in a sample of midlife women.
Methods
Study participants
SWAN is an ongoing, longitudinal, multi-ethnic study of the biological, physical, psychological, and social changes during the menopausal transition. The study design has been previously reported.17 In brief, between 1996 and 1997, 3,302 participants aged 42–52 years were recruited from seven designated sites (Boston, MA; Detroit, MI; Oakland, CA; Los Angeles, CA; Pittsburgh, PA; Chicago, IL; and Newark, NJ). The eligibility criteria for the SWAN study were 1) an intact uterus and at least 1 ovary, 2) not pregnant or breastfeeding, 3) at least 1 menstrual period within the past 3 months, 4) no HT use within the past 3 months. Participants of the current study were part of an ancillary study to SWAN at the Pittsburgh site where enrollment began between the 4th (1998) and 7th (2000) annual visit of the SWAN study. A random pilot sample of 100 participants was obtained based on availability of frozen blood specimens and menopausal status (50% were pre- or early perimenopausal and 50% were late peri- or postmenopausal).
Research protocols were approved by the University of Pittsburgh institutional review board and all the participants provided a written informed consent prior to enrollment.
Study measures
Blood assays
Phlebotomy was performed in the morning after an overnight fast. The participants were scheduled for venipuncture on days 2 to 5 of a spontaneous menstrual cycle. Two attempts were made to obtain a sample at days 2 to 5. If a timed sample could not be obtained (because menstrual cycles became less regular, samples tied to the early follicular phase were not feasible), a random fasting sample was taken within 90 days of the annual visit. Blood was maintained up to 1 hour at 4° C until separated and then frozen (−80°C) and sent on dry ice to the Medical Research Laboratories to measure glucose, circulating factor VII (factor VIIc), fibrinogen, plasminogen activator inhibitor-1 (PAI-1) and tissue plasminogen activator antigen (tPA-ag). Glucose was measured with a hexokinase-coupled reaction (Boehringer Mannheim Diagnostics). Hemostatic markers factor VIIc, fibrinogen, PAI-1 and tPA-ag were measured in plasma. Fibrinogen and FVIIc were measured in frozen citrated plasma (MLA ELECTRA 1400C; Medical Laboratory Automation Inc., Mt. Vernon, NY) using a turbidimetric detection system. Fibrinogen monthly interassay CVs were 2.3% to 3.5% and 2.6% to 3.6% at mean concentrations of 250 and 140 mg/dL, respectively, and FVIIc monthly interassay CVs were 7.8%, 5%, and 4% for mean activities of 8%, 45%, and 99%, respectively. PAI-1 was measured with a sandwich procedure using a solid-phased monoclonal antibody and enzyme-labeled goat second antiserum for detection (IMUBIND plasma PAI-1 enzyme-linked immunosorbent assay [ELISA]; American Diagnostica, Greenwich, CT). PAI-1 monthly interassay CVs were 5% to 9% and 4% to 9% at mean concentrations of 7 and 22.5 ng/dL, respectively. tPA-ag was measured in plasma using a double antibody in an ELISA (IMUBIND tPA-ag ELISA; American Diagnostica). The assay uses human single-chain tPA-ag as a standard calibrated against an international standard (NIBSAC, Hertfordshire, UK). Monthly interassay CVs were 4.7% to 8.7% and 3.8% to 7.8% at mean concentrations of 5.6 and 11 ng/dL, respectively. Both C3 and C4 were measured using frozen serum specimens by immunoturbidimetric assay.
Study covariates
Weight and height were measured annually. Body mass index (BMI) was calculated as weight/height2. Race/ethnicity was self-reported. Age was calculated using the date of the annual interview minus date of birth obtained upon screening. Smoking status was defined as current versus past/never if a participant answered yes to the following question: Since your last study visit, have you smoked cigarettes regularly (at least one cigarette a day). Diabetes was defined based on self-reported diabetes or fasting glucose levels ≥ 126 mg/dL, or reported any use of insulin or anti-diabetic agents. Self-reported uses of steroids, lipid lowering medications and/or anti-hypertensive during the last month of the study visit were also collected. Blood pressure was averaged after two sequential measures in the right arm with the participant seated after at least 5 minutes of rest and hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or self-reported use of anti-hypertensive medications in the last month of the study visit. Menopausal status was determined annually based on reports about frequency, regularity of menstrual bleeding and use of hormone therapy18 as follow: 1) Premenopause: monthly bleeding with no perceived change in cycle interval, 2) Early peri-menopause: monthly bleeding with a perceived change in cycle interval, but at least one menstrual period within the past 3 months, 3) Late peri-menopause: 3 consecutive months of amenorrhea, 4) Postmenopause: 12 consecutive months of amenorrhea. Both pre and early peri-menopausal were combined in one group while late peri- and postmenopausal were combined in a second group.
Statistical Analysis
Independent (C3, C4) and dependent variables (tPA-ag, PAI-1, factor VIIc, fibrinogen) as well as covariates were examined for distributions and outliers. Both factor VIIc and PAI-1 were log transformed. Both C3 and C4 were normally distributed. Separate linear regression models for each hemostatic marker and each compelemnt protein were fitted. Models adjusted for age, race, status and BMI based on univaraite analysis. Effect modification of BMI on associations between status and complement protein was tested. For a clearer presenation of the interaction between BMI and status, BMI was dictotomized into 2 categories as follow:obese if BMI ≥ 30 kg/m2 and none obese if BMI < 30 kg/m2. Bonferroni adjustement was used for multiple comparisons. Additionally, effect modification of menopausal status for associations between commplement proteins and each hemostatic marker was evaluated. Analyses were performed with SAS, version 9.2 (SAS Institute, Cary, NC).
Results
Characteristics of the study participants were summarized at Table 1. Per study design, 50% of the study participants were pre- or early peri-menopausal and 50% were late peri- or postmenopausal. The majority of the participants were Caucasian (73%). The mean age for the premenopausal group was 48.8 years old while for the postmenopausal group was 52.1 years old. In general, participants were overweight with mean ± SD for BMI=28.7 ± 5.9 kg/m2. The two menopausal groups significantly differed in terms of age and level of complement protein C3, with postmenopausal women have higher levels of C3 compared to premenopausal women (P=0.002). Of the participants, 15% were current smokers, 4% were classified as diabetic, 19% as hypertensive, and 5% were on lipid lowering medications, and 1% on steroids within the last month of the study visit.
Table 1.
Characteristics of the study sample by menopausal status
| Variables | Total N=100 |
Menopausal Status | P value | |
|---|---|---|---|---|
| Pre-/Early Peri-menopausal N=50 |
Late Peri-/Postmenopausal N=50 |
|||
| Age (years), Mean (SD) | 50.5 (2.6) | 48.8(1.9) | 52.1(2.2) | <0.0001 |
| Race, n (%) | 0.7 | |||
| African American | 27 (27) | 15 (30) | 12 (24) | |
| Caucasian | 73 (73) | 35(70) | 38(76) | |
| BMI (kg/m2), Mean (SD) | 28.7 (5.9) | 28.7(6.4) | 28.6(5.5) | 0.9 |
| C3 (mg/dL), Mean (SD) | 162.3 (33.3) | 152.3(28.6) | 172.3(34.9) | 0.002 |
| C4 (mg/dL), Mean (SD) | 31.9 (8.8) | 31.6(8.9) | 32.3(8.7) | 0.7 |
| PAI-1 (ng/mL), Median (IQR) | 14.8(6.4,23.5) | 12.4(6.4,22.8) | 16.0(6.4,26.1) | 0.7 |
| tPA-ag (ng/mL), Mean (SD) | 7.2(3.0) | 7.0(2.8) | 7.4(3.2) | 0.5 |
| Fibrinogen (mg/dL), Mean (SD)* | 273.1(57.5) | 269.3(62.1) | 275.0(56.1) | 0.8 |
| Factor VIIc (%), Median (IQR)* | 127.5(104.0,151.0) | 108.0(100.0,129.0) | 145.0(108.0,153.0) | 0.2 |
Factor VIIc and fibrinogen were available for 44% of the study participants
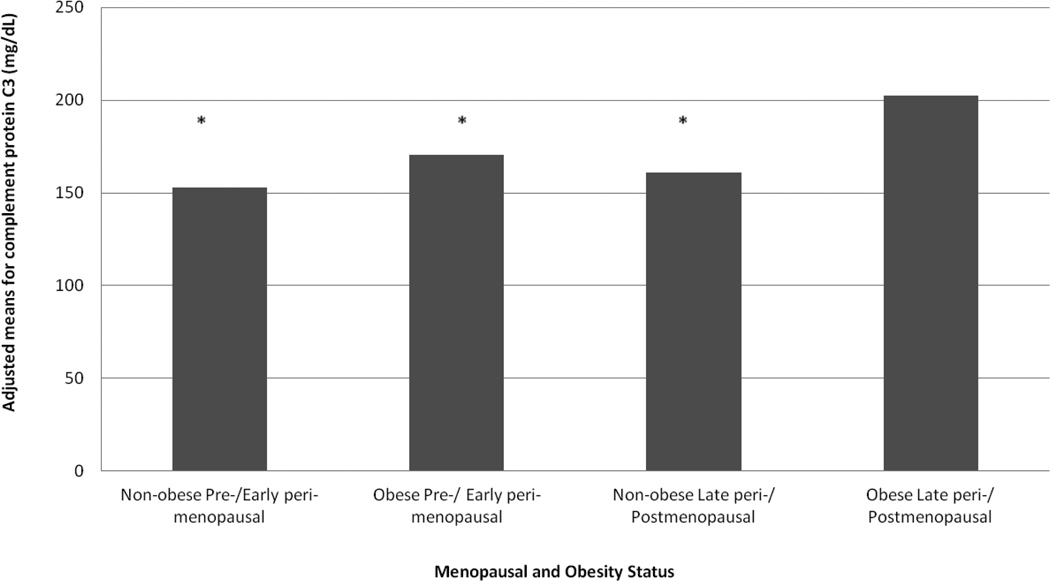
Table 2 presents unadjusted and adjusted analyses for the associations between menopausal status and complement proteins. Consistent with the summary statistics presented in Table 1, postmenopausal women reported significantly higher levels of C3 (P value=0.03) but not C4 (P value=0.2) compared to premenopausal women after adjusting for age, race and BMI. Significant interaction was found between obesity status (obese: BMI ≥30kg/m2, non obese: BMI<30kg/m2) and menopausal status in relation to complement protein C3 (P value=0.039) but not C4, Figure 1. Obese postmenopausal women had significantly higher levels of C3 (202.3 mg/dL vs 152.8 mg/dL), compared to all other groups non obese premenopausal women. Additionally, both obese premenopausal women and non obese postmenopausal women had significantly lower levels of C3 compared to obese postmenopausal women (Bonferroni adjusted p value for all <0.05. All significant results were independent of age, race and menopausal status.
Table 2.
Association between menopausal status and complement protein C3 and C4
| Complement Protein C3 (mg/dL) | Complement Protein C4 (mg/dL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Unadjusted | Model 1 | Model 2 | |||||||
| β(SE) | P value | β(SE) | P value | β(SE) | P value | β(SE) | P value | β(SE) | P value | β(SE) | P value | |
| Menopausal Status | ||||||||||||
| Pre-/Early peri- | --- | ---- | ---- | --- | ---- | --- | ||||||
| Late peri-/Post- | 20.0(6.4) | 0.002 | 13.6(7.6) | 0.08 | 15.0(6.6) | 0.03 | 0.7(1.8) | 0.7 | 2.7(2.2) | 0.2 | 2.9(2.2) | 0.2 |
| Age | ---- | 2.4(1.5) | 0.1 | 1.9(1.3) | 0.1 | --- | --- | −0.5(0.4) | 0.2 | −0.6(0.4) | 0.2 | |
| Race | ||||||||||||
| African American | ---- | ---- | ---- | ---- | ---- | ---- | ||||||
| Caucasian | ---- | −27.4(6.9) | 0.0002 | −15.9(6.2) | 0.01 | ---- | −4.6(1.9) | 0.02 | −3.4(2.0) | 0.1 | ||
| BMI | ---- | --- | 2.7(0.5) | <0.0001 | --- | --- | 0.3(0.1) | 0.05 | ||||
Figure 1. Adjusted Means of Complement Protein C3 by Menopausal Status and Obesity.
*P value for interaction between status and obesity = 0.039. Obese: BMI>=30 kg/m2. Models adjusted for age, race, and menopausal status. Non-obese Pre-/ Early peri-menopausal, obese Pre-/ Early peri-menopausal, non-obese Late peri-/ Postmenopausal significantly differ from obese Late peri-/ Postmenopausal (Bonferroni adjusted p value for all <0.05)
Table 3 shows results from multivariable linear regression models for associations between each complement protein and each hemostatic marker. In models adjusted for age, race and menopausal status, C3 was independently associated with higher levels of log PAI-1 (P value <0.0001), tPA-ag (P value<0.0001) and log factor VII c (P value=0.04); while C4 was independently associated with higher levels of log factor VIIc (P value=0.03), fibrinogen (P value=0.005) and log PAI-1 (P value=0.04). Additional adjustment for BMI attenuated the associations between C3 and factor VIIc and the association between C4 and log PAI-1. The remaining results did not change. Interactions between each hemostatic marker and menopausal status in relation to complement proteins C3 or C4 were not significant except for associations between factor VIIc and C4. Stratification analysis were performed by menopausal status and higher levels of C4 was significantly associated with greater level of factor VIIc in premenopausal women but not in postmenopausal women (P value=0.006), These results should be interpreted with caution given the small sample size of the current study. Additional analyses adjusting for smoking status, hypertension, diabetes and use of lipid lowering medications did not change the above detected associations between C3 and PAI-1 and tPA-ag as well as between C4 and fibrinogen. Although the p-value for the association between C4 and factor VIIc was attenuated, the magnitude and the direction of the association did not change. Smoking status, hypertention, diabetes and use of lipid lowering medications were not significant in final models and therefore, only parsimonious models were presented.
Table 3.
Associations between hemostatic markers and complement proteins C3 and C4 among midlife women
| Separate models | Complement Protein C3 (mg/dL) | Complement Protein C4 (mg/dL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| β(SE) | P value | β(SE) | P value | β(SE) | P value | β(SE) | P value | |
| PAI-1* | 0.02(0.003) | <0.0001 | 0.01(0.003) | 0.0009 | 0.03(0.01) | 0.04 | 0.01(0.01) | 0.3 |
| tPA-ag | 0.05(0.01) | <0.0001 | 0.04(0.01) | 0.0003 | 0.06(0.03) | 0.07 | 0.03(0.03) | 0.3 |
| Fibrinogen | 0.43(0.27) | 0.1 | 0.52(0.32) | 0.1 | 2.76(0.92) | 0.005 | 2.80(0.95) | 0.005 |
| Factor VIIc* | 0.003(0.001) | 0.04 | 0.002(0.002) | 0.2 | 0.01(0.01) | 0.03 | 0.01(0.01) | 0.04 |
log transformed
Model 1: adjusted for age, race and menopausal status
Model 2: Model 1 + BMI
Discussion
In the current study complement protein C3 but not C4 was found to be significantly related to menopausal status independent of age, race and BMI. Further, the association between C3 and late peri-/postmenopausal status was found to be more pronounced among obese women. Both complement proteins C3 and C4 were found to be significantly associated with hemostatic/coagulation markers in women at midlife. Complement protein C3 was independently associated with two important hemostatic markers, PAI-1 and tPA-ag. These markers have significant roles in thrombus development, stabilization and destabilization in lesion areas. C4 was independently associated with thrombus development factors, factor VIIc and fibrinogen.
To the best of our knowledge this is the first epidemiological study to show an association between menopausal status and C3 protein of the complement system. Estradiol, the main hormone that declines during the menopausal transition,19 has both anti-inflammatory and proinflammatory roles depending on several factors such as the immune stimulus and subsequent immune responses (for example, T cells are inhibited by estrogen while B cells are activated), variability in expression of estrogen receptors, estrogen circulating levels and reproductive status.20 Interestingly, complement protein C3 contains estrogen-responsive elements in the promoter region,21 which may suggest a direct hormonal effect on complement system. Moreover, recent studies evaluating the genomic response of estradiol replacement therapy in the frontal cortex of ovariectomized, middle-aged rats as a rodent model to mimic the low estradiol level in postmenopause show that E2 replacement therapy down-regulating most of the genes related to extracellular matrix and immunity including complement components C3 and C4b genes.22 Additionally, significant up-regulation of immune-competent gene expression including C3 and complement component receptor 3 alpha subunit was found in post- compared to premenopausal bone tissue.23
The significant interaction between menopausal status and obesity in relation to C3 levels, suggests a potential role of body fat accumulation, that accompany the menopausal transition,24–26 in the higher levels of C3 reported among postmenopausal women. The greater activation of the complement system, as marked by the higher level of C3 reported in obese postmenopausal women in the current study, could be a consequence of fat accumulation at midlife. Adipose tissue is widely recognized as an important source of several immune-related mediators that play critical roles in the inflammatory response. It produces proinflammatory cytokines that control the hepatic synthesis of acute phase inflammatory proteins such as complement factors C3 and C4.27 Interestingly, reduction in C3 level was reported among obese participants after being on 6-week very low calorie diet,28 which supports the above hypothesis. On the other hand, the current results also suggest that fat accumulation in postmenopausal women could be a consequence of the activation of the complement system as marked by the higher levels of C3 among obese midlife women. C3-deficient mice have been shown to have higher energy expenditure and lower body fat.29 In humans, higher levels of plasma C3 predict weight gain.30 Both C3 fragment, C3a, and acylation-stimulating protein (C3desArg) can stimulate triglycerides synthesis in adipocytes.29 Given the cross-sectional nature of the current study, we cannot determine if fat accumulation in postmenopausal women is a consequence of complement system activation or the reverse. It also needs to be stressed that at the current state of knwoledge, our findings represent simply an association, but not a mechanistic explanation.
We found significant associations between complement proteins C3 and C4 and hemostatic/coagulation markers in women at midlife. We are not aware of any previous epidemiological study that assessed similar associations between complement components and hemostatic/coagulation markers. However, our results are extending what have been reported from proteomic analysis in vitro and ex vivo studies.15 Complement C3 was identified in perfused, solubilised plasma clots as a novel clot component. It has been reported to have high affinity binding to fibrinogen and fibrin. Additionally, lysis analysis showed C3 to have concentration-dependent fibrinolysis impairment effect both in vitro and ex vivo. These data suggest a potential role of C3 in prolonging fibrinolysis.15 Our findings that C3 was independently associated with two important hemostatic/thrombus formation constituents, PAI-1 and tPA-ag suggesting a potential role of C3 in thrombus development, stabilization and destabilization in lesion areas. Additionally, we found that C4 was independently associated with thrombus development factors: factor VIIc and fibrinogen.
Increasing evidence suggests that the complement system is associated with atherosclerosis.3–10 Significant positive correlation was reported between C3 and intima-media thickness in overweight/obese young subjects.31 Patients with severe atherosclerotic lesions had higher levels of C4 than controls in multivariate analysis.7 Further, elevated C3 was able to predict major complications of atherosclerosis developed during 5-year long independent of other CV risk factors in women who had received aorto-coronary bypass graft surgery.9 Since acute thrombosis is the common feature of several CVD including myocardial infarction and ischemic stroke, our findings from this pilot study suggest novel pathways by which complement proteins may increase risk of CVD in women at midlife. The current study showed that the circulating level of C3 is significantly higher in postmenopausal women compared to premenopausal women and this association is more pronounced among obese postmenopausal women. Taken together, our study suggests that obese postmenopausal women may be at greater risk of thrombus formation due to higher circulating complement protein C3 when compared with premenopausal women. If our findings are substantiated by replicating the study using a larger sample size, monitoring circulating complement protein C3 may prove beneficial for determining the susceptibility of obese postmenopausal women to thrombotic events.
The main limitations of the current study are 1) the small sample size; 2) the cross-sectional design, which limits our ability to understand the temporality of the detected associations; 3) limited generalizability, as only women at midlife were included, and, therefore, our results may not be generalized to men or other age groups; 4) inability to compare results between Caucasians and African Americans due to small sample size, particularly for African American women; 5) unavailability of data about chronic inflammation. However, only one woman reported using steroids during the last month of the study visit. Despite the small sample size, the findings are novel and suggest new pathways for the development of higher risk of CVD in postmenopausal women.
Future studies should replicate our findings in a larger multi-racial population. Examination of reproductive sex hormones, in association with complement proteins, would shed light on possible mechanisms of action. Longitudinal studies are needed to understand roles of complement C3 in relation to fat accumulation during the menopausal transition and how that may impact the CVD risk after menopause.
Highlights.
C3 and C4 may be related to blood clots via their associations with hemostatic markers.
C3 is significantly higher in postmenopausal women independent of age, BMI and race.
Acknowledgments
We thank the study staff at each site and all the women who participated in SWAN. Souce of Funding:
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
C3 and C4 assays were funded through the small Department of Epidemiology grant program at the University of Pittsburgh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
Disclosures: The authors declare no conflict of interest.
Contributor Information
Samar R. El Khoudary, Email: elkhoudarys@edc.pitt.edu.
Kelly J. Shields, Email: kshield1@wpahs.org.
Hsiang-Yu Chen, Email: chenh@edc.pitt.edu.
Karen A. Matthews, Email: matthewska@upmc.edu.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Gorodeski GI. Impact of the menopause on the epidemiology and risk factors of coronary artery heart disease in women. Exp Gerontol. 1994;29:357–375. doi: 10.1016/0531-5565(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 3.Onat A, Uzunlar B, Hergenç G, Yazici M, Sari I, Uyarel H, Can G, Sansoy V. Cross-sectional study of complement C3 as a coronary risk factor among men and women. Clin Sci (Lond) 2005;108:129–135. doi: 10.1042/CS20040198. [DOI] [PubMed] [Google Scholar]
- 4.Bhakdi S, Torzewski M, Klouche M, Hemmes M. Complement and atherogenesis: binding of CRP to degraded, nonoxidized LDL enhances complement activation. Arterioscler Thromb Vasc Biol. 1999;19:2348–2354. doi: 10.1161/01.atv.19.10.2348. [DOI] [PubMed] [Google Scholar]
- 5.Bhakdi S, Lackner KJ, Han SR, Torzewski M, Husmann M. Beyond cholesterol: the enigma of atherosclerosis revisited. Thromb Haemost. 2004;91:639–645. doi: 10.1160/TH03-12-0733. [DOI] [PubMed] [Google Scholar]
- 6.Hansson GK, Libby P, Schönbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 7.Muscari A, Bozzoli C, Gerratana C, Zaca' F, Rovinetti C, Zauli D, La Placa M, Puddu P. Association of serum IgA and C4 with severe atherosclerosis. Atherosclerosis. 1988;74:179–186. doi: 10.1016/0021-9150(88)90204-3. [DOI] [PubMed] [Google Scholar]
- 8.Muscari A, Bozzoli C, Puddu GM, Sangiorgi Z, Dormi A, Rovinetti C, Descovich GC, Puddu P. Association of serum C3 levels with the risk of myocardial infarction. Am J Med. 1995;98:357–364. doi: 10.1016/S0002-9343(99)80314-3. [DOI] [PubMed] [Google Scholar]
- 9.Széplaki G, Prohászka Z, Duba J, Rugonfalvi-Kiss S, Karádi I, Kókai M, Kramer J, Füst G, Kleiber M, Romics L, Varga L. Association of high serum concentration of the third component of complement (C3) with pre-existing severe coronary artery disease and new vascular events in women. Atherosclerosis. 2004;177:383–389. doi: 10.1016/j.atherosclerosis.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Selzer F, Sutton-Tyrrell K, Fitzgerald S, Tracy R, Kuller L, Manzi S. Vascular stiffness in women with systemic lupus erythematosus. Hypertension. 2001;37:1075–1082. doi: 10.1161/01.hyp.37.4.1075. [DOI] [PubMed] [Google Scholar]
- 11.Speidl WS, Kastl SP, Huber K, Wojta J. Complement in atherosclerosis: friend or foe? J Thromb Haemost. 2011;9:428–440. doi: 10.1111/j.1538-7836.2010.04172.x. [DOI] [PubMed] [Google Scholar]
- 12.Niculescu F, Rus H. Complement activation and atherosclerosis. Mol Immunol. 1999;36:949–955. doi: 10.1016/s0161-5890(99)00117-0. [DOI] [PubMed] [Google Scholar]
- 13.Yasojima K, Schwab C, McGeer EG, McGeer PL. Complement components, but not complement inhibitors, are upregulated in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1214–1219. doi: 10.1161/hq0701.092160. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Holm J, Kral JG. Accumulation of IgG and complement factor C3 in human arterial endothelium and atherosclerotic lesions. Acta Pathol Microbiol Immunol Scand A. 1984;92:429–435. doi: 10.1111/j.1699-0463.1984.tb04424.x. [DOI] [PubMed] [Google Scholar]
- 15.Howes JM, Richardson VR, Smith KA, Schroeder V, Somani R, Shore A, Hess K, Ajjan R, Pease RJ, Keen JN, Standeven KF, Carter AM. Complement C3 is a novel plasma clot component with anti-fibrinolytic properties. Diab Vasc Dis Res. 2012;9:216–225. doi: 10.1177/1479164111432788. [DOI] [PubMed] [Google Scholar]
- 16.Muscari A, Massarelli G, Bastagli L, Poggiopollini G, Tomassetti V, Volta U, Puddu GM, Puddu P. Relationship between serum C3 levels and traditional risk factors for myocardial infarction. Acta Cardiol. 1998;53:345–354. [PubMed] [Google Scholar]
- 17.Sowers M, Crawford S, Sternfed B, Morganstein D, Gold EB, Greendale G, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathology. New York, NY: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 18.World Health Organization. Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1996. Research on the menopause in the 1990s. (WHO technical report series 866). [PubMed] [Google Scholar]
- 19.Tepper PG, Randolph JF, Jr, McConnell DS, Crawford SL, El Khoudary SR, Joffe H, Gold EB, Zheng H, Bromberger JT, Sutton-Tyrrell K. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women's Health across the Nation (SWAN) J Clin Endocrinol Metab. 2012;97:2872–2880. doi: 10.1210/jc.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 21.Fan JD, Wagner BL, McDonnell DP. Identification of the sequences within the human complement 3 promoter required for estrogen responsiveness provides insight into the mechanism of tamoxifen mixed agonist activity. Mol Endocrinol. 1996;10:1605–1616. doi: 10.1210/mend.10.12.8961270. [DOI] [PubMed] [Google Scholar]
- 22.Sárvári M, Kalló I, Hrabovszky E, Solymosi N, Tóth K, Likó I, Molnár B, Tihanyi K, Liposits Z. Estradiol replacement alters expression of genes related to neurotransmission and immune surveillance in the frontal cortex of middle-aged, ovariectomized rats. Endocrinology. 2010;151:3847–3862. doi: 10.1210/en.2010-0375. [DOI] [PubMed] [Google Scholar]
- 23.Kósa JP, Balla B, Kiss J, Podani J, Takács I, Lazáry A, Nagy Z, Bácsi K, Karsai A, Speer G, Lakatos P. Postmenopausal expression changes of immune system-related genes in human bone tissue. J Clin Immunol. 2009;29:761–768. doi: 10.1007/s10875-009-9321-9. [DOI] [PubMed] [Google Scholar]
- 24.Svendsen OL, Hassager C, Christiansen C. Age- and menopause-associated variations in body composition and fat distribution in healthy women as measured by dual-energy X-ray absorptiometry. Metabolism. 1995;44:369–373. doi: 10.1016/0026-0495(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 25.Toth MJ, Tchernof A, Sites CK, Poehlman ET. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes Relat Metab Disord. 2000;2:226–231. doi: 10.1038/sj.ijo.0801118. [DOI] [PubMed] [Google Scholar]
- 26.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 28.Hernández-Mijares A, Bañuls C, Bellod L, Jover A, Solá E, Morillas C, Víctor VM, Rocha M. Effect of weight loss on C3 and C4 components of complement in obese patients. Eur J Clin Invest. 2012;42:503–509. doi: 10.1111/j.1365-2362.2011.02606.x. [DOI] [PubMed] [Google Scholar]
- 29.Xia Z, Stanhope KL, Digitale E, Simion OM, Chen L, Havel P, Cianflone K. Acylation-stimulating protein (ASP)/complement C3adesArg deficiency results in increased energy expenditure in mice. J Biol Chem. 2004;279:4051–4057. doi: 10.1074/jbc.M311319200. [DOI] [PubMed] [Google Scholar]
- 30.Engström G, Hedblad B, Janzon L, Lindgärde F. Weight gain in relation to plasma levels of complement factor 3: results from a population-based cohort study. Diabetologia. 2005;48:2525–2531. doi: 10.1007/s00125-005-0021-6. [DOI] [PubMed] [Google Scholar]
- 31.De Pergola G, Ciccone MM, Guida P, Morea G, Giannuzzo E, Cortese F, Scicchitano P, Favale S, Silvestris F. Relationship between C3 levels and common carotid intima-media thickness in overweight and obese patients. Obes Facts. 2011;4:159–163. doi: 10.1159/000327893. [DOI] [PMC free article] [PubMed] [Google Scholar]