SUMMARY
Objective
To evaluate the cost-effectiveness of novel first-line treatment regimens for tuberculosis (TB).
Design
Using decision analysis, we projected costs and effectiveness, from the healthcare perspective, of treating a patient cohort in the public sector for active TB without known or suspected resistance to first-line drugs. We compared standard (six-month) therapy to hypothetical regimens of equal efficacy, higher cost, and shorter duration.
Results
For every 100 TB patients treated, replacing standard therapy with shorter-course regimens avert an estimated 2–4 failures/relapses, 0.2–0.4 deaths, and 8–14 disability-adjusted life years (DALYs), or 6–11% of all DALYs suffered. We identified three primary determinants of cost-effectiveness: drug price, continuation-phase treatment delivery costs, and deaths averted through fewer relapses. In a high treatment cost scenario (similar to Brazil), averted delivery costs outweighed higher drug costs, making novel regimens cost-saving. In a low treatment cost scenario (similar to the Philippines), a four-month regimen with a drug price of $1/day cost $66 per patient, or $840 per DALY averted, and became cost-saving if the drug price dropped below $0.37/day.
Conclusion
Although they avert a small proportion of total DALYs, novel shorter-course first-line regimens for TB are likely to be cost-effective or cost-saving in most settings.
Keywords: cost-benefit analysis, antitubercular agents, drugs, investigational
Background
Although highly efficacious,(1–4) existing standard “short-course” chemotherapy for tuberculosis (TB) requires that patients complete six months of supervised treatment.(5) This extended time course places strains on both patients and health systems;(6, 7) in the case of TB, a classic disease of poverty,(8) the resources available to both are often particularly constrained. Prolonged treatment increases the difficulty of maintaining adherence, particularly toward the end of therapy when patients’ symptoms have often improved.(9, 10) Development of novel first-line therapeutic regimens that could shorten the necessary treatment duration for TB is therefore a high priority.(11, 12) Regimens involving moxifloxacin-based treatment for four months have shown preliminary evidence of equivalent efficacy,(13) and many other duration-shortening regimens are in earlier stages of development.
One challenge to implementing new first-line regimens for TB is their cost. New regimens for drug-resistant TB may be immediately cost-saving, as existing regimens cost thousands of dollars per person, and any agent that shortens the course of therapy may generate substantial savings in drug costs alone. In contrast, the current standard first-line TB regimen consisting of isoniazid, rifampin, pyrazinamide, and ethambutol costs less than 25 cents per day,(14) so novel first-line TB regimens may multiply drug costs several-fold. Whether such regimens can be cost-effective, and the price point at which increased drug costs are counterbalanced by reduced costs for healthcare delivery (through shorter therapy), remain unclear. We therefore conducted a model-based cost-effectiveness analysis of hypothetical new first-line regimens for TB therapy as deployed in high-burden settings.
Methods
Model Structure and Population
We constructed a decision analysis of TB treatment from the perspective of a public sector TB control program (Figure 1). Since novel regimens are not currently available, our primary aim was to provide a simple, preliminary framework for initial decision-making; we did not seek to model any specific epidemiological situation into which novel regimens might be deployed. Therefore, we parameterized our model with globally-representative parameters including epidemiological and financial TB data notified to the World Health Organization (WHO), standard disability weights, and drug prices as reported by the WHO. All model parameters are shown in Table 1. Ethical approval was not required for this research, which did not involve human subjects.
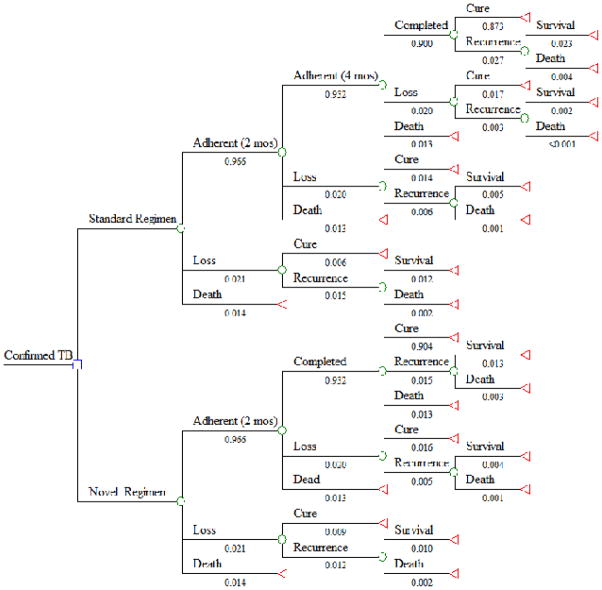
Figure 1. Decision Tree Comparing Standard and Novel Treatment Regimens for TB.
Cumulative probabilities are shown below each path; the probability of being registered as a cure/treatment success in this model (0.873) is consistent with the WHO-estimated treatment success rate among new cases diagnosed in public facilities in 2012 (87%).
Table 1.
Parameter Estimates for Cost-Effectiveness Analysis of Novel First-Line Treatment Regimens for Tuberculosis.
| Parameter | Value | Sensitivity Range | Notes/References |
|---|---|---|---|
| Adherence, per 2 months | 0.97 | 0.95–0.98 | Fit to global treatment success rate(15) |
| Probability of TB Cure (no recurrence) | |||
| No treatment | 0.3 | 0–0.5 | Natural history(20) |
| <2 months of standard therapy | 0.3 | 0–0.5 | Natural history(20) |
| 2–4 months of standard therapy | 0.69 | 0.3–0.86 | 2-month regimen(21) |
| 4–6 months of standard therapy | 0.87 | 0.7–0.95 | 4-month regimen(3) |
| Full course of treatment | 0.97 | 0.95–0.98 | 6-month regimen(19) |
| Probability of Death | |||
| Treated TB | 0.04 | 0.03–0.12 | WHO notifications(15) |
| Recurrent TB | 0.165 | 0.04–0.3 | Global TB mortality(15) |
| Costs | |||
| Drugs, standard regimen, per day | |||
| Intensive Phase (2 months) | $0.24 | $0.07-$0.60 | Global Drug Facility(23) |
| Continuation Phase (4 months) | $0.15 | $0.04-$0.29 | |
| Drugs, 4-month regimen, per day | $1.00 | See Fig. 3 | Assumed |
| Drugs, 2-month regimen, per day | $5.00 | Assumed | |
| Treatment Delivery costs, per month: | WHO notifications(15) | ||
| Low | |||
| Intensive Phase | $24.53 | See Fig. 3 | |
| Continuation Phase | $11.42 | See Fig. 3 | |
| Moderate | |||
| Intensive Phase | $49.53 | ||
| Continuation Phase | $23.92 | ||
| High | |||
| Intensive Phase | $238 | ||
| Continuation Phase | $118 | ||
| DALYs suffered | Disability 0.271(25), age | ||
| Cured TB (6 months disability) | 0.19 | 0–0.26 | 35, 35-year life |
| Recurrent TB (6 + 12 months) | 0.56 | 0–0.7 | expectancy, 3% |
| Never-treated TB (12 + 12 months) | 0.73 | 0–1.0 | discounting |
| TB Death | 24.36 | 15–30 | |
We took as our population a hypothetical cohort of 100 individuals with confirmed TB (e.g., through sputum smear or culture) presenting for treatment in the public sector (i.e., typical of those healthcare organizations that notify data to the WHO). For purposes of transparency and simplicity of model assumptions, we excluded patients whose TB was not bacteriologically confirmed, those seeking care in the private sector, and those (e.g., retreatment cases) for whom drug-sensitive TB could not be reasonably assumed. Thus, the present analysis speaks to the setting of new, bacteriologically-confirmed TB being treated in the public sector in an area of low background drug resistance or with known drug susceptibility.
Estimation of Clinical Outcomes and Treatment Efficacy
We assumed that patients could experience one of three outcomes following treatment: success (no recurrence within five years of treatment), recurrent TB (including both failure and relapse following default), and death. To estimate the probability of recurrence or death, we first used WHO notification data to estimate mortality and adherence to a full six-month course of standard therapy. Since 4% of all notified cases starting TB therapy die on treatment,(15) and deaths may occur evenly throughout the six-month course,(16) we assumed that the probability of death was 1 − (1 − 0.04)1/3 = 1.4% per two months for purposes of simplicity. We assumed that that the overall on-treatment mortality risk was not reduced by the novel regimen. Similarly, we assumed that 6% of notified cases (all notified defaults and 50% of patients “not evaluated”) leave treatment without completion, and there is no definitive evidence that default rates are differential over time;(9) thus, we modeled the two-month probability of leaving treatment as 1 − (1 − 0.06)1/3 = 2.1%. Alternative models in which 50% of all deaths occurred in the first 2 months,(17) and in which 50% of all default occurred in months 2–4,(18) were evaluated but did not change incremental cost-effectiveness estimates sufficiently to appear in our main sensitivity analysis (Figure 2).
Figure 2. Sensitivity Analysis: Incremental Cost-Effectiveness of a Novel 4-Month Regimen in a Low Treatment Cost Scenario.
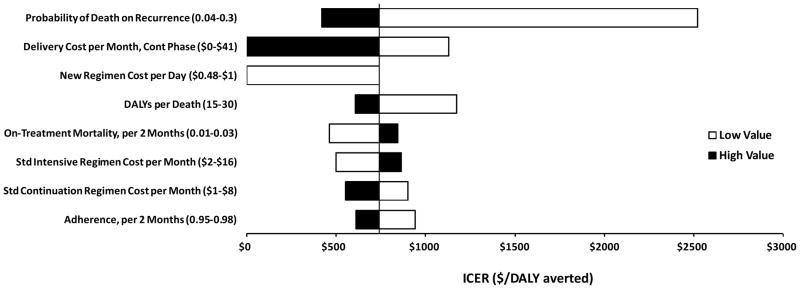
The vertical line corresponds to the estimated incremental cost-effectiveness ratio (ICER) of a four-month regimen, relative to the standard (six-month) regimen, in the low cost scenario ($740 per disability-adjusted life year [DALY] averted, Table 2). Black bars represent the high value of each parameter, as listed in Table 1 (e.g., 0.3 for probability of death on recurrence); white bars represent the low value (e.g., 0.04). Bars to the left represent more favorable ratios for the four-month regimen; sensitivity ranges of the delivery cost and new regimen cost were truncated at the point where they become cost-saving (i.e., ICER = 0). Only those parameters for which sensitivity analysis changed the estimated ICER by $200 in either direction are shown.
Among those who completed a full course of therapy, we assumed that 3% would experience recurrent TB;(19) this is similar to the 2% failure proportion among new cases notified to the WHO (which does not include relapses).(15) Among those who did not complete a full therapeutic course, we used long-term follow-up data from early clinical trials of shorter-course TB therapy to estimate the probability of recurrent TB as a function of the duration of therapy successfully completed. For patients who did not complete the two-month intensive phase, we assumed no benefit of treatment, but did assume that 30% of individuals would spontaneously resolve without treatment.(20) For those who received 2–4 months of standard treatment, we assumed that 31% would relapse, based on long-term efficacy of a 2-month intensive-phase regimen given alone.(21) For those who received 4–6 months of therapy, we assumed that 13% would relapse within 5 years, based on efficacy of a four-month regimen substituting streptomycin for ethambutol.(3)
For simplicity, we assumed that all recurrences occurred within one year (i.e., no discounting of costs or outcomes), and that all individuals with recurrent TB began a second course of treatment. Both of these assumptions were tested in sensitivity analysis and found not to substantively affect results. Since repeat diagnosis may be delayed, we assumed a probability of death after recurrent TB equal to the global mortality proportion (i.e., mortality/incidence) of all people with TB (16.5%(15)). We assumed that people who died of recurrent TB incurred diagnostic and treatment costs equal to half a treatment course before death (an assumption that also did not affect results).
We assumed that novel treatment regimens would achieve equivalent on-treatment outcomes as standard therapy, as it is unlikely that any trial of novel first-line treatment regimens will be powered for superiority. Thus, we assumed that novel regimens could reduce mortality by increasing the probability of treatment completion and thus reducing the likelihood of recurrent TB (which can, in turn, be fatal), but not by reducing the proportion of patients who die on treatment for the initial episode of TB. After two months of treatment, a 4-month regimen is half completed; we therefore assumed the same efficacy as completing three months of standard (6-month) therapy. For ease of comparison, we assumed that all treatment regimens were taken daily (not intermittently), and that all outcomes occurred at the midpoint of each time interval.
Costs and Cost-Effectiveness
Our primary outcomes were the incremental cost of treatment and the incremental cost-effectiveness ratio (cost per disability-adjusted life year, DALY, averted), comparing standard and novel treatment regimens. We adopted the perspective of a public-sector TB treatment program (healthcare perspective) with a lifetime time horizon, although as above, we assumed that all clinical events occurred in the first year. We started with WHO estimates of the per- patient cost (in 2012 US dollars) of first-line TB treatment,(22) and defined “delivery” costs as the per-patient cost of treatment minus the estimated cost of TB drugs.(14, 23) We then calculated the delivery cost per treatment month under the assumption that half of all costs occur during the two-month intensive phase, and the other half occur during the four-month continuation phase. We calculated DALYs assuming a 35-year-old patient (the median age of TB cases worldwide(15)), 35-year life expectancy at age 35,(24) standard disability weight for tuberculosis,(25) 6-month period of disability for the initial episode of TB, 12-month period of disability for recurrent TB, 3% discounting per year, and standard age weighting.
We constructed alternative scenarios to represent differences in novel drug regimen price ($1/day versus $5/day), duration of therapy required to achieve equivalent efficacy (2 months versus 4 months), and TB treatment costs. For the latter, we took WHO estimates of the total per-patient treatment cost in the 22 high-burden countries and ordered them from lowest to highest. We defined a “low treatment cost scenario” as the 25th percentile of those estimates (representative of Ethiopia, the Philippines, and Afghanistan), a “moderate-cost scenario” as the median (representative of China and Nigeria), and a “high-cost scenario” as the 95th percentile (representative of Brazil). These categories correlate with with income categories (e.g., low income, lower-middle income, upper-middle income)(15) but also include considerations such as lower delivery costs in large countries that benefit from economies of scale.
Under each scenario of treatment cost, we estimated the threshold drug price at which novel treatment regimens would become cost-saving. We performed one-way sensitivity analyses across reasonable ranges for all parameters, and multi-way sensitivity analyses across those parameters to which the outcomes were found to be most sensitive. Analyses were performed using TreeAge Pro Suite (TreeAge Software, Williamstown, MA) 2009/2011.
Results
Primary results are shown in Table 2. For every 100 patients initiating treatment for drug-sensitive TB under the standard regimen, 5.7 experienced recurrence after therapy (due either to relapse following default or primary failure), and 4.7 died. In this cohort, an estimated 135 DALYs were suffered, of which 85% represented years of life lost. In all, 16% of deaths occurred from recurrent TB, rather than the initial episode. By preventing 36% of recurrences through better treatment completion, the 4-month treatment regimen averted 6% of all deaths (also 6% of DALYs); shortening therapy to 2 months averted 70% of recurrences and thus 11% of DALYs. Although daily drug prices for a novel 4-month treatment regimen costing $1/day are four times higher than the daily intensive-phase drug prices for standard therapy, savings in healthcare delivery costs were such that this regimen only increased total TB treatment costs by 47% ($5,900 per 100 people) in the low-cost scenario and 15% ($3,400) in the moderate-cost scenario. Novel TB treatment regimens were more cost-effective in settings where treatment delivery costs (i.e., costs averted from shorter treatment courses) were higher. As a result, all novel regimens were cost-saving in the high-cost scenario. In the low-cost scenario, incremental cost-effectiveness ratios ranged from $740 per DALY averted with a 4-month, $1/day regimen to $1400 with a 2-month, $5/day regimen.
Table 2.
Costs and Effectiveness for 100 Patients Treated for Tuberculosis in the Public Sector
| Scenario | Fail/Relapse | Deatha | DALYs Suffered | Incremental DALYs Avertedb | Cost (2012 US$) | Incremental Cost | Incremental Cost per DALY Avertedb |
|---|---|---|---|---|---|---|---|
| Standard Treatment (6 Months, $0.24/day) | 5.7 | 4.7 | 135 | ||||
| Low treatment cost | $12,600 | ||||||
| Moderate treatment cost | $22,600 | ||||||
| High treatment cost | $98,500 | ||||||
| 4-Month Regimen, $1/day | 3.6 | 4.4 | 127 | 7.9 | |||
| Low treatment cost | $18,400 | $5,900 | $740 | ||||
| Moderate treatment cost | $26,000 | $3,400 | $430 | ||||
| High treatment cost | $83,200 | ($15,300) | Preferredd | ||||
| 2-Month Regimen, $5/dayc | 1.7 | 4.2 | 121 | 14.6 | |||
| Low treatment cost | $32,800 | $20,200 | $1400 | ||||
| Moderate treatment cost | $37,700 | $15,100 | $1000 | ||||
| High treatment cost | $75,300 | ($23,200) | Preferredd |
Includes deaths among patients who fail/relapse and are not subsequently cured. Since we assume equivalent efficacy for mortality among the three regimens, incremental mortality benefit occurs only among such individuals.
Relative to standard treatment
A 2-month regimen at $1/day is dominant (more effective and less costly) in all scenarios.
In the moderate treatment cost scenario, both novel regimens are less costly and more effective than standard treatment. The 2-month regimen is likewise less costly and more effective than the 4-month regimen.
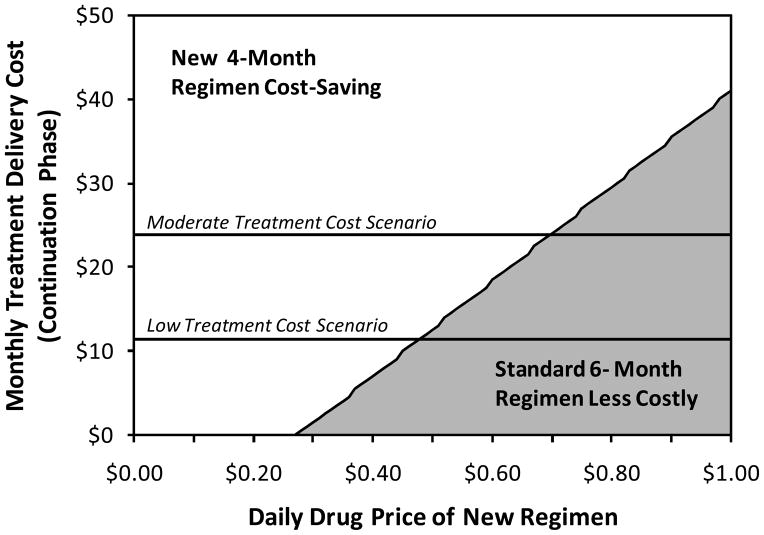
Sensitivity analyses revealed that there were three primary drivers of cost-effectiveness: drug cost, cost of treatment delivery in the continuation phase, and the ability of novel treatment regimens to avert TB deaths suffered during episodes of recurrent TB (Figure 2). Given that novel regimens may be less cost-effective than other alternatives for TB control (e.g., expansion of existing services), we reasoned that novel regimens may not be adopted in some areas unless they are cost-saving. Thus, we determined the cost-saving threshold as a function of regimen price and monthly cost of TB treatment delivery in the continuation phase (Figure 3). Under our pre-defined low-cost, moderate-cost, and high-cost scenarios, a 4-month regimen would become cost-saving at drug prices of less than $0.48/day, $0.69/day, and $2.38, respectively, and a 2-month regimen would be cost-saving at respective prices less than $1.37/day, $2.29/day, and $9.17/day.
Figure 3. Cost-Minimizing TB Therapeutic Regimen, by Treatment Delivery Cost and Novel Drug Price.
In scenarios above the diagonal line (white background), a novel 4-month TB treatment regimen costing $1 per day would be cost-saving relative to standard 6-month therapy. In scenarios below the diagonal line (grey shading), the standard regimen remains the least costly option, although the novel regimen is more effective.
Discussion
This decision model suggests that novel therapeutic regimens for treatment of drug-sensitive TB in the public sector are likely to be cost-effective or cost-saving in the majority of economic and epidemiological conditions. For example, we estimated that a 4-month regimen with a drug cost of $1/day and equivalent efficacy to current standard treatment will be cost-saving in any setting where the cost of treatment delivery in the continuation phase is more than $41 per month. In settings where novel first-line TB regimens are not cost-saving (i.e., treatment delivery costs are lower), cost-effectiveness is primarily driven by the balance between drug price, continuation-phase delivery costs, and the ability of novel regimens to avert mortality from recurrent TB through improved treatment completion.
There is no universally-accepted threshold for “cost-effective” interventions; however, a widely used metric is that of cost per DALY averted less than per capita gross domestic product (GDP) being “highly cost-effective,” and less than three times per capita GDP being “cost-effective.”(26) By this calculation, a 4-month, $1/day first-line regimen (projected to cost less than $750 per DALY averted in all scenarios) would be cost-effective in all high-burden countries,(27) and highly cost-effective in most. Cost-effectiveness is not the only relevant economic consideration, however; this regimen was projected to increase total first-line treatment costs by 47% in the low-income scenario (i.e., might be deemed unaffordable) while only averting 6% of DALYs (i.e., might be less cost-effective than other TB control options).
This preliminary analysis provides early insight into the likely cost-effectiveness of duration-shortening TB treatment regimens currently in development. For example, moxifloxacin has equivalent or superior efficacy to ethambutol(13) and isoniazid(28) in achieving 8-week culture conversion, and four-month moxifloxacin-based regimens are currently being evaluated in phase III trials.(29) Should these trials demonstrate equivalent efficacy, our analysis suggests that local estimates of treatment delivery costs, from both the healthcare and patient perspectives, will play an important role in determining whether this regimen will be cost-effective or cost-saving in different countries. This cost-saving potential is highest in areas with high delivery costs, such that novel regimens may paradoxically be least cost-effective in low-resource settings where delivery costs (which often reflect local wages) are also low. Given that shorter regimens will reduce patient costs and improve outcomes, the ethical implications of this situation – where the economic incentives to benefit patients are weakest in low-income areas – deserve further exploration. Further studies are also needed to quantify costs from the patient/family perspective (not included in this analysis), including local data collection across a variety of country-specific settings.
As with any model-based analysis, this preliminary study has certain limitations. In an attempt to provide early insight on a global level, we used a simplified model based on WHO country-wide estimates of treatment costs and arbitrary thresholds ($1 and $5) of drug price. To fully evaluate the cost-effectiveness of specific regimens, more definitive studies are needed that comprehensively measure continuation-phase costs across multiple settings, assess the potential of novel regimens to reduce TB transmission, and consider the implications of using novel drugs in other situations (e.g., non-confirmed cases, private sector, high background rates of MDR-TB). Such studies could evaluate the validity of many simplifying assumptions made here, including breakdown of costs in the intensive versus continuation phases, probability of treatment success on novel regimens, and early estimates of recurrence rates.
In summary, as we prepare to usher in new first-line TB therapeutic regimens for the first time in decades, economic considerations are of great importance. The ability of these regimens to save healthcare dollars depends strongly on treatment delivery costs in the continuation phase, making them most economically attractive in higher-income settings. Ultimately, however, novel treatment regimens for drug-susceptible TB are likely to be cost-effective – and often cost-saving – to the healthcare system in the majority of high-burden settings, even those with few available resources.
Acknowledgments
DWD and JPO designed the analysis. JPO constructed the initial model, and DWD revised the analytic model into its final form. MOF checked the model for accuracy and intellectual content. JPO wrote the first draft of the manuscript. MOF and DWD revised the manuscript for important intellectual content. All authors saw and approved the final manuscript version.
This work was funded in part by the U.S. National Institutes of Health (R21 AI101152, to DWD; and 3T32GM007309-38S1, to MOF).
Dr. Dowdy receives research funding from the Global Alliance for TB Drug Development; this relationship was established after, and independently of, the decision to publish this manuscript.
Footnotes
None of the other authors have any conflicts of interest to declare.
References
- 1.Hong Kong Chest Service/British Medical Research Council. Controlled trial of 4 three-times-weekly regimens and a daily regimen all given for 6 months for pulmonary tuberculosis second report: The results up to 24 months. Tubercle. 1982;63:89–98. doi: 10.1016/s0041-3879(82)80044-5. [DOI] [PubMed] [Google Scholar]
- 2.Hong Kong Chest Service/British Medical Research Council. Controlled trial of 2, 4, and 6 months of pyrazinamide in 6-month, three-times-weekly regimens for smear-positive pulmonary tuberculosis, including an assessment of a combined preparation of isoniazid, rifampin, and pyrazinamide. Results at 30 months. Am Rev Respir Dis. 1991;143(4 Pt 1):700–706. doi: 10.1164/ajrccm/143.4_Pt_1.700. [DOI] [PubMed] [Google Scholar]
- 3.Singapore Tuberculosis Service/British Medical Research Council. Long-term follow-up of a clinical trial of six-month and four-month regimens of chemotherapy in the treatment of pulmonary tuberculosis. Am Rev Respir Dis. 1986;133:779–783. [PubMed] [Google Scholar]
- 4.Iseman MD. Tuberculosis therapy: Past, present and future. Eur Respir J. 2002;36 (Suppl):87S–94S. doi: 10.1183/09031936.02.00309102. [DOI] [PubMed] [Google Scholar]
- 5.Snider DE, Jr, Cohn DL, Davidson PT, Hershfield ES, Smith MH, Sutton FD., Jr Standard therapy for tuberculosis: 1985. Chest. 1985;87(2 Suppl):117S–124S. doi: 10.1378/chest.87.2.117s. [DOI] [PubMed] [Google Scholar]
- 6.Steffen R, Menzies D, Oxlade O, et al. Patients’ costs and cost-effectiveness of tuberculosis treatment in DOTS and non-DOTS facilities in Rio de Janeiro, Brazil. PLoS ONE. 2010;5:e14014. doi: 10.1371/journal.pone.0014014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menzies D, Lewis M, Oxlade O. Costs for tuberculosis care in Canada. Can J Public Health. 2008;99:391–396. doi: 10.1007/BF03405248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spence DP, Hotchkiss J, Williams CS, Davies PD. Tuberculosis and poverty. BMJ. 1993;307:759–761. doi: 10.1136/bmj.307.6907.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kruk ME, Schwalbe NR, Aguiar CA. Timing of default from tuberculosis treatment: a systematic review. Trop Med Int Health. 2008;13:703–712. doi: 10.1111/j.1365-3156.2008.02042.x. [DOI] [PubMed] [Google Scholar]
- 10.Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med. 2007;4:e238. doi: 10.1371/journal.pmed.0040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginsberg AM. Emerging drugs for active tuberculosis. Semin Respir Crit Care Med. 2008;29:552–559. doi: 10.1055/s-0028-1085706. [DOI] [PubMed] [Google Scholar]
- 12.Spigelman MK. New tuberculosis therapeutics: A growing pipeline. J Infect Dis. 2007;196 (Suppl 1):S28–S34. doi: 10.1086/518663. [DOI] [PubMed] [Google Scholar]
- 13.Conde MB, Efron A, Loredo C, et al. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet. 2009;373:1183–1189. doi: 10.1016/S0140-6736(09)60333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. [Accessed 21 September 2012];Global price reporting mechanism. Available from: http://www.who.int/hiv/amds/gprm/en/
- 15.World Health Organization. Global tuberculosis control: WHO report 2011. Geneva: WHO Press; 2011. [Google Scholar]
- 16.Jonnalagada S, Harries AD, Zachariah R, et al. The timing of death in patients with tuberculosis who die during anti-tuberculosis treatment in Andhra Pradesh, South India. BMC Public Health. 2011;11:921. doi: 10.1186/1471-2458-11-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harries AD, Hargreaves NJ, Gausi F, Kwanjana JH, Salaniponi FM. High early death rate in tuberculosis patients in Malawi. Int J Tuberc Lung Dis. 2001;5:1000–1005. [PubMed] [Google Scholar]
- 18.Tekle B, Mariam DH, Ali A. Defaulting from DOTS and its determinants in three districts of Arsi zone in Ethiopia. Int J Tuberc Lung Dis. 2002;6:573–579. [PubMed] [Google Scholar]
- 19.Menzies D, Benedetti A, Paydar A, et al. Effect of duration and intermittency of rifampin on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS Med. 2009;6:e1000146. doi: 10.1371/journal.pmed.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJ. Natural history of tuberculosis: Duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS ONE. 2011;6:e17601. doi: 10.1371/journal.pone.0017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. A controlled trial of a 2-month, 3-month, and 12-month regimens of chemotherapy for sputum smear-negative pulmonary tuberculosis: rhe results at 30 months. Am Rev Respir Dis. 1981;124:138–142. doi: 10.1164/arrd.1981.124.2.138. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. [Accessed 21 September 2012];Tuberculosis country profiles. Available from: http://www.who.int/tb/country/data/profiles/en/index.html.
- 23.Stop TB Partnership. [Accessed 21 September 2012];Global drug facility: List of drugs available. Available from: http://www.stoptb.org/gdf/drugsupply/pc2.asp?CLevel=3&CParent=10.
- 24.World Bank. [Accessed 21 September 2012];Life expectancy. Available from: http://www.google.com/publicdata/explore?ds=d5bncppjof8f9_&ctype=l&strail=false&nselm=h&hl=en&dl=en#!ctype=l&strail=false&bcs=d&nselm=h&met_y=sp_dyn_le00_in&scale_y=lin&ind_y=false&rdim=region&ifdim=region&tdim=true&hl=en_US&dl=en&ind=false.
- 25.Murray CJL, Lopez AD. The global burden of disease: A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Boston: Harvard School of Public Health (on behalf of the World Health Organization and the World Bank); 1996. [Google Scholar]
- 26.Commission on Macroeconomics and Health. Macroeconomics and health: Investing in health for economic development. Geneva: World Health Organization; 2001. [Google Scholar]
- 27.World Bank. [Accessed 21 September 2012];World development indicators. Available from: http://www.google.com/publicdata/overview?ds=d5bncppjof8f9_&ctype=l&strail=false&nselm=h&hl=en&dl=en.
- 28.Dorman SE, Johnson JL, Goldberg S, et al. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am J Respir Crit Care Med. 2009;180:273–280. doi: 10.1164/rccm.200901-0078OC. [DOI] [PubMed] [Google Scholar]
- 29.Grosset JH, Singer TG, Bishai WR. New drugs for the treatment of tuberculosis: Hope and reality. Int J Tuberc Lung Dis. 2012;16:1005–1014. doi: 10.5588/ijtld.12.0277. [DOI] [PubMed] [Google Scholar]