Abstract
BACKGROUND
Blood and plasma cannabinoid stability is important for test interpretation and is best studied in authentic rather than fortified samples.
METHODS
Low and high blood and plasma pools were created for each of 10 participants after they smoked a cannabis cigarette. The stabilities of Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC), 11-nor-9-carboxy-THC (THCCOOH), cannabidiol (CBD), cannabinol (CBN), THC-glucuronide, and THCCOOH-glucuronide were determined after 1 week at room temperature; 1, 2, 4, 12, and 26 (±2) weeks at 4 °C; and 1, 2, 4, 12, 26 (±2), and 52 (±4) weeks at −20 °C. Stability was assessed by Friedman test.
RESULTS
Numbers of THC-glucuronide and CBD-positive blood samples were insufficient to assess stability. In blood, 11-OH-THC and CBN were stable for 1 week at room temperature, whereas THC and THCCOOH-glucuronide decreased and THCCOOH increased. In blood, THC, THCCOOH-glucuronide, THCCOOH, 11-OH-THC, and CBN were stable for 12, 4, 4, 12, and 26 weeks, respectively, at 4 °C and 12, 12, 26, 26, and 52 weeks at −20 °C. In plasma, THC-glucuronide, THC, CBN, and CBD were stable for 1 week at room temperature, whereas THCCOOH-glucuronide and 11-OH-THC decreased and THCCOOH increased. In plasma, THC-glucuronide, THC, THCCOOH-glucuronide, THCCOOH, 11-OH-THC, CBN, and CBD were stable for 26, 26, 2, 2, 26, 12, and 26 weeks, respectively, at 4 °C and 52, 52, 26, 26, 52, 52, and 52 weeks, respectively, at −20 °C.
CONCLUSIONS
Blood and plasma samples should be stored at −20 °C for no more than 3 and 6 months, respectively, to assure accurate cannabinoid quantitative results.
Drug storage stability is an important consideration for interpreting drug concentrations (1). To investigate pharmacokinetic parameters, detection windows (2), and last cannabis intake prediction (3-5), it is essential to establish the stabilities of cannabinoids. Stability in blood is critical for interpretation, especially if repeat analysis is requested months after initial testing. To our knowledge, cannabinoid stability in authentic blood and/or plasma samples after controlled smoked cannabis has not been systematically investigated. Cannabinoid stability in fortified drug-free blood and plasma samples has been reported (1, 6-9), sometimes with variable results (7, 8, 10). Δ9-Tetrahydrocannabinol (THC)3 is stable in blood stored at 5 °C, −5 °C, and −20 °C for 17 weeks (10). Temperature conditions and repetitive freeze–thaw cycles do not influence drug recovery. Johnson et al. (7) found no changes for 1 month at room temperature, 4 °C, or −10 °C for THC, 11-hydroxy-THC (11-OH-THC), and 11-nor-9-carboxy-THC (THCCOOH) in blood and plasma. However, THC and 11-OH-THC concentrations decreased at room temperature after 2 months. Cannabinoid concentrations in blood stored at 4 °C were unchanged for 4 months, but after 6 months, results were inaccurate due to inefficient extractions.
Skopp and Potsch (1) reported in vitro instability of THCCOOH-glucuronide in fortified serum samples stored at higher than −20 °C due to free THCCOOH formation. Fortified and authentic blood and plasma sample stability after cannabis smoking could differ significantly (1, 6-9). Pilot studies in our laboratory indicated that plasma protein binding in authentic samples could be critical for in vitro cannabinoid stability. We previously reported concentration decreases of ≤70% in fortified blood stored for 2 weeks at −20 °C (6). Here we report on the stability of THC, 11-OH-THC, THCCOOH, cannabidiol (CBD), cannabinol (CBN), THC-glucuronide, and THCCOOH-glucuronide in 10 different blood and plasma pools collected from 10 participants after controlled cannabis smoking. These authentic specimen cannabinoid stability data will aid scientists in interpreting blood and plasma cannabinoid results.
Materials and Methods
PARTICIPANTS
Detailed demographics for the 10 study participants (9 men and 1 woman) and inclusion and exclusion criteria were previously reported (11). The study was approved by the National Institute on Drug Abuse Institutional Review Board, and participants provided voluntary written informed consent.
SAMPLE COLLECTION AND ANALYSIS
After staying overnight on a secure residential unit, participants smoked one 6.8% THC cigarette ad libitum over 10 min. We collected blood and plasma for stability evaluation in sodium heparin Vacutainers at 0.25, 0.5, 1, 2, 3, and 4 h. Plasma was obtained after 4 °C centrifugation at 2000g for 10 min. Low and high pools were created for each participant (n = 10). High THC pools included samples collected at 0.25, 0.5, and 1 h, and low THC pools, at 2, 3, and 4 h. Pools were mixed and aliquots were placed into Nunc cryotubes (Thermo Scientific) for storage with duplicates at each condition and time, except triplicate aliquots analyzed within 24 h. Baseline 24-h samples were stored in the dark at room temperature, 4 °C, and −20 °C. We analyzed duplicate samples from each pool after 1 week at room temperature; 1, 2, 4, 12, and 26 (±2) weeks at 4 °C; and 1, 2, 4, 12, 26 (±2), and 52 (±4) weeks at −20 °C.
We quantified blood and plasma THC, 11-OH-THC, THCCOOH, CBD, CBN, THC-glucuronide, and THCCOOH-glucuronide concentrations using a previously published liquid chromatography/tandem mass spectometry blood method that was additionally cross-validated for plasma (12). Blood and plasma limits of quantification (LOQs) were 1 μg/L for THC, 11-OH-THC, THCCOOH, CBD, and CBN; 0.5 μg/L for THC-glucuronide; and 5 μg/L for THCCOOH-glucuronide.
STATISTICAL ANALYSES
Because visual data inspection and Kolmogorov–Smirnov tests indicated nonnormal distribution, we conducted statistical comparisons via nonparametric tests in Prism 5.02 (GraphPad Software). With repeated-measures Friedman tests, we compared concentrations and molar sums of free THCCOOH + THCCOOH-glucuronide at different storage times and temperatures to those at baseline. We used Dunn multiple comparisons tests for post hoc comparisons. Blood and plasma concentrations and molar sums of free THCCOOH + THCCOOH-glucuronide during room temperature stability studies were evaluated with Wilcoxon matched pairs test. Results with 2-tailed P<0.05 were considered significant.
Results
BLOOD CANNABINOID STABILITY
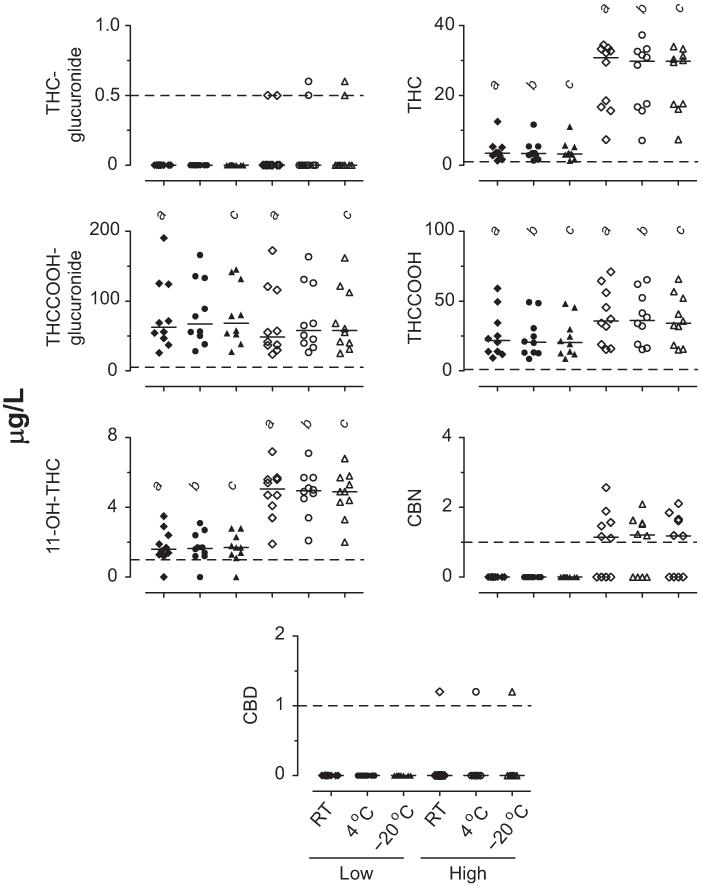
Baseline blood cannabinoid concentrations are shown in Fig. 1. Baseline THC, THCCOOH, and 11-OH-THC concentrations were higher in the high pools than in the low pools (χ2= 40.1, 45.2, and 41.4, respectively; df = 5). Post hoc tests indicated differences between the low and high concentrations for each storage condition. Overall differences in baseline THCCOOH-glucuronide low and high blood pools also occurred (χ2= 33.4, df = 5). Post hoc comparisons revealed that the THCCOOH-glucuronide low and high blood pool baseline concentrations were similar at 4 °C, but high pool baseline concentrations were lower than those of the low pools after 24 h at room temperature and −20 °C. Differences were not observed in THC-glucuronide, CBN, and CBD baselines because most low and high baseline concentrations were <LOQ (χ2= 9.7, 26.4, and 5.0, respectively; df = 5).
Fig. 1. Baseline concentrations in low and high THC blood pools prepared from samples collected after controlled cannabis smoking (n = 10 participant pools).
Horizontal short lines are medians, and dashed lines are LOQs. a,b,cSignificant differences between low and high pools at room temperature (RT), 4 °C, and −20 °C, respectively.
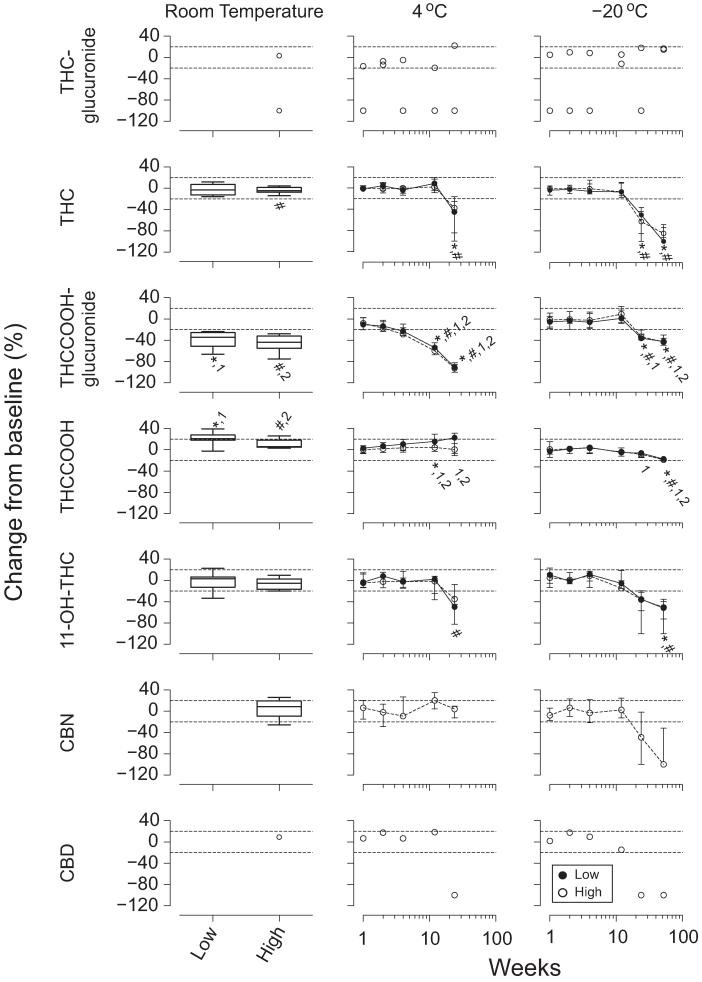
Blood cannabinoid stability results are shown in Fig. 2. THC-glucuronide blood concentrations exceeded the LOQ in only 2 participants, thus confounding statistical interpretation. Low THC blood concentrations were unchanged after 1 week at room temperature (T = 19.0), whereas high concentrations decreased (T = 39.0). THC blood concentrations decreased in the low and high pools beyond 12 weeks at 4 °C and −20 °C (χ2= 12.7–40.2, df = 5–6).
Fig. 2. Median (interquartile range) (n = 10) cannabinoid stability after 1 week of storage at room temperature and up to 26 and 52 weeks at 4 °C and −20 °C, respectively, in low and high THC blood pools.
No THC-glucuronide, CBN, or CBD low pools exceeded the LOQ; 2, 9, 6, and 1 high pools exceeded the LOQ for THC-glucuronide, 11-OH-THC, CBN, and CBD, respectively. Significant differences from baseline for *low pool; #high pool; 1low pool moles of THCCOOH + THCCOOH-glucuronide; or 2high pool moles of THCCOOH + THCCOOH-glucuronide.
THCCOOH-glucuronide concentrations decreased in the low and high blood pools during 1 week at room temperature(T=55and55),>4weeksat4 °C(χ2=38.9 and 41.2, df = 5), and >12 weeks at −20 °C (χ2= 40.2 and 39.3, df = 6).
THCCOOH increased in the low and high blood pools during 1 week at room temperature (T =−53 and −43) and in the low pools after >4 weeks of storage at 4 °C (χ2= 13.9, df = 5). High THCCOOH concentrations did not change significantly (χ2= 2.4, df = 5, P > 0.05). THCCOOH low and high blood concentrations decreased at −20 °C after >26 weeks of storage (χ2= 32.0 and 33.4, df = 6).
11-OH-THC blood concentrations did not change during 1 week at room temperature (T =−11.0 and 35). 11-OH-THC in the low pools decreased overall at 4 °C (χ2= 12.1, df = 5), although none of the pools’ concentrations differed from baseline at individual storage times. 11-OH-THC decreased beyond 12 weeks in the high pools at 4 °C (χ2= 20.4, df = 5) and beyond 26 weeks in both pools at −20 °C (χ2= 27.6 and 34.2, df = 6). Large intersubject variability in 11-OH-THC stability was observed in the low pools under all conditions, whereas samples from all participants followed similar trends in the high pools.
CBN concentrations did not change in the high pools during 1 week at room temperature (T =−3.0) or 26 weeks at 4 °C (χ2= 8.4, df = 5). CBN blood concentrations decreased overall across 52 weeks at −20 °C (χ2= 19.9, df = 6), although concentrations did not differ from baseline at any specific storage time.
Baseline CBD blood concentrations were >LOQ (1.2 μg/L) in only 1 participant; concentrations remained within 20% of baseline for 1 week at room temperature and for 12 weeks at 4 °C and −20 °C.
The molar sums of THCCOOH+THCCOOH-glucuronide in both blood pools decreased after 1 week at room temperature (T = 55 and 55), 26 weeks at 4 °C (χ2= 38.6 and 39.4, df = 5), and 52 weeks at −20 °C (χ2= 39.1 and 38.3, df = 6).
PLASMA CANNABINOID STABILITY
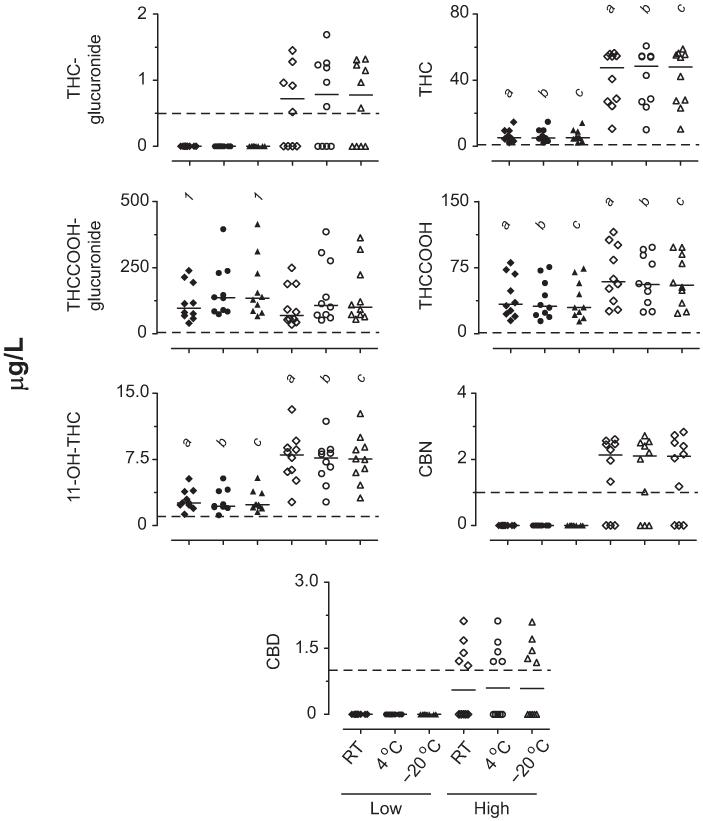
Baseline plasma cannabinoid concentrations are shown in Fig. 3. There were overall differences for all analytes’ baseline low and high plasma pools (χ2= 22.3–47.3, df = 5). Post hoc comparisons confirmed differences between the low and high pools for THC, THCCOOH, and 11-OH-THC under each storage condition. Post hoc comparisons showed no differences between low and high THC-glucuronide, THCCOOH-glucuronide, CBN, and CBD for any storage condition. THCCOOH-glucuronide baseline concentrations were lower after 24 h at room temperature than at −20 °C.
Fig. 3. Baseline concentrations in low and high THC plasma pools prepared from samples collected after controlled cannabis smoking (n = 10 participant pools).
Horizontal short lines are medians, and dashed lines are LOQs. a,b,cSignificant differences between low and high pools at room temperature, 4 °C, and −20 °C, respectively. 1Significant differences between room temperature (RT) and −20 °C pools.
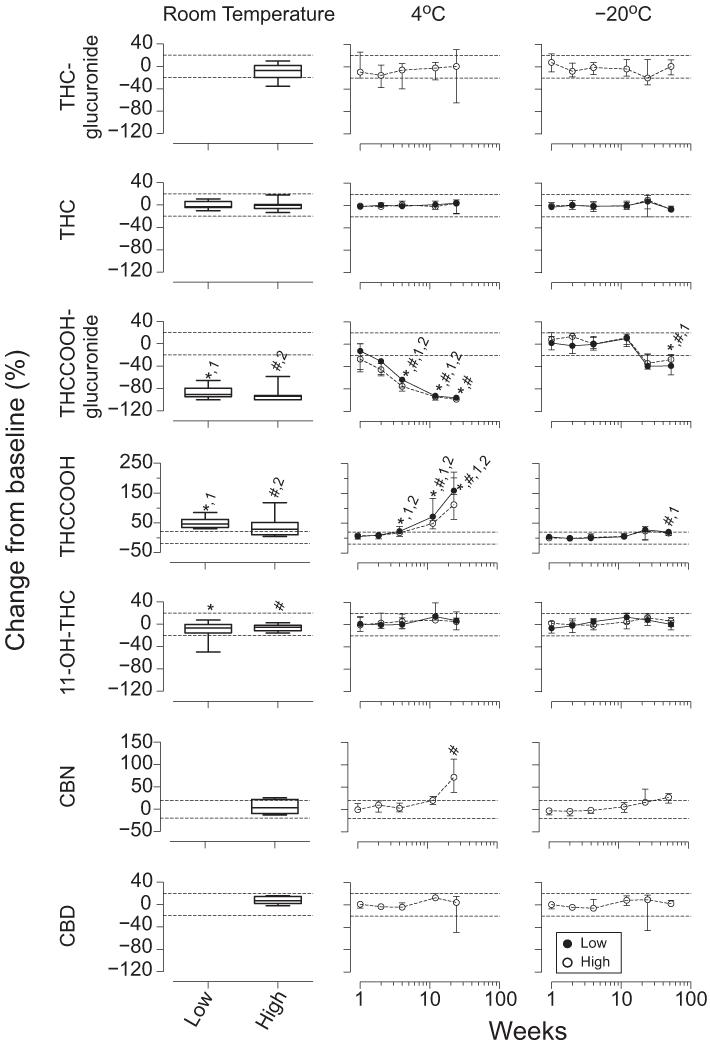
In vitro stability of cannabinoids and cannabinoid glucuronides at room temperature, 4 °C, and −20 °C are shown in Fig. 4. Plasma THC-glucuronide high concentrations were stable for 1 week at room temperature (T = 15), 26 weeks at 4 °C (χ2= 5.0, df = 5), and 52 weeks at −20 °C (χ2= 6.9, df = 6). One participant’s plasma THC-glucuronide concentration decreased 36% after 1 week at room temperature, whereas the 5 other participants’ remained within 20% of baseline. This participant’s THC-glucuronide concentrations decreased >20% at 4 °C, but were stable for 26 weeks at −20 °C.
Fig. 4. Median (interquartile range) (n = 10) cannabinoid stability after 1 week at room temperature and up to 26 and 52 weeks at 4 °C and −20 °C, respectively, in low and high THC plasma pools.
No THC-glucuronide, CBN, or CBD low pools exceeded the LOQ; 6, 7, and 6 high pools exceeded the LOQ for THC-glucuronide, CBN, and CBD, respectively. Significant differences from baseline for *low pool; #high pool; 1low pool moles of THCCOOH + THCCOOH-glucuronide; or 2high pool moles of THCCOOH + THCCOOH-glucuronide.
THC was stable in plasma pools for 1 week at room temperature (T = 9.0 and 11.0), 26 weeks at 4 °C (χ2= 2.1 and 4.4, df = 5), and 52 weeks at −20 °C (χ2= 7.1 and 11.6, df = 6).
THCCOOH-glucuronide decreased in both high and low plasma pools during 1 week at room temperature (T = 55 and 55), >2 weeks at 4 °C (χ2= 47.4 and 47.3, df = 5), and >26 weeks at −20 °C (χ2= 25.7 and 24.1, df = 6). Little intersubject variability occurred for the THCCOOH-glucuronide plasma pools at room temperature, 4 °C, and −20 °C, except for both high and low pools in 1 participant that decreased >20% by 1 week at −20 °C and continued to decrease with longer storage. All other participants’ pools remained within 20% of baseline for 12 weeks at −20 °C.
THCCOOH increased in both high and low plasma pools during 1 week at room temperature (T =−55 and −55), 26 weeks at 4 °C (χ2= 47.3 and 39.9, df = 5), and 52 weeks at −20 °C (χ2= 20.3 and 24.1, df = 6). THCCOOH increased beyond 2 and 4 weeks in the low and high plasma pools, respectively, at 4 °C. Little intersubject variability occurred for THCCOOH low plasma concentrations at room temperature, whereas 7 participants’ high pools increased >26%. Large intersubject variability occurred for THCCOOH low and high pools at 4 °C, whereas little variability was noted at −20 °C until 26 and 52 weeks in low pools.
11-OH-THC decreased in the low and high plasma pools during 1 week at room temperature (T = 43 and 45), with no other changes at 4 °C for 26 weeks (χ2= 8.8 and 7.9, df = 5), and in the high pool for 52 weeks at −20 °C (χ2= 11.0, df = 6). 11-OH-THC decreased in plasma low pools during 52 weeks at −20 °C (χ2= 15.9, df = 6), although no differences were detected by post hoc comparisons. More intersubject variability was observed in the 11-OH-THC plasma low pools than in the high pools at room temperature and 4 °C, with little intersubject variability at −20 °C.
No CBN changes occurred in plasma high pools during 1 week at room temperature (T =−4.0). CBN increases were observed in these pools beyond 12 weeks at 4 °C (χ2= 20.6, df = 5) and at 52 weeks at −20 °C (χ2= 23.0, df = 6), although all post hoc comparisons were nonsignificant at −20 °C.
Increases in CBD occurred in plasma high pools during 1 week at room temperature (T =−13.0) and overall at 26 weeks at 4 °C (χ2= 12.7, df = 5), although all post hoc comparisons were nonsignificant. No CBD changes occurred in plasma high pools for 52 weeks at −20 °C (χ2= 6.2, df = 6).
The molar sums of THCCOOH + THCCOOH-glucuronide significantly decreased in both plasma pools after 1 week at room temperature (T = 55 and 55), 26 weeks at 4 °C (χ2= 41.7 and 35.3, df = 5), and 52 weeks at −20 °C (χ2= 36.7 and 23.2, df = 6).
Discussion
THC adsorption to polystyrene tubes and minimal adsorption to glass tubes has been reported (8). Because of safety concerns, we conducted studies with polypropylene tubes, in which most laboratories store blood samples. Synthetic cannabinoids showed similar stability in glass and polypropylene tubes (13), suggesting that THC adsorption may not be as large for polypropylene as for polystyrene tubes. Previous fortified cannabinoid studies were conducted in polypropylene (6), glass (1), and silanized glass tubes (7, 9). Low analyte concentrations likely demonstrate more instability than higher concentrations, making analyte concentration another confounding factor for comparing stability studies. Acidifying samples limits acyl glucuronide hydrolysis (14) and may limit THC-glucuronide and THCCOOH-glucuronide hydrolysis; however, CBD is converted to THC under acidic conditions (15). Therefore we did not investigate stability under reduced pH conditions. We also did not study stability under alkaline pH conditions, since alkaline pH hydrolyzes THCCOOH-glucuronide to THCCOOH (16, 17 ).
These are the first plasma THC-glucuronide stability data. THC-glucuronide was stable in plasma throughout the study at room temperature, 4 °C, and −20 °C. These results support THC-glucuronide as an indicator of recent cannabis exposure (11, 12, 18). The presence of THC-glucuronide in blood and plasma is inclusionary, not exclusionary, for identifying recent cannabis intake. We previously reported low THC-glucuronide concentrations and short detection windows after cannabis smoking (11). Because of the limited number of positive blood THC-glucuronide samples, stability could not be assessed.
THC blood concentrations were stable for 1 week at room temperature, as in previous reports of fortified samples (6, 7), and for 12 weeks at 4 °C and −20 °C. Our results are consistent with those of Johnson et al. with fortified blood (7) showing THC stability for 16 weeks at 4 °C, but contrast with THC stability for 26 weeks at −10 °C at concentrations similar to those in our studies, although in silanized glass tubes (7). The study of Johnson et al. (7) with silanized glass tubes presents safety risks but may limit THC adsorption, thus extending frozen stability. Our current authentic THC sample results contrast with our previous report of stable THC concentrations in fortified blood (6). In that study, THC was stable at 4 °C but decreased 70% after 2 weeks at −20 °C for fortified blood at similar concentrations in polypropylene tubes (6). These data demonstrate the importance of conducting stability studies with authentic rather than fortified samples. It is possible that THC more readily binds proteins and other materials in vivo in authentic samples. Our current data in authentic blood samples strongly support sample analysis within 3 months, whether refrigerated or frozen, to ensure accurate concentrations. Decreasing THC blood concentrations after 3 months at 4 °C and −20 °C present challenges for reanalysis. THC concentrations most likely will be lower and should not be viewed as inconsistent with earlier reports.
Plasma THC concentrations were stable throughout the study at room temperature, 4 °C, and −20 °C. Johnson et al. (7) also observed stable THC plasma concentrations in fortified samples for 4 weeks at room temperature and for 6 months at 4 °C and −10 °C. Grauwiler et al. (9) reported 30% and 43% THC decreases in 100 μg/L fortified plasma stored in silanized glass vials at −70 °C for 3 and 6 weeks. It is uncertain why these results differ from fortified plasma results in Johnson et al. (7) and our authentic plasma samples at −10 °C and −20 °C, respectively. THC concentrations were similar and silanized glass vials should have limited adsorption. One possibility is that the lower −70 °C temperature may have enhanced protein precipitation during the initial freezing process, providing more solid material for THC adsorption. Most pharmacokinetic studies characterizing THC pharmacokinetics and duration of detection were conducted with plasma samples stored at −20 °C before analysis (19-27); our observed THC plasma stability at −20 °C for 52 weeks supports the validity of previously published pharmacokinetic results.
This is the first report of THCCOOH-glucuronide instability in blood at 1 week at room temperature, and beyond 4 weeks at 4 °C and 12 weeks at −20 °C. Our results are consistent with known acyl glucuronide instability (14), supporting sample storage at −20 °C for no more than 12 weeks for accurate THCCOOH-glucuronide measurement.
Skopp and Potsch (1) previously reported decreasing THCCOOH-glucuronide concentrations in fortified plasma after 8 days at 20 °C, but stability for 8 days at 4 °C. Similarly, we observed 80%–100% plasma THCCOOH-glucuronide decreases after 1 week at room temperature and no changes from baseline after 1 week at 4 °C.
THCCOOH blood stability was previously evaluated with only fortified samples (6, 7); the current study is the first to offer complete THCCOOH stability characterization when both THCCOOH-glucuronide and THCCOOH are quantified in authentic samples. Comparisons of our data to previous studies with only fortified THCCOOH is difficult, since THCCOOH-glucuronide hydrolysis to THCCOOH could not occur. Therefore, it is not surprising that at room temperature, Johnson et al. (7) and Schwilke et al. (6) observed THCCOOH concentrations within 20% of baseline for 8 and 7 days, respectively, contrasting with our 10%–20% increases after 1 week. We observed THCCOOH-glucuronide loss and/or hydrolysis (50%–100% of baseline) beyond 4 weeks at 4 °C, but found smaller increases in THCCOOH (<40%); the only significant changes in THCCOOH at 4 °C occurred when concentrations in the low pool increased after 12 weeks. THCCOOH increases were not significant in the high pool. Lower THCCOOH baseline concentrations in the low pool compared to the high pool may account for observed between-pool differences. Total moles of free THCCOOH did not match total moles of THCCOOH-glucuronide lost in blood at room temperature, 4 °C, or −20 °C, indicating that THCCOOH-glucuronide hydrolysis alone did not account for the observed changes. Skopp and Potsch (1) also observed that total moles of lost THCCOOH-glucuronide did not account for moles of THCCOOH gained at 20 °C and 40 °C. Adsorption of THCCOOH-glucuronide and THCCOOH to storage tubes, blood cells, or precipitant material in addition to THCCOOH-glucuronide hydrolysis may explain the results.
We observed increases in plasma THCCOOH concentrations after 1 week at room temperature, 4 weeks at 4 °C, and 12 weeks at −20 °C. Skopp and Potsch (1) fortified THCCOOH and THCCOOH-glucuronide in plasma and found <10% increases in THCCOOH concentrations after 8 days at 4 °C and 20 °C, similar to our observations after 1 week at these temperatures. Similar to observations for blood, the total moles of THCCOOH-glucuronide and THCCOOH decreased over time at room temperature,4 °C, and-20 °C, indicating adsorption of THCCOOH-glucuronide and/or THCCOOH or further degradation to another cannabinoid. Interestingly, blood and plasma THCCOOH-glucuronide profiles were similar at 4 °C and −20 °C, whereas THCCOOH concentrations were more stable in blood than plasma. It is possible that THCCOOH-glucuronide and/or THCCOOH binds to blood cells or other precipitants that are exclusive to blood and may explain the matrix differences.
THCCOOH-glucuronide/THCCOOH ratios have been proposed for identifying recent cannabis intake (2, 11) because THCCOOH concentrations increase more rapidly than THCCOOH-glucuronide 1–2 h after smoking. This approach could be useful for identifying new use or recent intake. Our results indicate that blood and plasma should be stored at −20 °C for no more than 12 weeks if THCCOOH-glucuronide/THCCOOH ratios are to identify new use or assess recent intake.
11-OH-THC was stable in blood for 1, 12, and 26 weeks at room temperature, 4 °C, and −20 °C, respectively; decreases were observed beyond 12 and 26 weeks at 4 °C and −20 °C. 11-OH-THC concentrations did not vary by >20% after 8 days at room temperature in previous studies with fortified samples (6, 7), similar to our observations. Fortified results showed that 11-OH-THC concentrations did not vary by >20% after 2 weeks (6) or 16 weeks at 4 °C (7), consistent with the current study. Similar to our findings, 11-OH-THC concentrations remained within 20% of baseline in fortified samples stored at −10 °C for 26 weeks (7) and also after 2 weeks at −20 °C (6).
During the current study, 11-OH-THC plasma concentrations decreased after 1 week at room temperature, although most samples remained within 20% of baseline, similar to previously reported results (7). Our results are the first investigating 11-OH-THC stability in plasma at 4 °C, finding no significant changes after 26 weeks. Consistent with our observation of stable 11-OH-THC plasma concentrations at −20 °C for 52 weeks, previous reports indicated that 11-OH-THC did not vary by >20% after 26 weeks at −10 °C and 6 weeks at −70 °C (7, 9).
There have been no previous investigations of cannabinol or cannabidiol stability in blood. In our study, only 1 participant’s cannabidiol high blood pool exceeded 1 μg/L, preventing stability determination, but concentrations remained >1μg/L (LOQ) for 1 week at room temperature and 12 weeks at 4 °C and −20 °C.
CBN and CBD were stable for 6 weeks at −70 °C in fortified plasma (9). Our −20 °C plasma results also showed CBN and CBD stability at 6 weeks and beyond to 52 weeks. We observed increases in plasma CBN concentrations after 26 weeks at 4 °C that could be due to hydrolysis of CBN-glucuronide. However, further studies are necessary to confirm this hypothesis.
CBN and CBD are minor cannabinoids in cannabis plant material that we previously suggested as possible indicators of recent cannabis intake (11). Samples should be stored at −20 °C for <3 months before analysis to obtain accurate CBN and CBD concentrations to document recent cannabis intake.
Understanding analyte stability is critical for obtaining accurate cannabinoid concentrations during blood or plasma pharmacokinetic studies, and for interpreting clinical and forensic tests. This study is the first characterizing THC, 11-OH-THC, THCCOOH, CBN, CBD, THC-glucuronide, and THCCOOH-glucuronide stability in authentic blood and plasma collected after controlled cannabis administration. These data describe required storage conditions for obtaining accurate blood and plasma cannabinoid concentrations. Our recommended blood and plasma storage durations at different temperatures for accurate cannabinoid measurement are presented in Table 1. Generally, we observed more intersubject blood and plasma variability at room temperature and 4 °C than at −20 °C. Blood 11-OH-THC was the only analyte with high intersubject variability at −20 °C. Generally, cannabinoids were equally or more stable in plasma compared with blood. THCCOOH-glucuronide, THCCOOH, and CBN were less stable in plasma than blood at 4 °C. It appears that blood may provide a buffering effect for THCCOOH-glucuronide, THCCOOH, and CBN, possibly limiting adsorption to polypropylene tubes at 4 °C. 11-OH-THC concentrations were unstable in plasma but unaltered in blood after 1 week at room temperature. These results may not be clinically significant, since only 1 participant had a >20% decrease in plasma 11-OH-THC at room temperature.
Table 1.
Duration of acceptable storage stability for cannabinoids in authentic blood and plasma samples.
| Analyte and temperature |
Blood | Plasma |
|---|---|---|
| THC-glucuronide | ||
| Room temperature | NAa | 1 week |
| 4 °C | NA | 6 months |
| −20 °C | NA | 12 months |
| THC | ||
| Room temperature | 1 week | 1 week |
| 4 °C | 3 months | 6 months |
| −20 °C | 3 months | 12 months |
| THCCOOH-glucuronide | ||
| Room temperature | <1 week | <1 week |
| 4 °C | 1 month | 2 weeks |
| −20 °C | 3 months | 6 months |
| THCCOOH | ||
| Room temperature | <1 week | <1 week |
| 4 °C | 1 month | 2 weeks |
| −20 °C | 6 months | 6 months |
| 11-OH-THC | ||
| Room temperature | 1 week | <1 week |
| 4 °C | 3 months | 6 months |
| −20 °C | 6 months | 1 year |
| CBN | ||
| Room temperature | 1 week | 1 week |
| 4 °C | 6 months | 3 months |
| −20 °C | 3 months | 1 year |
| CBD | ||
| Room temperature | NA | 1 week |
| 4 °C | NA | 6 months |
| −20 °C | NA | 1 year |
NA, inconclusive data (only 1 or 2 participant pools exceeded assay limit of quantification during baseline analysis).
Based on these findings, we recommend storage of blood and plasma at −20 °C for no more than 3 months before analysis. THC blood concentrations were stable for only 3 months at 4 °C and −20 °C, creating accuracy problems for blood testing if laboratory backlogs or court-ordered retesting requires analysis beyond this time. THC plasma concentrations were stable for 1 year at −20 °C.
Acknowledgments
Role of Sponsor: The funding organizations played a direct role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Research Funding: Intramural Research Program of the National Institute on Drug Abuse, NIH.
Footnotes
Portions of this work were previously presented as an oral presentation at the 2012 meeting of the Society of Forensic Toxicologists, Boston, MA.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Expert Testimony: None declared.
Nonstandard abbreviations: THC, Δ9-tetrahydrocannabinol; 11-OH-THC, 11-hydroxy-THC; THCCOOH, 11-nor-9-carboxy-THC; CBD, cannabidiol; CBN, cannabinol; LOQ, limit of quantification.
References
- 1.Skopp G, Potsch L. Stability of 11-nor-delta(9)-carboxy-tetrahydrocannabinol glucuronide in plasma and urine assessed by liquid chromatography-tandem mass spectrometry. Clin Chem. 2002;48:301–6. [PubMed] [Google Scholar]
- 2.Kelly P, Jones RT. Metabolism of tetrahydrocannabinol in frequent and infrequent marijuana users. J Anal Toxicol. 1992;16:228–35. doi: 10.1093/jat/16.4.228. [DOI] [PubMed] [Google Scholar]
- 3.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. II. Models for the prediction of time of marijuana exposure from plasma concentrations of delta-9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (THCCOOH) J Anal Toxicol. 1992;16:283–90. doi: 10.1093/jat/16.5.283. [DOI] [PubMed] [Google Scholar]
- 4.Huestis MA, Barnes A, Smith ML. Estimating the time of last cannabis use from plasma delta9-tetrahydrocannabinol and 11-nor-9-carboxy-delta9-tetrahydrocannabinol concentrations. Clin Chem. 2005;51:2289–95. doi: 10.1373/clinchem.2005.056838. [DOI] [PubMed] [Google Scholar]
- 5.Huestis MA, Elsohly M, Nebro W, Barnes A, Gustafson RA, Smith ML. Estimating time of last oral ingestion of cannabis from plasma THC and THCCOOH concentrations. Ther Drug Monit. 2006;28:540–4. doi: 10.1097/00007691-200608000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Schwilke EW, Karschner EL, Lowe RH, Gordon AM, Cadet JL, Herning R, Huestis MA. Intra- and intersubject whole blood/plasma cannabinoid ratios determined by 2-dimensional, electron impact GC-MS with cryofocusing. Clin Chem. 2009;55:1188–95. doi: 10.1373/clinchem.2008.114405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson JR, Jennison TA, Peat MA, Foltz RL. Stability of delta-9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC in blood and plasma. J Anal Toxicol. 1984;8:202–4. doi: 10.1093/jat/8.5.202. [DOI] [PubMed] [Google Scholar]
- 8.Christophersen AS. Tetrahydrocannabinol stability in whole blood: plastic versus glass containers. J Anal Toxicol. 1986;10:129–31. doi: 10.1093/jat/10.4.129. [DOI] [PubMed] [Google Scholar]
- 9.Grauwiler SB, Scholer A, Drewe J. Development of a LC/MS/MS method for the analysis of cannabinoids in human EDTA-plasma and urine after small doses of Cannabis sativa extracts. J Chromatogr B. 2007;850:515–22. doi: 10.1016/j.jchromb.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 10.Wong AS, Orbanosky MW, Reeve VC, Beede JD. Research Monograph. National Institute on Drug Abuse; Rockville: 1982. Stability of delta-9-tetrahydrocannabinol in stored blood and serum: analysis of cannabinoids; pp. 119–24. [PubMed] [Google Scholar]
- 11.Schwope DM, Karschner EL, Gorelick DA, Huestis MA. Identification of recent cannabis use: whole-blood and plasma free and glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis administration. Clin Chem. 2011;57:1406–14. doi: 10.1373/clinchem.2011.171777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwope DM, Scheidweiler KB, Huestis MA. Direct quantification of cannabinoids and cannabinoid glucuronides in whole blood by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401:1273–83. doi: 10.1007/s00216-011-5197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dresen S, Kneisel S, Weinmann W, Zimmermann R, Auwarter V. Development and validation of a liquid chromatography-tandem mass spectrometry method for the quantitation of synthetic cannabinoids of the aminoalkylindole type and meth-anandamide in serum and its application to forensic samples. J Mass Spectrom. 2011;46:163–71. doi: 10.1002/jms.1877. [DOI] [PubMed] [Google Scholar]
- 14.Shipkova M, Armstrong VW, Oellerich M, Wie-land E. Acyl glucuronide drug metabolites: toxicological and analytical implications. Ther Drug Monit. 2003;25:1–16. doi: 10.1097/00007691-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Andrews R, Paterson S. Production of identical retention times and mass spectra for delta-9-tetrahydrocannabinol and cannabidiol following derivatization with trifluoracetic anhydride with 1,1,1,3,3,3-hexafluoroisopropanol. J Anal Toxicol. 2012;36:61–5. doi: 10.1093/jat/bkr017. [DOI] [PubMed] [Google Scholar]
- 16.Abraham TT, Lowe RH, Pirnay SO, Darwin WD, Huestis MA. Simultaneous GC-EI-MS determination of delta9-tetrahydrocannabinol, 11-hydroxy-delta9-tetrahydrocannabinol, and 11-nor-9-carboxy-delta9-tetrahydrocannabinol in human urine following tandem enzyme-alkaline hydrolysis. J Anal Toxicol. 2007;31:477–85. doi: 10.1093/jat/31.8.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kemp PM, Abukhalaf IK, Manno JE, Manno BR, Alford DD, McWilliams ME, et al. Cannabinoids in humans. II. The influence of three methods of hydrolysis on the concentration of THC and two metabolites in urine. J Anal Toxicol. 1995;19:292–8. doi: 10.1093/jat/19.5.292. [DOI] [PubMed] [Google Scholar]
- 18.Mareck U, Haenelt N, Geyer H, Guddat S, Kamber M, Brenneisen R, et al. Temporal indication of cannabis use by means of THC glucuronide determination. Drug Test Anal. 2009;1:505–10. doi: 10.1002/dta.106. [DOI] [PubMed] [Google Scholar]
- 19.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–82. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- 20.Cocchetto DM, Owens SM, Perez-Reyes M, Di Guiseppi S, Miller LL. Relationship between plasma delta-9-tetrahydrocannabinol concentration and pharmacologic effects in man. Psychopharmacology. 1981;75:158–64. doi: 10.1007/BF00432179. [DOI] [PubMed] [Google Scholar]
- 21.Lindgren JE, Ohlsson A, Agurell S, Hollister L, Gillespie H. Clinical effects and plasma levels of delta-9-tetrahydrocannabinol (delta-9-THC) in heavy and light users of cannabis. Psychopharmacology. 1981;74:208–12. doi: 10.1007/BF00427095. [DOI] [PubMed] [Google Scholar]
- 22.Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma levels of delta-9-tetrahydrocannabinol after intravenous, oral and smoke administration. Clin Pharmacol Ther. 1980;28:409–16. [Google Scholar]
- 23.Johansson E, Halldin MM, Agurell S, Hollister LE, Gillespie HK. Terminal elimination plasma half-life of delta-1-tetrahydrocannabinol (delta-1-THC) in heavy users of marijuana. Eur J Clin Pharmacol. 1989;37:273–7. doi: 10.1007/BF00679783. [DOI] [PubMed] [Google Scholar]
- 24.Heishman SJ, Huestis MA, Henningfield JE, Cone EJ. Acute and residual effects of marijuana: profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol Biochem Behav. 1990;37:561–5. doi: 10.1016/0091-3057(90)90028-g. [DOI] [PubMed] [Google Scholar]
- 25.Goodwin RS, Gustafson RA, Barnes A, Nebro W, Moolchan ET, Huestis MA. Delta(9)-tetrahydrocannabinol, 11-hydroxy-delta(9)-tetrahydrocannabinol and 11-nor-9-carboxy-delta(9)-tetrahydrocannabinol in human plasma after controlled oral administration of cannabinoids. Ther Drug Monit. 2006;28:545–51. doi: 10.1097/00007691-200608000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Schwilke EW, Schwope DM, Karschner EL, Lowe RH, Darwin WD, Kelly DL, et al. Delta9-Tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clin Chem. 2009;55:2180–9. doi: 10.1373/clinchem.2008.122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral delta-9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem. 2011;57:66–75. doi: 10.1373/clinchem.2010.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]