Abstract
The DNA cytosine deaminase APOBEC3G (A3G) is a two-domain protein that binds single-stranded DNA (ssDNA) largely through its N-terminal domain and catalyzes deamination using its C-terminal domain. A3G is considered an innate immune effector protein, with a natural capacity to block the replication of retroviruses such as HIV and retrotransposons. However, knowledge about its biophysical properties and mechanism of interaction with DNA are still limited. Oligomerization is one of these unclear issues. What is the stoichiometry of the free protein? What are the factors defining the oligomeric state of the protein? How does the protein oligomerization change upon DNA binding? How stable are protein oligomers? We address these questions here using atomic force microscopy (AFM) to directly image A3G protein in a free-state and in complexes with DNA, and using time-lapse AFM imaging to characterize the dynamics of A3G oligomers. We found that the formation of oligomers is an inherent property of A3G and that the yield of oligomers depends on the protein concentration. Oligomerization of A3G in complexes with ssDNA follows a similar pattern: the higher the protein concentrations the larger oligomers sizes. The specificity of A3G binding to ssDNA does not depend on stoichiometry. The binding of large A3G oligomers requires a longer ssDNA substrate; therefore, much smaller oligomers form complexes with short ssDNA. A3G oligomers dissociate spontaneously into monomers and this process primarily occurs through a monomer dissociation pathway.
Keywords: APOBEC3G, single-stranded DNA binding proteins, site search mechanisms, atomic force microscopy, AFM, high-speed AFM
Introduction
APOBEC3G (A3G) is a single-stranded DNA deaminase that converts cytosine into uracil, leading to extensive deamination of HIV cDNA and eventually inhibiting HIV reproduction (Harris et al., 2012; Malim and Bieniasz, 2012). A3G and other APOBEC proteins are considered natural defenses against AIDS; therefore, understanding the mechanisms of A3G’s enzymatic activity and its interactions with its target will help in developing antiviral A3G based treatments. A3G is a two-domain protein that interacts with single-stranded DNA (ssDNA) largely via its N-terminal binding domain and carries out catalytic activity with its C-terminal domain (reviewed in (Harris et al., 2012)). Various biophysical and imaging techniques showed that A3G is capable of forming oligomers (Bennett et al., 2008; Chelico et al., 2010; Chelico et al., 2008), prompting questions about the role of A3G oligomerization in its catalytic activity. However, the data currently available are rather controversial. According to some studies (McDougall et al., 2011), the full-length native A3G protein has to form higher order oligomers to be catalytically active. It was shown that oligomers as large as tetramers are catalytically active, whereas dimers have no deaminase activity. In contrast, according to other studies (Chelico et al., 2010; Opi et al., 2006), monomeric A3G is both catalytically active and possesses antiviral activity. Another important question that has arisen concerns the role of RNA or ssDNA in the A3G multimerization process: does the DNA substrate promote multimerization of A3G? It was demonstrated in (Huthoff et al., 2009) that RNA promotes A3G oligomerization, and according to (Friew et al., 2009), A3G monomers form oligomers upon binding to RNA. The early work of Chelico et al (Chelico et al., 2008) claimed that the oligomeric state of A3G is defined by the its interaction with ssDNA. Later, the same group reported that A3G oligomers exist in solution but change dramatically upon binding to ssDNA (Chelico et al., 2010). The mechanism by which the A3G oligomeric forms are stabilized is an unanswered question; though one publication (Bennett et al., 2008) suggested that A3G forms RNA-independent oligomers through interactions within its C-terminus.
We used Atomic Force Microscopy (AFM) to study the oligomerization process of free A3G and the structure and dynamics of A3G in complex with ssDNA. We employed our recently developed hybrid DNA approach that enables us to unambiguously identify complexes of A3G with ssDNA (Shlyakhtenko et al., 2011; Shlyakhtenko et al., 2012a). Additionally, we utilized time-lapse high-speed AFM (HS-AFM) to directly visualize the dynamics of A3G oligomers in a free state and in complexes with ssDNA. Our results show that oligomerization is an inherent property of A3G and its oligomeric state is strongly influenced by protein concentration. A3G oligomers specifically bind to ssDNA, but the size of A3G oligomers present in complexes depends on the length of the single-stranded stretch of the substrate. Free A3G oligomers and A3G-ssDNA complexes are dynamic and dissociate spontaneously, primarily into monomers. We discuss the functional role of this dynamic feature of A3G.
Material and Methods
DNA substrates
Hybrid DNA substrates with a ssDNA length of 69 nt (69ss-5’- tail DNA or 69ss-gap DNA) were prepared as described (Shlyakhtenko et al., 2011; Shlyakhtenko et al., 2012b). The substrate with a ssDNA length of 9 nt (9ss-3’-tail DNA; ACC AGT GGC-3’ attached to 169 bp DNA) was obtained after restriction of the PCR fragment by TspRI, followed by standard gel purification procedures (Shlyakhtenko et al., 2011; Shlyakhtenko et al., 2012a; Shlyakhtenko et al., 2012b).
Preparation of the A3G-DNA complex
Full-length human A3G was purified from 293 cells using a C-terminal hexahistidine tag, as described in (Li et al., 2012) and (Shlyakhtenko et al., 2011; Shlyakhtenko et al., 2012b). The DNA-protein complexes of 69ss-5’- tail DNA, 69ss-gap DNA or 9ss-3’- tail DNA and A3G were prepared as described (Shlyakhtenko et al., 2011; Shlyakhtenko et al., 2012a; Shlyakhtenko et al., 2012b). Briefly, DNA substrates were mixed with different concentrations of A3G in the reaction buffer, containing 50 mM HEPES, pH 7.5, 100 mM NaCl, 5 mM Mg2+ and 1 mM DTT, incubating at 37°C for 10 min, followed by purification using Montage UFC spin-columns.
Sample preparation and AFM imaging in air
AFM experiments were performed with the use of functionalized APS-mica as described (Lyubchenko and Shlyakhtenko, 2009; Lyubchenko et al., 2011; Shlyakhtenko et al., 2011; Shlyakhtenko et al., 2012a; Shlyakhtenko et al., 2012b). Briefly, a 5 µl sample was deposited on APS-mica for 2 min, rinsed with deionized water and dried with Argon gas. Images were acquired in tapping mode in air using the Multimode Nanoscope IV system (Bruker-Nano, Santa Barbara, CA). Silicon probes with 42N/m at resonance frequencies between 310– 340 Hz were used.
Sample preparation and HS-AFM imaging in liquid
The HS-AFM images were acquired by using the HS-AFM instrument developed by the Ando group (Ando et al., 2013). The data were acquired by operating the instrument in tapping mode in liquid. Carbon tips were grown on the small silicon nitride AFM cantilevers (BioLever Fast AC10DS, Olympus) using the electron beam deposition (EBD) procedure, and were sharpened with the TE 2000 plasma etcher, as described (Lyubchenko et al., 2011; Shlyakhtenko et al., 2012b). The spring constant of the AFM probes was between 0.1 and 0.2 N/m, with the resonance frequency between 400 and 1000 kHz in water. Continued scanning over the selected area (200 nm ×200 nm) was performed to follow the dynamics of A3G-DNA complexes. The scan rate corresponded to data acquisition at 353ms/frame and 720 ms/frame.
Data Analysis
For each protein-ssDNA complex, height and diameter were measured using Femtoscan Online with the cross-section option (Advanced technologies Center, Moscow, Russia), as described (Shlyakhtenko et al., 2011; Shlyakhtenko et al., 2012a; Shlyakhtenko et al., 2012b). The data for measurements of protein volume and the length of the DNA arms were analyzed and collected as histograms using Origin 6.0 (Originlab, Northampton, MA). The A3G volume data were converted into molecular mass using a conversion coefficient, as described (Shlyakhtenko et al., 2012a).
Results
1. The effect of A3G concentration on oligomerization in complex with 69ss-tail-DNA
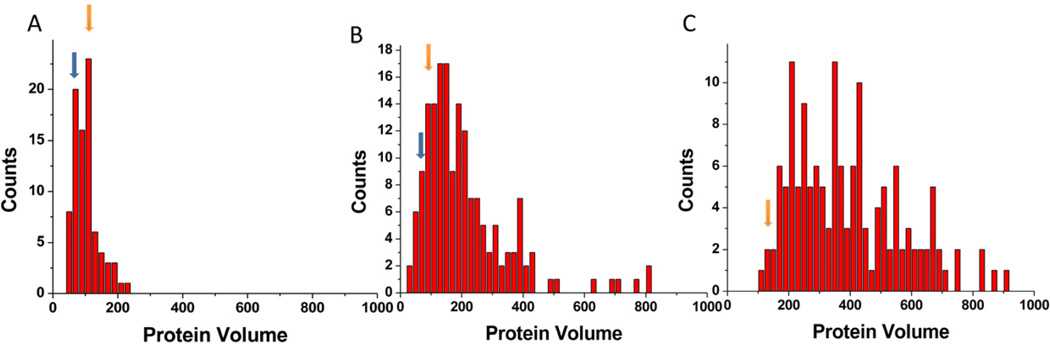
Previously, we have shown that A3G exists primarily as a dimer when in complex with 69ss-tail-DNA (5:1 protein /DNA ratio) (Shlyakhtenko et al., 2011; Shlyakhtenko et al., 2012a; Shlyakhtenko et al., 2012b). Here, we used the same DNA substrate, but performed the experiments in a range of A3G protein concentrations from 7.5 nM to 144 nM (corresponds to 1.8:1 to 36:1 molar protein/DNA ratio, respectively) and kept the DNA concentration constant at 4 nM in all experiments. Figure 1 shows representative AFM images of complexes between 69ss-tail DNA with 20 nM (A) and 144 nM (B) A3G protein (5:1 and 36:1 protein-to-DNA ratio, respectively). DNA molecules on both images have bright blobs at one end corresponding to the formation of specific A3G-ssDNA complexes. The primary difference between these two images is the size of the blobs. The blobs are brighter and larger for complexes with high, 144nM, A3G concentration (B) than for 20 nM A3G (A). Importantly, at both low and high protein concentrations, A3G does not bind double-stranded segments of the DNA substrate, demonstrating that A3G retains its specificity for ssDNA regardless of the protein concentration. Note that the yield of the complexes depends on the A3G protein concentration in the solution, with yields being approximately 3 – 4 times lower at 7.5nM than at 75 nM.
Figure 1.
AFM images of complexes of 69-tail-DNA with A3G at different concentrations. (A) 20 nM and (B) 144 nM A3G. Bar size is 200nm.
Next, we measured the volume of the proteins in the complexes with ssDNA and calculated the protein molecular weight using the conversion coefficient 1.3 (Shlyakhtenko et al., 2012a). From these measurements, we estimated the A3G stoichiometry in the complexes and labeled monomers as 1, dimers as 2, trimers as 3, etc. (Figures 1A and B). This analysis was performed for complexes with different concentrations of A3G and the data are shown as histograms in Figures 2A, B, and C. The DNA concentration remained the same, therefore, the molar monomer protein-to-DNA ratios were 1.8:1, 18:1 and 36:1 for the histograms in Figures 2 A, B and C, respectively. Figure 2A shows that in the presence of 7.5 nM A3G (1.8:1 protein/DNA ratio, the lowest protein concentration in this dataset), the vast majority of A3G-ssDNA complexes are comprised of monomers and dimers, with roughly similar yields (shown with arrows on the histogram). A ten-fold increase in the protein concentration dramatically changes the yield of the protein oligomers (Figure 2B), and is accompanied by a drop in the amount of monomers in the complex. At the highest A3G concentration, 144 nM, a small fraction of dimers is still apparent but the size distribution becomes much more heterogeneous with large oligomers predominating (Figure 2C).
Figure 2.
Volume measurements for A3G-DNA complexes obtained at different protein concentrations for 69-tail-DNA substrate. (A)- 7.5 nM (protein/DNA ratio 2:1), (B)- 75 nM (protein/DNA ratio 20:1) and (C)- 144 nM of A3G (protein/DNA ratio 36:1) in the reaction mixture. Blue and orange arrows denote volume values corresponding to monomers and dimers, respectively.
2. The efficiency of A3G binding with short 9ss-tail-DNA
Next, we performed experiments with a shorter ssDNA substrate to understand the effect of ssDNA length on A3G multimerization within complexes. We designed the hybrid DNA substrate with the tail as short as 9 nucleotides, 9ss-tail DNA. AFM images of the complexes prepared in the presence of 72 nM A3G protein are shown in Figure 3. We used a high protein concentration to obtain a yield of complexes comparable to the experiment performed with 69ss-tail DNA in Figure 1A. Regardless of the short length of the ssDNA, A3G binds to the ends of DNA only and does not form non-specific complexes. The blob sizes vary in this image, demonstrating that monomers, dimers and larger A3G oligomers can also form under these conditions. Volume measurements under these conditions were compared to those from experiments performed with two times higher protein concentrations of A3G, and are presented as histograms in Figure 4A, B. The increase in the A3G concentration leads to a decrease in the number of monomers and an increase in the formation of larger complexes, as observed by the prevalence of tetramers under these conditions (Figure 4B). Oligomers as large as octamers can also be found (protein volume ~480 nm3), but the overall yield of large oligomers was low. Qualitatively, the results obtained with the short 9ss-tail-DNA substrate (Figure 4) are similar to those obtained for the larger 69ss-tail-DNA substrate (Figure 2): higher protein concentrations are associated with the formation of larger oligomers in the protein-DNA complexes; however, the sizes of oligomers are considerably smaller for the short substrate than the longer substrate. Indeed, a comparison of the same A3G concentration between the two substrates (cf. Figures 2B & C and Figures 4A & B, respectively) shows that the distribution of oligomer sizes is much broader for the 69ss-tail-DNA substrate than for the 9ss-tail-DNA substrate. This finding suggests that the size of the ssDNA substrate determines the binding capability of large A3G oligomers. These can be pre-assembled large A3G aggregates, but DNA-mediated assemblies cannot be excluded. Also, the overall yield of the complexes at the identical protein concentration is ~3 times higher for longer ssDNA than for shorter ssDNA, which can be explained by the lower binding affinity of large pre-assembled A3G aggregates by the shorter 9ss-tail-DNA substrate.
Figure 3.
AFM images of complexes between 9ss-tail-DNA and A3G protein at 72nM. Bar size 500nm. Numbers indicate the complexes with different A3G stoichiometry: 1- monomers, 2- dimers and 3- trimers.
Figure 4.
Volume measurements for A3G in complexes with 9ss-tail DNA at different protein concentrations: (A) 72 nM (protein/DNA ratio 20:1) and (B) 150 nM (protein/DNA ratio 38:1) of A3G in the reaction mixture. Blue and orange arrows correspond to the positions of monomers and dimers and green to the position of trimers respectively.
3. A3G multimerization in the absence of DNA
The results shown above demonstrate that A3G oligomers bind ssDNA, but whether these oligomers are assembled first in solution and then bind to ssDNA or whether they are formed on ssDNA is not clear from these experiments. To address this question, we analyzed A3G oligomerization in the absence of DNA at different protein concentrations. AFM images of the protein at 2 nM and 8 nM are shown in Figure 5A and B, respectively. These images show that, in addition to monomers, A3G oligomers exist in solution and that oligomerization increases with protein concentration. The volume measurements of more than 100 blobs were assembled as histograms, which clearly demonstrate that monomers are the predominant species of A3G at 2 nM (Figure 6A). However, monomers become the minor species at the higher protein concentration of 8 nM, as evidenced by the maximum in the volume distribution shifted towards dimers with the accumulation of large oligomers (Figure 6B). Thus, a four-fold difference in protein concentration facilitates A3G oligomerization. A similar analysis for higher A3G concentrations is complicated by the dense coverage of the surface with the protein. Even at 8 nM A3G, the density of coverage is high. Importantly, the data for the protein volume distribution in Figure 6B are very close to those obtained for the protein volume distribution for the complex with DNA at 7.5 nM A3G (Figure 2A). This suggests that ssDNA can be bound by both monomers as well as by preformed A3G oligomers; however, DNA length clearly influences the size of oligomers that can be accommodated.
Figure 5.
AFM images of free A3G protein at different concentrations deposited on APS mica: (A) 2 nM and (B) 8 nM A3G. Bar size 200 nm.
Figure 6.
Protein volumes distributions of free A3G protein deposited on APS mica for two different protein concentrations: (A) 2nM and (B) 8nM. Blue arrow points to the monomers and orange arrows to the dimers.
4. Dynamics of A3G-ssDNA complexes
The data shown above demonstrate that various oligomeric forms of A3G can exist in ssDNA bound complexes. How stable are the complexes with different protein stoichiometry? What are the pathways for the oligomers dissociation? To answer these questions, we applied time-lapse AFM to A3G in complexes with 69ss-gap DNA to visualize directly dynamics of A3G oligomeric forms. Complexes with ~5:1 protein-to-DNA ratio (5 nM DNA and 26 nM A3G) were imaged with high-speed AFM (HS-AFM), as described (Shlyakhtenko et al., 2012a). The set of images in Figure 7 represent 7 selected frames out of 148 total frames (full montage in Supplementary Movie S1). This set illustrates the dissociation of a tetramer shown in frame 18. The separation of one monomer is seen in frame 32 and this monomer later translocates to a distant position from the DNA (frame 54). Shortly thereafter (frame 59), another monomer dissociates and moves away (frame 64). The third monomer dissociates from the substrates at frame 83, and frame 148 shows the fully dissociated tetramer with monomers indicated by arrows.
Figure 7.

High-speed AFM of A3G protein complexed with 69-gap-DNA illustrates the dissociation of the complex into four monomer units (frame 148) suggesting the tetrameric A3G stoichiometry in initial frame 18. Selected numbered frames from the movie (movie S1 in the supplement) are shown. The data acquisition corresponds to 720 msec per frame. The bar size is 50 nm.
As a second example, the dissociation pattern for an A3G trimer is shown in Figure 8. The initial image (frame 10) already shows a sign of dissociation, and the first monomer moves away from the DNA rather quickly (frame 12). The dissociation of the second monomer is shown in frame 21 and the last image (frame 49) shows the final monomer dissociated (full montage in Supplementary Movie S2).
Figure 8.
High-speed AFM of A3G protein complexed with 69-gap-DNA illustrates the trimer dissociation process. Selected numbered frames from the movie (movie S2 in the supplement) are shown. The data acquisition corresponds to 720 msec per frame. The bar size is 50 nm.
Several conclusions emerge from these studies. First, oligomerization and de-oligomerization are clearly dynamic processes. Monomeric A3G as well as larger oligomeric subcomplexes (Figures 7 and 8 and data not shown) were clearly capable of both associating with and dissociating from A3G-ssDNA complexes. Second, ssDNA bound A3G oligomers predominantly dissociate by losing mostly monomeric subunits (representative data in Figures 7 and 8 and Supplementary Movie S1 and S2). Dissociation of dimers and higher oligomers was also observed, but the monomeric dissociation pathway appeared to be the major mechanism.
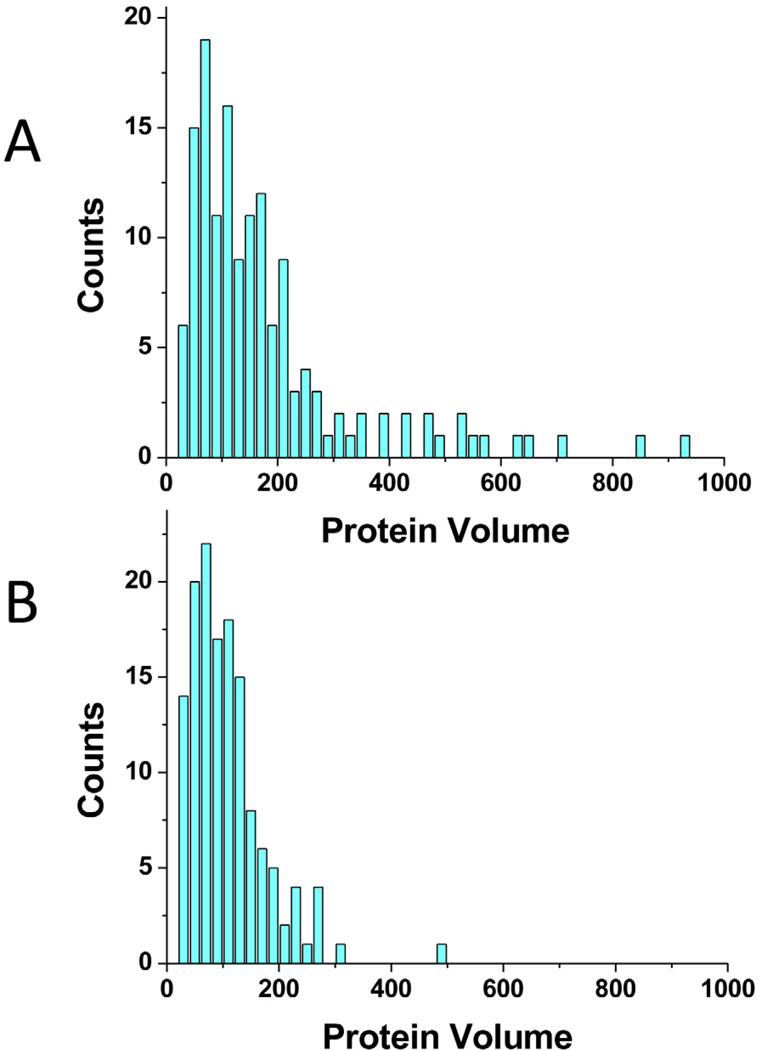
If this is indeed the case and the monomeric dissociation pathway is the primary mechanism, then we should be able to observe the accumulation of monomers on the AFM images of the dried sample during the sample preparation. According to the time-lapse images (Figures 7 and 8), A3G-ssDNA complexes can dissociate in a few minutes. Therefore, to test this model, the complex of A3G with ssDNA was deposited for two minutes, rinsed, dried, imaged and the volumes of free protein blobs on the field were measured. The volume measurements for proteins dissociated from complexes with the high protein concentration A3G and the hybrid DNA substrates with different sizes of ssDNA are shown in Figure 9. The data indicate that for both substrates, 69ss-tail DNA and 9ss-tail DNA, the volumes of the dissociated proteins are very similar and are primarily monomers and dimers. Note, that the sizes of A3G oligomers in complexes (Figures 2C & 4B, respectively) are much larger. For example, A3G monomers and dimers are rarely found complexed with 69-ss-tail DNA (Figure 2C). Thus, A3G oligomers are dynamic and undergo spontaneous dissociation, primarily via a monomer dissociation pathway.
Figure 9.
The histogram of the volume of the protein dissociated from the complexes of A3G with two substrates: (A) 69ss-tail-DNA in complex with A3G at 144nM and (B) 9ss-tail-DNA in complex with A3G at 150nM as appeared on the AFM images of the complexes.
The third major conclusion of the time-lapse data is derived from the lifetimes of A3G oligomers with various stoichiometries. The data in Figures 7 and 8 show that the lifetimes of the oligomers are in the range between 10 and 20 frames, whereas the last monomer can remain bound to ssDNA for a considerably longer time. For example, the dimer appeared stable from frames 65 and 83, compared to the ensuing monomer which persisted from frames 83 and 148 (Figure 7 and Supplementary Movie S1). Lifetime of the monomeric complex in Figure 8 is 28 frames (between frames 21 and 49) compared to 10 and 9 frames for the dissociation of the trimer and dimer, respectively (Figure 8 and Supplemetary movie S2). A similar analysis performed for an additional data set (Supplementary Figures S1A, B, C, D, E) showed even larger values for monomeric forms, varying between 85 and 224 frames. Therefore, monomeric complexes are characterized by longer lifetime values than oligomeric complexes.
Similar time-lapse experiments were performed to follow the dynamics of free A3G oligomers in solution. Figure 10A shows the dynamics of a trimer (frame 94), which dissociates into two blobs of different sizes (frame 107), reassociates (frame 109), and dissociates with a monomer remaining in the area (frame 130). The plot in Figure 10B shows the changes in the volume for the protein during the dissociation and association processes. The arrows show the frames where the changes in the protein volumes occurred. Additional observations for oligomer dynamics are shown in Figure 11A. Two blobs, a monomer and a dimer (frame 298 marked with circles), associate to make a trimer (frame 301) that remains stable for about 37 frames and then dissociates into the monomer and dimer (frame 338). These dynamics are graphically illustrated in Figure 11B in which the time-dependent volume changes for each blob are plotted. Therefore, oligomeric A3G undergoes dissociation dynamics resembling those observed for A3G in complex with ssDNA, in which primarily monomers dissociate from the oligomer.
Figure 10.
High-speed AFM of free A3G protein. (A) Selected frames from Movie S3. (B) The protein volume values correspond to proteins on the field for each frame. The arrows show the changes in the protein volume during the separation and association processes. The data acquisition corresponds to 353 msec per frame.
Figure 11.
High-speed AFM of free A3G protein. (A) Selected frames from Movie S4. (B) The protein volume values corresponding to proteins on the field for each frame. The arrows show the changes in the protein volume during separation and association processes. The data acquisition corresponds to 353 msec per frame. Image size 150 nm.
Discussion
Results presented here clarify a number of properties of A3G: the protein state in solution, its interaction with ssDNA substrates, and the dynamics of these interactions. We elaborate on these points in the sections below.
A3G oligomerization is a concentration dependent process
The AFM data demonstrate unambiguously that A3G monomers can exist in solution. This result agrees with prior reports of A3G stoichiometry in vivo and in vitro (e.g., (Bennett et al., 2008; Opi et al., 2006; Salter et al., 2009; Wedekind et al., 2006)). Our data further indicate that the formation of oligomers is a concentration-dependent process, with the dimer formation occurring at the protein concentrations as low as 2 nM and higher oligomers predominating at higher protein concentrations. We characterized the oligomerization process through the protein volume measurements from our AFM analysis. Images in Figure 6A show that at A3G concentrations of 2 nM, monomers and dimers are the primary species. The approximation of the protein volume histogram with tree Gaussians, and the yield of monomers, dimers and trimers are shown in Supplementary Figure S2. According to this approximation, the population of monomers is three times greater than that of dimers for the experiments performed at 2 nM A3G. The yield of trimers is more than two times less than dimers. Given the low yield of trimers, we assume that this protein concentration corresponds primarily to a monomer-to-dimer equilibrium. Therefore, we used the data in Supplementary Figure S2 to estimate a value for dimer dissociation, KD = 1 ± 0.2 nM. The data at higher A3G concentrations (8 nM, Figure 6B), demonstrate that dimers are the predominant species with a substantial drop in the monomer concentration. These data are in agreement with even lower dissociation constant values for the dimers, KD = 0.4 ± 0.3 nM. However, these are very rough estimates because the overlap between the distribution of dimers and trimers complicates histogram deconvolution.
Therefore, the data shown in Figure 6A and B strongly indicate that A3G oligomerizes in a concentration-dependent manner. There is a primarily monomer-dimer equilibrium at 2 nM A3G, allowing us to estimate the KD value as low as 1 ± 0.2 nM, but increasing the protein concentration four-fold resulted in a more complex system consisting of dimers, trimers and even tetramers. Higher order A3G oligomers have been reported in numerous prior studies (e.g., (Bennett et al., 2008; Chelico et al., 2008; McDougall et al., 2011; Opi et al., 2006; Salter et al., 2009; Wedekind et al., 2006)).
A3G oligomerization in complex with ssDNA
The role of the DNA substrate in the A3G oligomerization process has been widely discussed (e.g., (Friew et al., 2009; Huthoff et al., 2009; McDougall et al., 2011)). A key question is whether A3G oligomers form in solution and bind to the template, or is this oligomerization process initiation by the protein binding to the substrate. The latter mechanism was favored by (McDougall et al., 2011), who suggested that the formation of higher order oligomers could be enhanced by A3G homodimers binding nucleic acids. An RNA-dependent oligomerization of A3G has also been shown in a number of studies (e.g., (Huthoff et al., 2009)). For instance, one study suggested that A3G is a monomer in vivo and forms oligomers upon binding to RNA (Friew et al., 2009). Our data on A3G oligomerization in a free state and in complex with ssDNA clarifies this issue. First, A3G oligomerization appears to be a concentration-dependent process that can occur when the protein is in a free state or when it is in complex with DNA. Second, the size distribution of the free protein oligomers closely approximates that for A3G in complex with DNA, when measured at similar A3G concentrations (cf. Figures 2A and 6B). These data suggest that binding to DNA does not alter protein oligomerization. Additional support for this model comes from the comparison of the dissociation constants for free A3G protein and A3G protein in complex with DNA. According to our estimates, the dissociation constant for the dimer-monomer equilibrium of free protein is ~1 nM, while this value for A3G-ssDNA complexes is considerably higher, with KD estimates ranging from 52 to 238 nM (reviewed in (Imahashi et al., 2012)). These data show that A3G dimers in solution are more stable compared to the dimers in protein-DNA complexes. Such a high stability of the free protein is in favor of the model in which the oligomers of A3G are formed in solution prior to the protein binding to ssDNA; so the ssDNA substrate does not drive A3G oligomerization.
The length of ssDNA is an important factor modulating oligomer binding. Monomers are primarily found for the complexes formed with 9-nt ssDNA and 72 nM A3G; whereas dimers, trimers and tetramers are the most representative species in the complexes with the same A3G concentration and the longer 69-nt ssDNA (cf. Figures 2B and 4A). Larger oligomers are found as well, but their population is low. At higher protein concentrations (150 nM), trimers and tetramers are the highly populated species (Figure 4B), but these and larger oligomers are present only if the 69 nt substrate is used (Figure 2C). These data suggest that the binding constant for oligomers depends on substrate size. Therefore, if the DNA substrate is shorter, the affinity for binding large A3G oligomers will be lower. This assumption is supported by the decrease in the complex yield for short ssDNA compared to the long ssDNA. Importantly, regardless of the protein concentration and the size of the oligomer formed by the protein, the specificity of A3G binding to ssDNA remains very high. We did not find A3G bound to DNA duplexes or the blunt end of the substrate. Additionally, a short ssDNA length, as low as 9 nt, did not lead to a loss of A3G binding specificity, suggesting that the length of the ssDNA is not a critical parameter for specific protein binding.
Dynamics of A3G-ssDNA complexes
Additional insights into the dynamics of the A3G-ssDNA interaction come from time-lapse imaging Figures 7and 8 and Supplementary Movies S1 and S2. These data indicate that the A3G-ssDNA complex is dynamic, which is in agreement with our previous publication (Shlyakhtenko et al., 2012a). Our current observations show that A3G oligomers primarily dissociate as monomers in a step-wise fashion. These data are supported by the volume analysis of free protein on the AFM images of the A3G-DNA complexes. The samples are prepared by filtering out the free protein; therefore, the free protein appearing on the images should be due to dissociation of complexes during sample preparation, which takes approximately 2 minutes. Therefore, the volume measurements of free protein in these samples allow us to measure the stoichiometry of protein dissociation from DNA. The amount of dissociated proteins is small, suggesting that the A3G-ssDNA complex is rather stable. Therefore, statistical analysis requires a large number of images. The results of our analysis for the A3G dissociated from the complex with 9ss-tail-DNA (Supplementary Figure S3B) demonstrate that A3G dissociates primarily as a monomer whereas it predominantly exists as trimers and tetramers when in complex with the substrate, under the assay conditions described here (Figure 4B; see also Supplementary Figure S3A). The difference in volumes of the dissociated protein and the protein in complexes with DNA is more striking for longer ssDNA. If the stoichiometry of A3G in complex with ssDNA is large, consisting of tetramers as the smallest species (Figure 2C), the volume distribution reveals that the proteins dissociated from the complex are monomers (Supplementary Figure S4B; the distribution for the protein in the complex is shown in frame A for comparison). This distribution is very similar to that of the previous sample (Figures S3 A & B, respectively). Altogether, these analyses support the time-lapse observations that show that the multistep dissociation process primarily involves the dissociation of monomeric units; although, whole dimers, trimers and larger oligomers can also occasionally dissociate from the multimers.
We observed through time-lapse studies that free A3G protein can assemble from monomers into dimers (e.g., Figure 10). These are rather rare events and were observed for the protein bound to the surface. The association was observed for protein in close proximity, thus providing direct evidence for the propensity of A3G to oligomerize. A more interesting event is observed in Figure 11, where a monomer and a dimer associate into a trimer, and the exchange of monomers can also be seen.
The stoichiometry of enzymatically active A3G is another issue widely discussed in the literature. The data reported in (Chelico et al., 2010) indicate that an A3G mutation that disrupts dimerization is capable of binding with ssDNA and catalyzes deamination similar to native A3G, suggesting that oligomerization is not necessary for DNA deamination. This finding agrees with the earlier work from the same group (Chelico et al., 2008), suggesting that deamination correlates with the monomeric state of A3G. The importance of A3G monomeric binding for its catalytic activity is also mentioned in (Nowarski et al., 2008). These data are in contrast to a recent paper (McDougall et al., 2011) in which the enzymatic activity of various oligomeric forms of A3G was analyzed. The primary conclusion of that paper is that tetramerization of A3G is required for the deamination process. However, according to our data, such large A3G oligomers are formed in a high yield in a concentrated protein solution (144 nM A3G; Figure 2C), which is equivalent to ~36:1 protein-to-DNA ratio. An additional requirement for the complex formation of A3G tetramers is a long DNA substrate, as evidenced by our observation that large oligomers are rare in the presence of a 9-nt DNA substrate (Figure 4B) suggesting that no deaminase activity should be observed for a short DNA substrate. However, the results obtained in (Chelico et al., 2008) show that A3G is active on similarly short substrates. So the claim on the requirement of tetramerization for A3G deaminase activity (McDougall et al., 2011) is not in accord with most other studies.
According to recent publications (Chelico et al., 2006; Chelico et al., 2010; Chelico et al., 2008), A3G is a highly processive enzyme performing deamination of multiple cytosine residues within a single ssDNA substrate (analogous to the ssDNA intermediate in HIV reverse transcription). This high processivity suggests that A3G substrate binding should be dynamic. We did observe A3G monomer sliding in our previous paper (Shlyakhtenko et al., 2012a), but the dynamics of oligomeric forms of A3G suggest that dissociated monomers can bind to another target and perform the reaction. The dissociation-association of free protein occurs in the second time-scale, allowing the deamination reaction to occur at a rate observed in biochemical experiments.
Supplementary Material
Acknowledgements
We thank P. Beal, M. Kotler, H. Matsuo, J. Mueller and C. Schiffer, for critical review of the data and useful suggestions and the Lyubchenko lab members for the discussion of the results and critical comments.
Funding
The work was supported by grants from National Institutes of Health (1P01GM091743-02, 3R01GM096039-02 and 1R01GM100156-01A1), and National Science Foundation (EPS – 1004094).
Abbreviations
- APOBEC
apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like
- A3G
APOBEC3G
- AFM
Atomic force microscopy
- ssDNA
single-stranded DNA
- APS-mica
aminopropyl silatrane treated mica
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Data
Additional AFM images of A3G-DNA complexes, graphs characterizing A3G-DNA complexes, and movies of animated time-lapse AFM images of the complexes. This material is available free of charge via the ScienceDirect: http://www.sciencedirect.com.
References
- Ando T, Uchihashi T, Kodera N. High-Speed AFM and Applications to Biomolecular Systems. Annu Rev Biophys. 2013;42:393–414. doi: 10.1146/annurev-biophys-083012-130324. [DOI] [PubMed] [Google Scholar]
- Bennett RP, Salter JD, Liu X, Wedekind JE, Smith HC. APOBEC3G subunits self-associate via the C-terminal deaminase domain. J Biol Chem. 2008;283:33329–33336. doi: 10.1074/jbc.M803726200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelico L, Pham P, Calabrese P, Goodman MF. APOBEC3G DNA deaminase acts processively 3' -->5' on single-stranded DNA. Nat Struct Mol Biol. 2006;13:392–399. doi: 10.1038/nsmb1086. [DOI] [PubMed] [Google Scholar]
- Chelico L, Sacho EJ, Erie DA, Goodman MF. A model for oligomeric regulation of APOBEC3G cytosine deaminase-dependent restriction of HIV. J Biol Chem. 2008;283:13780–13791. doi: 10.1074/jbc.M801004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelico L, Prochnow C, Erie DA, Chen XS, Goodman MF. Structural model for deoxycytidine deamination mechanisms of the HIV-1 inactivation enzyme APOBEC3G. J Biol Chem. 2010;285:16195–16205. doi: 10.1074/jbc.M110.107987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friew YN, Boyko V, Hu WS, Pathak VK. Intracellular interactions between APOBEC3G, RNA, and HIV-1 Gag: APOBEC3G multimerization is dependent on its association with RNA. Retrovirology. 2009;6:56. doi: 10.1186/1742-4690-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RS, Hultquist JF, Evans DT. The restriction factors of human immunodeficiency virus. J Biol Chem. 2012;287:40875–40883. doi: 10.1074/jbc.R112.416925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huthoff H, Autore F, Gallois-Montbrun S, Fraternali F, Malim MH. RNA-dependent oligomerization of APOBEC3G is required for restriction of HIV-1. PLoS Pathog. 2009;5:e1000330. doi: 10.1371/journal.ppat.1000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imahashi M, Nakashima M, Iwatani Y. Antiviral Mechanism and Biochemical Basis of the Human APOBEC3 Family. Front Microbiol. 2012;3:250. doi: 10.3389/fmicb.2012.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Shandilya SM, Carpenter MA, Rathore A, Brown WL, Perkins AL, Harki DA, Solberg J, et al. First-in-class small molecule inhibitors of the single-strand DNA cytosine deaminase APOBEC3G. ACS Chem Biol. 2012;7:506–517. doi: 10.1021/cb200440y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubchenko YL, Shlyakhtenko LS. AFM for analysis of structure and dynamics of DNA and protein-DNA complexes. Methods. 2009;47:206–213. doi: 10.1016/j.ymeth.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubchenko YL, Shlyakhtenko LS, Ando T. Imaging of nucleic acids with atomic force microscopy. Methods. 2011;54:274–283. doi: 10.1016/j.ymeth.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH, Bieniasz PD. HIV Restriction Factors and Mechanisms of Evasion. Cold Spring Harb Perspect Med. 2012;2:a006940. doi: 10.1101/cshperspect.a006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall WM, Okany C, Smith HC. Deaminase activity on ssDNA occurred in vitro when APOBEC3G forms homotetramers and higher-order complexes. J Biol Chem. 2011 doi: 10.1074/jbc.M111.269506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowarski R, Britan-Rosich E, Shiloach T, Kotler M. Hypermutation by intersegmental transfer of APOBEC3G cytidine deaminase. Nat Struct Mol Biol. 2008;15:1059–1066. doi: 10.1038/nsmb.1495. [DOI] [PubMed] [Google Scholar]
- Opi S, Takeuchi H, Kao S, Khan MA, Miyagi E, Goila-Gaur R, Iwatani Y, Levin JG, et al. Monomeric APOBEC3G is catalytically active and has antiviral activity. J Virol. 2006;80:4673–4682. doi: 10.1128/JVI.80.10.4673-4682.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter JD, Krucinska J, Raina J, Smith HC, Wedekind JE. A hydrodynamic analysis of APOBEC3G reveals a monomer-dimer-tetramer self-association that has implications for anti-HIV function. Biochemistry. 2009;48:10685–10687. doi: 10.1021/bi901642c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlyakhtenko LS, Lushnikov AY, Li M, Lackey L, Harris RS, Lyubchenko YL. Atomic force microscopy studies provide direct evidence for dimerization of the HIV restriction factor APOBEC3G. J Biol Chem. 2011;286:3387–3395. doi: 10.1074/jbc.M110.195685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlyakhtenko LS, Lushnikov AY, Miyagi A, Li M, Harris RS, Lyubchenko YL. Nanoscale Structure and Dynamics of ABOBEC3G Complexes with Single-Stranded DNA. Biochemistry. 2012a doi: 10.1021/bi300733d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlyakhtenko LS, Lushnikov AY, Miyagi A, Lyubchenko YL. Specificity of binding of single-stranded DNA-binding protein to its target. Biochemistry. 2012b;51:1500–1509. doi: 10.1021/bi201863z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedekind JE, Gillilan R, Janda A, Krucinska J, Salter JD, Bennett RP, Raina J, Smith HC. Nanostructures of APOBEC3G support a hierarchical assembly model of high molecular mass ribonucleoprotein particles from dimeric subunits. J Biol Chem. 2006;281:38122–38126. doi: 10.1074/jbc.C600253200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.