Abstract
During eye lens development, regulation of Wnt/β-catenin signaling is critical for two major processes: initially it must be silent in the lens placode for lens development to proceed, but subsequently it is required for maintenance of the lens epithelium. It is not known how these different phases of Wnt/β-catenin activity/inactivity are regulated. Secreted frizzled related protein-2 (Sfrp2), a putative Wnt-Fz antagonist, is expressed in lens placode and in lens epithelial cells and has been put forward as a candidate for regional Wnt/β-catenin pathway regulation. Here we show its closely-related isoform, Sfrp1, has a complimentary pattern of expression in the lens, being absent from the placode and epithelium but expressed in the fibers. As mice with single knockouts of Sfrp1 or Sfrp2 had no defects in lens formation, we examined lenses of Sfrp1;Sfrp2 double knockout (DKO) mice and showed that they formed lens placode and subsequent lens structures. Consistent with this we did not observe ectopic TCF/Lef activity in lens placode of DKOs. This indicates that Sfrp1 and Sfrp2 individually, or together, do not constitute the putative negative regulator that blocks Wnt/β-catenin signaling during lens induction. In contrast, Sfrp1 and Sfrp2 appear to have a positive regulatory function because Wnt/β-catenin signaling in lens epithelial cells was reduced in Sfrp1;Sfrp2 DKO mice. Lenses that formed in DKO mice were smaller than controls and exhibited a deficient epithelium. Thus Sfrps play a role in lens development, at least in part, by regulating aspects of Wnt/β-catenin signaling in lens epithelial cells.
Keywords: Lens development, Wnt/β-catenin, Secreted frizzled-related protein, TCF/Lef activity, lens epithelial cells
Introduction
Canonical Wnt/β-catenin signaling is one of the fundamental signaling pathways that is required for embryogenesis and maintenance of the adult body. Development of conditional alleles of β-catenin in the form of constitutively-active and loss-of-function mutations, as well as availability of tissue-specific Cre transgenic mice have enabled researchers to identify critical roles for canonical Wnt pathways in many tissues (reviewed in Grigoryan et al., 2008)). These studies have shown that the canonical Wnt pathway is essential in many systems for cell fate determination and progenitor cell expansion during embryogenesis and for controlling stem cell populations in adult tissues/organs. Since Wnt/β-catenin signaling controls such fundamental processes, understanding its regulation is an important step towards opening up pathways for tissue regeneration, disease prevention and treatment.
The eye lens is derived from a region of head ectoderm. Initially a broad region of surface ectoderm has lens-forming potential but lens is formed only at a specific site and this is now known to be determined by the signaling activities of members of the BMP and FGF growth factor families (Gunhaga, 2011). Proper positioning of the lens placode in the ectoderm depends on BMPs and FGFs playing roles in medial-lateral restriction, whilst rostral-caudal restriction is determined by Wnt signaling activity at the late gastrula stage. Wnt promotes generation of neural crest cells at the caudal region so that the presumptive lens/olfactory placode is derived from the rostral Wnt-free region. For lens induction Wnt activity is not required, rather the evidence points to it suppressing the acquisition of lens fate. A conditional knockout (KO) of β-catenin in surface ectoderm does not block lens induction (Kreslova et al., 2007; Smith et al., 2005); interestingly, in this case caudal expansion of lens induction is observed and ectopic lens lineage cells are detected in the vicinity of the future nose region (Kreslova et al., 2007; Smith et al., 2005). Conversely, activation of the Wnt/β-catenin pathway in surface ectoderm by expressing constitutively-active β-catenin completely inhibits lens formation (Kreslova et al., 2007; Miller et al., 2006; Smith et al., 2005). Thus, taken together these observations indicate that for lens induction to take place, the β-catenin pathway needs to be turned off whereas for non-lens regions, β-catenin signaling must be activated in order to prevent acquisition of a lens lineage. This regional activation/inactivation of the β-catenin pathway appears to be a key process that is required for lens lineage specification but currently it is not understood how this patterning is regulated in the ectoderm.
The mature lens is comprised of two types of cells, the anterior lens epithelium and the differentiated lens fibers. The fibers are the main cellular constituent and form the bulk of the lens. The epithelial cells are proliferative and provide new cells that differentiate into fibers as the lens grows throughout life. In contrast to its inhibitory role in lens induction, Wnt/β-catenin signaling activity has been shown to be required for formation and maintenance of a normal lens epithelial monolayer. A complete epithelium does not form in the absence of the Lrp6 co-receptor that is required for Wnt/β-catenin signaling (Stump et al., 2003). Also, depletion of β-catenin after the lens induction stage results in a diminished layer of lens cells that do not express characteristic epithelial cell markers and proceed to premature differentiation (Cain et al., 2008). In contrast, forced activation of β-catenin signaling causes expansion of the epithelial layer and delays fiber differentiation (Martinez et al., 2009). These early and late embryonic stage experiments indicate that during lens development there are two phases of Wnt/β-catenin pathway activity; the first phase negatively regulates lens induction whereas the second phase is required for the formation and maintenance of a normal epithelial sheet. Since β-catenin is expressed both in lens placode and lens epithelial cells some regulatory factor(s) must play a role in determining temporal and spatial patterns of Wnt/β-catenin signaling activity.
As several Wnt ligands, Frizzled (Fz) receptors and co-factors that are required for Wnt/β-catenin signaling are expressed in the surface ectoderm, changes in their expression patterns may be responsible for the regional activation/inactivation of the canonical Wnt/β-catenin pathway in the presumptive lens area. Similarly, the β-catenin pathway may be regulated by spatial and temporal patterns of expression of Wnt/Fz suppressors. For example, Sfrp2, a putative Wnt/Fz antagonist, is expressed in the mouse lens placode at E9.5 and has been recognised as one of the earliest marker of lens induction (Wawersik et al., 1999). Sfrp2 expression appears to be regulated by Pax6, an essential transcriptional factor for lens placode formation, and it has been proposed that Pax6 induces Sfrp2 which in turn suppresses β-catenin pathway activity during lens placode formation (Duparc et al., 2006; Machon et al., 2010; Shaham et al., 2009; Wawersik et al., 1999). Consistent with this, abnormal Wnt/β-catenin signaling activity in the lens placode has been reported in the Pax6 KO mouse (Machon et al., 2010).
If Sfrp2 is the putative suppressor of β-catenin activity in the lens placode, then its depletion may activate the β-catenin pathway and in this case lens induction would be blocked. However, our finding here is that the conventional double knockout (DKO) of Sfrp2 with its functionally redundant isoform, Sfrp1, did not block lens induction and subsequent lens development. No ectopic Wnt/β-catenin signaling was detected in the lens placode of the DKO mice, rather this pathway remained silent as observed in lens of wild type mice. Thus Sfrp1 and Sfrp2 do not appear to provide the putative negative regulatory influence that downregulates Wnt/β-catenin activity during lens induction. However, these Sfrps do exert some regulatory function because the DKO mice showed reduced Wnt/β-catenin signaling activity in the lens epithelial layer around E13.5. This was not accompanied by a change in proliferation rate, although the DKO lenses were small and the epithelium was incompletely formed. Taken together, these results indicate that Sfrps regulate lens development, specifically through controlling the proportion of Wnt/β-catenin signaling cells in the epithelium.
Materials and methods
Animals
The use of animals in this study conformed to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the use of animals in Ophthalmic and Vision Research. Generation of Sfrp1 and Sfrp2 KO mice was described previously (Satoh et al., 2006). The Sfrp1 allele was knocked-out by replacement of the first exon of the Sfrp1 allele with a nuclear-localisation-signal-tagged LacZ cassette. Sfrp2 was knockedout by deletion of the 5' transcription regulation region and the following first exon. Generation of the TCF/Lef-lacZ transgenic mice was described previously (Mohamed et al., 2004). The reporter lacZ is driven by the hsp68 promoter and upstream x6 TCF/Lef response elements. To distinguish the lacZ allele of the TCF/Lef line from that of the Sfrp1 line we used the following primers designed specifically for the TCF/Lef-lacZ construct for genotyping; Hsp68-F2 agatcatcgccaacgaccag; gal-R2 gggttttcccagtcacgacg. The PCR program was optimized to; 95°C 1 min x1; 95°C 15 secs, 60°C 15 secs, 72°C 10 secs (x40); 72°C 7 mins. The PCR product size is about 200bp.
In situ hybridization
Dissected embryos were fixed with 4% PFA overnight and frozen in OCT compound (Tissue Tek) after gradual cryoprotection with 15% and 30% sucrose/PBS. Cryosections were hybridized with DIG-labelled riboprobes at 72°C overnight. Positive signals were detected by BCIP/NBT AP Substrate kit (SK-5400 Vector Lab). PCR fragments amplified from a cDNA library or genomic DNA with T7-tagged primer sets were used for riboprobe synthesis by T7 RNA polymerase (10881767001, Roche) with DIG RNA Labeling Mix (11277073910, Roche). PCR primer sets used were; Sfrp1 911/930 ACGAGTGTCCCACCTTCCAG and Sfrp1 T7 -635/−616 TAATACGACTCACTATAGGGCTCCCACCCAAACCCTTAGC, which extends exon 3 to 3' UTR of Sfrp1 transcript; Sfrp1 -2320/−2339 GGCCCGAAAGCTGCATGATC and Sfrp1 T7 -2857/−2838 TAATACGACTCACTATAGGGAGCAGCCAACATGCCATCC, which reside in 3' UTR of Sfrp1; Sfrp2 90/109 GCCCGACTTCTCCTACAAGC and Sfrp2 T7 654/635 TAATACGACTCACTATAGGGCTCTTTGTCTCCAGGATGA, which extends exon 1–3 of Sfrp2.
X-gal staining
To detect β-galactosidase (β-gal) activity we followed the X-gal staining method previously described (Robinson et al., 1995). Briefly whole embryos (E9.5 – E11.5), embryonic heads (E12.5 – E16.5) or eyes (E18.5 and P1) were fixed with 2% PFA/1xPBS for 30 min at RT and rinsed twice in PBS for 5 min each. Fixed samples were incubated in staining solution (4 mM potassium ferricyanide /4 mM potassium ferrocyanide/1 mg/ml X-gal (Sigma B4252)/0.1% deoxycholic acid sodium salt (Sigma D6750)/0.2% Nonidet P-40 /2 mM MgCl2 in PBS for overnight at RT with light shield on rotator. Next morning samples were rinsed in PBS for 1 hr and then followed by post-fixation with 10 % NBF for 1 hr at RT before paraffin embedding and sectioning. Images were observed with Zeiss Axioskop 2 mot/AxioCam CCD camera or Leica M60/DFC480 CCD camera.
Immunofluorescent staining
Immunofluorescent staining of paraffin sections was performed by the method described previously (Sugiyama et al., 2009). Antibodies used in this study were as follows; rabbit antibodies against CP49 (2981, (Sandilands et al., 1995)), Pax6 (Covance PRB-278P, 1:300), β-catenin (H102, Santa Cruz sc-7199, 1:200) and c-Maf (M-153, Santa Cruz sc-7866, 1:40), and mouse monoclonal antibodies against β-actin (clone AC15, Sigma A5441, 1:1,000), E-cadherin (clone 36, TDL/BD 610182, 1:800), Prox1 (Chemicon/Merck AB5475, 1:2,000), AP-2α (3B5, DSHB, supernatant 1:1) and BrdU (clone G3G4, DSHB, concentrate, 1:50). For nuclear staining PI (Molecular Probes/Invtrogen 1:5,000) was used. Coverslips were mounted with Aqua Poly/Mount (Polysciences 18606, diluted with dH2O as Aqua Poly: dH2O = 4:1) and images were taken by Zeiss LSM/Pascal confocal system. For BrdU counting analysis, pregnant mice were injected intraperitoneally with BrdU (100 µg/g body weight) 1 hr before euthanasia and embryo collection.
Results
Sfrp expression patterns
Because Sfrps regulate important signaling pathways, and their distribution patterns provide clues about their domains of activity, they have generated considerable interest and several studies have reported on their expression in mouse eyes.
Expression of Sfrp1 is restricted to lens fibers
For Sfrp1 in the mouse eye, a variety of different expression patterns have been described but no consensus has yet been reached (Chen et al., 2004; Esteve et al., 2011a; Leimeister et al., 1998; Liu et al., 2003). Initially we obtained several antibodies against Sfrp1 for immunostaining but none of the staining showed depletion in our KO mouse tissue samples (data not shown). Since this discrepancy may indicate technical difficulties for Sfrp1 detection by immunostaining, we examined Sfrp1 expression using a different approach. In a reporter mouse model we detected β-gal activity which is driven by an endogenous Sfrp1 promoter/enhancer and has been shown to reflect endogenous expression of Sfrp1 (Satoh et al., 2006). We also conducted an in situ hybridization analysis for Sfrp1 to see how this related to the results from the reporter study.
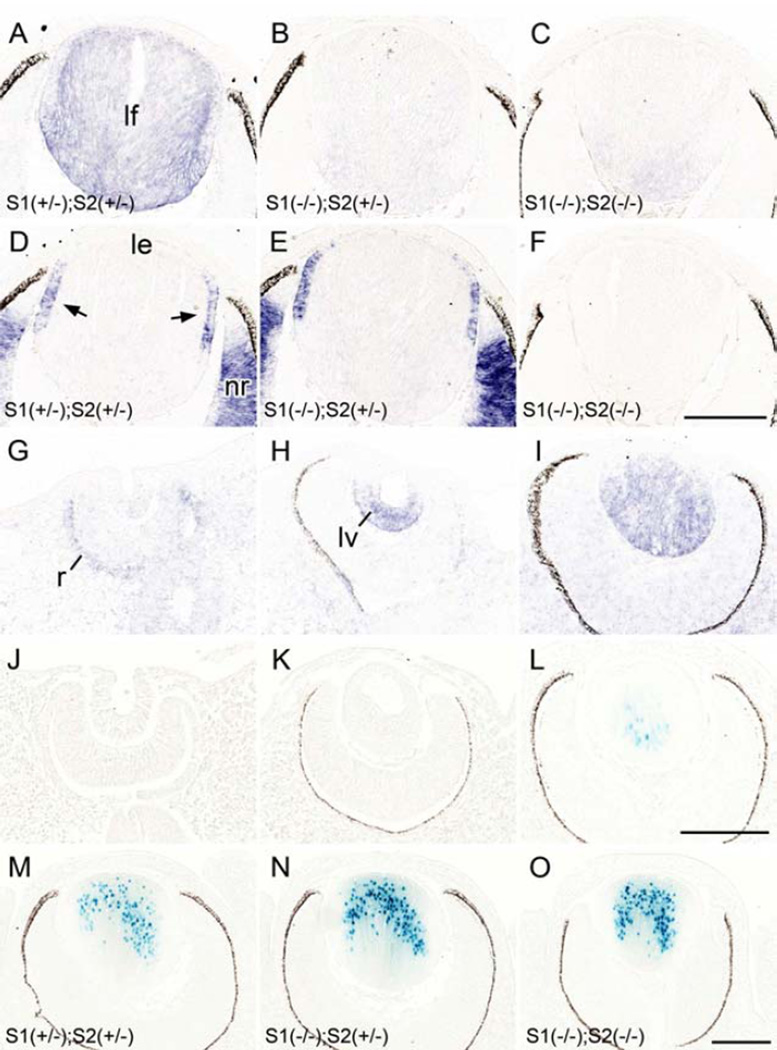
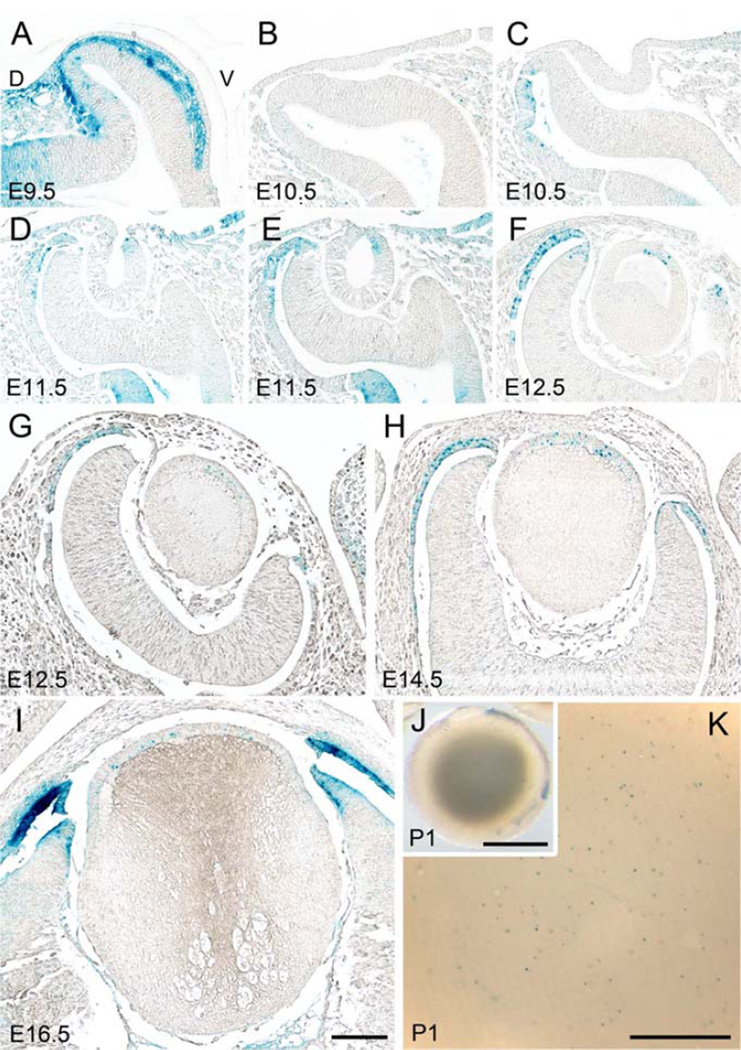
First, to assess the specificity of our in situ hybridization probe we examined Sfrp1 expression in the lens of E14.5 mice. We showed that Sfrp1 was consistently detected in lens fibers with slightly enhanced intensity in the cortical region (Fig. 1A). This signal was absent from Sfrp1 KO samples (Fig. 1B, C); thus confirming the specificity of the Sfrp1 probe. For the developmental pattern we showed that Sfrp1 was barely detected in the lens cells at lens pit stage (Fig. 1G), whereas a weak signal was evident in the outer layer of the developing retina. By the time of lens pit/vesicle separation from the surface ectoderm (E11.5), a strong signal for Sfrp1 was detected in the posterior cells (Fig. 1H). After closure of the lens vesicle lumen at E12.5, Sfrp1 was detected mainly in the primary lens fibers (Fig. 1I). Using the reporter mouse we did not detect Xgal labeling (blue signal) during lens placode, lens pit or lens vesicle stages (Fig. 1J, K). The earliest signal was observed in the fully elongated innermost primary fibers around the time of lens vesicle lumen closure (Fig. 1L). The number of X-gal positive fibers increased as new fibers were added and the signal was most prominently detected in the older (innermost) fibers (Fig. 1M-O). Since the β-gal induced in this mouse line was tagged with a nuclear localization signal, the X-gal staining was mainly detected in nuclei but with stronger staining some cytoplasmic signal was also detected. β-gal activity was highly specific to lens fibers and we did not detect any X-gal staining in other parts of the eye (i.e. retina, cornea etc.), at least until postnatal day 0. Thus, in situ and reporter methods show essentially similar results in that the expression of Sfrp1 was restricted to the fibers. The main difference was that the in situ method appears to be more sensitive because it detects Sfpr1 expression in the presumptive primary fibers at E11.5, whereas the reporter method did not detect signal in the primary fibers until elongation was well advanced at E12.5. Also at later developmental stages the observation that the signal was most prominent in the more mature fibers indicates that a certain amount of transcription or translation (ie. β-galactosidase) product needs to accumulate to produce blue staining.
Fig. 1. Sfrp expression pattern in lens.
(A-I) In situ hybridization for Sfrp1 (A-C, G-I) and Sfrp2 (D-F) expression in the developing eye. Sfrp1 was consistently detected in lens fibers (lf) with slightly enhanced intensity in the cortical region (A). This signal was depleted from Sfrp1 KO samples (B, C). Sfrp2 was detected in the germinative zone (arrows) of the lens epithelium (le) and the neural retina (nr; D, E) and these signals were absent in the Sfrp2 KO sample (F). Note the sections A and D, B and E, and C and F, respectively were obtained from the same embryos (at E14.5). Sfrp1 was barely detected in the lens cells at lens pit stage (E10.5, G) whereas a weak signal was evident in the outer layer of the developing retina (r). By the time of lens pit/vesicle (lv) separation from the surface ectoderm a strong signal for Sfrp1 was detected in the posterior cells (at E11.5, H). After closure of the lens vesicle lumen Sfrp1 was detected mainly in the primary lens fibers (at E12.5, I). (J-O) The β-galactosidase derived from the Sfrp1 knock-in locus was monitored by X-gal staining. The nuclear-localisation signal conjugated to this enzyme resulted in blue stain development that was predominantly localized to the nucleus. (J, K) No X-gal staining was observed at E10.5 (J) and E11.5 (K) during lens pit and lens vesicle stages. (L) The first positive signal was detected at E12.5 in centrally located primary lens fibers. (M-O). Intensity of X-gal staining correlated with the copy number of the Sfrp1 knock-in allele; the lens with a single copy (i.e. heterozygote) showed weaker X-gal stain (M) compared to the lens with two copies of the Sfrp1 knock-in allele (i.e. homozygote, N). Removal of the Sfrp2 gene did not affect the intensity (or pattern) of X-gal staining because a similar intensity of X-gal staining was evident in Sfrp2 heterozygote (N) and Sfrp2 KO (O) lenses. Images shown were obtained from E14.5 embryos. Scale bars for A-F, G-L, M-O 200 µm.
It was also noted that the intensity of X-gal staining was relatively weak in lenses of Sfrp1 heterozygotes (Fig. 1M) compared to stronger staining in lenses of Sfrp1 homozygote mice (Fig. 1N). We did not see any compensatory activation of the Sfrp1 enhancer/promoter with removal of a Sfrp2 allele; i.e. intensity of the Sfrp1 X-gal signal was not altered in Sfrp2+/+, +/− or −/−, but rather reflected the copy number of the modified Sfrp1 allele. This is evident in Figure 1N and O (that shows two lenses from mice homozygous for the Sfrp1-lacZ transgene but heterozygous (N) or homozygous (O) for the Sfrp2 deletion allele); however, the intensity of X-gal signal was not affected by the change of Sfrp2 allele number as both lenses had similarly strong staining. The intensity of X-gal staining in brain also correlated with transgene copy number in whole mounts but again was independent of Sfrp2 allele number (Supplementary Fig. S1E-G). This result indicates that the expression level of Sfrp1 mRNA is stoichiometric to its copy number and Sfrp2 depletion does not alter the level of Sfpr1 transcription.
Sfrp2 expression in lens is transitory
Several studies have also reported on expression of Sfrp2 in the mouse eye but, unlike Sfrp1, they are all in general agreement. A distinctively localised and temporally regulated expression pattern for Sfrp2 in lens has been described by in situ hybridization experiments (Chen et al., 2004; Esteve et al., 2011a; Leimeister et al., 1998; Liu et al., 2003; Wawersik et al., 1999) (see summary in Fig. 8). These studies show that Sfpr2 expression is first detected in lens placode (Chen et al., 2004; Wawersik et al., 1999) and then throughout the lens pit-stage of morphogenesis (Chen et al., 2004; Esteve et al., 2011a). On the formation of the lens vesicle, Sfrp2 mRNA becomes restricted to anterior lens epithelial cells and is absent from primary lens fibers (Chen et al., 2004). This polarised expression of Sfrp2 is maintained after lens vesicle lumen closure and during secondary fiber cell induction (Esteve et al., 2011a; Leimeister et al., 1998; Liu et al., 2003) but becomes predominantly restricted to the incipient germinative zone of the epithelium just above the lens equator (Chen et al., 2004) before being undetectable in the lens epithelium around E16.5 and beyond (Chen et al., 2004; Leimeister et al., 1998; Liu et al., 2003). During the current studies we confirmed this pattern of Sfrp2 expression by in situ hybridisation. We also confirmed the veracity of this result by showing that the characteristic lens expression pattern for Sfrp2 in the germinative zone of the E14.5 lens was absent in the Sfrp2 KO sample (Fig. 1D-F).
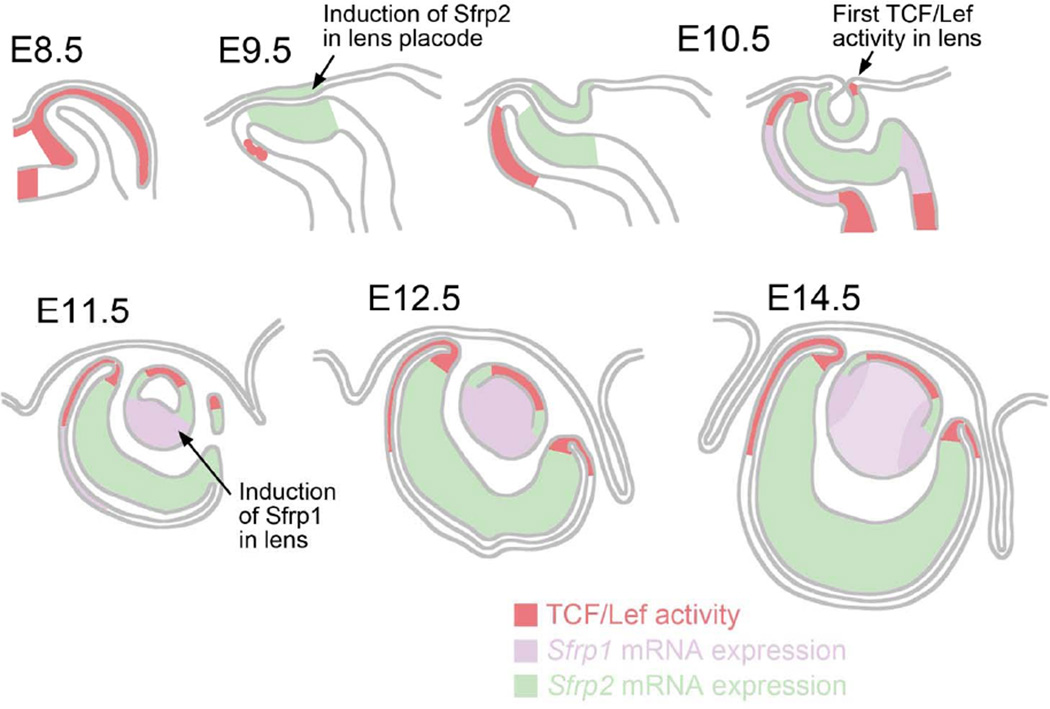
Fig. 8. Non-overlapping distribution of Sfrp1 and Sfrp2 expression and TCF/Lef activity.
Sfrp2 mRNA is detected in developing lens cells at lens placode (E9.5) and lens pit stages (E10.5). It becomes restricted to a band of cells above the lens equator at E11.5 and is not detectable in lens after E16.5 (see Chen et al, 2004). Sfrp1 expression is not detected in lens cells until the lens pit stages and then becomes strongly expressed in the elongating primary fibers of the lens vesicle at E11.5. Strong expression persists in fibers through all embryonic stages with slightly more prominent expression detected in cortical fibers. TCF/Lef activity is first detected at the ventral edge of lens pit at E10.5 and then found in the central lens epithelial cells after E11.5. The number of cells showing TCF/Lef activity in the lens epithelium diminishes after E16.5. Sfrp2 expression and TCF/Lef activity show complementary patterns in the developing retina. Although Sfrp1 and Sfrp2 do not show overlapping patterns of expression, it is only in DKOs of Sfrp1 and Sfrp2 that abnormal development of both lens and retina is evident. Moreover, analysis of DKOs reveals that Sfrp1 and Sfrp2 do not have a suppressive effect on TCF/Lef activity in eye but rather appear to act as enhancers of TCF/Lef activity in adjacent cells.
Taken together, these results indicate that Sfrp1 transcripts have non-overlapping expression patterns with Sfrp2 in the lens, at least during embryogenesis. Non-overlapping expression is also evident in other eye tissues and head structures (Supplementary Fig. S1A-D). A diagrammatic overview of Sfrp1 and Sfrp2 expression in relation to TCF/Lef activity in the developing eye is presented in Figure 8.
Double knockout of Sfrp1 and 2 does not block lens induction
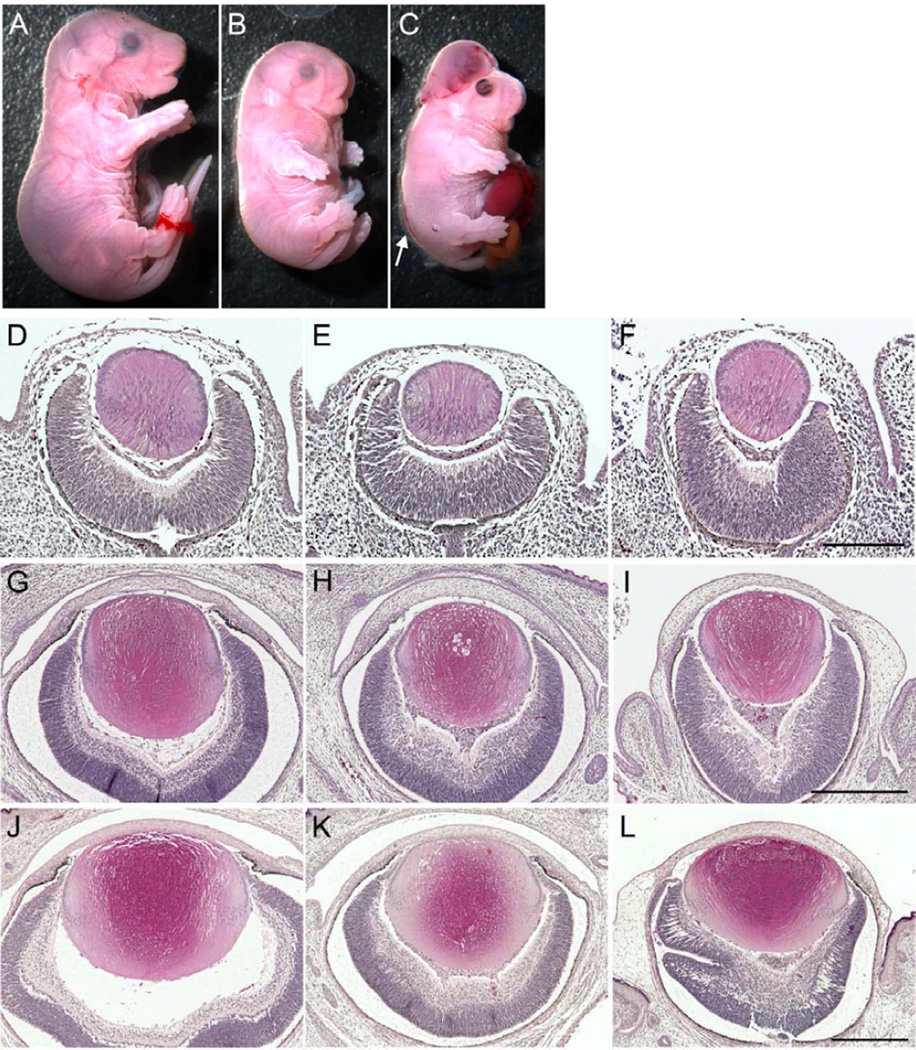
Previous analysis of Sfrp1 and Sfrp2 KO mice have shown that single gene KO (Sfrp1−/−;Sfrp2+/+, Sfrp1+/+;Sfrp2−/−, Sfrp1−/−;Sfrp2+/−, or Sfrp1+/−;Sfrp2−/−) did not exhibit any particular defects but DKOs (Sfrp1−/−;Sfrp2−/−) were embryonic lethal by E16.5 displaying a small-sized body with short snout, truncated back bones and short limbs (Satoh et al., 2006). In our colony, some DKO embryos survived until E18.5 (Fig. 2A-C). In these mice we frequently observed exencephaly, eyelid closure defects and protruded gut (Fig. 2C). We also noticed that on occasion, DKO embryos displayed an open neural tube at the lower part of the back (Misra and Matise, 2010) (Fig. 2C arrow).
Fig. 2. Lens formation in Sfrp1;Sfrp2 DKOs.
(A-C) Embryonic appearance at E18.5. In contrast to the normal appearance of Sfrp1−/−;Sfrp2+/− control embryo (A), Sfrp1;Sfrp2 DKOs (B, C) are small and have characteristically truncated snouts, short limbs and round backs. Occasionally, DKOs also show exencephaly, eyelid closure defect, open neural tube (arrow) and protruded guts (C). (D-L) H&E staining of transverse paraffin sections of eyes at E12.5 (D-F), E16.5 (G-I) and E18.5 (J-L) for controls (D, G, J), DKOs with closed eyelid (E, H, K) and with open eyelid defect (F, I, L). At E16.5 and E18.5, the open eyelid DKO eyes were not covered by a developing eyelid (I, L). Compared to control lenses, DKO lenses were small and had abnormal shapes. DKOs with closed eyelid tended to have round-shaped lenses whereas DKOs with open eyelid defect tended to be more variable but progressively developed a more flattened shape. Defects were also seen in development of the retina and vitreous and these tended to be more prominent in the mice with open eyelids. Scale bars for D-F 200 µm, G-I 400 µm, J-L 400 µm.
We examined lens formation in Sfrp1;Sfrp2 DKO embryos at E12.5 (Fig. 2D-F), E16.5 (Fig. 2G-I) and E18.5 (Fig. 2J-L). We did not see any defect in lens formation in Sfrp1 and Sfrp2 single KO (Sfrp1−/−;Sfrp2+/+, Sfrp1−/−;Sfrp2+/−, Sfrp1+/+;Sfrp2−/−, or Sfrp1+/−;Sfrp2−/−) so that these genotypes served as control tissues (Fig. 2D, G, J). In contrast, DKO lenses were small in size and showed severe and variable eye development defects; DKO embryos with closed eyes tended to have mildly affected lenses that tended to be round in shape (Fig. 2E, H, K), whilst DKO embryos with an open eyelid defect had smaller lenses that progressively developed a more flattened shape (Fig. 2F, I, L). In addition to the lens defects, severe defects were evident in the layering of retina. The iris, vitreous and cornea also developed abnormalities as previously reported (Esteve et al., 2011a; Esteve et al., 2011b). Overall eye defects tended to be more severe in embryos with open eyelid defects compared with those with closed eyelids. Importantly, all of the DKO embryos we analysed formed lenses, albeit, abnormally.
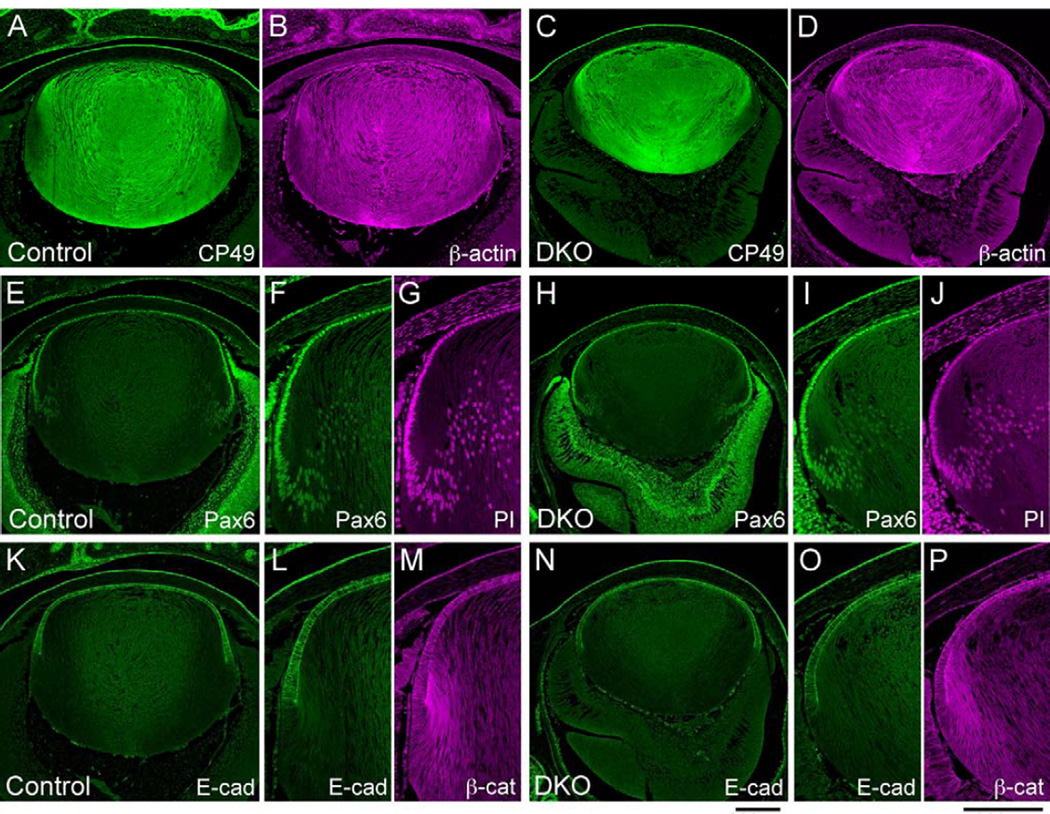
The lenses of Sfrp1;Sfrp2 DKOs expressed lens epithelial and fiber cell markers similar to control lenses at E18.5 (Fig. 3). CP49 (also known as BFSP2) is a lens fiber-specific intermediate filament that is expressed during lens fiber differentiation (Ireland and Maisel, 1984; Sandilands et al., 1995). We detected CP49 expression in Sfrp1;Sfrp2 DKO lens fibers as well as in control tissues along with prominent β-actin staining (Fig. 3A-D). Pax6 is one of the lens epithelial cell markers that is critical for lens induction and lens epithelial cell maintenance/differentiation (Grindley et al., 1995; Shaham et al., 2009). Although the lens epithelial cell layer of DKOs was invariably thinner than in controls, we detected Pax6 expression in the epithelium of DKO lenses with a similar intensity to control tissues and, as in controls, this immunoreactivity similarly diminished during fiber differentiation (Fig. 3E-J). We also found E-cadherin was expressed in both control and DKO lenses. In controls, down regulation of E-cadherin occurred sharply at the lens equator (Fig. 3K-M) (Nose and Takeichi, 1986). This loss of E-cadherin was also observed in DKO lenses (Fig. 3N-P). Thus, the shift from the epithelial state to the onset of fiber differentiation, as well as the spatial patterns of protein synthesis appear to be normal in DKO lenses.
Fig. 3. Lens fiber and epithelial cell markers are expressed normally in Sfrp1;Sfrp2 DKO lenses at E18.5.
Transverse paraffin sections of control and DKO lenses were immunostained with the antibodies indicated and fluorescent signals were imaged by confocal microscopy. (A-D) CP49 is a lens-specific intermediate filament protein which is expressed during fiber differentiation (A). In DKO lenses (C) CP49 expression is detected in differentiated fibers as observed in controls. Expression pattern of β-actin also appeared similar in control and DKO lenses (B, D). (E-J) Pax6 is a lens epithelial cell marker which is highly expressed in lens epithelial cells and diminishes gradually during fiber differentiation (E). Normal expression pattern of Pax6 is maintained in Sfrp1;Sfrp2 DKO lens (H). Higher magnification images (F,G, I,J), counter stained with PI to visualise nuclei, show that Pax6 is localised to nuclei. (K-P) E-cadherin is another lens epithelial cell marker that disappears immediately at the equator at the commencement of fiber differentiation (K). In the DKO lens, E-cadherin is similarly strongly expressed in the epithelium and is lost immediately below the equator (N). In the higher magnification views (L, M, O, P), the sharp loss of E-cadherin at the lens equator is evident. Immunostaining for β-catenin also shows a similar distribution pattern between control and DKO. Scale bars for A-E, H, K, N 200 µm, F, G, I, J, L, M, O, P 200 µm.
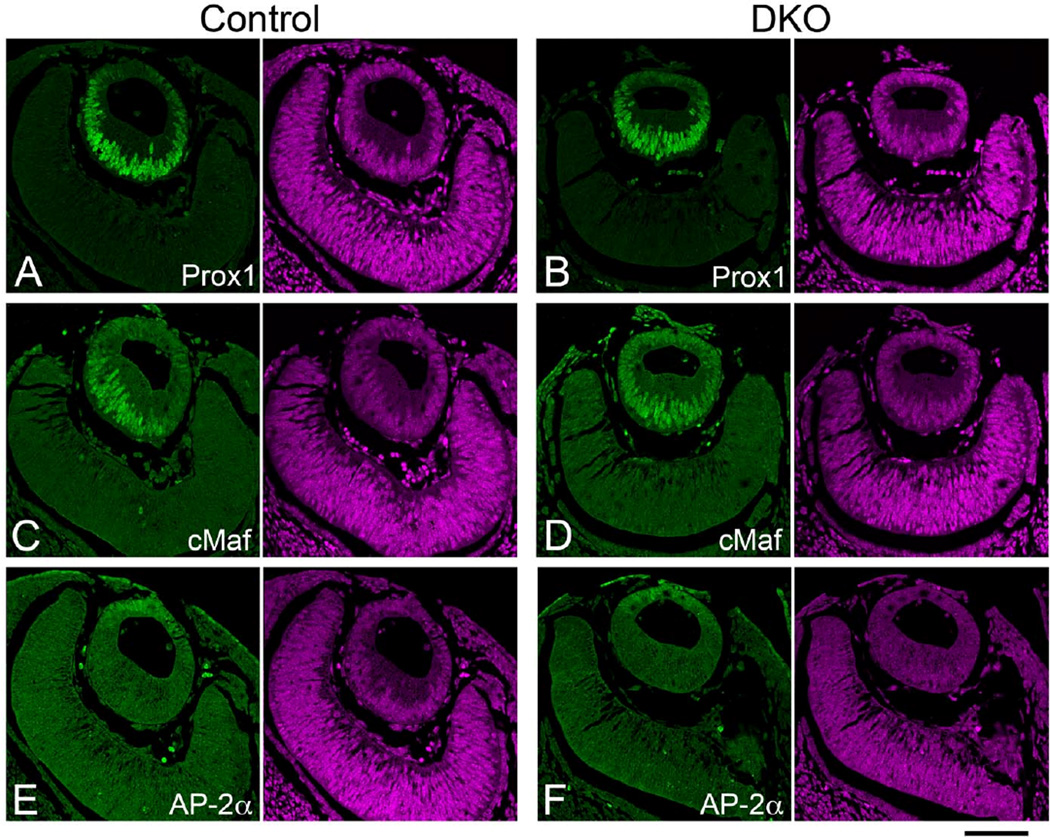
We next proceeded to examine the induction of early lens marker expression in Sfrp1;Sfrp2 DKO lenses (Fig. 4). A prospero-related homeobox protein, Prox1, and a Maf family transcription factor, cMaf, are essential for lens fiber differentiation starting from E11.5 (Kawauchi et al., 1999; Ring et al., 2000; Wigle et al., 1999). In control lenses these proteins were detected predominantly in the nuclei of elongating primary lens fibers and to a lesser extent in the anterior lens epithelial cells at E11.5 (Fig. 4A, C). Similar distribution of Prox1 and cMaf was detected in DKO lenses (Fig. 4B, D). A retinoic acid responsive protein family member, AP-2α transcription factor, is essential for lens vesicle separation from the surface ectoderm that occurs around E11 to form a lens vesicle (Pontoriero et al., 2008). AP-2α expression was confined to the anterior lens epithelium and overlying surface ectoderm in control embryos (Fig. 4E) and this expression pattern was retained in DKO embryos at E11.5 (Fig. 4F). In addition to the normal expression of Pax6 in early lens (Esteve et al., 2011a), the normal induction and distribution pattern of these lens markers suggests fundamental lens induction/formation processes are maintained in Sfrp1;Sfrp2 DKO embryos.
Fig. 4. Induction of early lens markers in Sfrp1;Sfrp2 DKO lenses (E11.5).
Confocal images of paraffin sections immunostained with antibodies indicated in the panels (green). Each panel was paired with PI counterstaining to show nuclei (purple). (A, B) Prox1 is induced in the lens placode and at the lens vesicle stage Prox1 is detected in the elongating primary fibers (A). A normal pattern of expression of Prox1 is maintained in Sfrp1;Sfrp2 DKO lenses (B). (C, D) cMaf is another lens marker with its expression induced in the lens placode and which is subsequently detected in the anterior lens epithelial cells as well as being significantly increased in elongating fibers of the lens vesicle (C). cMaf expression is also maintained in Sfrp1;Sfrp2 DKO lenses (F). (E, F) AP-2α is detected in the anterior lens cells in both control (E) and DKO lenses (F). Scale bar 100µm.
TCF/Lef activity in lens
So far transcriptional activity of the canonical Wnt/β-catenin pathway in lens has been reported using 4 independently established reporter lines [TOPGAL (DasGupta and Fuchs, 1999), BAT-gal (Maretto et al., 2003), BATlacZ (Nakaya et al., 2005) and TCF/Lef-lacZ (Mohamed et al., 2004)]. Consistent with the fact that Wnt/β-catenin signaling is not required during lens induction, reporter activity has never been detected in the presumptive lens region of surface ectoderm or lens placode [TOPGAL (Miller et al., 2006; Smith et al., 2005; Zhou et al., 2008), BAT-gal (Kreslova et al., 2007), BATlacZ (Song et al., 2007) and TCF/Lef-lacZ (Liu et al., 2006)]. However, results from analysis of conditional KOs of β-catenin or conventional KOs of Lrp6 in the lens has indicated a role for the Wnt/β-catenin pathway in the formation/maintenance of the lens epithelium during lens morphogenesis (Kreslova et al., 2007; Smith et al., 2005; Stump et al., 2003). Although both TOPGAL and BAT-gal reporter lines have not shown transcriptional activity in lens epithelial cells (Kreslova et al., 2007; Miller et al., 2006; Smith et al., 2005), the TCF/Lef-lacZ line showed transient activity in lens epithelial cells between E11.5 and E14.5 (Liu et al., 2003; Liu et al., 2006).
Here we extend previous studies and provide a detailed analysis of the TCF/Lef-lacZ reporter line. In the TCF/Lef-lacZ transgenic mouse, no β-gal activity was detected in the presumptive lens region at E9.5 (Fig. 5A). In contrast, strong X-gal labeling (blue stain) was detected in the mesenchymal cells that lie between the surface ectoderm and the neuroectodermal outpocketing as was similarly observed in the BATlacZ line (Song et al., 2007) (Fig. 5A). However, by E10.5 these mesenchymal cells have been excluded from this area as surface ectoderm and optic vesicle make close contact (Fig. 5B). The first β-gal activity was detected in the dorsal outer layer of the optic vesicle around E10.5 and at this stage no activity was seen in the lens pit cells (Fig. 5C). Interestingly the first β-gal activity in lens was found asymmetrically in the cells at the rim of the lens pit on the ventral side (Fig. 5D, E). After closure of lens pit, the β-gal positive cells were detected in the lens epithelial cells around the anterior pole at E12.5 (Fig. 5F). After secondary fiber differentiation, β-gal positive cells were still predominantly present in the central region of the epithelium; few, if any, positive cells were present towards the epithelial periphery where the germinative zone develops (Fig. 5G, H). This asymmetric pattern was reciprocal to the β-gal pattern in developing retina where the positive cells were found at the rim of the optic cup opposite the peripheral lens epithelium (see also Fig. 8 for schematic summary). The proportion of cells with β-gal activity in the lens epithelium gradually diminished after E16.5 (Fig. 5I). However, in postnatal lenses a small population of lens epithelial cells were still positive for β-gal activity. These were most readily detected in a whole lens view of the anterior lens surface (Fig. 5J, K). This analysis of TCF/Lef activity indicates that the Wnt/β-catenin pathway is active in lens epithelial cells throughout embryogenesis, longer than previously reported, and this activity persists in postnatal lenses.
Fig. 5. TCF/Lef reporter activity during lens formation (E9.5-P1).
Coronal paraffin sections of TCF/Lef-lacZ transgenic embryos were reacted with X-gal to detect β-gal activity (A-I). Dorsal to left and ventral to right in all images. (A) At E9.5, strong β-gal activity is detected in neural crest-derived mescenchymal cells between surface ectoderm and optic vesicle but signal is absent in presumptive lens region. (B, C) At E10.5, β-gal reactivity is still not evident in lens placode (B) and lens pit (C). At this stage, X-gal staining is evident in the outer layer of the optic cup on its dorsal side. (D, E) At E11.5, two slices from a same eye through the centre of the lens vesicle (D) and slightly off-centre where lens vesicle is still fused with surface ectoderm (E) are shown. At the neck of lens pit/vesicle blue β-gal activity is asymmetric, being predominantly present on the ventral side. (F) At early E12.5, β-gal activity is present centrally in the anterior layer of the lens vesicle. In the optic cup, X-gal staining is detected at the distal half of the outer layer and the distal tip of the inner layer (presumptive neural retina). (G, H) At late E12.5 and E14.5, X-gal staining is present in the central lens epithelial cells, distal tips of neural retina and distal portion of RPE. (I) At E16.5, X-gal staining in the central lens epithelial cells becomes weaker but is clearly stronger at the distal tips of the optic cup where the ciliary body and iris forms. (J, K) At P1, the whole lens viewed at high magnification shows that cells with β-gal activity are scattered throughout the epithelium. Scale bars for A-I 100 µm, J 1 µm, K 100 µm.
The spatiotemporal distribution of TCF/Lef activity in lens appears to be accompanied by the expression of Lrp6, a co-receptor of Frizzled for Wnt/β-catenin signal activation (Supplementary Fig. S2). The Lrp6 signal was first detected in the epithelial cells of the lens pit (Fig. S2B), about the time as when the first TCF/Lef activity became detectable. Then Lrp6 signal was observed in most cells of the lens vesicle at E11.5 (Fig. S2C) and then in cells of the lens epithelium from E12.5 onwards (Fig. S2D-F). The expression of Lrp6 generally overlapped with TCF/Lef activity but did not match completely; for example we noticed the Lrp6 signal was slightly stronger in the periphery of the lens epithelium (germinative zone) whilst TCF/Lef activity was more intense in the centrally situated epithelial cells. Lrp5 expression was not detected in the lens at any stage examined (E9.5 and E18.5; S2G, I, and data not shown).
TCF/Lef activity in Sfrp1 and Sfrp2 double knockout lenses
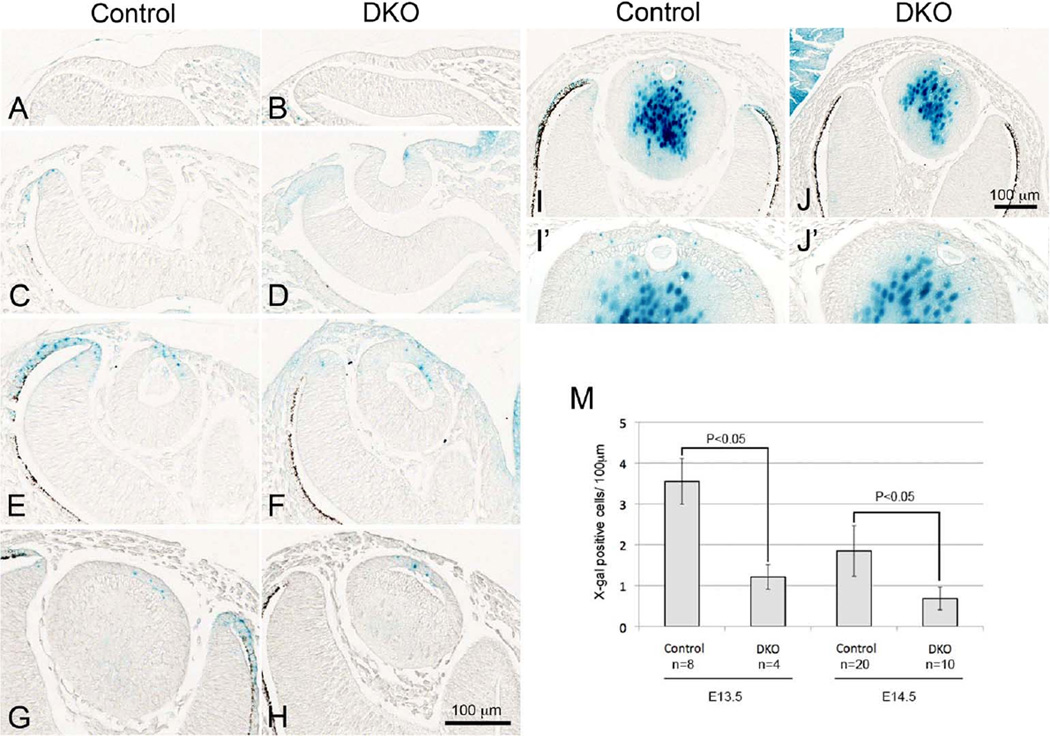
If Sfrp1 or Sfrp2 is a putative suppressor of Wnt/β-catenin activity in presumptive lens region, it would be expected that TCF/Lef-lacZ activity would be enhanced in Sfrp DKO lenses. To test this we examined β-gal activity in lens placode by generating Sfrp1;Sfrp2;TCF/Lef-lacZ mice. As shown in Fig. 6A and B, no β-gal activity was detected in the lens placode at E9.5 of DKO mice similar to the situation in control lines of mice. Between E10.5 and E11.5, when lens pit develops and pinches off, β-gal activity was detected in DKOs in some cells on the ventral side of the developing lens and then in the central region of the epithelial layer just as in control lenses (Fig. 6C-F). In contrast, reduction of β-gal activity in the dorsal retina tips of the DKO mice was clearly observed at E11.5 with this reporter line (Fig. 6E, F). This is consistent with a previous report by Esteve and colleagues that showed the absence of expression of Lef1 and Axin2, as well as the lack of the non-phosphorylated active form of nuclear β-catenin, in the optic cup (Esteve et al., 2011b). After E12.5 when secondary fiber cells have started differentiating, β-gal activity from the Sfrp1 KO allele was also detected in the central lens fibers (cf. Fig. 1). However β-gal activity of the TCF/Lef-lacZ allele was readily distinguishable from the Sfrp1 allele-derived β-gal activity because of its different localisation (i.e., TCF/Lef-lacZ is expressed in lens epithelial cells). We detected TCF/Lef-lacZ activity in lens epithelial cells of DKO lenses from E12.5 to E15.5 and then observed that the signal was reduced after E16.5 as was the case in controls (Fig. 6G-J and data not shown). However, we noticed that X-gal positive epithelial cells in the DKOs appeared to be reduced in number compared with controls (Fig. 6I, I’, J, J’). Statistical analysis showed that there were significantly fewer X-gal positive cells in DKO lenses at E13.5 and E14.5 (Fig. 6M). Again reduction of X-gal staining was consistently observed at the distal tips of the optic cup of DKOs from E12.5 to E16.5 (Fig. 6G-J, data not shown). These results indicate that Sfrp1 and Sfrp2 individually, or together, do not constitute the putative negative regulator that blocks Wnt/β-catenin signaling during lens induction. In contrast, the presence of Sfrp1 and Sfrp2 appear to have a positive regulatory function towards maintaining the episode of prominent β-catenin signaling that occurs in the differentiating epithelium between E11.5 and E15.5.
Fig. 6. TCF/Lef activity in Sfrp1;Sfrp2 DKO lenses detected by X-gal staining.
Staining results were shown for controls (A, C, E, G, I) and Sfrp1;Sfrp2 DKO (B, D, F, H, J). (A, B) At E9.5, no ectopic activation of TCF/Lef activity is evident in DKO lens placode. (C, D) At E10.5, normal induction of TCF/Lef activity is detected in cells on the ventral side of the neck of the lens pit in the DKO eye (D). (E-H) At E11.5 and 12.5, TCF/Lef activity is present in the anterior lens epithelial cells of the DKO lens vesicle (F, H) similar to controls (E, G); however, the X-gal staining at the distal tips of optic cup is dramatically reduced in DKO eyes. (I, J) At E13.5, in contrast to relatively abundant X-gal staining in control lens epithelial cells (I), fewer positive cells are found in DKO lens epithelium (J). Enlarged images are shown in I' and J'. (M) X-gal positive cells were counted and shown as numbers per 100 µm of epithelium. There are significantly fewer X-gal positive cells in DKO lenses at E13.5 and E14.5 than in controls. X-gal stained cells were counted from at least three non-sequential sections per samples. Total length examined per sample was about 900 µm (control E13.5), 600 µm (DKO E13.5), 1,800 µm (control E14.5) and 1,000 µm (DKO E14.5). Counted sample numbers were indicated in the chart (n). The mean value of each sample was analysed by the two-tailed t-test. Scale bars for A-H 100 µm, I, J 100 µm.
Deletion of Sfrp1 and Sfrp2 does not affect proliferation rate
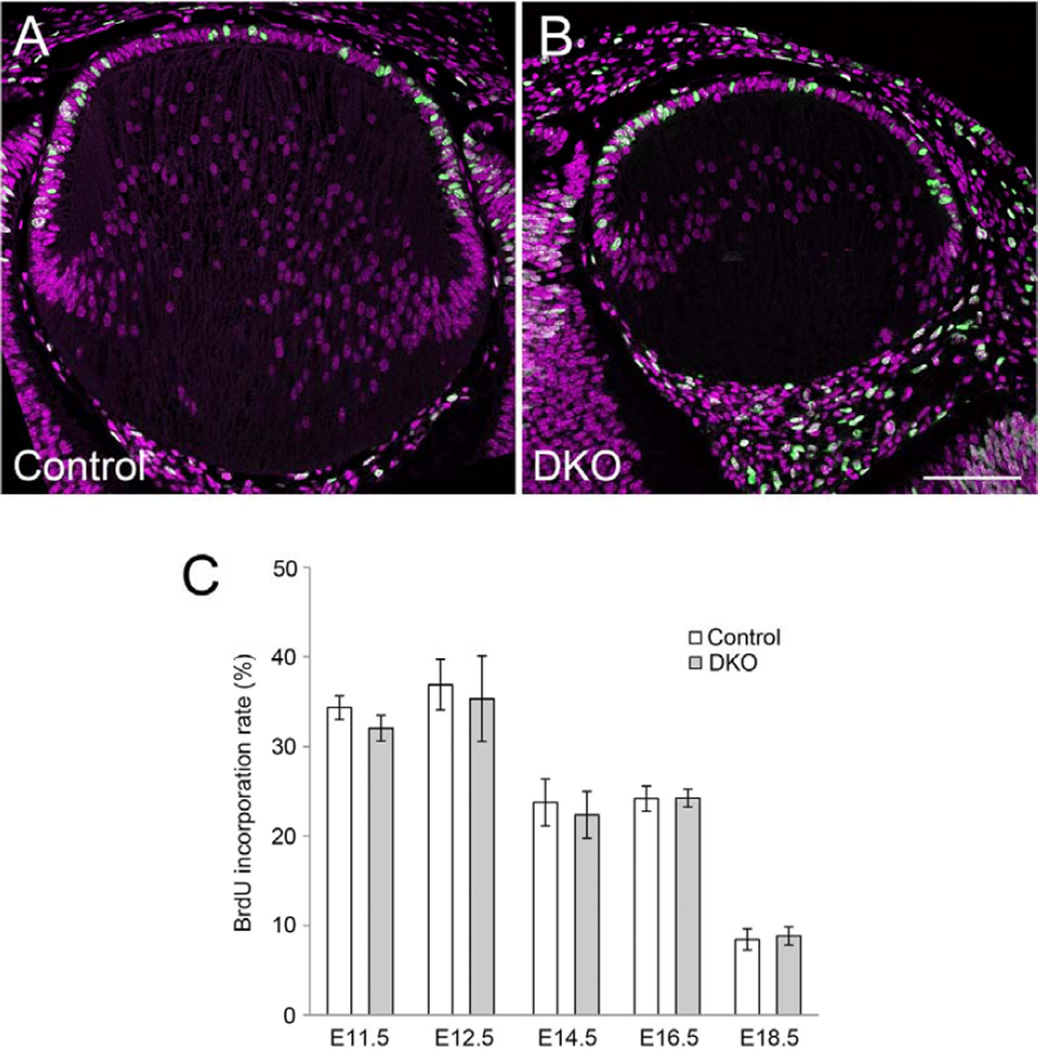
Given the earlier studies that showed conditional KO of β-catenin in lens epithelial cells around E12.5 caused a significant reduction in proliferation rate (Cain et al., 2008), it was thought that the reduced Wnt/β-catenin signaling activity in the epithelium of the DKO mice might be associated with a reduced rate of proliferation. To investigate this possibility, BrdU was injected into pregnant mice and embryos were collected after one hour. Counts of BrdU-labeled cells showed that there was no statistical difference in rate of BrdU incorporation between controls and DKOs at all the ages examined between E11.5 to E18.5 (Fig. 7). These observations showed that knocking out both Sfrp1 and Sfrp2 did not significantly alter the rate of lens epithelial cell proliferation; consequently, the reduction in numbers of TCF/Lef active cells in the epithelium of DKO mice could not simply be attributed to a reduced rate of cell proliferation in this region.
Fig. 7. BrdU incorporation rate does not change in Sfrp1;Sfrp2 DKO lenses.
Pregnant mice were injected with BrdU (100 µg/g body weight) 1 hour before collection of embryos. Incorporated BrdU was visualised by immunostaining with anti-BrdU antibody. (A, B) Confocal images of incorporated BrdU (green) and counterstained PI (purple) in the E14.5 lens. BrdU positive cells and total cell numbers were counted in the central epithelium. Scale bar 200 µm. (C) Mean value of incorporated BrdU (%) in lens cells of lens pit (E11.5), lens epithelial cells located anterior to lens lumen/lens fibers (E12.5) and central lens epithelial cells (E14.5, E16.5 and E18.5). Statistical analysis (two-tailed t-test, α=0.001) does not show any significant differences between controls and DKOs at all ages. Sample number (n) and cells counted were as follows; E11.5 control 1,054 (6), DKO 1,207 (8), E12.5 control 4,748 (18), DKO 2,140 (10), E14.5 control 1,814 (8), DKO 720 (4), E16.5 control 1,617 (6), DKO 1,103 (4), E18.5 control 2, 347 (10), DKO 1,534 (7). Scale bar 200 µm.
Discussion
Inactivation of Wnt/β-catenin pathway in lens placode is not regulated by Sfrp1 and Sfrp2
Because of the highly localised and strong expression of Sfrp2 in lens placode and its reported antagonistic activity on Wnt/Fz signaling, we and others conjectured that this Sfrp was important for suppression of Wnt/β-catenin signaling at this stage of lens development. However, the current study has shown that depletion of both Sfrp1 and Sfrp2 did not block lens induction. Consistent with this we did not observe ectopic Wnt/β-catenin signaling activation in the lens placode in DKO embryos. Thus Sfrp1 and Sfrp2 are not involved in the suppression of the Wnt/β-catenin pathway that is required for lens morphogenesis to proceed.
This leaves the question open as to how Wnt/β-catenin signaling is suppressed early in lens morphogenesis. It is possible that other members of the Sfrp family, such as the closely related Sfrp5, could contribute to regulation of the Wnt/β-catenin pathway during lens placode stage. Note that among the five members of the vertebrate Sfrp family, phylogenetic analysis has shown that Sfrp1, Sfrp2 and Sfrp5 form a subgroup that is distinct from other family members (Bovolenta et al., 2008; Mii and Taira, 2011). However, the observation that Sfrp1;Sfrp5 DKO or Sfrp2;Sfrp5 DKO mice are normal (Satoh et al., 2008), as well as the fact that we could not detect Sfrp5 transcripts from embryonic eye samples by RT-PCR indicates that Sfrp5 is unlikely to be a key regulator of ocular developmental processes. Another possibility is that other inhibitor families, such as the Dkks, may be involved in suppressing Wnt/β-catenin signaling. In addition, other mechanisms could involve down-regulation or absence of Lrp5 or 6 as the presence of a member of this family of co-receptors is required for Wnt/β-catenin pathway activation. Our in situ hybridization experiments supports this idea since no expression of Lrp6 was detected in the lens placode (Fig. S2A). Consistent with this, Lrp6 depletion did not block lens induction (Smith et al., 2005; Stump et al., 2003; Zhou et al., 2008). Thus absence of Lrp6 may contribute to keeping the Wnt/β-catenin pathway inactive during early stages of lens development. Lrp5 is known to have a role in hyaloid vessel regression in postnatal eyes (Kato et al., 2002) but so far there is no evidence for involvement of Lrp5 in early eye development. We also did not detect distinct Lrp5 expression in lens during development between E9.5 to E18.5 (Fig. S2G and data not shown). One further possibility is that the non-canonical Wnt/planar cell polarity (PCP) pathway, that is purported to be inhibitory to the canonical pathway (Grumolato et al., 2010; Torres et al., 1996)), and known to operate at later stages of lens development (Chen et al., 2008; Sugiyama et al., 2010), may predominate at this stage in lens morphogenesis. Further work will be required to address this range of possibilities.
It has been known for some time now that Pax6 is critical for eye development and much attention has been focussed on understanding mechanisms of Pax6 regulation and how this influences a wide range of developmental processes. In recent studies of Pax6-depleted embryos with no lenses (as is consistent with earlier studies), it was reported that Wnt/β-catenin pathway activity was upregulated (Machon et al., 2010). Given earlier studies that indicated Sfrp2 expression in lens placode is regulated by Pax-6 (Duparc et al., 2006; Machon et al., 2010; Shaham et al., 2009; Wawersik et al., 1999), it was suggested that the upregulation of Wnt/β-catenin signaling activity was due to the absence of Pax6-induced Sfrp2. Since we have shown that Sfrps are not involved in the suppression of Wnt/β-catenin signaling activity in the eye, Sfrps are unlikely to be the mediators downstream of Pax6 that suppress this pathway.
Sfrps regulate TCF/Lef activity in lens epithelial cells
Although it appears that Sfrp1 and Sfrp2 do not play a role in Wnt/β-catenin pathway regulation during lens induction our studies have shown that they are involved in the maintenance of Wnt/β-catenin signaling in lens epithelial cells. Specifically, we detected a significant reduction in the number of cells positive for TCF/Lef activity in lens epithelial cells at E13.5 and E14.5. Moreover, the observation that depletion of Sfrp1 and Sfrp2 did not impact on cell proliferation rate in the central epithelium (where the TCF/Lef activity was detected; see Fig 5) indicates that the reduction in numbers of cells with TCF/Lef activity was not simply a consequence of a reduced rate of cell proliferation in this region. This also raises the question of how to explain the small size of the lens in DKO embryos. To ensure that reduced proliferation in DKO lenses was not a contributing factor, we extended our BrdU incorporation studies to include the germinative zone of the epithelium. Statistical analysis showed, as with the central epithelium, there were no significant differences in BrdU incorporation rates between control and DKO lenses at either E14.5 or E16.5 [E14.5 control 38.3% (1,671, n=8), DKO 39.0% (610, n=4); E16.5 control 28.6% (1,613, n=6), DKO 30.5% (817, n=4). P<0.05]. Furthermore, apoptosis in the DKO lenses is unlikely to be the cause of their small size since we did not find any evidence for enhancement of apoptosis in DKO lenses (Supplementary Fig. S3). One possibility could be that, given the role of the canonical Wnt pathway for controlling stem cell populations in adult tissues/organs (reviewed in Grigoryan et al., 2008), the TCF/Lef active cells represent a stem-like cell population. Adult stem cells are recognised as a cell population that can self-renew and also produce transiently amplifying cells for tissue regeneration or long term homeostasis. Given the reduced numbers of these putative stem cells, this could also lead to reduced numbers of fast dividing transiently amplifying cells available to differentiate into fibers, and in this way may account for the small lens formation in DKO with no change of proliferation rate.
Sfrp1 and Sfrp2 expression pattern and TCF/Lef activity
By using a LacZ reporter driven by the endogenous Sfrp1 enhancer/promoter construct we showed that Sfrp1 is expressed only in differentiating and mature lens fibers (Figure 8, purple). This expression pattern is complementary to Sfrp2 as its expression is restricted to the developing germinative zone epithelium (Figure 8, green). Furthermore in lens and retina, Wnt/β-catenin signaling activity (Figure 8, red) is excluded from, but bordered by, the regions of Sfrp1 and Sfrp2 expression. Other studies have shown that Sfrp1 and Sfrp2 are essential for optic cup periphery specification and their removal caused inactivation of the Wnt/β-catenin pathway and transdifferentiation of optic cup periphery into neural retina (Esteve et al., 2011b). Despite their obvious importance for development of this region, both Sfrps were shown to be absent from the optic cup periphery. In our current study, we showed that removal of Sfrp1 and Sfrp2 resulted in reduced Wnt/β-catenin signaling activity in the central lens epithelium and it was also noted that both Sfrps were absent from this region. A common feature of these two studies is that the KO of Sfrp1 and Sfrp2 caused a reduction of Wnt/β-catenin signaling activity in the region adjacent to where expression of these two proteins was detected. These results fit well with the proposal by Mii and colleagues that Sfrps, contrary to the commonly promoted view that they are inhibitory, act as carriers of Wnt ligands to spread and enhance their diffusion and increase their signaling range (Mii and Taira, 2009). Further studies will be required to determine if this intriguing possibility is applicable to the lens.
Supplementary Material
Highlights.
Sfrps play a role in lens development
Sfrps function by regulating aspects of Wnt/β-catenin signaling
Sfrp1/2 do not negatively regulate Wnt/β-catenin signaling in lens
Sfrp1 and Sfrp2 have a positive regulatory function for Wnt/β-catenin signaling
Acknowledgments
The authors would like to acknowledge the support for this work from the National Institutes of Health (R01 EY0-3177), the National Health and Medical Research Council (NHMRC), the Sydney Foundation for Medical Research and the Ophthalmic Research Institute of Australia. Thanks also go to Daniel Dufort for his generous gift of the TCF/Lef-lacZ reporter mice and to Fumiyasu Imai for helpful advice on the in situ hybridization procedures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- Cain S, Martinez G, Kokkinos MI, Turner K, Richardson RJ, Abud HE, Huelsken J, Robinson ML, de Iongh RU. Differential requirement for beta-catenin in epithelial and fiber cells during lens development. Dev Biol. 2008;321:420–433. doi: 10.1016/j.ydbio.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stump RJ, Lovicu FJ, McAvoy JW. Expression of Frizzleds and secreted frizzled-related proteins (Sfrps) during mammalian lens development. Int J Dev Biol. 2004;48:867–877. doi: 10.1387/ijdb.041882yc. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stump RJ, Lovicu FJ, Shimono A, McAvoy JW. Wnt signaling is required for organization of the lens fiber cell cytoskeleton and development of lens three-dimensional architecture. Dev Biol. 2008;324:161–176. doi: 10.1016/j.ydbio.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Duparc RH, Boutemmine D, Champagne MP, Tetreault N, Bernier G. Pax6 is required for delta-catenin/neurojugin expression during retinal, cerebellar and cortical development in mice. Dev Biol. 2006;300:647–655. doi: 10.1016/j.ydbio.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Esteve P, Sandonis A, Cardozo M, Malapeira J, Ibanez C, Crespo I, Marcos S, Gonzalez-Garcia S, Toribio ML, Arribas J, Shimono A, Guerrero I, Bovolenta P. SFRPs act as negative modulators of ADAM10 to regulate retinal neurogenesis. Nat Neurosci. 2011a;14:562–569. doi: 10.1038/nn.2794. [DOI] [PubMed] [Google Scholar]
- Esteve P, Sandonis A, Ibanez C, Shimono A, Guerrero I, Bovolenta P. Secreted frizzled-related proteins are required for Wnt/beta-catenin signalling activation in the vertebrate optic cup. Development. 2011b;138:4179–4184. doi: 10.1242/dev.065839. [DOI] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley JC, Davidson DR, Hill RE. The role of Pax-6 in eye and nasal development. Development. 1995;121:1433–1442. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunhaga L. The lens: a classical model of embryonic induction providing new insights into cell determination in early development. Philos Trans R Soc Lond B Biol Sci. 2011;366:1193–1203. doi: 10.1098/rstb.2010.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland M, Maisel H. A cytoskeletal protein unique to lens fiber cell differentiation. Exp Eye Res. 1984;38:637–645. doi: 10.1016/0014-4835(84)90182-9. [DOI] [PubMed] [Google Scholar]
- Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S, Takahashi S, Nakajima O, Ogino H, Morita M, Nishizawa M, Yasuda K, Yamamoto M. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J Biol Chem. 1999;274:19254–19260. doi: 10.1074/jbc.274.27.19254. [DOI] [PubMed] [Google Scholar]
- Kreslova J, Machon O, Ruzickova J, Lachova J, Wawrousek EF, Kemler R, Krauss S, Piatigorsky J, Kozmik Z. Abnormal lens morphogenesis and ectopic lens formation in the absence of betacatenin function. Genesis. 2007;45:157–168. doi: 10.1002/dvg.20277. [DOI] [PubMed] [Google Scholar]
- Leimeister C, Bach A, Gessler M. Developmental expression patterns of mouse sFRP genes encoding members of the secreted frizzled related protein family. Mech Dev. 1998;75:29–42. doi: 10.1016/s0925-4773(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–334. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- Liu H, Thurig S, Mohamed O, Dufort D, Wallace VA. Mapping canonical Wnt signaling in the developing and adult retina. Invest Ophthalmol Vis Sci. 2006;47:5088–5097. doi: 10.1167/iovs.06-0403. [DOI] [PubMed] [Google Scholar]
- Machon O, Kreslova J, Ruzickova J, Vacik T, Klimova L, Fujimura N, Lachova J, Kozmik Z. Lens morphogenesis is dependent on Pax6-mediated inhibition of the canonical Wnt/beta-catenin signaling in the lens surface ectoderm. Genesis. 2010;48:86–95. doi: 10.1002/dvg.20583. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez G, Wijesinghe M, Turner K, Abud HE, Taketo MM, Noda T, Robinson ML, de Iongh RU. Conditional mutations of beta-catenin and APC reveal roles for canonical Wnt signaling in lens differentiation. Invest Ophthalmol Vis Sci. 2009;50:4794–4806. doi: 10.1167/iovs.09-3567. [DOI] [PubMed] [Google Scholar]
- Mii Y, Taira M. Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development. 2009;136:4083–4088. doi: 10.1242/dev.032524. [DOI] [PubMed] [Google Scholar]
- Mii Y, Taira M. Secreted Wnt "inhibitors" are not just inhibitors: regulation of extracellular Wnt by secreted Frizzled-related proteins. Dev Growth Differ. 2011;53:911–923. doi: 10.1111/j.1440-169X.2011.01299.x. [DOI] [PubMed] [Google Scholar]
- Miller LA, Smith AN, Taketo MM, Lang RA. Optic cup and facial patterning defects in ocular ectoderm beta-catenin gain-of-function mice. BMC Dev Biol. 2006;6:14. doi: 10.1186/1471-213X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra K, Matise MP. A critical role for sFRP proteins in maintaining caudal neural tube closure in mice via inhibition of BMP signaling. Dev Biol. 2010;337:74–83. doi: 10.1016/j.ydbio.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Mohamed OA, Clarke HJ, Dufort D. Beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev Dyn. 2004;231:416–424. doi: 10.1002/dvdy.20135. [DOI] [PubMed] [Google Scholar]
- Nakaya MA, Biris K, Tsukiyama T, Jaime S, Rawls JA, Yamaguchi TP. Wnt3a links left-right determination with segmentation and anteroposterior axis elongation. Development. 2005;132:5425–5436. doi: 10.1242/dev.02149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A, Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986;103:2649–2658. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontoriero GF, Deschamps P, Ashery-Padan R, Wong R, Yang Y, Zavadil J, Cvekl A, Sullivan S, Williams T, West-Mays JA. Cell autonomous roles for AP-2alpha in lens vesicle separation and maintenance of the lens epithelial cell phenotype. Dev Dyn. 2008;237:602–617. doi: 10.1002/dvdy.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring BZ, Cordes SP, Overbeek PA, Barsh GS. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development. 2000;127:307–317. doi: 10.1242/dev.127.2.307. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, Friesel R, Maciag T, Thompson JA. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995;121:505–514. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Prescott AR, Carter JM, Hutcheson AM, Quinlan RA, Richards J, FitzGerald PG. Vimentin and CP49/filensin form distinct networks in the lens which are independently modulated during lens fiber cell differentiation. J Cell Sci. 1995;108(Pt 4):1397–1406. doi: 10.1242/jcs.108.4.1397. [DOI] [PubMed] [Google Scholar]
- Satoh W, Gotoh T, Tsunematsu Y, Aizawa S, Shimono A. Sfrp1 and Sfrp2 regulate anteroposterior axis elongation and somite segmentation during mouse embryogenesis. Development. 2006;133:989–999. doi: 10.1242/dev.02274. [DOI] [PubMed] [Google Scholar]
- Satoh W, Matsuyama M, Takemura H, Aizawa S, Shimono A. Sfrp1, Sfrp2, and Sfrp5 regulate the Wnt/beta-catenin and the planar cell polarity pathways during early trunk formation in mouse. Genesis. 2008;46:92–103. doi: 10.1002/dvg.20369. [DOI] [PubMed] [Google Scholar]
- Shaham O, Smith AN, Robinson ML, Taketo MM, Lang RA, Ashery-Padan R. Pax6 is essential for lens fiber cell differentiation. Development. 2009;136:2567–2578. doi: 10.1242/dev.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AN, Miller LA, Song N, Taketo MM, Lang RA. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol. 2005;285:477–489. doi: 10.1016/j.ydbio.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Song N, Schwab KR, Patterson LT, Yamaguchi T, Lin X, Potter SS, Lang RA. pygopus 2 has a crucial, Wnt pathway-independent function in lens induction. Development. 2007;134:1873–1885. doi: 10.1242/dev.001495. [DOI] [PubMed] [Google Scholar]
- Stump RJ, Ang S, Chen Y, von Bahr T, Lovicu FJ, Pinson K, de Iongh RU, Yamaguchi TP, Sassoon DA, McAvoy JW. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev Biol. 2003;259:48–61. doi: 10.1016/s0012-1606(03)00179-9. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Akimoto K, Robinson ML, Ohno S, Quinlan RA. A cell polarity protein aPKClambda is required for eye lens formation and growth. Dev Biol. 2009;336:246–256. doi: 10.1016/j.ydbio.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Stump RJ, Nguyen A, Wen L, Chen Y, Wang Y, Murdoch JN, Lovicu FJ, McAvoy JW. Secreted frizzled-related protein disrupts PCP in eye lens fiber cells that have polarised primary cilia. Dev Biol. 2010;338:193–201. doi: 10.1016/j.ydbio.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Yang-Snyder JA, Purcell SM, DeMarais AA, McGrew LL, Moon RT. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol. 1996;133:1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 acts in murine lens placode development. Dev Biol. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fiber elongation. Nat Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- Zhou CJ, Molotkov A, Song L, Li Y, Pleasure DE, Pleasure SJ, Wang YZ. Ocular coloboma and dorsoventral neuroretinal patterning defects in Lrp6 mutant eyes. Dev Dyn. 2008;237:3681–3689. doi: 10.1002/dvdy.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.