Abstract
Kynurenic acid is a tryptophan metabolite that is synthesized and released in the brain by astrocytes and acts as an antagonist of nicotinic acetylcholine receptors and N-methyl-D-aspartate glutamate receptors, both of which are critically involved in cognition as well as neural plasticity and brain development. The concentration of kynurenic acid is increased in the brains of persons with schizophrenia and this increase has been implicated in the cognitive and social impairments associated with the disease. In addition, growing evidence suggests that the increase in kynurenic acid may begin early in life. For example, exposure to influenza A virus during development results in a transient increase in kynurenic acid concentration that could disrupt normal brain development and lead to cognitive deficits later in life. Changes in kynurenic acid may thus provide a link between developmental exposure to viruses and the increased risk of subsequently developing schizophrenia. To test this, we mimicked the effects of influenza A exposure by treating rats with kynurenine, the precursor of kynurenic acid, on postnatal days 7-10. We observed a transient increase in both kynurenic acid and quinolinic acid during treatment. When rats were subsequently behaviorally tested as adults, those previously treated with kynurenine exhibited decreased social behavior and locomotor activity. In contrast, attentional function and fear conditioning were not affected. Together with other recent findings, these findings have several implications for understanding how viral-induced changes in tryptophan metabolism during development may contribute to schizophrenia-related symptoms later in life.
Keywords: schizophrenia, glia, attention, learning, memory, social
1. Introduction
Kynurenic acid (KYNA), a final product of tryptophan metabolism, is synthesized and released in the brain by astrocytes (Schwarcz & Pellicciari, 2002) and acts as an endogenous antagonist of α7 nicotinic acetylcholine receptors (α7-nACh-Rs) and N-methyl-D-aspartate (NMDA) glutamate receptors (Hilmas et al., 2001; Parsons et al., 1997; Pereira et al., 2002; Stone, 1993). The concentrations of KYNA and its precursor kynurenine are significantly increased in the brains and cerebral spinal fluid of persons with schizophrenia (Erhardt et al., 2001; Linderholm et al., 2012; Schwarcz et al., 2001) and experimentally-induced elevations in KYNA concentration in adult rats reproduce the impairments in social behavior, attentional function, and contextual memory associated with this disease (Chess and Bucci, 2006; Chess et al., 2007, 2009; Erhardt et al., 2004; Geyer et al., 2001; Sams-Dodd, 1999; Shepard et al., 2003; Silver et al, 2003; Waters et al., 2004). Growing evidence now suggests that the increase in KYNA concentration in schizophrenia may begin early in life. For example, there is evidence that exposing mice to viruses during development can induce changes in KYNA levels in neonates. Indeed, exposure to influenza A virus on postnatal day (PND) 3 or 4 (which is comparable to late 2nd/early 3rd trimester in humans, Rice and Barone, 2000) produces a transient increase in KYNA concentration one week later (Asp et al., 2010; Holtze et al., 2008). In addition, genetic studies in humans have identified mutations associated with schizophrenia that result in increased KYNA levels, potentially at a very early age (Miller et al., 2004, 2006, 2008, 2009). These findings are significant because both α7-nACh-Rs and NMDA-Rs are critically involved in cognitive function and neural plasticity, as well as neural development (Bast et al., 2003; Broide and Leslie, 1999; Komuro and Rakic, 1993; Levin, 2002). Thus, early increases in KYNA concentration could disrupt normal brain development and lead to cognitive deficits later in life.
Consistent with this hypothesis, recent studies have shown that treating rats with kynurenine throughout adolescence (PND 27-53) increases KYNA levels during the treatment period, and produces memory deficits and decreased social behavior when rats are tested as adults, despite having normal KYNA levels at the time of behavioral testing (Akagbosu et al., 2012; Trecartin & Bucci, 2011). Similarly, treating rat dams with kynurenine-enriched food from gestational day 15 to PND 21 increased KYNA concentration in the offspring, which later exhibited impaired attention and memory as adults (Alexander et al., 2013; Pocivavsek et al., 2012). However, little research has focused on the long-term behavioral effects of increased KYNA concentration at earlier and more circumscribed time periods of development, as occurs following exposure to influenza A in mice. Indeed, it may be that the brain is particularly sensitive to increased KYNA levels at a specific time during development. Determining the effects of early exposure to KYNA is particularly important because developmental exposure to viruses has long been associated with an increased risk of subsequently developing schizophrenia (Karlsson, 2003; Yolken and Torrey, 2008), yet the underlying mechanism has remained unclear. Moreover, previous studies have revealed that a reduced immune response in persons with schizophrenia is associated with an increase in KYNA levels (Müller and Schwarz, 2010). An increase in KYNA levels at early ages may thus provide a significant link between viral exposure and subsequent cognitive dysfunction.
In the only published behavioral studies to date, Asp et al. (2009, 2010) exposed wild-type and immuno-compromised mice to influenza A on PND 3 or 4 and observed deficits in sensory gating and working memory in adulthood that were reminiscent of impairments observed in schizophrenia (Geyer et al., 2001; Silver et al., 2003), but only in the adult mice that were immuno-compromised. Although these data suggest that a viral-induced increase in KYNA concentration may not be sufficient to impact behavior in adults with normal immune functioning, there are several unresolved questions. For example, the studies by Asp and colleagues (2009, 2010) were conducted in mice while most other studies of developmental KYNA exposure and behavior have been carried out in rats (Akagbosu et al., 2012; Trecartin & Bucci, 2011; Alexander et al., 2013; Pocivavsek et al., 2012), making it difficult to draw firm conclusions because of potential species differences as well as strain-specific differences in behavior in mice (e.g., Falls et al., 1997). In addition, not all of the cognitive and behavioral symptoms of schizophrenia are modulated by KYNA exposure, and the effects have also been shown to depend on age of treatment (Akagbosu et al., 2012; Chess et al., 2009; Trecartin and Bucci, 2011). Thus, it remains unclear whether neonatal KYNA exposure affects other types of behavior and cognitive function.
To address these issues, we treated neonatal rats with 100mg/kg of kynurenine (or vehicle solution) on PND 7-10 to mimic the effects of influenza exposure on KYNA levels. This dose of kynurenine results in a 2-3 fold increase in KYNA concentration in juvenile and adult rats (Akagbosu et al., 2012; Erhardt et al., 2004), mirroring the magnitude of the increase observed in schizophrenia (Erhardt et al., 2001; Schwarcz et al., 2001). Using kynurenine to manipulate KYNA concentration also has high translational value since an increase in kynurenine level is a primary factor leading to the increased KYNA concentration associated with schizophrenia (Miller et al., 2006; Linderholm et al., 2012). Upon reaching adulthood, rats were tested in a fear conditioning task and a social interaction paradigm previously shown to be sensitive to kynurenine treatment during adolescence (Chess et al., 2009; Trecartin and Bucci, 2011). Another set of rats was tested in an attentional orienting procedure that has features in common with the sensory gating paradigm used by Asp et al. (2010). We also measured the concentrations of KYNA and quinolinic acid (QUIN, another product of tryptophan/kynurenine metabolism) during kynurenine treatment as well as at the end of behavioral testing.
2. Materials and Methods
2.1. Subjects
Seven pregnant Long-Evans rats (~4 months old) were obtained from Harlan Laboratories (Indianapolis, IN) and maintained in individual cages on a 14:10 light-dark cycle with food (Purina standard rat chow; Nestle Purina, St. Louis, MO) and water available ad libitum throughout pregnancy and after giving birth. Pups were maintained on the same light-dark cycle and had free access to food and water throughout the experiment. At 25 days of age, the pups were weaned and group-housed (3-4 rats/cage) for the remainder of the study. All procedures were approved by the Dartmouth College Institutional Animal Care and Use Committee and carried out according to AAALAC guidelines.
2.2. Drug preparation
L-kynurenine (L-KYN; Sigma, St Louis, MO) was prepared fresh daily as described previously (Akagbosu et al., 2012; Chess et al., 2009).
2.3. Treatment regimen
On PND 7-10, an equal number of pups from each of the litters received daily intraperitoneal (i.p.) injections of either L-KYN (100 mg/kg; 2 ml/kg) or a comparable volume of 0.1 M HEPES buffer (vehicle, pH 7.0). In total, 22 pups were treated with L-KYN and another set 22 pups were treated with vehicle.
2.4. Experimental design
Two hours after the injection of L-KYN (or vehicle) on PND 10, four rats from each treatment condition were sacrificed to determine the concentrations of KYNA and QUIN resulting from LKYN treatment. Rats were euthanized using isofluorane followed by rapid decapitation and brains were rapidly dissected and frozen on dry ice for analysis using high-performance liquid chromatography (HPLC; described below). The remaining 18 rats in each treatment condition were maintained until they reached adulthood and began behavioral testing at age 70 days.
One cohort of adult rats (10/group) was tested in the fear conditioning and social interaction tasks described below. A second cohort of adult rats (8/group) was tested in the attentional orienting procedure. After the last day of behavioral testing, 4 rats in each of the drug treatments conditions were sacrificed and brains processed to determine the concentrations of KYNA and QUIN at the time of behavioral testing as adults.
2.5. Behavioral apparatus, procedure, and observations
2.5.1. Social behavior and locomotor activity
Social behavior and locomotor activity were assessed as described previously (File, 1980; Hopkins et al., 2009; Trecartin and Bucci, 2011). Briefly, rats were individually placed in a white plastic tub containing an unfamiliar (target) rat in a restraint tube and allowed to explore for 10 minutes. The number of interactions with the target rat (placing the nose inside the restrainer) was recorded as measure of social behavior. In addition, the number of times the rat crossed perpendicular lines that were superimposed on the video image of the tub were counted and served as a measure of general locomotor activity.
2.5.2. Fear conditioning
Rats were trained in a standard fear conditioning task as described previously (Akagbosu et al., 2012; Keene & Bucci, 2008). Briefly, rats were placed in individual operant conditioning chambers and presented with 3 tone-shock pairings during the acquisition session. Twenty-four hours later, contextual fear memory was assessed by returning the rats to the chambers and measuring freezing behavior, which served as the indicator of conditioned fear (no shocks or tones were presented). Tone-specific fear memory was subsequently tested by placing the rats in a different chamber and replaying the tone (no shocks delivered).
2.5.3. Attentional orienting behavior
As described previously (Bucci and Burwell, 2004; Keene and Bucci, 2008), rats were placed in individual operant conditioning chambers and received 12 non-reinforced presentations of a light (10 sec in duration). Normal rats typically rear up on their hind legs and orient toward the visual stimulus (Holland, 1977, 1984), which is often-used indicator of attentional processing (Gallagher et al., 1990; Kaye and Pearce, 1984; Lang et al., 1997). Rearing behavior rapidly decreases (habituates) when the cue is not followed by reinforcement, reflecting a decrease in attention to a behaviorally irrelevant stimulus (Gallagher et al., 1990; Holland, 1997; Kaye & Pearce, 1984).
2.6. Behavioral Data Analyses
Analyses of the behavioral data were carried out as described previously (Keene and Bucci, 2008; Akagbosu et al., 2012; Trecartin and Bucci, 2011). Briefly, the number of social interactions and line crossings exhibited by rats previously treated with vehicle or L-KYN were analyzed using an independent samples t-test. For the fear conditioning task, freezing behavior during the training session, context test session, and tone test session was analyzed using a repeated measures analysis of variance (ANOVA) with Group (Vehicle, L-KYN) as the between subjects variable. For the acquisition session and tone test session, Trial was used as the within subjects variable. For the context test session data, freezing was measured in 64-second blocks and Block served as the within-subjects variable. In the attentional orienting task, the number of rears was analyzed using a repeated measures ANOVA with Group as the between subjects variable and Blocks of 4-trials as the within subjects variable.
2.7 Biochemistry
Whole brain tissue (minus the cerebellum) was used to determine the levels of KYNA and QUIN following L-KYN treatment.
2.7.1. Determination of KYNA concentration
The concentration of KYNA was determined using HPLC as described previously (Akagbosu et al., 2012) and group differences analyzed using an independent samples t-test.
2.7.2. Determination of QUIN concentration
QUIN levels in brain homogenate were quantified by electron- capture negative chemical ionization gas chromatography using a minor modification of the procedure described by Heyes and Markey (1988). The modifications included a different internal standard (13C7)-QUIN and precipitation with perchloric acid followed by liquid /liquid extraction using prepurified ethyl acetate. This extract was evaporated in a vacuum centrifuge and the QUIN and (13C7)-QUIN esterified to their dihexafluoroisopropanol esters. After extraction into heptane, the samples were quantified using the molecular ions at m/z 467 from QUIN and m/z 474 from (13C7)-QUIN. Intra- and inter-assay variations at plasma concentrations of 240, 600, and 1200 pmol/mL were <6% and <7%, respectively. The lower limit of quantification was ~20 pmol/mL (CV = 10.4%, n=5). Group differences in QUIN concentration were analyzed using an independent samples t-test.
3. Results
3.1. KYNA and QUIN Concentration
The concentrations of KYNA and QUIN measured after the L-KYN injection on PND 10 and after behavioral testing on PND 75 are shown in Table 1. On PND 10, there was significant increase in the level of KYNA [t(6)=4.3, p<0.005)] and in the level of QUIN [t(6)=2.6, p<0.04] in the brains of rats treated with L-KYN compared to vehicle-treated controls. After behavioral testing, the concentrations of KYNA and QUIN did not differ significantly between L-KYN-treated and vehicle-treated rats (p=0.28, p=0.09, respectively).
Table 1.
Concentrations (pmol/gm) of KYNA and QUIN in brain tissue 2 hours after L-KYN on PND 10, and immediately after behavioral testing in adults.
| PND 10 | Adult | |||
|---|---|---|---|---|
| Treatment group | KYNA (n=4) | QUIN (n=4) | KYNA (n=4) | QUIN (n=4) |
| Vehicle | 11.2 ± 0.9 | -----† | 19.2 ± 5.3 | 58.7 ± 15.3 |
| L-KYN | 32.3 ± 4.8* | 64.6 ± 12.9* | 35.4 ± 12.8 | 95.5 ± 9.0 |
Data are means ± SEM.
p<0.05 compared to Vehicle group
levels below limit of detection
3.2. Social interaction and locomotor activity
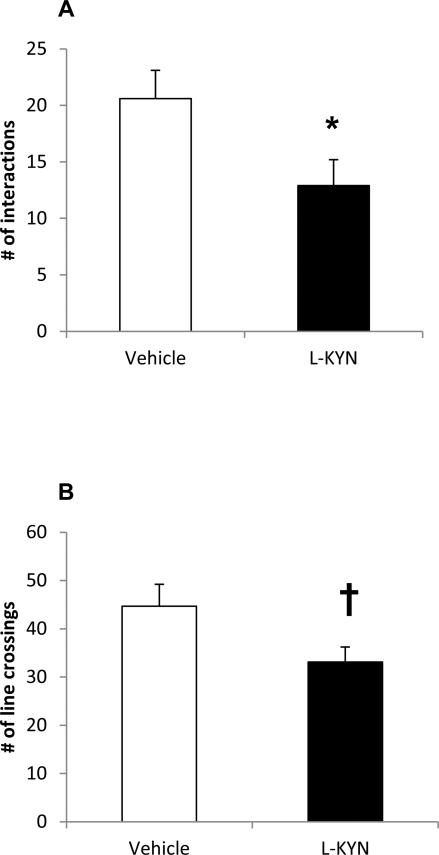
The number of social interactions exhibited by adult rats in each group is shown in Figure 1A. Compared to rats that were previously treated with vehicle on PND 7-10, those treated with L-KYN displayed significantly fewer social interactions with an unfamiliar rat [t(17)=2.3, p<0.04]. In addition, rats treated L-KYN exhibited a marginally significant decrease in the number of lines crossed while exploring the test chamber [t(17)=2.2, p=0.05], as shown in Figure 1B. There was no significant correlation between the number of social interactions and the line crossings in the data set as a whole (r2=0.1, p=0.2) or in the individual groups (Vehicle: r2=0.003, p=0.9; L-KYN, r2=0.05, p=0.4).
Figure 1.
(A) The number of social interactions displayed by adult rats that had been treated with either vehicle or L-KYN on PND 7-10 (n=10/group). The number of social interactions reflects the number of contacts made by an experimental rat with holes in the cylinder containing the target rat during a 10-min session. The number of social interactions was significantly decreased in rats that had been treated with L-KYN on PND 7-10. (B) Locomotor activity measured during the social interaction task. Data reflect the number of times rats in each group crossed a line that separated the arena into thirds. There was a marginally significant decrease in line crossings in adult rats that had received L-KYN on PND 7-10. Data are means ± SEM. *p<0.05, †p=0.05.
3.3. Fear conditioning
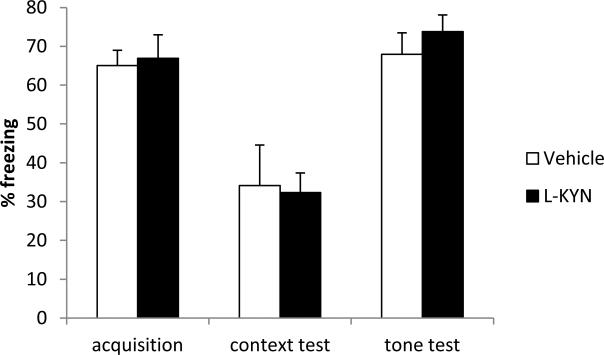
As summarized in Figure 2, freezing behavior was comparable in adult rats that had been previously treated with vehicle or L-KYN as neonates. There were no significant group differences in post-shock freezing during the acquisition session (p=0.8) and no significant differences during the context (p=0.9) or tone test sessions (t=0.4).
Figure 2.
Summary of the amount of freezing behavior observed during the acquisition session, context test session, and tone test session of the fear conditioning task. There were no group differences during any phase of training (n=10/group). Data are means ± standard error.
3.4. Attentional orienting behavior
Orienting behavior during presentations of a non-reinforced visual cue is shown in Figure 3. A repeated measures ANOVA revealed a significant main effect of Block [F(2,28)=9.9, p<0.001], indicating that the amount of rearing behavior decreased across trials. There was no significant main effect of Group (p=0.3) and no significant Group X Block interaction (p=0.5), indicating that rats previously treated with vehicle or L-KYN exhibited comparable patterns of orienting behavior and habituation.
Figure 3.
Unconditioned orienting (rearing behavior) observed during repeated presentation of a non-reinforced visual stimulus. The number of breaks in the photobeams used to detect rearing behavior is shown on the y-axis. Blocks of trials (first 4, middle 4, and last 4 trials) are shown on the x-axis. Rearing behavior habituated across trials and there were no significant group differences (n=8/group). Data are means ± standard error.
4. Discussion
The present study was designed to examine how a neonatal increase in KYNA concentration, similar to that which occurs following an influenza A infection (Holtze et al., 2008), effects cognition and social behavior later in adulthood in rats. Administering L-KYN to rats on PND 7-10 mimicked the effects of neonatal exposure to influenza A by transiently elevating the concentration of KYNA in whole-brain tissue (Asp et al., 2010; Holtze et al., 2008). Indeed, the concentration of KYNA measured on PND 10 was ~3X higher in rats that were treated with L-KYN compared to vehicle-treated control rats, mirroring the magnitude of the increase observed following viral exposure (Asp et al., 2010). When rats were subsequently tested as adults, a number of behavioral measures were impacted in rats that had previously been treated with L-KYN. These findings have several implications for understanding how viral-induced changes in tryptophan metabolism during development may contribute to schizophrenia-related symptoms later in life.
First, the results indicate that cognition and social behavior in adulthood are not uniformly affected by exposure to increased KYNA during PND 7-10. For example, attentional orienting behavior was unaffected in L-KYN-treated rats, a finding that is consistent with the observation of normal sensory gating in wild-type mice exposed to influenza A as neonates (Asp et al. 2010). In contrast, we observed fewer social interactions in rats previously exposed to L-KYN. Together with our previous finding that social behavior was decreased following treatment with L-KYN during adolescence, but not by L-KYN treatment during adulthood (Trecartin and Bucci, 2011), these data suggest that social behavior is particularly sensitive to changes in the level of KYNA during development. Still other types of behavior, such as conditioned freezing (i.e., fear learning/memory), were unaffected by neonatal treatment (present study) but were instead found to be sensitive to L-KYN treatment during adolescence (Akagbosu et al., 2012) and adulthood (Chess et al., 2009). Collectively, as illustrated in Table 2, these findings indicate that the effects of L-KYN treatment on behavior in adult rats depends on the age at which L-KYN is administered as well as the behavioral domain that is being assessed by a particular task. Although the basis of these age and task-dependent effects is currently unknown, they may reflect differences in the maturation of the brain systems that underlie specific functions (e.g., hippocampus and contextual memory; prefrontal cortex and attention) and/or region-specific differences in the distribution of α7-nACh-Rs and NMDA receptors during development.
Table 2.
Behavioral effects in adult rats previously treated with L-KYN at different ages
| Age when L-KYN was administered | |||
|---|---|---|---|
| Adult | Adolescent | PND 7-10 | |
| Contextual fear memory | Decreased1 | Decreased2 | No effect |
| Social behavior | No effect3 | Decreased3 | Decreased |
| Locomotor behavior | No effect3 | No effect3 | Decreased |
| Attentional set shifting | Decreased4 | Decreased5* | Decreased5* |
rats were exposed to L-KYN from gestational day 15 through PND 21 (Alexander et al., 2013; Alexander et al., 2012)
We also found that locomotor behavior in adults was impacted by treatment with L-KYN on PND 7-10, as evidenced by a decrease in line crossings in the social interaction chamber. Although it is possible that the decrease in locomotor behavior contributed to the reduction in social interactions exhibited by rats previously treated with L-KYN in the present study, several observations suggest that is unlikely. For instance, the correlation between the number of social interactions and line crossings was not statistically significant. In addition, freezing behavior during the fear conditioning task, which could also be affected by a basal change in locomotor activity, was not different between the vehicle-treated rats and L-KYN-treated rats. Regardless, the decrease in locomotor activity observed here provides additional evidence that behavioral changes following L-KYN treatment depend on the time of intervention. Indeed, there was no change in locomotor behavior in adult rats that had been treated with L-KYN during adolescence or adulthood (Akagbosu et al., 2012).
Another explanation for the observed decreases in social interaction and locomotor behavior is that they were due to changes in tryptophan metabolites other than KYNA. An additional new finding in the current study was that the QUIN concentration was also increased by L-KYN administration on PND 10. This is consistent with the observation that levels of the transcripts of the biosynthetic enzymes leading to QUIN production are increased in mice treated with influenza A (Holtze et al., 2008). Like KYNA, QUIN is also a downstream metabolic product of kynurenine in the tryptophan degradation pathway. QUIN acts as an NMDA agonist (Stone and Perkins, 1981) and it has been previously shown that intracerebroventricular infusions of QUIN reduce social interaction and locomotor behavior (Lapin et al., 1996). Interestingly, L-KYN during adulthood does not have a significant effect on QUIN levels (Shepard et al., 2003) nor did it affect locomotor behavior (Akagbosu et al., 2012), further suggesting that the changes in locomotor behavior observed following neonatal exposure to L-KYN may be due to changes in QUIN concentration.
Moreover, the quantity of NMDA-Rs in the brain has been shown to reach adult levels by embryonic day 19 (Sanchez et al., 2010), whereas the development of nACh-Rs is more protracted and peak levels are not observed until the 3rd postnatal week (Adams, 2003). Thus, the behavioral effects of increased KYNA and/or QUIN concentration following flu exposure (Holtze et al., 2008; Asp et al., 2009, 2010), or following treatment with L-KYN on PND 7-10, are likely mediated more by NMDA-Rs than nACh-Rs. In contrast, previous studies have shown that L-KYN administration during adulthood impairs sensory gating (Erhardt et al., 2004; Shepard et al., 2003), an effect that was not mediated by NMDA-Rs (Shepard et al., 2003) and likely involves the antagonism of nACh-Rs instead. Thus, it is possible that the specific behavioral effects observed following L-KYN treatment depend in part on the respective expression levels of NMDA-Rs and nACh-Rs. This is underscored by the finding that KYNA potently inhibits α7-nAChR activation at an IC50 of 7μM in cultured hippocampal neurons, while the IC50 for KYNA-induced blockade of NMDA-Rs is 235μM (Hilmas et al., 2001). Future studies could investigate this notion further by comparing the present findings to those obtained when L-KYN is administered around PND 21, when nACh-Rs are more numerous.
It is possible that effects on fear learning/memory or attentional function would have been observed in the present study if the L-KYN treatment extended beyond PND 10. However, we specifically chose to limit the L-KYN treatment to PND 7-10 for several reasons. First, a primary goal of the study was to mimic the effects of neonatal influenza exposure on KYNA levels. In the influenza studies (Asp et al., 2010; Holtze et al., 2008), rats were exposed to the virus on PND 3 or 4 and KYNA was found to be elevated on PND 13, but not PND 7 or 24 (no other days were tested). Thus, we chose to begin injections late in the day on PND 7 so that we could elevate KYNA levels as early as possible during development (in an attempt to maximize the influence of KYNA on the developing brain), while retaining translational relevance to the influenza studies. Secondly, continuing beyond day PND 10 was impractical since systemic L-KYN administration no longer increases KYNA concentration after 3-4 days of treatment because of metabolic changes (Vescei et al., 1992). Future studies are therefore needed to more precisely characterize the time course of changes in KYNA concentration following influenza A exposure and to assess corresponding behavioral effects. If the metabolic changes associated with repeated treatment can be surmounted, using L-KYN to mimic the effects of viral exposure on KYNA and QUIN remains an attractive method since it eliminates the potential confounding effects of viral exposure on other organ systems.
Importantly, the behavioral changes we observed in adults that had been treated with L-KYN as neonates could not be attributed to an increase in KYNA at the time of testing. Indeed, the concentration of KYNA was not significantly different in L-KYN-treated rats and vehicle-treated rats when they were reached adulthood, as was also the case following neonatal influenza A exposure (Asp et al., 2010). From an experimental perspective, this is ideal in that it allows us to distinguish the behavioral effects of early exposure to increased levels of KYNA from the effects of high KYNA levels at the time of testing. This is significant since we have shown previously that an acute increase in KYNA concentration on the day of behavioral training impairs contextual fear memory (Chess et al., 2009). On the other hand, the transient nature of the increase in KYNA after neonatal viral exposure (Asp et al. 2009, 2010) or neonatal treatment with L-KYN (present study) indicates that these early changes in KYNA may not contribute to the increases in KYNA levels observed in adults with schizophrenia (Schwarcz et al., 2001).
In summary, the present findings provide new evidence that exposure to increased levels of tryptophan metabolites, such as KYNA and QUIN, during development can affect behavior in adulthood (Akagbosu et al., 2012; Alexander et al., 2013; Pocivavsek et al., 2012; Trecartin and Bucci, 2011). Both of these substances act on neurotransmitter systems (glutamatergic and cholinergic) that are critically involved in cognitive function as well as normal brain development and neural plasticity, and may thus contribute to cognitive and behavioral deficits associated with various neuropsychiatric disorders, such as schizophrenia. Moreover, changes in KYNA concentration can modulate dopamine levels, and vice versa (Rassoulpour et al., 2005; Wu et al., 2000, 2007). Thus, alterations in tryptophan metabolites can influence the levels of several of the major neurotransmitters that have been implicated in schizophrenia. However, the current findings together with those of Asp et al. (2009, 2010) indicate that early exposure to KYNA and QUIN may only affect certain types of behavior. Moreover, the resulting behavioral consequences may be more apparent in immuno-compromised animals than in animals with normal immune systems (Asp et al., 2009, 2010). This may be particularly important to consider for the development and eventual use of new ‘kynurenergic’ therapies for schizophrenia (Erhardt et al., 2009; Schwarcz et al., 2010, 2012; Thevandavakkam et al., 2010; Wonodi & Schwarcz, 2010) since growing evidence suggests that schizophrenia may be associated with immune dysfunction that could results in elevated levels of kynurenine and KYNA (Müller and Schwarz, 2010).
Acknowledgements
Role of Funding Source. Funding for this study was provided by NIH Grant R01DA027688 (DJB). The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors. H.F.S. and D.J.B. participated in the design of the study, writing the protocol, conducting literatures searches and data analyses, and writing drafts of the manuscript. R.F.S. and S.X. carried out the biochemical analyses. All authors contributed to and have approved the final version of the manuscript.
Conflict of Interest. All authors declare that they have no conflicts of interest.
References
- Adams CE. Comparison of alpha7 nicotinic acetylcholine receptor development in the hippocampal formation of C3H and DBA/2 mice. Brain Res. Dev. Brain Res. 2003;143(2):137–49. doi: 10.1016/s0165-3806(03)00106-8. [DOI] [PubMed] [Google Scholar]
- Akagbosu CO, Evans GC, Gulick D, Suckow RF, Bucci DJ. Exposure to kynurenic acid during adolescence produces memory deficits in adulthood. Schizophrenia Bulletin. 2012;38:769–778. doi: 10.1093/schbul/sbq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KS, Pocivavsek A, Wu HQ, Pershing ML, Schwarcz R, Bruno JP. Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: reversal with galantamine. Neuroscience. 2013;238:19–28. doi: 10.1016/j.neuroscience.2013.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KS, Wu HQ, Schwarcz R, Bruno JP. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology. 2012;220(3):627–37. doi: 10.1007/s00213-011-2539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asp L, Beraki S, Kristensson K, Ogren SO, Karlsson H. Neonatal infection with neurotropic influenza A virus affects working memory and expression of type III Nrg1 in adult mice. Brain Behav. Immun. 2009;23(6):733–41. doi: 10.1016/j.bbi.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Asp L, Holtze M, Powell SB, Karlsson H, Erhardt S. Neonatal infection with neurotropic influenza A virus induces the kynurenine pathway in early life and disrupts sensorimotor gating in adult Tap1−/− mice. Int. J. Neuropsychopharmacol. 2010;13(4):475–85. doi: 10.1017/S1461145709990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: Effects of local NMDA-receptor blockade and stimulation. Hippocampus. 2003;13:657–675. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Broide RS, Leslie FM. The alpha7 nicotinic acetylcholine receptor in neuronal plasticity. Mol. Neurobiol. 1999;20(1):1–16. doi: 10.1007/BF02741361. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Burwell RD. Deficits in attentional orienting following damage to postrhinal or perirhinal cortex. Behavioral Neuroscience. 2004;118:1117–1122. doi: 10.1037/0735-7044.118.5.1117. [DOI] [PubMed] [Google Scholar]
- Chess AC, Bucci DJ. Increased levels of cerebral kynurenic acid alter stimulus processing and conditioned responding in rats. Behavioural Brain Research. 2006;170:326–332. doi: 10.1016/j.bbr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Chess AC, Landers AM, Bucci DJ. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav. Brain Res. 2009;201:325–331. doi: 10.1016/j.bbr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophrenia Bulletin. 2007;33:797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci. Lett. 2001;313:96–98. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Olsson SK, Engberg G. Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders. CNS Drugs. 2009;23(2):91–101. doi: 10.2165/00023210-200923020-00001. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol. Psychiatry. 2004;56:255–260. doi: 10.1016/j.biopsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Falls WA, Carlson S, Turner JG, Willott JF. Fear-potentiated startle in two strains of inbred mice. Behav. Neurosci. 1997;111(4):855–61. [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J. Neurosci. Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. J. Neurosci. 1990;10(6):1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156(2-3):117–54. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Markey SP. Quantification of quinolinic acid in rat brain, whole blood, and plasma by gas chromatography and negative chemical ionization mass spectrometry: effects of systemic L-tryptophan administration on brain and blood quinolinic acid concentrations. Anal. Biochem. 1988;174(1):349–59. doi: 10.1016/0003-2697(88)90556-8. [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EFR, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: Physiopathological implications. J. Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtze M, Asp L, Schwieler L, Engberg G, Karlsson H. Induction of the kynurenine pathway by neurotropic influenza A virus infection. J. Neurosci. Res. 2008;86(16):3674–83. doi: 10.1002/jnr.21799. [DOI] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3(1):77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC. Unblocking in Pavlovian appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10(4):476–497. [PubMed] [Google Scholar]
- Hopkins ME, Sharma M, Evans GC, Bucci DJ. Voluntary physical exercise alters attentional orienting and social behavior in rat model of attention-deficit/hyperactivity disorder. Behav. Neurosci. 2009;123:599–606. doi: 10.1037/a0015632. [DOI] [PubMed] [Google Scholar]
- Karlsson H. Viruses and schizophrenia, connection or coincidence? Neuroreport. 2003;14(4):535–42. doi: 10.1097/00001756-200303240-00001. [DOI] [PubMed] [Google Scholar]
- Kaye H, Pearce JM. The strength of the orienting response during Pavlovian conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10(1):90–109. [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behav. Neurosci. 2008;122:89–97. doi: 10.1037/0735-7044.122.1.89. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993;260(5104):95–7. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Simons RG, Balaban M. Attention and orienting: Sensory and motivational processes. Erlbaum, Mahwah, NJ: 1997. [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53(4):633–40. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, Samuelsson M, Erhardt S. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr. Bull. 2012;38(3):426–32. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Llenos IC, Dulay JR, Barillo MM, Yolken RH, Weis S. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobio. of Disease. 2004;15:618–629. doi: 10.1016/j.nbd.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073-1074:25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Miller CL, Llenos IC, Cwik M, Walkup J, Weis S. Alterations in kynurenine precursor and product levels in schizophrenia and bipolar disorder. Neurochem. Int. 2008;52:1297–303. doi: 10.1016/j.neuint.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Miller CL, Murakami P, Ruczinski I, Ross RG, Sinkus M, Sullivan B, Leonard S. Two complex genotypes relevant to the kynurenine pathway and melanotropin function show association with schizophrenia and bipolar disorder. Schizophr. Res. 2009;113:259–67. doi: 10.1016/j.schres.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N, Schwarz MJ. Immune System and Schizophrenia. Curr. Immunol. Rev. 2010;6(3):213–220. doi: 10.2174/157339510791823673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G, Hartmann S, Lorenz B, Wollenburg C, Baran L, Przegalinski E, Kostowski W, Krzascik P, Chizh B, Headley PM. Novel systemically active antagonists of the glycine site of the N-methyl-D-aspartate receptor: electrophysiological, biochemical and behavioral characterization. J. Pharmacol. Exp. Ther. 1997;283(3):1264–75. [PubMed] [Google Scholar]
- Pereira EF, Hilmas C, Santos MD, Alkondon M, Maelicke A, Albuquerque EX. Unconventional ligands and modulators of nicotinic receptors. J. Neurobiol. 2002;53(4):479–500. doi: 10.1002/neu.10146. [DOI] [PubMed] [Google Scholar]
- Pocivavsek A, Wu HQ, Elmer GI, Bruno JP, Schwarcz R. Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. Eur. J. Neurosci. 2012;35(10):1605–12. doi: 10.1111/j.1460-9568.2012.08064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulpour A, Wu HQ, Ferre S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J Neurochem. 2005;93(3):762–5. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health. Perspect. 2000;108(Suppl 3):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams-Dodd F. Phencyclidine in the social interaction test: an animal model of schizophrenia with face and predictive validity. Rev. Neurosci. 1999;10(1):59–90. doi: 10.1515/revneuro.1999.10.1.59. [DOI] [PubMed] [Google Scholar]
- Sanchez JT, Wang Y, Rubel EW, Barria A. Development of glutamatergic synaptic transmission in binaural auditory neurons. J. Neurophysiol. 2010;104(3):1774–89. doi: 10.1152/jn.00468.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: Glial targets, neuronal effects, and clinical opportunities. J. Pharmacol. Exp. Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 2012;13(7):465–77. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu H-Q, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatry. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Guidetti P, Sathyasaikumar KV, Muchowski PJ. Of mice, rats and men: Revisiting the quinolinic acid hypothesis of Huntington's disease. Prog. Neurobiol. 2010;90(2):230–45. doi: 10.1016/j.pneurobio.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard PD, Joy B, Clerkin L, Schwarcz R. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharm. 2003;28:1454–1462. doi: 10.1038/sj.npp.1300188. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am. J. Psychiatry. 2003;160:1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 1993;45:309–379. [PubMed] [Google Scholar]
- Thevandavakkam MA, Schwarcz R, Muchowski PJ, Giorgini F. Targeting kynurenine 3-monooxygenase (KMO): implications for therapy in Huntington's disease. CNS Neurol. Disord. Drug Targets. 2010;9(6):791–800. doi: 10.2174/187152710793237430. [DOI] [PubMed] [Google Scholar]
- Trecartin KV, Bucci DJ. Administration of kynurenine during adolescence, but not during adulthood, impairs social behavior in rats. Schizophrenia Research. 2011;133:156–158. doi: 10.1016/j.schres.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsei L, Miller J, MacGarvey U, Beal FM. Effects of kynurenine and probenecid on plasma and brain tissue concentrations of kynurenic acid. Neurodegeneration. 1992;1:17–26. [Google Scholar]
- Waters FAV, Mayberry MT, Badcock JC, Michie PT. Context memory and binding in schizophrenia. Schizophr. Res. 2004;68:119–125. doi: 10.1016/S0920-9964(03)00221-4. [DOI] [PubMed] [Google Scholar]
- Wonodi I, Schwarcz R. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in Schizophrenia. Schizophr. Bull. 2010;36(2):211–8. doi: 10.1093/schbul/sbq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HQ, Rassoulpour A, Schwarcz R. Effect of systemic L-DOPA administration on extracellular kynurenate levels in the rat striatum. J Neural Transm. 2002;109(3):239–49. doi: 10.1007/s007020200020. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm. 2007;114(1):33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF. Are some cases of psychosis caused by microbial agents? A review of the evidence. Mol. Psychiatry. 2008;13(5):470–9. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]