Abstract
A breakdown in intestinal homeostasis results in inflammatory bowel diseases including coeliac disease and allergy. Galectins, evolutionarily conserved β-galactoside-binding proteins, can modulate immune-epithelial cell interactions by influencing immune cell fate and cytokine secretion. In this study we investigated the ‘glycosylation signature’ as well as the regulated expression of galectin-1 and -3 in human duodenal samples of allergic and non-allergic children. Whereas galectin-1 was predominantly localized in the epithelial compartment (epithelial cells and intraepithelial lymphocytes) and the underlying lamina propria (T cells, macrophages and plasma cells), galectin-3 was mainly expressed by crypt epithelial cells and macrophages in the lamina propria. Remarkably, expression of these galectins was not significantly altered in allergic versus non-allergic patients. Investigation of the glycophenotype of the duodenal inflammatory microenvironment revealed substantial α2–6-linked sialic acid bound to galactose in lamina propria plasma cells, macrophages and intraepithelial lymphocytes and significant levels of asialo core 1 O-glycans in CD68+ macrophages and enterocytes. Galectin-1 preferentially bound to neutrophils, plasma cells and enterocytes, while galectin-3 binding sites were mainly distributed on macrophages and intraepithelial lymphocytes. Notably, galectin-3, but not galectin-1 binding, was substantially increased in intraepithelial gut lymphocytes of allergic patients compared to non-allergic subjects, suggesting a potential role of galectin-3-glycan interactions in shaping epithelial-immune cell connections during allergic inflammatory processes.
Keywords: allergy, galectin-1, galectin-3, gut, lymphocytes
Food allergies are becoming increasingly common worldwide. They consist of a spectrum of disorders that result from adverse immune responses to dietary antigens (1). Hypersensitivity to cow milk allergy is one of the most common food allergies in childhood and constitutes a pathological condition in which oral tolerance against luminal cow’s milk proteins is abrogated (1). Although the precise pathogenic mechanisms are unknown, immunological responses to food may be IgE- or non-IgE- mediated. It seems possible that during the chronic phase of an originally IgE-initiated allergic inflammation, the pathogenic immune reaction responsible for clinical symptoms is dominated by allergen-specific T lymphocytes. In this context, it has become increasingly evident that allergen-specific Th2 cells play a central role in the genesis and maintenance of the allergic inflammatory reactions in both human subjects and mouse models (2–3). On the other hand, cell mediated immunity has been implicated in IgE-independent gastrointestinal food hypersensitivity. Activation of cytotoxic T lymphocytes has been described as the main pathogenic mechanism in cow’s milk protein sensitive enteropathy (CMSE) (4).
Glycan structures, which decorate the surfaces of all mammalian cells, can substantially change under different physiological and pathological conditions (5). In fact, glycosylation of cell surface proteins can control critical immunological processes, including T-cell activation, migration and apoptosis (6–7). Therefore, the physiology and pathology of a cell is determined to a great extent by the complex information encoded by its cell surface glycosylation pattern, which is decoded by various glycan-binding proteins, including C-type lectins, siglecs and galectins (5, 8–9).
Galectins, a family of highly conserved carbohydrate-binding proteins, are characterized by their ability to recognize multiple N-acetyllactosamine residues, which can be displayed on both N- and O-glycans on cell surface glycoconjugates (8). Galectins have emerged as novel regulators of immune cell homeostasis. Results from our laboratory demonstrated that galectin-1 (Gal-1) contributes to immune cell tolerance by selectively eliminating Th1 and Th17 pathogenic cells, thus promoting a shift toward a Th2-dominant cytokine profile (6, 10) or by favoring the expansion of regulatory T cells (11–12). On the other hand, Gal-3 induces activation of various inflammatory cell types (13), modulates T-cell apoptosis (13–14) and induces a pro-inflammatory response (13, 15). However, Gal-3 can also have anti-inflammatory activity under certain circumstances (16).
Although the role of galectins in inflammatory and allergic processes has been extensively described (13, 15–16), the relevance of these glycan-binding proteins within the intestinal microenvironment is poorly understood. Considering that the duodenum is an intestinal region critical during the development of allergic responses to food antigens, and that Th2 cells and IELs are deeply involved in their pathogenesis, we investigated the binding of Gal-1, Gal-3 and plant lectins to human duodenum samples of allergic and non-allergic patients, as well as the regulated expression of these glycan-binding proteins, in an attempt to characterize the human intestinal ‘glycosylation signature’ under physiological and pathological conditions, and to further delineate the potential role of galectin-glycan lattices within the intestinal microenvironment.
MATERIALS AND METHODS
Human samples and patients
The study group consisted of 16 patients (8 females and 8 males; mean age 1.5; range 1 month to 5 years) diagnosed as cow milk allergic (n: 8; mean age, 1.8 years; range, 1.5 to 2.5 years) or non-allergic (n: 8; sex- and age-matched). All patients were assisted at the Gastroenterology Unit in the Sor María Ludovica Children’s Hospital (La Plata, Argentina). Upper intestinal endoscopy was performed under general anesthesia. Biopsy samples were taken for routine histology, immunohistochemistry, lectin-histochemistry and confocal microscopic analysis.
Cow milk allergy (CMA) diagnosis was performed on the basis of clinical suspicion of milk-related symptoms (prolonged diarrhea, atopic dermatitis or broncoespasm after the ingestion of cow′s milk), elevated total serum IgE levels and the presence of cow milk specific IgE antibodies. Parasitic infections and lactose intolerance were discarded. Serum IgA levels were normal in all patients (0.15–1.59 g/l). IgG and IgA anti-gliadin and anti-transglutaminase were found to be negative, and two of the allergic patients had anemia and blood in stools. After CMA diagnosis was performed, a strict milk-free diet was indicated. All patients had clinical remission and showed a significant increase in weight and height after 6 months of allergen avoidance. An extensively hydrolyzed cow′s milk formula or a probiotic were indicated as a dairy substitute during the elimination diet. Written parental consent was obtained for all patients and the project was approved by the local Ethics Committee.
Histopathology and immunohistochemical methods
Specimens were fixed in 5% neutral buffered formalin and embedded in paraffin. Samples were cut and mounted onto charged glass slides and heated at 60°C for 30 min. Briefly, xylene and ethanol were used to deparaffinize and endogenous peroxidase activity was blocked with 3% H2O2/methanol. Antigen retrieval was performed in buffer citrate (pH 6) and slides were microwaved. Afterwards, non-specific sites were blocked with 1% BSA in PBS and incubated with the primary antibody: anti-CD3 monoclonal antibody (1:25, DAKO, CLONE F7.2.38; Glostrup, Denmark), biotinylated anti-human IgE (human ε chain specific) (1:500, Vector Labs, Burlingame, CA, USA), anti-CD45RO monoclonal antibody (1:300, DAKO, Glostrup, Denmark), anti-ST6Gal1 polyclonal antibody (1:200) (Sigma), anti-Gal-1 rabbit antibody (1:200) generated as described (17) and polyclonal anti-Gal-3 (1:300). The anti-Gal-3 antibody was raised in rabbits using the purified recombinant Gal-3 (see below), affinity-purified on Protein A-Sepharose, and its specificity was validated by Western blot. This was followed by the appropriate secondary antibodies: anti-mouse IgG-biotin, anti-rabbit IgG-biotin and strepavidin-HRP conjugate (DAKO, Glostrup, Denmark). 3,3’-diaminobenzidine tetrahydrochloride chromogen (DAKO, Glostrup, Denmark) was used for up to 2 min at room temperature for immunostaining. A serum from a non-immunized rabbit was used as primary antibody for specificity controls. Controls omitting the primary antibody were also performed. Sections were subsequently counterstained with 10% hematoxylin and mounted with Eukit. Stained sections were evaluated by two independent pathologists blinded to the clinical or endoscopic data.
For routine histology examination the length of duodenal crypt and villi was assessed using an ocular micrometer. Giemsa staining was performed to quantify mast cells, while eosinophils were counted on hematoxylin-eosin stained sections. Different types of infiltrating cells were counted per 6 villi.
For lectin-histochemistry, biotinylated lectins were used as the primary binding reagents: Sambucus nigra agglutinin (SNA) (25 μg/ml, Vector Labs, Burlingame, CA, USA), peanut agglutinin (PNA) (25 μg/ml, Vector Labs, Edelberry; Burlingame, CA, USA), recombinant Gal-1 (10 μg/ml) generated as described (17) and recombinant Gal-3 (10 μg/ml). The recombinant Gal-3 was expressed in E. coli from a construct based in the pET 30 Ek/Lic vector (Novagen, Madison, WI), and purified on a lactosyl-Sepharose column. Streptavidin-HRP (DAKO, Glostrup, Denmark) was used as secondary reagent. Controls were performed by omitting the biotinylated lectins. A Nikon Eclipse E400 microscopy was used to analyze the slides.
Tissue staining and analysis by confocal microscopy
Immunofluorescence and confocal microscopy were performed with deparaffinized sections. After antigen retrieval, samples were incubated with the primary antibody: anti-CD3 (1:25; DAKO, CLONE F7.2.38, Glostrup, Denmark), anti-CD68 as a macrophage marker (1:50; DAKO Clone M7228, Glostrup, Denmark), anti-CD138 as a plasma cell marker (1:25; DAKO, Clone MI 15, Glostrup, Denmark), or biotinylated lectins (PNA, SNA, Gal-1 and Gal-3) (Vector Labs, Burlingame, CA, USA). Anti-mouse-Cy3 (1:300; Jackson Immunoresearch Laboratorioies Inc., PA, USA) or streptavidin-FITC (1:200; Sigma-Aldrich, St Louis, MO, USA) were used as secondary reagents. DAPI (Invitrogen, Oregon, USA) was used for nucleus staining. An LSM 510 Carl Zeiss Laser Scanning microscope and an LSM5v3.2 software were used. For negative control staining the primary antibodies or the biotinylated lectins were replaced by phosphate-buffered saline.
Validation of lectin binding
Different sugars were used as competitive inhibitors to validate lectin binding to the glycosylated receptors. SNA, PNA and galectin binding were inhibited with 100 mM lactose, 200 mM galactose, and 100 mM lactose respectively.
Statistical analysis
The data collected were analyzed using the Student′s t test for unpaired data to estimate the significance of the differences between groups.
RESULTS
Expression and cellular distribution of Gal-1 and Gal-3 in the gut of allergic and non-allergic patients
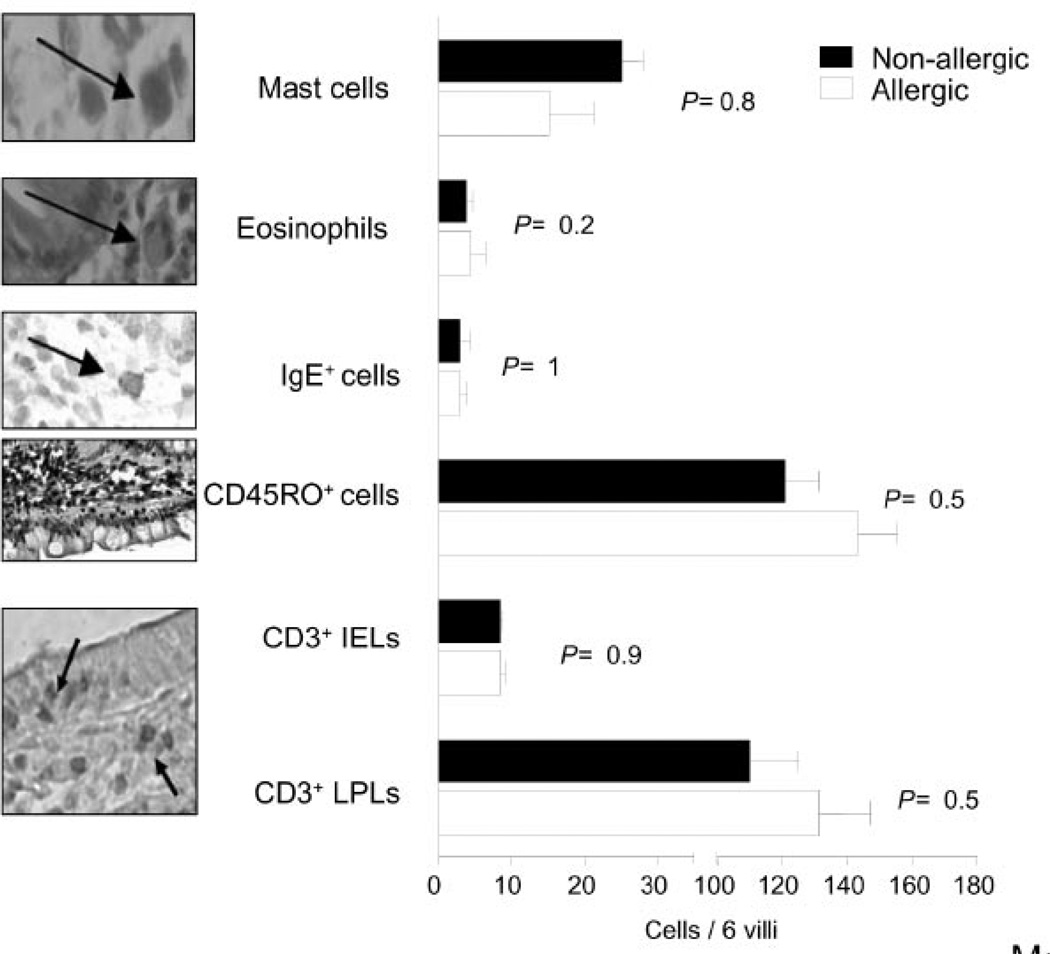
To determine the expression of Gal-1 and Gal-3 in duodenal samples of allergic and non-allergic patients, we first performed a routine histological examination. Histopathological analysis revealed no significant differences between samples of non-allergic and allergic patients (Fig. 1). No specific cellular infiltrates were observed in the gut of allergic patients, and CD3+ cell, mast cell, eosinophil and IgE-positive cell counts were not statistically different when both groups were compared. In addition, there was no villous atrophy, and villous or crypt heights were normal in all samples (villi/crypt ratio of 2.1 ± 0.6).
Fig. 1. Histopathologic analysis of duodenal samples of allergic and non-allergic patients.
Mast cells, eosinophils, IgE+ cells, CD45RO+ cells, CD3+ intraepithelial (IELs) and CD3+ lamina propria (LPLs) cells were counted in 6 villi of duodenal samples of allergic (N: 8) and non-allergic patients (N: 8). Cells were detected by Giemsa or hematoxylin/eosin staining (mast cells and eosinophils, respectively) or by immunohistochemistry using anti-IgE, anti-CD45RO or anti-CD3 monoclonal antibodies. Statistical analysis revealed no significant differences between different groups of patients. (Original magnification: 1000x except for CD45RO).
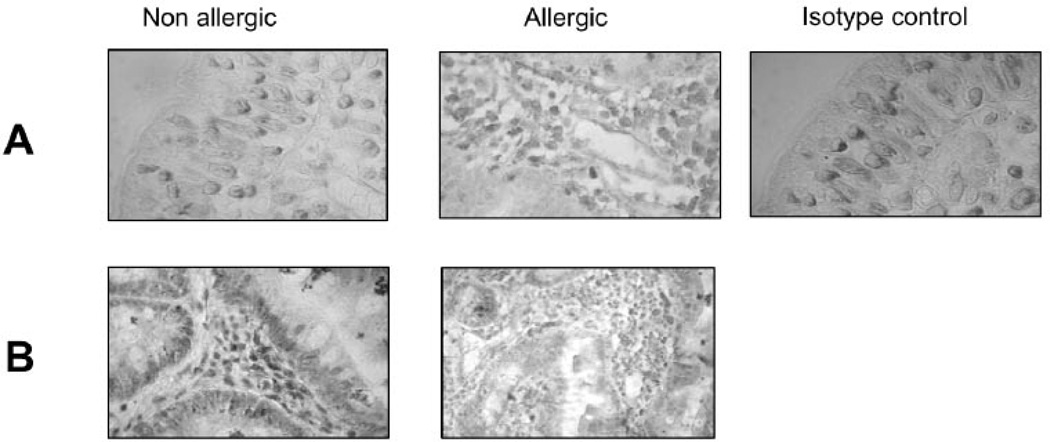
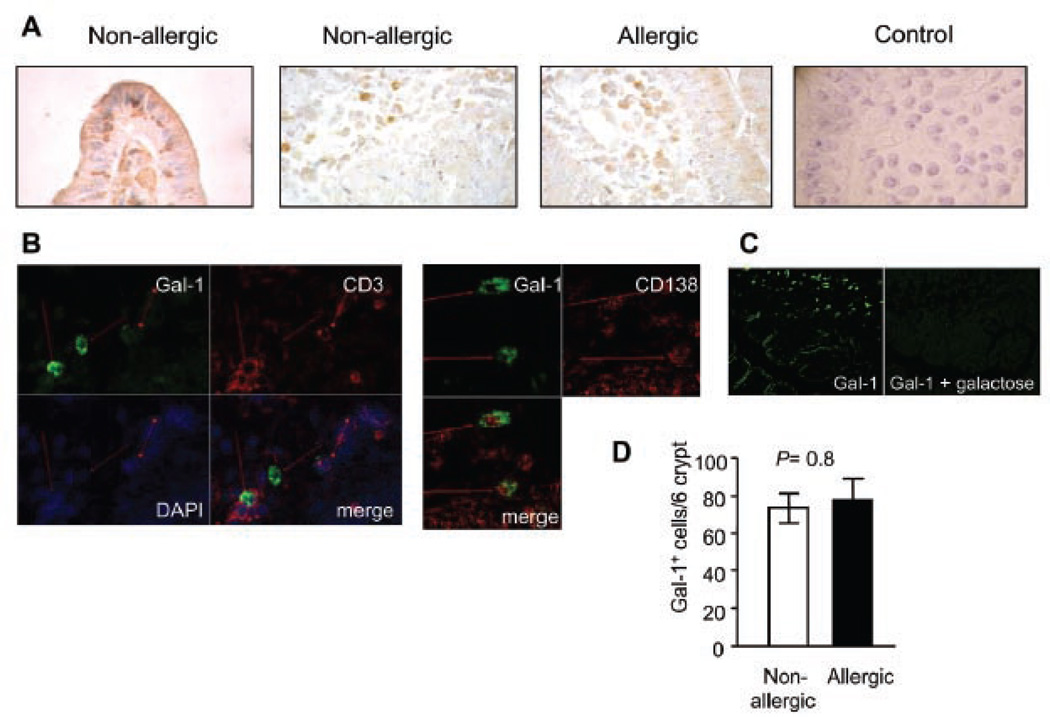
Several sources of endogenous Gal-1 and Gal-3 were found (Fig. 2). Galectin-1 was predominantly expressed in the epithelial compartment, mainly epithelial cells and intraepithelial lymphocytes (IELs) and in the underlying lamina propria (T cells, macrophages and plasma cells), whereas Gal-3 was mainly found in crypt epithelial cells and macrophages in the lamina propria (Fig. 2). Remarkably, expression of these galectins was not significantly altered in the gut of allergic patients (Fig. 2), suggesting that the contribution of galectin-glycan interactions in the transition to allergy may not be regulated at the level of galectin expression in the intestinal inflammatory microenvironment.
Fig. 2. Expression of Gal-1 and Gal-3 in duodenal specimens of allergic and non-allergic patients.
Gal-1 and Gal-3 expression was assessed by immuno-histochemistry using specific polyclonal antibodies against Gal-1 and Gal-3 (purified rabbit IgGs) in duodenal samples of allergic and non-allergic patients. Representative photographs of mucosa from allergic and non allergic patients were selected to show Gal-1 (A) and Gal-3 (B) positive cells. Control with serum from non-immunized rabbits is depicted. All samples were analyzed at least three times in independent experiments (Original magnification: 100x and 400x).
Lectin binding sites within the duodenal microenvironment in allergic and non-allergic subjects
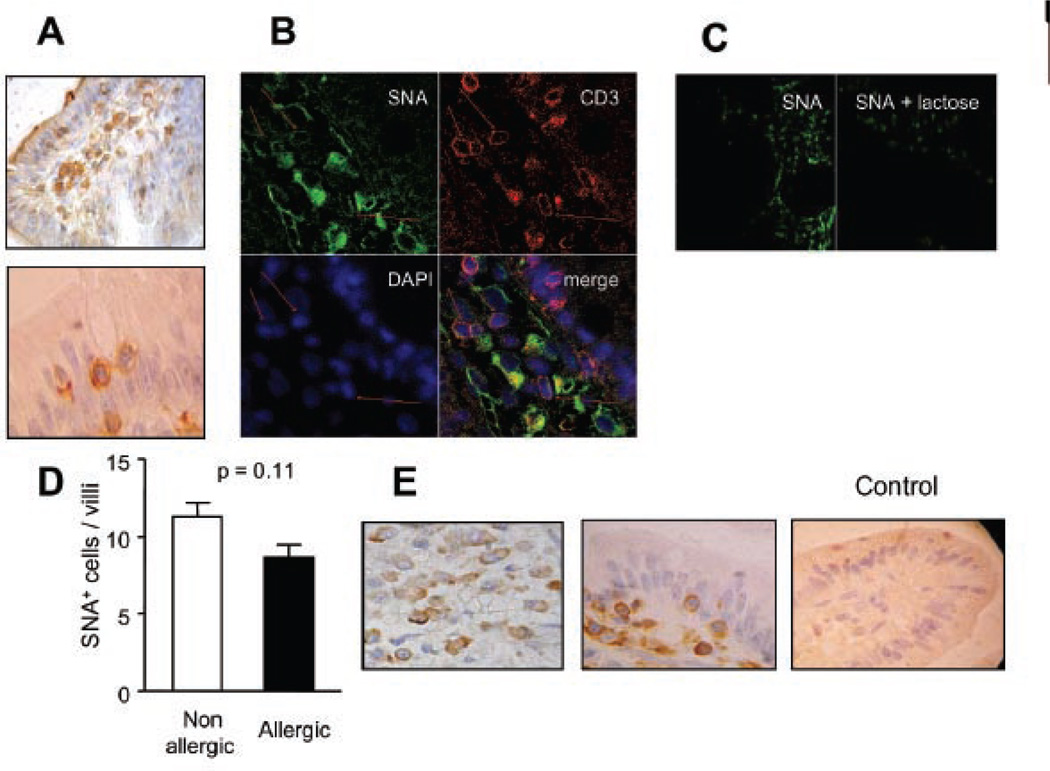
To determine whether the transition from ‘non-allergic’ to ‘allergic’ inflammatory human gut is accompanied by changes in cell surface glycosylation that could directly affect galectin binding and immunoregulation, we studied the binding of exogenous biotin-labeled lectins to mucosal tissues. It has been demonstrated that incorporation of α2–6-linked sialic acid to N-glycans may be more restrictive for the binding of Gal-1 but not Gal-3 to cell surface glycoproteins (6, 18). As depicted in Fig. 3 biotinylated SNA, a plant lectin that recognizes the SAα2–6-galactose sequence on N-glycans (6), preferentially bound macrophages of the lamina propria, although a clear membrane staining was also observed in intraepithelial lymphocytes. Double-staining analysis was performed by confocal microscopy to evaluate the binding of SNA to CD3+ T lymphocytes. As shown in Fig. 3b, SNA bound strongly to lamina propria CD3+ T cells. However, not all CD3+ T cells were positive for SNA. Remarkably, no significant differences were found between samples of allergic and non-allergic patients in the number of CD3+ SNA+ cells (10 ± 1 SNA+CD3+ cell/villi). Importantly, SNA binding was completely inhibited in the presence of 100 mM lactose, demonstrating that lectin binding was saccharide-dependent (Fig. 3c). In addition, the presence of α2–6-linked sialic acid was confirmed by immunohistochemical analysis of the expression of α2–6 sialyltransferase 1 (ST6Gal 1), an enzyme responsible for generating these glycan structures (Fig 3e). Expression of this glycosyltransferase was found in plasma cells, T cells and, to a lesser extent, in epithelial cells of duodenal mucosa. Notably, no differences were found between allergic and non-allergic samples.
Fig. 3. SNA binding pattern on duodenal samples: Pattern of α2,6 sialylation in mucosal tissue of allergic versus non-allergic patients.
A) Lectin-histochemistry using biotinylated-SNA and hematoxylin staining was done to identify SAα2–6-galactose residues. A photograph with stained IEL from an allergic patient is shown. B) Co-localization studies using a Cy3-conjugated anti-CD3 antibody (red), biotinylated SNA/ FITC-conjugated streptavidin (green) and DAPI (blue) was performed by confocal microscopy. C) Lectin binding inhibition assessed by confocal microscopy. Cells were incubated with biotinylated SNA or biotinylated SNA in the presence of 100 mM lactose. D) SNA+ cells were quantified by lectin-histochemistry in biopsy specimens of allergic (N=8) and non-allergic (N=8) patients and no significant difference was found (p= 0.11). E) ST6Gal1 expression was detected using a polyclonal specific antibody by immunohistochemistry. A control of immunoreactivity is shown using sera from non-immunized rabbits. All samples were analyzed at least three times in independent experiments and representative images are shown (Original magnifications: 200x and 1000x).
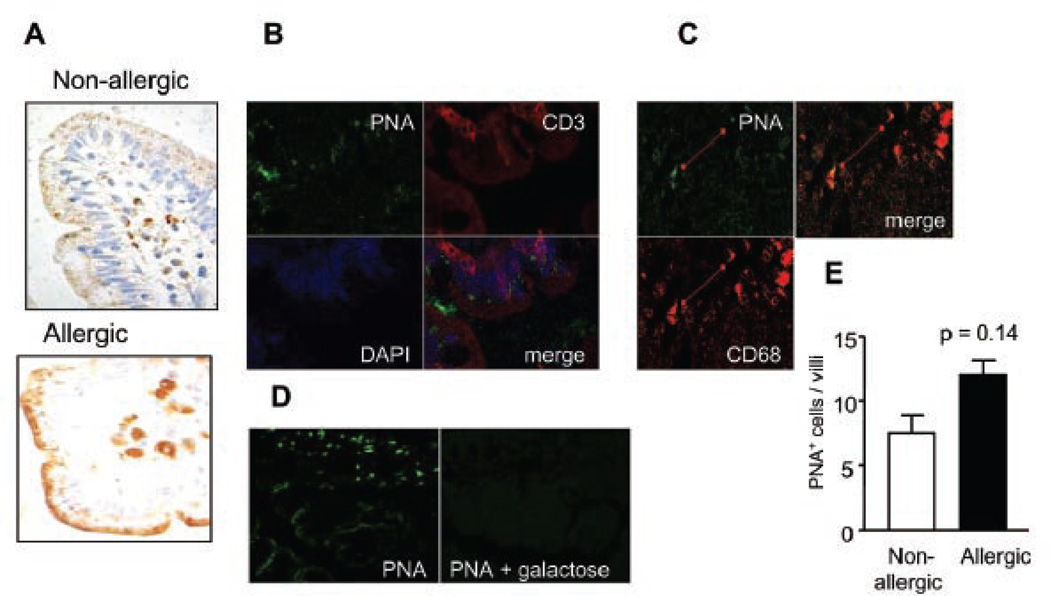
Next, we probed cell surface glycoreceptors for the presence of asialo-core-1-O-glycans using the lectin peanut agglutinin (PNA), which binds to galactose-β1–3-N-acetylgalactosamine core-1-O-glycans and thus indicates lack of terminal α2–3-linked sialic acid on these glycans. Interestingly, PNA binding correlated well with galectin binding to the surface of different cell types (6). We found high PNA binding to intestinal macrophages and enterocytes (supranuclear granules) (Fig. 4a). Remarkably, the glycocalyx coat was highly stained by biotinylated PNA, demonstrating the abundance of asialo-core 1-O-glycans on this structure (Fig 4a). Using confocal microscopy we found that CD68+ macrophages were highly positive for PNA staining (Fig. 4b). In contrast, no PNA+CD3+ cells could be detected, suggesting that CD3+ T cells are highly sialylated in the intestinal mucosa (Fig. 4c). Specific binding was demonstrated by confocal microscopy using 100 mM galactose (Fig. 4d)
Fig. 4. PNA binding pattern in duodenal samples: Detection of asialo core-1-O-glycans in intestinal glycoproteins of allergic and non-allergic patients.
A) Lectin-histochemistry with biotinylated PNA in gut biopsies. B) Co-localization studies by confocal microscopy using Cy3-conjugated anti-CD3 antibody (red), biotinylated PNA/ FITC-conjugated streptavidin (green) and DAPI (blue nuclear staining); (C) Co-localization studies by confocal microscopy using biotinylated PNA/ FITC-conjugated streptavidin (green) and PE-conjugated anti-CD68 antibody (red). D) Lectin binding inhibition studies assessed by confocal microscopy using biotinylated PNA/ FITC-labeled streptavidin or biotinylated PNA/ FITC-labeled streptavidin in the presence of 200 mM galactose. E) PNA+ cells were quantified by lectin-histochemistry in biopsy specimens of allergic and non-allergic patients; no significant differences were found (P:0.14). All samples were analyzed at least three times in independent experiments and representative images are shown (Original magnification: 200x).
To further demonstrate the availability of glycan ligands for galectin recognition and immunoregulation, we next performed lectin-histochemical analysis using biotinylated Gal-1 and Gal-3. Staining with biotinylated Gal-1 revealed the presence of Gal-1 binding sites predominantly on plasma cells, neutrophils and enterocytes (Fig. 5). Galectin-1 binding strongly co-localized with CD138 (Syndecan-1), a transmembrane heparan sulfate proteoglycan highly expressed by plasma cells. Importantly, Gal-1 binding was inhibited by addition of 100 mM lactose, clearly demonstrating that this glycan-binding protein binds to plasma cells in a saccharide-specific manner. Remarkably, we found no significant differences in the extent of Gal-1 binding between gut samples of allergic and non-allergic patients (Fig. 5). Of note, biotinylated Gal-1 was recognized by T cells in spleen biopsies of control subjects (data not shown), yet it did not bind to CD3+ T cells from intestinal biopsies, suggesting that the presence of α2–6-linked sialic acid on intraepithelial lymphocytes (Fig. 3 b, c) might restrict Gal-1 recognition of its specific glycan ligands and limit its subsequent functional effects.
Fig. 5. Gal-1-specific ligands on duodenal samples of allergic and non-allergic patients.
A) Lectin-histochemistry with biotinylated Gal-1/ HRP-conjugated streptavidin in gut biopsies. B) Co-localization studies by confocal microscopy using biotinylated Gal-1/ FITC-conjugated streptavidin (green), Cy3-labeled anti-CD3 or PE-labeled anti-CD138 (red) antibodies and DAPI (blue nuclear staining). C) Lectin binding inhibition studies assessed by confocal microscopy with biotinylated Gal-1/ FITC-labeled streptavidin or biotinylated Gal-1/ FITC-conjugated streptavidin in the presence of 100 mM lactose. D) Gal-1+ cells were quantified by lectin-histochemistry in biopsy specimens of allergic and non-allergic patients; no significant differences were found (P= 0.8). All samples were analyzed at least three times in independent experiments and representative images are shown (Original magnifications: 200x and 400x).
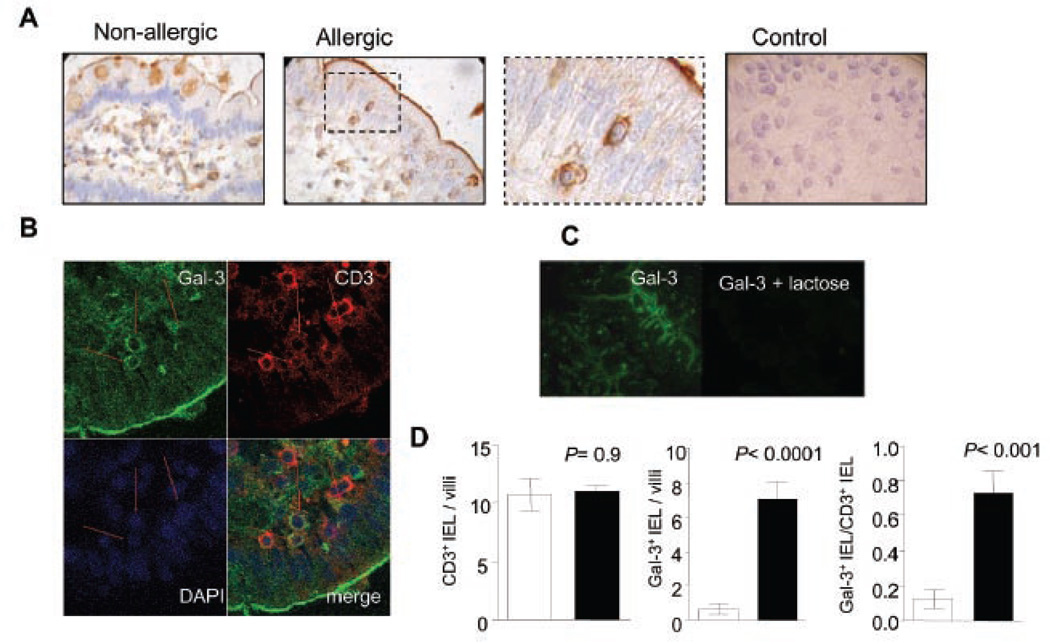
In contrast, biotinylated Gal-3 clearly bound to macrophages, intraepithelial and lamina propria T cells (Fig. 6). Also, the glycocalyx contained glycosylated components capable of recognizing Gal-3, as illustrated in Fig. 6a. Noteworthy, lectin-histochemistry and confocal microscopic analyses demonstrated substantially higher binding of Gal-3 to the surface of CD3+ intraepithelial T lymphocytes in biopsies of allergic patients compared to non-allergic subjects. Importantly, the total number of IELs did not statistically differ between normal and allergic patients: 8.25 ± 0.78 and 8.37 ± 0.24 IELs/villi (P= 0.9), respectively. However, the ratio of Gal-3+ IELs/total IELs per villi was 0.13 ± 0.05 for normal subjects and 0.73 ± 0.14 for allergic patients, clearly demonstrating that the transition from non-allergic to allergic mucosal tissue results in selective up-regulation of galectin-3-specific glycans on the surface of IELs.
Fig. 6. Gal-3-specific ligands in duodenal samples of allergic versus non-allergic patients.
A) Lectin-histochemistry with biotinylated Gal-3/ HRP-conjugated streptavidin in gut biopsies from allergic and non-allergic patients. A control omitting biotinylated Gal-3 is shown. B) Co-localization studies by confocal microscopy using biotinylated Gal-3/ FITC-labeled streptavidin (green), Cy3-conjugated anti-CD3 (red) and DAPI (blue nuclear staining). C) Lectin binding inhibition studies by confocal microscopy with biotinylated Gal-3/ FITC-labeled streptavidin or biotinylated Gal-3 / FITC-labeled streptavidin in the presence of 100 mM lactose. D) CD3+ IELs (upper panel; P= 0.9) and Gal-3+ IELs (middle panel; P<0.0001) were quantified in samples of allergic and non-allergic patients. In the lower panel the ratio of Gal-3+ IELs/total CD3+IELs is depicted (P<0.001). All samples were analyzed at least three times in independent experiments and representative images are shown (Original magnifications: 200x, 400x and 1000x)
DISCUSSION
Research during the past decade has shed light on the role of galectin-glycan interactions at different stages of the inflammatory responses (5, 8, 13). In addition, regulated expression of galectins, as well as modulation of their specific ligands, is a common feature of the transition from normal to inflamed or neoplastic tissues (19–20). In this study we characterized the pattern of Gal-1 and Gal-3 expression and their glycan-binding profiles in mucosal duodenum of normal individuals and allergic patients with a clinical outcome compatible with hypersensitivity to cow milk proteins, in an attempt to delineate the function of galectin-glycan lattices within the intestinal inflammatory microenvironment. Our results clearly demonstrate that, in the absence of evident histopathologic alterations, an early manifestation of cow milk allergy is represented by an up-regulated expression of galectin-3-binding sites on the surface of IELs.
It still remains controversial whether the gut mucosa is histologically altered as a result of food allergy. Although no histological alteration was found by Augustin and colleagues (21) in CMSE patients, the authors observed a higher density of TUNEL+ CD3+ IELs. This may reflect a compensatory mechanism to eliminate IELs during impaired immune responses to food allergens. In our study we found no villous atrophy in the duodenum of allergic patients. However, the presence of CD3+ IELs expressing the repertoire of glycans critical for Gal-3 binding might contribute to alterations of gut homeostasis and permeability. As Gal-3 has important pro-inflammatory properties (13), it is possible that Gal-3-activated IELs might drive inflammation and an uncontrolled response to food antigens. Interestingly, in our study, samples of allergic patients showed no considerable increase in the number of CD3+ IELs, or in the frequency of CD3+ cells infiltrating the lamina propria, although most T cells were activated (CD3+CD45RO+ cells). Kokkonen and colleagues (22) found similar results in CMA patients with no villous atrophy or cell infiltration. However, they found a higher density of CD3+ γδ+ IEL. Taken together, these results suggest an increased number of IELs might be a marker of young adult duodenum with CMSE.
Galectins and their specific glycan ligands are abundant within the gastrointestinal tract (23–24). Brinck and colleagues studied inflammation- and neoplasia-associated alterations in human large intestine and found critical differences in the lectin binding profile following acute peritonitis and tumorigenesis in the goblet cell population (24). Previous studies reported that Gal-1 and Gal-3 mRNA are weakly expressed in the lamina propria of the small intestine (25). Conversely, we found that epithelial cells and IELs were a major source of Gal-1. This striking observation suggests that this lectin might be involved in host-pathogen/commensal interactions, in epithelial cell homeostasis, or in the modulation of mucosal tolerance and inflammation. This assumption is supported by the ability of recombinant Gal-1 to suppress inflammatory bowel disease in mice (26). On the other hand, Gal-3, which is mainly produced in the crypt, may also be involved in epithelial cell proliferation or in immune cell activation. Strikingly, we did not find differences in Gal-1 or Gal-3 expression between tissues from non-allergic and allergic patients. As galectins are regulated during pathological conditions (27–29), further studies are warranted to analyze whether galectin expression is modified in response to allergic inflammation or following immunotherapy in experimental models or clinical settings.
According to our results, Gal-1 may not be involved in lamina propria T cell (LPTC) homeostasis although it clearly binds to peripheral T cells (6) or to Th1/Th17-skewed inflammatory mucosal T cells (26). Thus, one might speculate that under steady-state conditions or Th2-driven allergic responses, mucosal T cells are decorated by α2–6-linked sialic acid, thus restricting Gal-1 binding and its subsequent immunoregulatory effects (6). However, Gal-3 may successfully regulate Th2-driven inflammation in the mucosa during allergic inflammation, as has been previously demonstrated in mouse models (15–16). Intriguingly, Gal-2, a proto-type galectin structurally related to Gal-1, might play a compensatory role in mucosal homeostasis as it is widely expressed within the gastrointestinal tract (27).
Regarding the ligand selectivity of galectins, it is apparent that neither Gal-1 nor Gal-3 would bind to glycans containing only one lactosamine (Galβ1,3(4)GlcNAc) with α2,6 sialyation on its non-reducing terminal galactose residues, as the 6-OH of the galactose residue is critical for galectin binding. However, if a glycan contains more than two lactosamine residues, one of which bearing α2,6 sialyation, a difference between Gal-1 and Gal-3 glycan binding would then become evident. As Gal-1 preferentially recognizes peripheral (non-reducing terminal) β-galactoside residues, the incorporation of sialic acid in α2,6 linkage to the non-reducing terminal galactoside may significantly reduce binding of this lectin. In contrast, as Gal-3 binds to internal β-galactoside residues, the impact of sialyl modification with α2,6 linkage is relatively lower than for Gal-1. As most glycans found on cell surface glycoproteins contain more than two lactosamine residues, some of which are not sialylated in α2,6, the overall affinity of Gal-3 for α2,6 sialylated glycans looks higher than Gal-1 (9, 18).
In our study, SNA, which recognizes α2–6-linked sialic acid, bound to membrane glycoproteins in lamina propria macrophages, plasma cells and IELs at the same extent in allergic and non-allergic patients. This was confirmed by immunohistochemistry using a specific anti-ST6Gal1 antibody supporting the fact that Th2-polarized cells as well as other immune cell types display abundant cell surface expression of α2–6-linked sialic acid (5–6). On the other hand, PNA, that specifically recognizes asialo-core-1-O-glycans, labeled macrophages, epithelial cells and mucin produced by goblet cells. In this regard, PNA is a classical marker of immature thymocytes and activated T cells (6), but can also recognize macrophage glycoproteins (30). Of note, no PNA binding to LPTC could be detected in our study. Interestingly, we previously found that human and mouse CD4+ T cells, polarized toward Th1 or Th17 phenotypes, displayed abundant PNA-reactive asialo-core-1-O-glycans. On the contrary, Th2 cells showed limited PNA binding (6). In this study, we found high PNA binding to intestinal macrophages but not to T cells, an effect probably associated with the high frequency of tolerogenic and Th2-polarized cells in the mucosa of control individuals or allergic patients.
The role of Gal-3 in allergic inflammation is still controversial. Cortegano and colleagues showed an anti-allergic effect by using recombinant Gal-3 to suppress IL5 gene transcription in eosinophils and allergen-specific T cell lines (16). On the contrary, using Gal-3-deficient (Lgals3−/−) mice, Zuberi et al. assigned a pro-inflammatory role for Gal-3 in a mouse model of ovalbumin-induced asthma (15). Interestingly, we found that IELs from allergic patients differentially bind Gal-3, suggesting a potential role of this glycan-binding protein in modulating T cell homeostasis within the intestinal mucosa in allergy. Further studies are necessary to understand the effects of Gal-3 on IELs of CMSE patients.
As previously mentioned, other groups have reported that the density of IELs is often increased in CMSE patients. A differential recognition of these cells by Gal-3 in allergic patients may represent a compensatory mechanism to restore tolerance and homeostasis. Gal-3, although mostly described as pro-inflammatory mediator, can also act as an immunosuppressive agent by inducing T cell apoptosis or negatively regulating T cell signaling (5, 14). Hence, Gal-3 might exert a biphasic effect acting as a pro-inflammatory molecule during the first steps of allergic inflammation in early childhood, while it might have a regulatory role in limiting allergic processes in early adulthood. These opposite functions might be exerted through the recognition of differentially-glycosylated membrane glycoproteins (14). Our findings suggest that the transition from non-allergic to allergic inflammatory intestinal microenvironment may result in selective glycosylation changes mainly on the surface of IELs.
In conclusion, our findings provide the first evidence of a selective role of galectin-3 recognition of IELs within the allergic inflammatory mucosa, which may help to unveil the dominant mechanisms underlying an impaired immune response to food allergens. Future research is warranted to examine the biological role of galectin-3-glycan lattices within the normal and inflamed mucosal tissue, and the relevance of these multifunctional proteins in positively or negatively regulating immunity and/or tolerance within the intestinal microenvironment.
ACKNOWLEDGEMENTS
We are grateful to L. Baum for providing plasmid for recombinant galectin-1. This work was supported by grants from the National Agency for Promotion of Science and Technology to G.D. and G.A.R., grants from Fundación Sales, Cancer Research Institute to G.A.R. and grant R01 GM070589-01 from the National Institutes of Health to G.R.V.
REFERENCES
- 1.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2006;117:S470–S475. doi: 10.1016/j.jaci.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 2.Romagnani S. The role of lymphocytes in allergic disease. J Allergy Clin Immunol. 2000;105:399–408. doi: 10.1067/mai.2000.104575. [DOI] [PubMed] [Google Scholar]
- 3.Frydas S, Karagouni E, Hatzistilianou M, et al. Cytokines and allergic disorders: revisited study. Int J Immunopathol Pharmacol. 2004;17:233–235. doi: 10.1177/039463200401700302. [DOI] [PubMed] [Google Scholar]
- 4.Augustin M, Karttunen TJ, Kokkonen J. TIA1 and mast cell tryptase in food allergy of children: increase of intraepithelial lymphocytes expressing TIA1 associates with allergy. J Pediatr Gastroenterol Nutr. 2001;32:11–18. doi: 10.1097/00005176-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 5.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9:593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 6.Toscano MA, Bianco GA, Ilarregui JM, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez MI, Rubinstein N, Ilarregui JM, Toscano MA, Sanjuan NA, Rabinovich GA. Regulated expression of galectin-1 after in vitro productive infection with Herpes Simplex virus type 1: Implications for T-cell apoptosis. Int J Immunopathol Pharmacol. 2005;18:615–623. doi: 10.1177/039463200501800402. [DOI] [PubMed] [Google Scholar]
- 8.Rabinovich GA, Toscano MA, Jackson SS, Vasta GR. Functions of cell surface galectin-glycoprotein lattices. Curr Opin Struct Biol. 2007;17:513–520. doi: 10.1016/j.sbi.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasta GR, Ahmed H, editors. Animal Lectins: A Functional View. Boca Raton, FL: Taylor and Francis (CRC Press); 2008. [Google Scholar]
- 10.Rubinstein N, Alvarez M, Zwirner NW, et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241–251. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 11.Toscano MA, Commodaro AG, Ilarregui JM, Bianco GA, Liberman A, Serra HM, Hirabayashi J, Rizzo LV, Rabinovich GA. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant TH2- and T regulatory-mediated anti-inflammatory responses. J Immunol. 2006;176:6323–6332. doi: 10.4049/jimmunol.176.10.6323. [DOI] [PubMed] [Google Scholar]
- 12.Blois SM, Ilarregui JM, Tometten M, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 13.Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 14.Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176:778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- 15.Zuberi RI, Hsu DK, Kalayci O, et al. Critical role for galectin-3 in airway inflammation and bronchial hyperresponsiveness in a murine model of asthma. Am J Pathol. 2004;165:2045–2053. doi: 10.1016/S0002-9440(10)63255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.del Pozo V, Rojo M, Rubio ML, et al. Gene therapy with galectin-3 inhibits bronchial obstruction and inflammation in antigen-challenged rats through interleukin-5 gene downregulation. Am J Respir Crit Care Med. 2002;166:732–737. doi: 10.1164/rccm.2111031. [DOI] [PubMed] [Google Scholar]
- 17.Rabinovich GA, Daly G, Dreja H, Tailor H, Riera CM, Hirabayashi J, Chernajovsky Y. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J Exp Med. 1999;190:385–398. doi: 10.1084/jem.190.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, Smith DF, Cummings RD. Galectin-1,-2, and-3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008;283:10109–10123. doi: 10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danguy A, Camby I, Kiss R. Galectins and cancer. Biochim Biophys Acta. 2002;1572:285–293. doi: 10.1016/s0304-4165(02)00315-x. [DOI] [PubMed] [Google Scholar]
- 20.D’Haene N, Maris C, Sandras F, Dehou MF, Remmelink M, Decaestecker C, Salmon I. The differential expression of galectin-1 and galectin-3 in normal lymphoid tissue and non-Hodgkin`s and Hodgkin`s lymphomas. Int J Immunopathol Pharmacol. 2005;18:431–443. doi: 10.1177/039463200501800304. [DOI] [PubMed] [Google Scholar]
- 21.Augustin MT, Kokkonen J, Karttunen TJ. Evidence for increased apoptosis of duodenal intraepithelial lymphocytes in cow’s milk sensitive enteropathy. J Pediatr Gastroenterol Nutr. 2005;40:352–358. doi: 10.1097/01.mpg.0000151748.07469.bf. [DOI] [PubMed] [Google Scholar]
- 22.Kokkonen J, Haapalahti M, Laurila K, Karttunen TJ, Mäki M. Cow’s milk protein-sensitive enteropathy at school age. J Pediatr. 2001;139:797–803. doi: 10.1067/mpd.2001.118882. [DOI] [PubMed] [Google Scholar]
- 23.Mizoguchi E, Mizoguchi A. Is the sugar always sweet in intestinal inflammation? Immunol Res. 2007;37:47–60. doi: 10.1007/BF02686089. [DOI] [PubMed] [Google Scholar]
- 24.Brinck U, Korabiowska M, Bosbach R, Gabius HJ. Detection of inflammation- and neoplasia-associated alterations in human large intestine using plant/ invertebrate lectins, galectin-1 and neoglycoproteins. Acta Anat (Basel) 1998;161:219–233. doi: 10.1159/000046460. [DOI] [PubMed] [Google Scholar]
- 25.Nio J, Kon Y, Iwanaga T. Differential cellular expression of galectin family mRNAs in the epithelial cells of the mouse digestive tract. J Histochem Cytochem. 2005;53:1323–1334. doi: 10.1369/jhc.5A6685.2005. [DOI] [PubMed] [Google Scholar]
- 26.Santucci L, Fiorucci S, Rubinstein N, Mencarelli A, Palazzetti B, Federici B, Rabinovich GA, Morelli A. Galectin-1 suppresses experimental colitis in mice. Gastroenterology. 2003;124:1381–1394. doi: 10.1016/s0016-5085(03)00267-1. [DOI] [PubMed] [Google Scholar]
- 27.Paclik D, Berndt U, Guzy C, et al. Galectin-2 induces apoptosis of lamina propria T lymphocytes and ameliorates acute and chronic experimental colitis in mice. J Mol Med. 2008;86:1395–1406. doi: 10.1007/s00109-007-0290-2. [DOI] [PubMed] [Google Scholar]
- 28.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137–157. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 29.Jensen-Jarolim E, Gscheidlinger R, Oberhuber G, et al. The constitutive expression of galectin-3 is downregulated in the intestinal epithelia of Crohn’s disease patients, and tumour necrosis factor alpha decreases the level of galectin-3-specific mRNA in HCT-8 cells. Eur J Gastroenterol Hepatol. 2002;14:145–152. doi: 10.1097/00042737-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Howard DR, Batsakis JG. Peanut agglutinin: a new marker for tissue histiocytes. Am J Clin Pathol. 1982;77:401–408. doi: 10.1093/ajcp/77.4.401. [DOI] [PubMed] [Google Scholar]