Abstract
Quantifying the impact of different modifiable behavioral and biological risk factors on socioeconomic disparities in coronary heart disease (CHD) may help inform targeted, population-specific strategies to reduce the unequal distribution of the disease. Previous studies have used analytic approaches that limit our ability to disentangle the relative contributions of these risk factors to CHD disparities. The goal of this study was to assess mediation of the effect of low education on incident CHD by multiple risk factors simultaneously. Analyses are based on 15,067 participants of the Dutch Monitoring Project on Risk Factors for Chronic Diseases aged 20–65 years examined 1994–1997 and followed for events until January 1, 2008. Path analysis was used to quantify and test mediation of the low education-CHD association by behavioral (current cigarette smoking, heavy alcohol use, poor diet, and physical inactivity) and biological (obesity, hypertension, diabetes, and hypercholesterolemia) risk factors. Behavioral and biological risk factors accounted for 56.6% (95% CI: 42.6%–70.8%) of the low education-incident CHD association. Smoking was the strongest mediator, accounting for 27.3% (95% CI: 17.7%–37.4%) of the association, followed by obesity (10.2%; 95% CI: 4.5%–16.1%), physical inactivity (6.3%; 95% CI: 2.7%–10.0%), and hypertension (5.3%; 95% CI: 2.8%–8.0%). In summary, in a Dutch cohort, the majority of the relationship between low education and incident CHD was mediated by traditional behavioral and biological risk factors. Addressing barriers to smoking cessation, blood pressure and weight management, and physical activity may be the most effective approaches to eliminating socioeconomic inequalities in CHD.
Keywords: socioeconomic status, coronary heart disease, health behaviors, risk factors
INTRODUCTION
Coronary heart disease (CHD) is the leading cause of death in the Netherlands [1]. Studies in the Netherlands have shown low socioeconomic position (SEP) is associated with higher risk of adverse cardiovascular outcomes [2, 3]. Individuals with low SEP may have higher CHD risk due to greater adoption of unhealthy behaviors. This may be the result of living in environments that promote the adoption and maintenance of unhealthy behaviors and offer limited access to health-promoting options [4–8]. In addition, exposure to psychosocial stressors is generally higher among low SEP individuals, and many CHD behavioral risk factors, including cigarette smoking, heavy alcohol use, and consuming high-fat, high-carbohydrate foods, may be anxiety-reducing adaptations to the financial and psychological stress of having limited socioeconomic resources [9–12].
Low SEP may also lead to higher CHD risk due to higher prevalence of biological risk factors, including obesity, hypertension, hypercholesterolemia, and diabetes. These risk factors may be elevated in low SEP individuals due to the high prevalence of unhealthy behaviors or via more purely physiological mechanisms. For example, chronic exposure to physical or psychosocial stressors such as those associated with low SEP may lead to physiologic damage, or allostatic load, via increased sympathetic nervous system activity and dysregulation of stress-responsive neuroendocrine systems [13]. This physiologic wear and tear may in turn lead to several adverse outcomes that could increase CHD risk including hypertension, increased insulin resistance, increased visceral adiposity, and altered metabolism of low-density lipoprotein [14, 15].
Previous studies have estimated the overall contribution of various behavioral and biological mediators of the association between low SEP and CHD risk to range from 12.5% and 91% [16–25]. These differences may be due to variation in the risk factors that were adjusted for in the analyses and/or differences in how risk factors were measured. Fewer studies have quantified the specific contributions of different risk factors to associations of low SEP with CHD risk, and those studies have used analytic approaches that limit our ability to accurately quantify the independent contributions of these proposed mediators. Some have included all risk factors in one model [18, 23, 26]. However, this makes it difficult to distinguish the independent roles of these different mediating pathways in socioeconomic disparities in cardiovascular disease risk. Others adjust for one mediator at a time [17, 23, 27], but this ignores the propensity for risk factors to cluster. Furthermore, neither approach takes into account the likelihood that some CHD risk factors may be on the causal pathway of other risk factors (e.g. obesity is a risk factor for hypertension). Accounting for the unique contributions of co-occurring risk factors may provide a better approximation of the relative impact of each risk factor on socioeconomic disparities in incident CHD.
Using data from the Dutch Monitoring Project on Risk Factors for Chronic Diseases (MORGEN), we expanded upon previous analyses of the contributions of behavioral and biological risk factors to socioeconomic disparities in CHD risk by using path analysis [28, 29] to quantify the relative contribution of each risk factor, while accounting for hypothesized causal relationships among the mediating behavioral and biological factors. In the absence of unmeasured confounding, path analysis allows for the simultaneous assessment of the contributions of several risk factors to the relationship between low SEP and CHD risk. Moreover, while several studies have examined behavioral and biological pathways linking various indicators of low SEP with incident CHD [16–25], very few have examined these in a Dutch cohort [3]. Given that risk factor prevalence as well as the social patterning of risk factors has recently been shown to vary across European cohorts [30], a better understanding of the relative importance of these risk factors in the MORGEN cohort may aid in the selection of the most effective interventions to address social disparities in coronary heart disease in the Netherlands.
METHODS
Study population
MORGEN is a sex- and age-stratified random sample of individuals aged 20–65 years at baseline living in three towns in the Netherlands (Doetinchem, Maastricht, and Amsterdam) between 1993 and 1997 [31, 32]. Each year a new random sample of approximately 5000 subjects was examined. The average response rate was 45%. Participants received two questionnaires by mail: 1) a general questionnaire on socio-demographic factors, lifestyle and health indicators, and 2) a food frequency questionnaire. Participants also visited the local Public Health Service for a physical examination and to draw a 30-ml non-fasting blood sample. Since then, participants have been followed for disease occurrence through registries and for certain lifestyle changes through questionnaires sent in 3- to 5-year intervals.
Because the physical activity questionnaire was not administered in 1993, the present study was restricted to participants who were interviewed between 1994 and 1997. Of the 18,008 eligible men and women, participants were excluded for the following reasons: refusal of consent for follow-up of vital status and hospital discharge data (n=2,078); having prevalent CVD (n=224); being pregnant at baseline (n=96); and missing data on any of the other study covariates (n=466). Excluded participants were similar to those included in the study population in age, marital status, and sex. After exclusions, 15,067 participants remained for analyses.
Measures
Socioeconomic position was assessed using educational attainment, which was classified as low (primary school, lower vocational training, or intermediate secondary school), medium (intermediate vocational training or higher secondary school), and high (reference group; higher vocational training or university). Age was modeled continuously. Sex was dichotomized as male vs. female. Marital status was dichotomized as currently married vs. not currently married (including those who reported being single and never married, widowed, and separated).
Current cigarette smoking was based on self-report and dichotomized as yes or no. Alcohol use was based on self-reported consumption and dichotomized as heavy (> 3 drinks/day for men and > 2 drink/day for women) vs. not heavy. This dichotomization was used based on previous literature showing an inverse association between education and excessive or risky drinking [33, 34] and that heavy alcohol use is a risk factor for CHD. However, given substantial prior research suggesting the protective effect of moderate alcohol use on CHD risk [35, 36], we also explored dichotomizing alcohol use as moderate vs. heavy and none in sensitivity analyses. Study participants were asked several questions on occupational physical activity and time spent engaging in different forms of leisure-time activity in the summer and winter [37]. Participants were classified as physically inactive (vs. physically active) if they reported having sedentary jobs and not engaging in any recreational activity.
Dietary intake was assessed using a validated food frequency questionnaire developed for the European Prospective Investigation into Cancer and Nutrition study [31]. Dietary quality was operationalized using the modified Mediterranean diet score; this measure has been used in previous studies of Dutch cohorts [38, 39]. Values of 0 or 1 were assigned to 8 different nutritional components (vegetables, fruits, legumes and nuts, grains, fish and seafood, ratio of unsaturated to saturated fatty acids, dairy products, and meat) using sex-specific medians as cut-off values, with 1 indicating healthier consumption levels. This score was dichotomized as poor (scores 0–2) or not poor (scores 3–8).
BMI (kg/m2) was calculated based on measured height (in m) and weight (in kg). Obesity was defined as having BMI ≥ 30 kg/m2. Non-fasting plasma total cholesterol was measured using standardized enzymatic methods [32]. Hypercholesterolemia was defined as having total cholesterol ≥ 6.21 mmol/l or self-reported use of cholesterol lowering medication [40]. Resting blood pressure was recorded twice on the seated participant with a random zero sphygmomanometer [32]. The average of the two blood pressure measurements was used to estimate mean blood pressure. Participants were defined as hypertensive if they had mean systolic blood pressure ≥ 140 mm Hg, mean diastolic blood pressure ≥ 90 mm Hg, or they reported using antihypertensive medications [41]. Random, non-fasting plasma glucose levels were measured using the hexokinase method [32]. Participants were considered diabetic if they reported being diagnosed with diabetes or they had a random glucose > 11.0 mmol/l [42].
Ascertainment of fatal and nonfatal events
Incident coronary heart disease was defined as the first fatal or nonfatal coronary event that occurred during follow-up. Mortality and morbidity follow-up information was available up to January 1, 2008 (mean follow-up 11.5 years). Vital status was identified using the municipal population register; loss-to-follow-up was below 0.1%. Cause of death information was obtained from Statistics Netherlands. Morbidity data were collected from the national hospital discharge register. Fatal (primary or secondary cause of death) and non-fatal coronary heart disease was defined as ICD-9 [43] codes 410–414 and ICD-10 [44] codes I20–I25 for fatal cases after 1996.
Statistical analyses
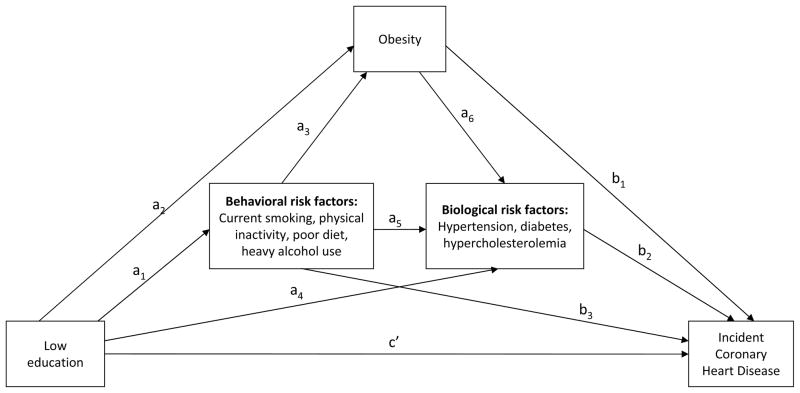
The distribution of all study covariates as well as age- and sex-adjusted CHD incidence rates were examined by educational attainment. The path analysis model described in Figure 1 was used to estimate the extent to which behavioral and biological cardiovascular disease risk factors mediated the association of education with incident CHD. This approach allows for the decomposition of the total effect of education on CHD risk into direct effects (independent of all measured mediating risk factors and confounders) and indirect effects (through each of the measured mediating risk factors).
Fig. 1.
Graphic display of the estimated path analysis model. Regression equations are represented by single-headed arrows. Coefficients a1–6 are based on estimates computed using logistic regression models with each potential mediator as separate outcomes. Coefficients b1–3 and c′ represent estimates generated using parametric accelerated failure time modeling with time to coronary heart disease as the outcome.
The arrows in Figure 1 represent regression equations used to assess mediation. To account for the fact that some risk factors may be on the causal pathway of others, we allowed for behavioral risk factors to act as mediators on their own paths and through obesity and the other biological risk factors (diabetes, hypertension, and hypercholesterolemia). Similarly, we examined obesity as a mediator on pathways with other biological risk factors.
Separate associations of education with each of the behavioral risk factors (a1); of education and behavioral risk factors with obesity (a2–3); and of education, behavioral risk factors, and obesity with each of the other 3 biological risk factors (a4–6) were each estimated using logistic regression. Parametric accelerated failure time modeling was used to estimate associations of all behavioral and biological risk factors with incident CHD independent of education (b1–3) as well as the direct effect of education on incident CHD (c′). The accelerated failure time model was chosen because previous studies suggest it may be a more robust approach for assessing causal mediation with survival data than the proportional hazards model [45, 46]. All models were adjusted for age, sex, and marital status as potential confounders. All path coefficients were estimated using SAS 9.2 (SAS Institute, Inc., Cary, North Carolina).
The a path coefficients were calculated as difference between the log odds of each mediator among those with low education and the log odds of each mediator among those with high education (see Appendix for more details). This was done to account for the fact that the mediators were binary, not continuous [47]. The b path coefficients were simply the beta coefficient for the association between the hypothesized mediator and incident CHD after adjusting for education and confounders. For hypertension, diabetes, and cholesterol, the product of the coefficient for the associations of education with each mediator and the coefficient for the association of each mediator with incident CHD after adjusting for education represents the indirect effect (a4*b2 in Figure 1). Mediation of the education-CHD association by obesity could occur directly (a2*b1) as well as through each of the other biological risk factors (a2*a6*b2). This combined indirect effect was estimated by taking the sum of the two products [48]. Estimation of the combined indirect effect of behavioral risk factors was calculated in a similar manner (a1*b3 + a1*a3*b1 + a1*a5*b2 + a1*a3*a6*b2).
The proportion mediated by each risk factor was defined as the risk factor-specific indirect effect divided by the total education-incident CHD effect, where the total effect is calculated as the sum of the total indirect effect (i.e., the indirect effect of all mediators combined) and the direct effect. These proportions were multiplied by 100 to approximate the percentage of the total education-incident CHD association mediated by each risk factor. Statistical significance was determined using 95% confidence intervals (CI) that were calculated around the indirect effects and percentages using the percentile bootstrap method with 2,000 re-samplings [48, 49].
In supplementary analyses, we compared our findings with those that would be obtained using more traditional epidemiologic approaches. Specifically, we used accelerated failure time modeling to estimate associations of education with incident CHD before and after adjusting for each risk factor separately as well as before and after adjusting for all risk factors in one model. The proportion of the education-CHD relationship attenuated by adjustment for the risk factor(s) was calculated as the beta coefficient for the model with the risk factor(s) subtracted from the beta coefficient for the model without the risk factor(s) divided by the beta coefficient for the model without the risk factor(s). This was multiplied by 100 to get the percent attenuation.
RESULTS
Baseline prevalence of current smoking, poor diet, physical inactivity, obesity, hypertension, diabetes, and hypercholesterolemia were all higher, but prevalence of heavy alcohol use was lower, among individuals with low versus high levels of education (Table 1). Compared to participants with high education levels, those with medium education levels were more likely to smoke, have a poor diet, and be obese; they also had a slightly higher prevalence of hypertension. There was no difference in physical inactivity, diabetes, or hypercholesterolemia prevalence between medium and high education study participants. However, heavy alcohol use was lower for those medium versus high education levels.
Table 1.
Baseline characteristics by educational attainment among participants in the Dutch Monitoring Project on Risk Factors for Chronic Diseases (MORGEN) 1994–1997
| Characteristics | Educational attainment (N = 15,067)
|
||
|---|---|---|---|
| Low (n=7068) | Medium (n=4286) | High (n=3713) | |
| Age in years, mean (SD) | 45.5 (10.5) | 37.2 (11.6) | 40.9 (9.9) |
| Male sex, % | 41.4 | 49.3 | 51.3 |
| Married, % | 71.4 | 53.0 | 50.8 |
| Current smoking, % | 41.5 | 34.0 | 28.2 |
| Heavy alcohol use, % | 12.6 | 13.7 | 18.1 |
| Poor diet, % | 19.2 | 16.8 | 11.0 |
| Physical inactivity, % | 10.1 | 5.5 | 5.3 |
| Obesity, % | 15.1 | 7.1 | 4.2 |
| Hypertension, % | 25.0 | 15.6 | 14.1 |
| Diabetes, % | 2.1 | 0.9 | 0.7 |
| Hypercholesterolemia, % | 23.5 | 14.1 | 13.9 |
Heavy alcohol use is defined as consuming > 3 drinks/day for men and > 2 drinks/day for women.
Poor diet is defined as having a modified Mediterranean Diet Score 0–2.
Physical inactivity is defined as having a sedentary job and not engaging in any recreational activity.
Table 2 presents age- and sex-adjusted CHD incidence rates that were highest for individuals with low levels of education, but similar for those with medium and high levels of education. There was no difference in CHD incidence between medium and high education individuals in unadjusted analyses or after adjustment for risk factors and very weak evidence of any mediation (not shown). Thus, results for the mediation analysis of the association of medium vs. high education with incident CHD are not presented.
Table 2.
Incidence of coronary heart disease by level of educational attainmenta among participants in the follow-up of the Dutch Monitoring Project on Risk Factors for Chronic Diseases (MORGEN) 1994–2008
| Educational attainment | No. of events/No. of participants | Incidence per 1000 person-years (95% CI) |
|---|---|---|
| Low | 419/7068 | 7.31 (6.40, 8.35) |
| Medium | 134/4286 | 5.86 (4.88, 7.03) |
| High | 122/3713 | 5.10 (4.21, 6.17) |
Poisson regression was used to estimate age- and sex-adjusted incidence rates by level of educational attainment.
The estimated total indirect effect of all behavioral and biological risk factors on the association between low (vs. high) education and incident CHD was −0.155, and the direct effect was −0.119 (Table 3). The negative sign of the coefficients indicates that those with low education levels had shorter times to incident CHD than those with high education levels. In terms of percentage mediated, this means behavioral and biological risk factors mediated 56.6% (95% CI: 42.6%–70.8%) of the total association between low education and incident CHD. The strongest mediators of the low education-incident CHD relationship were current smoking and obesity. Current smoking mediated 27.3% (95% CI: 17.7%–37.4%) of the association of education with incident CHD and obesity mediated 10.2% (95% CI: 4.5%–16.1%). Physical inactivity (6.3%; 95% CI: 2.7%–10.0%) and hypertension (5.3%; 95% CI: 2.8%–8.0%) were both substantial mediators as well. Diabetes and hypercholesterolemia were modest but significant mediators, while the indirect effects of poor diet and heavy alcohol use was non-significant. Results for the mediation analysis were similar when moderate alcohol use was used instead of heavy alcohol use in the sensitivity analyses.
Table 3.
Percentage of the association between education and incident coronary heart disease mediated by each risk factor determined from the path analysis model in Figure 1 among participants in the follow-up of the Dutch Monitoring Project on Risk Factors for Chronic Diseases (MORGEN) 1994–2008
| Potential mediating risk factor | Percentage of total association mediated by risk factor (95% CI)a,b |
|---|---|
| All mediators (total indirect effect) | 56.6 (42.6, 70.8) |
| Current smoking | 27.3 (17.7, 37.4) |
| Heavy alcohol usec | 2.3 (−0.6, 5.7) |
| Poor dietd | 1.2 (−4.2, 6.3) |
| Physical inactivitye | 6.3 (2.7, 10.0) |
| Obesity | 10.2 (4.5, 16.1) |
| Hypertension | 5.3 (2.8, 8.0) |
| Diabetes | 0.5 (0.1, 1.0) |
| Hypercholesterolemia | 3.5 (1.6, 5.9) |
Calculated as [indirect effect/(total indirect effect + direct effect)]*100
Based on regression models adjusted for age, sex, and marital status
Heavy alcohol use is defined as consuming > 3 drinks/day for men and > 2 drinks/day for women.
Poor diet is defined as having a modified Mediterranean Diet Score 0–2.
Physical inactivity is defined as having a sedentary job and not engaging in any recreational activity.
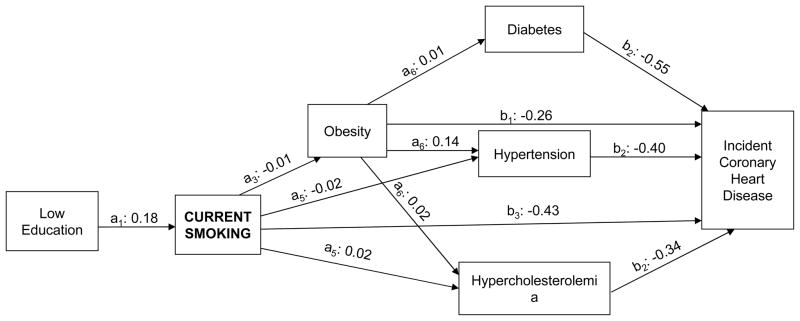
Figure 2 illustrates the statistically significant pathways through which current smoking mediated the low education-incident CHD relationship. Low education was significantly associated with higher smoking after adjusting for confounders and behavioral risk factors (a1: 0.18), and obesity was associated with higher incident CHD after adjusting for education and all other risk factors (b3: −0.43) (indirect effect a2*b1: −0.08). Current smoking also mediated the education-incident CHD relationship through its associations with obesity, both independently (a1*a3*b1: 0.0005) and through the other 3 biological risk factors (a1*a3*a6*b2: −0.0006), which were each associated with higher CHD risk. Current smoking mediated the low education-incident CHD association through hypertension (a1*a6*b2: 0.001) and hypercholesterolemia (a2*a6*b2: −0.001) as well.
Fig. 2.
Graphic display of significant pathways through which current smoking mediates the association of low education with incident coronary heart disease. All path coefficients were estimated from models adjusted for age, sex, and marital status.
Using more traditional approaches, we found that the combination of all behavioral and biological risk factors attenuated the association between low education and incident CHD by 62.0% (Supplementary Table 1). Current smoking and obesity remained two of the strongest mediators (20.5% and 17.4%). However, in the single risk factor-adjusted model we found hypertension explained a much higher percentage of the low education-incident CHD relationship (13.3%) than we found using path analysis.
DISCUSSION
In this study, we used path analysis to quantify and rank the mediating role of different behavioral and biological risk factors in the relationship between low educational attainment and incident CHD in a Dutch cohort. We assessed mediation by multiple risk factors simultaneously while taking into account that these factors may influence CHD serially rather than through parallel pathways (i.e. some are hypothesized to increase risk of others). There was a substantial difference in CHD risk over the follow-up period for low education individuals compared with high education individuals, and behavioral and biological risk factors accounted for 56.6% of the low education-incident CHD relationship.
Few studies have quantified the specific contributions of different risk factors to associations of education with CHD risk [3, 17, 20, 21], and none to our knowledge have estimated indirect effects of multiple risk factors simultaneously. The strongest mediator in this study was current cigarette smoking, accounting for 27.3% of the total association between low education and incident CHD, followed by obesity, physical inactivity, and hypertension (accounting for 10.2%, 6.3%, and 5.3% of the total association, respectively). Consistent with these findings, studies of cohorts in several countries have found smoking to be a strong mediator [3, 17, 20, 21]. However, there was less consistent evidence of mediation by blood pressure, BMI, or physical activity across these studies. This may be due to differences in the methodological approach we used to assess mediation (i.e., investigating the impact of all risk factors simultaneously). This may also reflect variation in the prevalence and the social patterning of potential mediating risk factors across countries [30].
Current cigarette smoking was the strongest mediator of the association between low education and incident CHD. An examination of the significant pathways through which these behavioral risk factors mediated the low education-incident CHD association indicates that the majority of the effect occurred independent of biological risk factors. It is likely that a larger proportion of the indirect effect of cigarette smoking on the low education-incident CHD association occurs through biological risk factors; the magnitude of these intermediate pathways may be underestimated in our model due to the fact that behavioral and biological risk factors were measured at the same point in time.
Obesity was also a strong mediator of the association between low education and incident CHD, even after accounting for diet and physical activity. One explanation for this is that our dietary measure assesses dietary quality, not energy imbalance. Thus, obesity may be reflecting the indirect effect of energy imbalance on CHD risk. Another explanation is that dietary assessments are often prone to measurement error, which may result in an underestimation of the contribution of dietary quality to CHD risk and an overestimation of the independent contribution of obesity. In addition, although we modeled the relationships between behavioral risk factors and obesity as if they occurred in series to lead to incident CHD, they were all measured at the same point in time. Therefore, the available data may not adequately reflect the long-term influence of these health behaviors on obesity risk. Alternatively, exposure to psychosocial stressors, such as those correlated with low educational attainment, may impact obesity independent of health behaviors. Epidemiologic studies have shown psychosocial factors are associated with higher visceral adiposity [50, 51]. Animal studies suggest social stress may lead to increased visceral adiposity through elevated glucocorticoid levels and hypercortisolemia [15, 52].
In our supplementary analyses we found that comparisons of mediation by all risk factors combined produced similar results using path analysis compared with the more traditional approach of calculating the difference in beta coefficients before and after adjusting for all risk factors (56.6% vs. 62.0%). However, comparisons of the estimated contributions of the individual mediators using path analysis vs. calculating the difference before and after adjustment for single risk factors were less consistent, particularly for the biological risk factors. The higher estimated mediation by obesity, hypertension, and diabetes using the single risk factor adjustment method may reflect the fact that our path analysis model took into account that behavioral risk factors might increase risk for the biological risk factors.
This study is not without limitations. One is that there may be measurement error in the self-reported health behaviors that could have influenced the estimated strength of each risk factor as a mediator. Also, because risk factors were only measured at one point in time, the strength of these mediating pathways as they change over time could be underestimated [53]. Moreover, while the relationships between behavioral risk factors, obesity, and the other biological risk factors were modeled as if they occurred in series to lead to incident CHD, they were all measured at the same single point in time. Therefore, the process through which behavioral risk factors impact CHD risk through the subsequent development of biological risk factors may not have been adequately captured.
In addition, recent research on causal mediation has demonstrated the importance of accounting for unmeasured confounding of both the exposure-outcome association and the mediator-outcome association to obtain unbiased estimates of direct and indirect effects [46, 54]. These assumptions cannot be tested using the data; they must be evaluated using a priori knowledge. Major confounders were controlled for, but there may be others that we were not able to account for that could bias our findings (e.g. early life illnesses or parental/neighborhood SEP which might influence educational attainment and increase CHD risk). While examining multiple mediators in one model could increase the chances that these assumptions are violated, this is less likely to be an issue in our study given how closely related the mediators are (i.e., they are likely to have overlapping confounders).
In summary, we used path analysis to show that low education study participants had significantly higher CHD risk compared with high education individuals and that cigarette smoking and obesity were the strongest risk factors underlying this disparity. Material and environmental constraints as well as psychosocial factors may contribute to the social patterning of these disparities and the mediating risk factors [3, 55]. Therefore, while these findings highlight specific risk factors as targets, effective long-term solutions to socioeconomic disparities in cardiovascular outcomes must also focus on eliminating the upstream correlates of these inequalities.
Supplementary Material
Acknowledgments
KN Kershaw was supported by grant T32-HL-069771-07. The Monitoring Project on Risk Factors and Chronic Diseases in the Netherlands (MORGEN) Study was supported by the Ministry of Health, Welfare and Sport of the Netherlands, the National Institute of Public Health and the Environment, Bilthoven, the Netherlands and the Europe Against Cancer Program of the European Union. The authors thank the epidemiologists and field workers of the Municipal Health Services in Amsterdam, Doetinchem, and Maastricht for their important contribution to the data collection for this study.
References
- 1.van der Lucht F, Polder JJ. The Dutch 2010 Public Health Status and Forecasts Report. National Institute for Public Health and the Environment; Bilthoven, The Netherlands: [Google Scholar]
- 2.Louwman WJ, et al. A 50% higher prevalence of life-shortening chronic conditions among cancer patients with low socioeconomic status. Br J Cancer. 2010;103(11):1742–8. doi: 10.1038/sj.bjc.6605949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Lenthe FJ, et al. Material and behavioral factors in the explanation of educational differences in incidence of acute myocardial infarction: the Globe study. Ann Epidemiol. 2002;12(8):535–42. doi: 10.1016/s1047-2797(01)00279-4. [DOI] [PubMed] [Google Scholar]
- 4.LaVeist TA, Wallace JM., Jr Health risk and inequitable distribution of liquor stores in African American neighborhood. Soc Sci Med. 2000;51(4):613–7. doi: 10.1016/s0277-9536(00)00004-6. [DOI] [PubMed] [Google Scholar]
- 5.Wilson DK, et al. Socioeconomic status and perceptions of access and safety for physical activity. Ann Behav Med. 2004;28(1):20–8. doi: 10.1207/s15324796abm2801_4. [DOI] [PubMed] [Google Scholar]
- 6.Hackbarth DP, Silvestri B, Cosper W. Tobacco and alcohol billboards in 50 Chicago neighborhoods: market segmentation to sell dangerous products to the poor. J Public Health Policy. 1995;16(2):213–30. [PubMed] [Google Scholar]
- 7.Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009;36(1):74–81. doi: 10.1016/j.amepre.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Moore LV, Diez Roux AV. Associations of neighborhood characteristics with the location and type of food stores. Am J Public Health. 2006;96(2):325–31. doi: 10.2105/AJPH.2004.058040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson JS, Knight KM. Race and self-regulatory health behaviors: The role of the stress response and the HPA axis in physical and mental health disparities. In: Schaie KW, Cartensen L, editors. Social structures, aging, and self-regulation in the elderly. Springer; New York: 2006. pp. 189–207. [Google Scholar]
- 10.Krueger PM, V, Chang W. Being poor and coping with stress: health behaviors and the risk of death. Am J Public Health. 2008;98(5):889–96. doi: 10.2105/AJPH.2007.114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng DM, Jeffery RW. Relationships between perceived stress and health behaviors in a sample of working adults. Health Psychol. 2003;22(6):638–42. doi: 10.1037/0278-6133.22.6.638. [DOI] [PubMed] [Google Scholar]
- 12.Dallman MF, et al. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100(20):11696–701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 14.Pickering T. Cardiovascular pathways: socioeconomic status and stress effects on hypertension and cardiovascular function. Ann N Y Acad Sci. 1999;896:262–77. doi: 10.1111/j.1749-6632.1999.tb08121.x. [DOI] [PubMed] [Google Scholar]
- 15.Tamashiro KL. Metabolic syndrome: links to social stress and socioeconomic status. Ann N Y Acad Sci. 2011;1231:46–55. doi: 10.1111/j.1749-6632.2011.06134.x. [DOI] [PubMed] [Google Scholar]
- 16.Kivimaki M, et al. Socioeconomic position, co-occurrence of behavior-related risk factors, and coronary heart disease: the Finnish Public Sector study. Am J Public Health. 2007;97(5):874–9. doi: 10.2105/AJPH.2005.078691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuper H, et al. Psychosocial determinants of coronary heart disease in middle-aged women: a prospective study in Sweden. Am J Epidemiol. 2006;164(4):349–57. doi: 10.1093/aje/kwj212. [DOI] [PubMed] [Google Scholar]
- 18.Lawlor DA, Ebrahim S, Davey Smith G. Adverse socioeconomic position across the lifecourse increases coronary heart disease risk cumulatively: findings from the British women’s heart and health study. J Epidemiol Community Health. 2005;59(9):785–93. doi: 10.1136/jech.2004.029991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch JW, et al. Do cardiovascular risk factors explain the relation between socioeconomic status, risk of all-cause mortality, cardiovascular mortality, and acute myocardial infarction? Am J Epidemiol. 1996;144(10):934–42. doi: 10.1093/oxfordjournals.aje.a008863. [DOI] [PubMed] [Google Scholar]
- 20.Marmot MG, et al. Biological and behavioural explanations of social inequalities in coronary heart disease: the Whitehall II study. Diabetologia. 2008;51(11):1980–8. doi: 10.1007/s00125-008-1144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strand BH, Tverdal A. Can cardiovascular risk factors and lifestyle explain the educational inequalities in mortality from ischaemic heart disease and from other heart diseases? 26 year follow up of 50,000 Norwegian men and women. J Epidemiol Community Health. 2004;58(8):705–9. doi: 10.1136/jech.2003.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suadicani P, Hein HO, Gyntelberg F. Strong mediators of social inequalities in risk of ischaemic heart disease: a six-year follow-up in the Copenhagen Male Study. Int J Epidemiol. 1997;26(3):516–22. doi: 10.1093/ije/26.3.516. [DOI] [PubMed] [Google Scholar]
- 23.Woodward M, et al. Contribution of contemporaneous risk factors to social inequality in coronary heart disease and all causes mortality. Prev Med. 2003;36(5):561–8. doi: 10.1016/s0091-7435(03)00010-0. [DOI] [PubMed] [Google Scholar]
- 24.Yarnell J, et al. Education, socioeconomic and lifestyle factors, and risk of coronary heart disease: the PRIME Study. Int J Epidemiol. 2005;34(2):268–75. doi: 10.1093/ije/dyh267. [DOI] [PubMed] [Google Scholar]
- 25.Rosengren A, et al. Education and risk for acute myocardial infarction in 52 high, middle and low-income countries: INTERHEART case-control study. Heart. 2009;95(24):2014–22. doi: 10.1136/hrt.2009.182436. [DOI] [PubMed] [Google Scholar]
- 26.Ramsay SE, et al. Socioeconomic inequalities in coronary heart disease risk in older age: contribution of established and novel coronary risk factors. J Thromb Haemost. 2009;7(11):1779–86. doi: 10.1111/j.1538-7836.2009.03602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McFadden E, et al. Occupational social class, risk factors and cardiovascular disease incidence in men and women: a prospective study in the European Prospective Investigation of Cancer and Nutrition in Norfolk (EPIC-Norfolk) cohort. Eur J Epidemiol. 2008;23(7):449–58. doi: 10.1007/s10654-008-9262-2. [DOI] [PubMed] [Google Scholar]
- 28.Wolfe LM. Sewall Wright on the method of path coefficients: An annotated bibliography. Structural Equation Modeling. 1999;6:280–291. [Google Scholar]
- 29.Wright S. The Relative Importance of Heredity and Environment in Determining the Piebald Pattern of Guinea-Pigs. Proc Natl Acad Sci U S A. 1920;6(6):320–32. doi: 10.1073/pnas.6.6.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stringhini S, et al. Health behaviours, socioeconomic status, and mortality: further analyses of the British Whitehall II and the French GAZEL prospective cohorts. PLoS Med. 2011;8(2):e1000419. doi: 10.1371/journal.pmed.1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beulens JW, et al. Cohort profile: the EPIC-NL study. Int J Epidemiol. 2010;39(5):1170–8. doi: 10.1093/ije/dyp217. [DOI] [PubMed] [Google Scholar]
- 32.Smit HA, et al. Monitoring van Risicofactoren en Gezondheid in Nederland (MORGEN-project): Doelstellingen en werkwijze. RIVM; Bilthoven, The Netherlands: 1994. [Google Scholar]
- 33.Van Oers JAM, et al. Alcohol consumption, alcohol-related problems, problem drinking, and socioeconomic status. Alcohol & Alcoholism. 1999;34(1):78–88. doi: 10.1093/alcalc/34.1.78. [DOI] [PubMed] [Google Scholar]
- 34.Grittner U, et al. Alcohol consumption and social inequality at the individual and country levels--results from an international study. Eur J Public Health. 2012 doi: 10.1093/eurpub/cks044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maclure M. Demonstration of deductive meta-analysis: ethanol intake and risk of myocardial infarction. Epidemiol Rev. 1993;15(2):328–51. doi: 10.1093/oxfordjournals.epirev.a036124. [DOI] [PubMed] [Google Scholar]
- 36.Sesso HD, Gaziano JM. Alcohol intake and cardiovascular morbidity and mortality. Curr Opin Nephrol Hypertens. 1999;8(3):353–7. doi: 10.1097/00041552-199905000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Wareham NJ, et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003;6(4):407–13. doi: 10.1079/PHN2002439. [DOI] [PubMed] [Google Scholar]
- 38.Trichopoulou A, et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ. 2005;330(7498):991. doi: 10.1136/bmj.38415.644155.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoevenaar-Blom MP, et al. Mediterranean style diet and 12-year incidence of cardiovascular diseases: the EPIC-NL cohort study. PLoS One. 2012;7(9):e45458. doi: 10.1371/journal.pone.0045458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 41.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Archives of Internal Medicine. 1997;157(21):2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 42.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization. International classification of diseases, 9th revision (ICD-9) World Health Organization; Geneva, Switzerland: 1977. [Google Scholar]
- 44.World Health Organization. International classification of diseases, 10th revision (ICD-10) World Health Organization; Geneva, Switzerland: 1990. [Google Scholar]
- 45.Tein J-Y, MacKinnon DP. Estimating mediated effects with survival data. In: Yanai H, et al., editors. New developments on psychometrics. Springer-Verlag Tokyo Inc; Tokyo, Japan: 2003. pp. 405–412. [Google Scholar]
- 46.VanderWeele TJ. Causal mediation analysis with survival data. Epidemiology. 2011;22(4):582–5. doi: 10.1097/EDE.0b013e31821db37e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valeri L, Vanderweele TJ. Mediation Analysis Allowing for Exposure-Mediator Interactions and Causal Interpretation: Theoretical Assumptions and Implementation With SAS and SPSS Macros. Psychol Methods. 2013 doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor AB, MacKinnon DP, Tein JY. Tests of the three-path mediated effect. Organizational Research Methods. 2008;11:241–269. [Google Scholar]
- 49.Barker N. A practical introduction to the bootstrap using the SAS system. Pharmaceutical Users Software Exchange Annual Meeting; 2005; Heidelberg, Germany. [Google Scholar]
- 50.Lewis TT, et al. Hostility is associated with visceral, but not subcutaneous, fat in middle-aged African American and white women. Psychosom Med. 2009;71(7):733–40. doi: 10.1097/PSY.0b013e3181ad13a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis TT, et al. Self-reported experiences of discrimination and visceral fat in middle-aged African-American and Caucasian women. Am J Epidemiol. 2011;173(11):1223–31. doi: 10.1093/aje/kwq466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shively CA, Register TC, Clarkson TB. Social stress, visceral obesity, and coronary artery atherosclerosis: product of a primate adaptation. Am J Primatol. 2009;71(9):742–51. doi: 10.1002/ajp.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stringhini S, et al. Association of socioeconomic position with health behaviors and mortality. JAMA. 2010;303(12):1159–66. doi: 10.1001/jama.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychological Methods. 2010;15(4):309–334. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 55.van Oort FV, van Lenthe FJ, Mackenbach JP. Material, psychosocial, and behavioural factors in the explanation of educational inequalities in mortality in The Netherlands. J Epidemiol Community Health. 2005;59(3):214–20. doi: 10.1136/jech.2003.016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.