Abstract
Bordetella pertussis is the bacterial agent of the human disease, whooping cough. In many bacteria, the extracellular function sigma factor σE is central to the response to envelope stress, and its activity is negatively controlled by the RseA anti-sigma factor. In this study, the role of RseA in B. pertussis envelope stress responses was investigated. Compared with the wild-type strain, an rseA mutant showed elevated resistance to envelope stress and enhanced growth at 25°C. rpoH and other predicted σE target genes demonstrated increased transcription in the rseA mutant compared with the wild type parent. Transcription of those genes was also increased in wild type B. pertussis and Escherichia coli under envelope stress, whereas no stress-induced increase in transcription was observed in the rseA mutant. rseA inactivation was also associated with altered levels of certain proteins in culture supernatant fluids, which showed increased adenylate cyclase toxin (CyaA) levels. The increased CyaA in the mutant was correlated with an apparent increased stability of the extracellular toxin and increased production of CyaA-containing outer membrane vesicles. Consistent with this, compared with the wild type strain, rseA mutant cells produced increased numbers of large surface-associated vesicles.
Keywords: Bordetella pertussis, envelope stress, RseA, σ E
INTRODUCTION
Bordetella pertussis is a Gram negative bacterium that is an obligate pathogen of humans, causing the acute respiratory disease pertussis (whooping cough) that manifests as a prolonged coughing illness that is more severe in infants (von Konig et al., 2002; Hewlett & Edwards, 2005; Mattoo & Cherry, 2005). B. pertussis produces multiple virulence factors, including a variety of adhesins and toxins (Locht et al., 2001; Mattoo & Cherry, 2005), which contribute to the complex pathology of pertussis. Most of the major virulence factors, including adhesins, pertussis toxin, and adenylate cyclase toxin (CyaA), are positively and coordinately regulated at the transcriptional level by the BvgAS two-component phosphorelay system (Weiss et al., 1983; Arico et al., 1989; Mattoo & Cherry, 2005).
Bacterial alternative sigma factors of RNA polymerase play important roles in the transcriptional response to a variety of environmental stimuli and stresses (Raivio & Silhavy, 2001). σE (RpoE), an extracytoplasmic function (ECF) sub-family sigma factor present in many bacteria, is involved in the cell’s stress response to perturbations in the outer membrane and the presence of misfolded proteins in the periplasm (Raivio & Silhavy, 2001; Alba & Gross, 2004; Hayden & Ades, 2008). Proteins encoded by σE regulon genes are diverse and have been linked to bacterial processes such as the synthesis, assembly and homeostasis of lipopolysaccharides and outer membrane proteins, maintenance of periplasmic proteases and outer membrane proteins, and energy metabolism (Humphreys et al., 1999; Alba & Gross, 2004; Rhodius et al., 2006).
The molecular mechanisms of σE activation by cell envelope stress have been elucidated in Escherichia coli, a species in which rpoE is apparently essential (De Las Penas et al., 1997). In the absence of stress, σE is inactivated via binding by RseA, an anti-sigma factor located in the cytoplasmic membrane. Upon detection of envelope stress in the periplasm, the DegS protease cleaves the periplasmic region of RseA, initiating what is known as regulated intra-membrane proteolysis (Akiyama et al., 2004; Chaba et al., 2007). Subsequently, RseP proteolytically cleaves RseA in its transmembrane region, releasing the N-terminal RseA fragment with its associated σE. RseA is then completely digested by the ClpPX cytoplasmic protease, freeing σE, allowing it to function in transcription (Flynn et al., 2004).
In other bacteria, σE has been implicated in the regulation of genes involved in pathogenesis. For example, htrA (also known as degP), which encodes a periplasmic serine protease, is transcribed in a σE -dependent manner (Clausen et al., 2002) and is known to be required for survival of Salmonella enterica subgroup Typhimurium (Johnson et al., 1991), Yersinia enterocolitica (Yamamoto et al., 1996) and Legionella pneumophila (Pedersen et al., 2001) in macrophages. Furthermore, σE has been shown to be important for the growth of S. Typhimurium in mice (Humphreys et al., 1999) and macrophages (Yoon et al., 2009), and is necessary for Vibrio cholerae survival in the intestine of experimentally infected mice (Kovacikova & Skorupski, 2002).
B. pertussis typically colonizes the ciliated respiratory epithelium and the bacteria have also been observed within epithelial cells and leukocytes (Hellwig et al., 1999; Mattoo & Cherry, 2005). Little is known about B. pertussis stress responses that may be activated in the host environment. A recent study examined σE function of Bordetella bronchiseptica, which is primarily a respiratory pathogen of nonhuman mammals (Barchinger et al., 2012). The authors demonstrated that mutation of rpoE in B. bronchiseptica resulted in increased sensitivity to heat, certain antibiotics and other stressors. A B. bronchiseptica rpoE mutant was more susceptible to being killed by polymorphonuclear neutrophils. In E. coli, the B. bronchiseptica rpoE gene functioned to increase expression of an E. coli rpoHP3-lacZ fusion, and it complemented an rpoE mutation, which is normally lethal in E. coli.
In the present study, an rseA mutant in the human-pathogenic species, B. pertussis, was constructed. Consistent with the role of RseA as an anti-sigma factor that antagonizes σE function, the rseA mutant exhibited increased resistance to envelope stressors, elevated transcription levels of rpoH and other genes, and also showed an enhanced capacity to grow at low temperature when compared with wild-type cells. The mutant was altered in its profile of extracellular proteins; in particular, levels of CyaA were highly elevated. The rseA mutant produced more numerous outer membrane vesicles than wild type cells and exhibited a remarkable number of large surface-associated vesicles.
MATERIALS AND METHODS
Bacterial strains and cultivation
The bacterial strains used in this study are listed in Table 1. B. pertussis strains were grown for two days at 35°C on Bordet Gengou agar (Bordet J & Gengou O, 1906). Liquid culture of B. pertussis used modified Stainer-Scholte (SS) medium (Stainer & Scholte, 1970; Schneider & Parker, 1982) supplemented with 0.5% casamino acids at 35°C with shaking at 300 rpm. SS cultures for ß-galactosidase measurements also contained 500 μg ml−1 of heptakis(2,6-di-O-methyl)-β-cyclodextrin (Imaizumi et al., 1983). E. coli strains were cultured on Luria Bertani agar or broth. As necessary, 15 μg ml−1 of tetracycline and 10 μg ml−1 of gentamicin were added to the media for plasmid selection. For selection of B. pertussis strains, streptomycin was used at 200 μg ml−1.
Table 1.
Bacterial strains and plasmids used in this study
| Bacterial strain | Relevant genotype or description | Reference |
|---|---|---|
|
B. pertussis UT25Sm1 |
Spontaneous streptomycin-resistant derivative of wild type isolate B. pertussis UT25 |
(Field & Parker, 1978; Brickman & Armstrong, 1996) |
| B. pertussis PM18 | UT25Sm1; ΔrseA | This study |
| B. pertussis PMK22 | PM18; wild type rseA allele reconstructed by allelic exchange |
This study |
| E. coli DH5α | Host strain for general genetic methods | Invitrogen |
| Plasmids | ||
| pRK2013 | Helper plasmid for conjugation, kanr | (Figurski & Helinski, 1979) |
| pRK415 | Low copy broad-host-range cloning vector, tetr | (Keen et al., 1988) |
| pRK59 | pRK415 with BamHI and EcoRI insert fragment containing B. pertussis rseA |
This study |
| pRK60 | pRK415 with 1.8-kb fragment containing lacI and Ptac-malE’ | This study |
| pRK601 | pRK60 with promoterless B. pertussis rseA for IPTG-inducible expression construction |
This study |
| pMAL-c2 | Source of 1.82-kb fragment containing lacI and Ptac-malE’ | New England Biolabs Inc. |
| pSS1129 | Allelic exchange plasmid genr, ampr | (Stibitz, 1994) |
| pSSK26 | pSS1129 with 2.0-kb DNA fragment encoding wild type rseA |
This study |
| pSS20 | pSS1129 with 1.86-kb DNA fragment encoding ΔrseA allele; genr |
This study |
| pSS22 | pSS1129 with 1.43-kb DNA fragment encoding ΔrseA allele; genr |
This study |
| pMP220 | Contains promoterless lacZ for transcriptional fusion construction |
(Spaink et al., 1987) |
DNA manipulation
Standard molecular biological techniques were performed as described previously (Sambrook et al. 1989). B. pertussis DNA fragments were amplified using PrimeSTAR® GXL DNA Polymerase (Takara Bio Inc., Otsu, Japan). TaKaRa Ex Taq ™ (Takara Bio Inc., Otsu, Japan) was used for the polymerase chain reaction (PCR) when the template DNA was from E. coli. Conjugal transfer of plasmids was performed as described by Brickman & Armstrong (1996). The sequences of oligonucleotide primers used for PCR in this study are shown in Table 2.
Table 2.
Oligonucleotide primers used for PCR amplification in this study
| PCR primer | Nucleotide sequence |
|---|---|
| rpoEl | 5′-GGCCAAGCTTCCGGAGTGCGACCTGGATTAC-3′ |
| rpoE2 | 5′-AACCGACTGGCAATGGCGGCTTCCTGCGCGACATCCT-3′ |
| rpoE3 | 5′-ATGTCGCGCAGGAAGCCGCCATTGCCAGTCGGTTGC-3′ |
| rpoE4 | 5′-GGCGGCCTGCTGGATGCTGGAGA-3′ |
| rseAl | 5′-GGCCGAATTCAGGCGGGGTCTCGGGTTAC-3′ |
| rseA2 | 5′-TCAACGTCCTGCTCCGGCGAGGGACTTGGCTGCGGTTTG-3′ |
| rseA3 | 5′-ACCGCAGCCAAGTCCCTCGCCGGAGCAGGACGTTGATGAC-3′ |
| rseA4 | 5′-GGCCGGATCCCCTTTGCGGGCTTATTGTTGTTT-3′ |
| rseA-BamHI | 5′-GGCCGGATCCGGTTCGTTCGCGCATATTCAGAGC-3′ |
| rseA-EcoRI | 5′-GGCCGAATTCGGCGCATGTAAAGCAACGGTGTCA-3′ |
| rseA-HindIII | 5′-GGCCAAGCTTGGCGCATGTAAAGCAACGGTGTCA-3′ |
| pMal-EcoRI/F | 5′-GGCCGAATTCCCAGCCTAGCCGGGTCCTCAAC-3′ |
| pMal-EcoRI/R | 5′-GGCCGAATTCAGCGGTCGTGTGCCCAGAAGATAA-3′ |
| cyaA-EcoRI | 5′-GGCCGAATTCCGCTTGCTCGCTTATTTATC-3′ |
| cyaA-XbaI | 5′-GGCCTCTAGATGTGTAGCGCTCAGAACCTC-3′ |
| cyaB-EcoRI | 5′-GGCCGAATTCGGTCGAGGCAATGGCGCAGTATCC-3′ |
| cyaB-Xbal | 5′-GGCCTCTAGACCAGCATGACCAGGCAGAGCAACC-3′ |
| rpoH-EcoRI | 5′-GGCCGAATTCGGCTGGTAAATAAGGGACGCATAG-3′ |
| rpoH-XbaI | 5′-GGCCTCTAGACCGGAAAGGGCCAACGAAC-3′ |
| BP0893-EcoRI | 5′-GGCCGAATTCCATCGCCAATAACAGTTTCTTTTC-3′ |
| BP0893-XbaI | 5′-GGCCTCTAGAATGTTCTCGCGCAGCCAATCC-3′ |
| BP2014-EcoRI | 5′-GGCCGAATTCGCGGCGGCTGGCTGTTCTGAGTC-3′ |
| BP2014-XbaI | 5′-GGCCTCTAGACCCAGGGCCGGCAGCGAATAGA-3′ |
| BP3218-EcoRI | 5′-GGCCGAATTCCCTGACAACCGGCTGAACTATTT-3′ |
| BP3218-XbaI | 5′-GGCCTCTAGATCTGGCCGGACTGCAACAACTGAT-3′ |
Restriction site adapter sequences used for cloning are underlined.
Sequence analysis
Nucleotide sequence data were from the genome sequence of B. pertussis Tohama I produced by the Bordetella Sequencing Group at The Wellcome Trust Sanger Institute (http://www.sanger.ac.uk/Projects/B_pertussis) and analyzed using GeneDB (http://www.genedb.org) (Parkhill et al., 2003). Sequence analyses were also conducted using the Lasergene sequence analysis software package (DNASTAR, Inc., Madison, WI). Database searches were accomplished using the BLAST servers provided by the Sanger Centre and the National Center for Biotechnology Information at the National Library of Medicine and Gene Context Tool from the Computational Genomic Group, Instituto de Biotecnologia, Universidad Nacional Autonoma de Mexico (http://bioinfo.ibt.unam.mx/gecont/index.cgi?second).
Construction of B. pertussis mutant strains and complementation plasmids
An rpoE in-frame deletion mutation of 381 nt was constructed by overlap extension PCR as described (Ho et al., 1989; Horton et al., 1989) using the rpoE1, rpoE2, rpoE3 and rpoE4 primers listed in Table 2. The DNA fragment was cloned into allelic exchange plasmid pSS1129 (Stibitz, 1994) and the resulting ΔrpoE plasmid, pSS22, was conjugally transferred to B. pertussis UT25Sm1, with selection on gentamicin. Transconjugants were scored for streptomycin sensitivity, and the modest number of subsequent streptomycin resistant and gentamicin sensitive allelic exchange mutant candidates were analyzed by PCR for the presence of the ΔrpoE allele. Of the candidates obtained, all retained the wild-type rpoE gene. The conjugation and allelic exchange experiment was performed three times (assessing over 100 candidates in total), with similar negative results. Other allelic exchange attempts, using a different DNA fragment where both rpoE and rseA were deleted, also failed to yield mutants.
To construct the B. pertussis rseA mutant strain PM18, a 1.86-kb DNA fragment containing a 459-bp in-frame rseA deletion mutation was synthesized using the rseA1 and rseA2, and rseA3 and rseA4 primer pairs by overlap extension PCR. The fragment was cloned into the pSS1129 suicide vector using the EcoRI and BamHI restriction sites. The resulting plasmid, pSS20, was transferred to B. pertussis UT25Sm1 by conjugation. After allelic exchange, the B. pertussis strain carrying the ΔrseA mutation was confirmed by PCR using the primers rseA-BamHI and rseA-EcoRI.
For use in genetic complementation experiments, the 632-bp DNA fragment containing the wild type rseA gene from B. pertussis UT25Sm1 was amplified by PCR with the primer pair rseA-BamHI and rseA-EcoRI, and cloned into pRK415 downstream of the lac promoter, resulting in plasmid pRK59. Additionally, the wild type rseA allele on a 2.0-kb DNA fragment synthesized by PCR using the rseA1 and rseA4 primers was cloned into pSS1129 to produce pSSK26. The plasmid was conjugated to the B. pertussis ΔrseA strain PM18 to reconstruct the wild type rseA allele by allelic exchange, resulting in strain PMK22. PCR was performed to confirm the allele replacement. To construct a plasmid containing the rseA gene expressed from a promoter inducible by isopropylthio-β-galactoside (IPTG), the 1.82-kb DNA fragment of pMALc2™ (New England Biolabs Inc., Ipswich, MA, USA) containing lacI and Ptac promoter was synthesized by PCR using the pMal-EcoRI/F and pMal-EcoRI/R primers, and cloned into pRK415 resulting in plasmid pRK60. The 632-bp DNA fragment containing the rseA gene from B. pertussis UT25Sm1 was amplified by PCR with the primer pair rseA-BamHI and rseA-HindIII, and cloned into pRK60 downstream of the Ptac promoter, resulting in plasmid pRK601. Stress sensitivity assays. An overnight B. pertussis SS culture was diluted to an OD600 of 0.1 with fresh SS medium and then sub-cultured to obtain bacteria in the exponential growth phase. When the turbidity reached an OD600 of approximately 0.4, either H2O2 or polymyxin B was added to the final concentrations indicated in the text. Parallel control cultures were untreated. After incubation at 35°C at the indicated times, the number of viable bacteria was determined by plate count on Bordet Gengou agar and percent survival was calculated. Means and standard deviations of a representative of three experiments are shown. The data were analyzed by Student’s t test (p values are shown).
Construction of transcriptional fusions with putative σE -dependent promoters and cyaA and cyaB promoters
The DNA regions upstream of the BP0893, BP2014 (acnA), BP3218, and BP3748 (rpoH) genes of B. pertussis UT25Sm1 were amplified by PCR from genomic DNA using the primer pairs: BP0893-EcoRI and BP0893-XbaI, BP2014-EcoRI and BP2014-XbaI, BP3218-EcoRI and BP3218-XbaI, and rpoH-EcoRI and rpoH-XbaI, respectively (Table 2). The primer pairs: cyaA-EcpRI and cyaA-Xba, and cyaB-EcoRI and cyaB-XbaI were used for PCR reactions generating the fragments containing the cyaA and cyaB promoters. The resulting fragments were digested with EcoRI and XbaI and subsequently ligated into the pMP220 lacZ fusion plasmid (Spaink et al., 1987).
Measurement of ß-galactosidase activity
E. coli and B. pertussis strains carrying transcriptional fusion plasmids were grown in defined M9 and SS media with selection, respectively. To assess transcription under stress conditions, overnight cultures of E. coli strains were subcultured and grown for 6.5 h to an OD600 of approximately 0.4 and treated with the indicated concentrations of polymyxin B for 30 min prior to ß-galactosidase measurement, as described previously (Miller, 1972; Brickman et al., 1990). B. pertussis 15-hr SS cultures were treated for 3 h with either H2O2, polymyxin B or sucrose, added to the indicated concentrations, prior to measurement of ß-galactosidase. The assays were performed in triplicate and the results reported are representative of at least three experiments.
SDS-PAGE and immunoblotting
Proteins were electrophoretically separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 12.5% polyacrylamide gels (Laemmli et al., 1970). For standard protein gels, each lane was loaded with culture fluid proteins corresponding to a culture with an OD600 of 1.5, and periplasmic protein samples corresponding to a culture with an OD600 of 7.0, and equivalent amounts of membrane protein fractions (0.1 units of OD280). For immunoblotting, the amount of protein sample that was loaded was doubled; the proteins were electrophoretically separated and transferred to PVDF membranes (Clear Blot Membrane-p; ATTO Co., Tokyo, Japan). Immunoblot membranes were probed with a 1:200 dilution of mouse monoclonal antibody specific for mouse monoclonal antibody to the B. pertussis CyaA (sc-13582; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). After reaction with a goat anti-mouse IgG and IgM-alkaline phosphatase conjugate (Sigma, St. Louis, MO) at a dilution of 1:10,000, the blots were washed and developed using ECL Plus Western Blotting Detection Reagents (GE Healthcare Life Sciences, Little Chalfont, Buckinghamshire, UK).
Preparation of B. pertussis protein samples
To obtain proteins from culture fluids, spent culture supernatant was obtained from a 24-h culture with an OD600 of approximately 1.2 by centrifugation (8,000 × g, 10 min) and passed through a 0.45-μm sterile membrane filter (Milex®GV, Millipore, Carrigtwohill, Ireland) to remove residual bacterial cells. Proteins in the supernatant fluids were precipitated with 10% trichloroacetic acid (TCA) in acetone and resuspended in loading buffer for SDS-PAGE.
Bacterial cells were disrupted by ultrasonication and the lysate centrifuged at 100,000 × g for 1 h to obtain the soluble (cytosol and periplasm) and membrane fractions. After the addition of Triton X-100 to a final concentration of 2 %, solubilized inner membrane proteins were isolated from the insoluble outer membrane fraction by ultracentrifugation. Proteins in the inner membrane fraction were concentrated by methanol precipitation. Periplasmic fractions were prepared using osmotic shock and separation from spheroplasts. Briefly, B. pertussis strains were grown in SS medium to an OD600 of 1. Cells were harvested, washed twice with a solution containing 30 mM NaCl, 10 mM Tris HCl (pH 7.5) and resuspended in 2.5 ml of a solution of 20% sucrose and 30 mM Tris–HCl (pH 7.5) at room temperature. After addition of EDTA to a final concentration of 1 mM, the cell suspensions were incubated at room temperature for 10 min. Bacterial cells were collected by centrifugation at 8,000 × g at 4°C for 10 min and resuspended in 10 ml of ice cold 0.5 mM MgCl2. After 10 min of incubation on ice, the cells were removed by centrifugation for 10 min at 8,000 × g, followed by filtration. The periplasmic proteins were concentrated by precipitation with 10% TCA in acetone.
To prepare outer membrane vesicles, cell-free culture fluids were concentrated by ultrafiltration (Millipore Amicon Ultra 50K, Millipore). Proteins in the ultrafiltrate were concentrated 100-fold by precipitation using 10% TCA in acetone (fraction 1). The concentrated samples retained by the membrane were fractionated by ultracentrifugation at 150,000 × g for 3 h. Proteins in the resulting supernatant fraction were precipitated by 10% TCA in acetone (fraction 2); the pellets contained crude outer membrane vesicles. Samples were analyzed by SDS-PAGE and immunoblotting. Protein amounts corresponding to a culture with an OD600 of 6.0 were applied to each lane.
Scanning electron microscopy
B. pertussis strains were grown on a layer of BG agar solidified onto a sterile glass slide. The colonies were fixed in situ using 2.5% glutaraldehyde prior to examination by scanning electron microscopy using a JSM 6330F microscope (JEOL Ltd. Tokyo, Japan).
RESULTS
Identification of the B. pertussis rpoE gene cluster and construction of an rseA deletion mutant
Barchinger and colleagues reported recently that the genomes of B. bronchiseptica, Bordetella parapertussis and B. pertussis contain gene clusters predicted to encode RpoE-related functions (Barchinger et al., 2012). In the present study, we specifically identified in the B. pertussis Tohama I genome sequence the genes BP2437, BP2436, BP2435 and BP2434 which are predicted orthologs of the known bacterial genes rpoE, rseA, rseB, and mucD, respectively (Fig. 1). Upstream of rpoE are genes encoding a putative 3-oxoacyl-[acyl-carrier-protein] synthase II (BP2439, FabF) and BP2438, encoding a membrane protein of unknown function. The mucD (BP2434) product is a predicted member of the HtrA family of serine proteases that includes MucD of Pseudomonas aeruginosa and E. coli DegP, which are involved in degrading damaged proteins; degP transcription is dependent on σE and enhanced under heat shock stress (Clausen et al., 2002). No obvious rseC homolog was identified in the B. pertussis genome sequence; however a gene annotated as degQ (BP0280) specifies a protein that exhibits significant similarity to E. coli DegS and DegQ. Based on the predicted transcriptional organization (Cummings et al., 2006), as well as short intergenic regions and overlapping coding sequences suggestive of transcriptional coupling, the B. pertussis rpoE, rseA, rseB and mucD genes are likely cotranscribed from the fabF promoter. This gene organization is conserved in B. parapertussis, B. bronchiseptica and Bordetella avium. Bioinformatic analysis revealed that other ß-proteobacteria, including Burkholderia species, possess rpoE gene clusters that are also adjacent to fabF and other fatty acid biosynthesis genes.
Figure 1. Genetic organization of the B. pertussis rseA gene cluster.
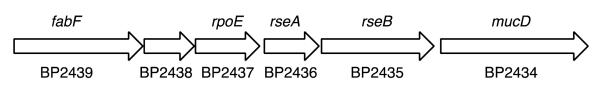
A schematic of the B. pertussis strain Tohama I DNA region is shown; arrows denote the spatial limits and transcriptional orientation of the genes. Gene locus tags are indicated.
In contrast to the B. bronchiseptica rpoE mutant results recently described (Barchinger et al., 2012), in this study, multiple efforts to construct a B. pertussis rpoE null mutant were unsuccessful. To facilitate the study of stress responses in this human-adapted Bordetella species, the gene encoding the predicted RseA anti-sigma factor was mutated. An rseA mutant would be expected to exhibit a σE-constitutive phenotype, regardless of envelope stress. An in-frame rseA deletion mutation in B. pertussis was constructed that resulted in the removal of 153 amino acids, comprising the majority of RseA polypeptide.
Stress sensitivity of B. pertussis
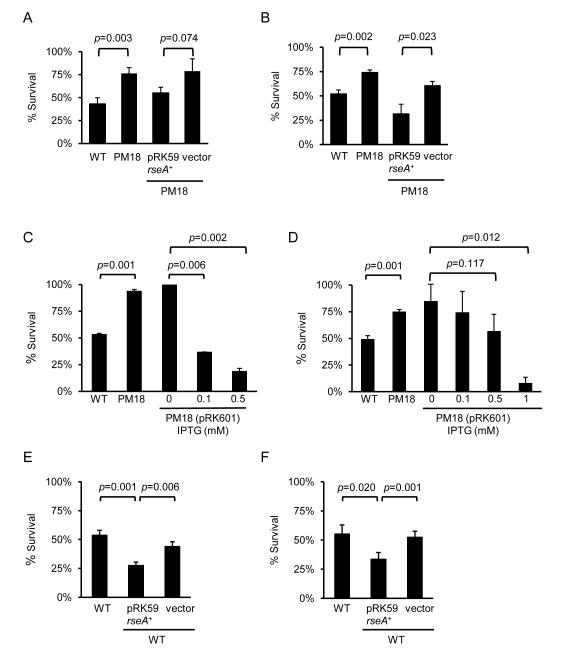
Both wild-type B. pertussis strain UT25Sm1 and its isogenic rseA mutant derivative PM18 were assessed for susceptibility to hydrogen peroxide stress and the bactericidal cationic peptide, polymyxin B, which are known activators of the σE –dependent envelope stress response (Humphreys et al., 1999; Testerman et al., 2002; Korbsrisate et al., 2005; Mathur et al., 2007). Exposure of the wild-type strain to 40 mM hydrogen peroxide for 1 h decreased its survival to 43%, relative to untreated cells, whereas the percent survival of the rseA mutant was 76% (Fig. 2A). Similarly, the rseA mutant showed moderately decreased sensitivity to polymyxin B (Fig. 2B). Sensitivity to both hydrogen peroxide and polymyxin B approaching wild-type levels was restored to mutant PM18 by complementation in trans using a wild type copy of the rseA gene (pRK59). Overexpression of rseA from an IPTG-inducible promoter (pRK601) in mutant PM18 led to markedly decreased resistance to hydrogen peroxide and polymyxin B (Fig. 2C, D). This decreased survival was not due to rseA overexpression itself since, in the absence of stress compounds, there was no difference in viable cell counts of uninduced cultures versus those induced with IPTG for 6 h (data not shown). Overexpression of rseA on multicopy plasmid pRK59 in wild-type B. pertussis UT25Sm1 increased its sensitivity to stress (Fig. 2E, F). In sum, these results suggest that the activation of σE caused by the loss of rseA, encoding the cognate anti-sigma, leads to increased tolerance to envelope stress. These results are also consistent with the idea that rseA overexpression leads to unrelieved cell envelope stress, since excess RseA may overwhelm the predicted protease that normally cleaves it; this would allow RseA to bind the available σE, preventing it from activating stress regulon genes.
Figure 2. Sensitivity of B. pertussis strains to stress conditions and effect of rseA overexpression.
Cells were untreated or treated for 1 h with 40 mM hydrogen peroxide (A) or 5 μg ml−1 polymyxin B (B). Wild-type UT25Sm1; WT, isogenic rseA mutant strain PM18, and PM18 carrying the rseA+ plasmid pRK59 or the pRK415 vector control were cultured as described in Materials and Methods. Wild-type strain UT25Sm1 (WT), rseA mutant PM18, strain PM18 carrying the IPTG-inducible rseA+ plasmid pRK601, and UT25Sm1 carrying the pRK59 (rseA+) or the pRK415 vector control plasmid were cultured as described in Materials and Methods. Cells were treated with hydrogen peroxide (C, D) and polymyxin B (E, F). Panels C, D: pRK601-carrying cells were supplemented with the denoted concentrations of IPTG or were unsupplemented (control). Percent survival indicates the ratio of the numbers of stressed bacterial cells per ml compared with the cell numbers from untreated cultures. Means and standard deviations of a representative of three experiments are shown. The data were analyzed by Student’s t test (p values are shown).
Effect of rseA deletion on growth
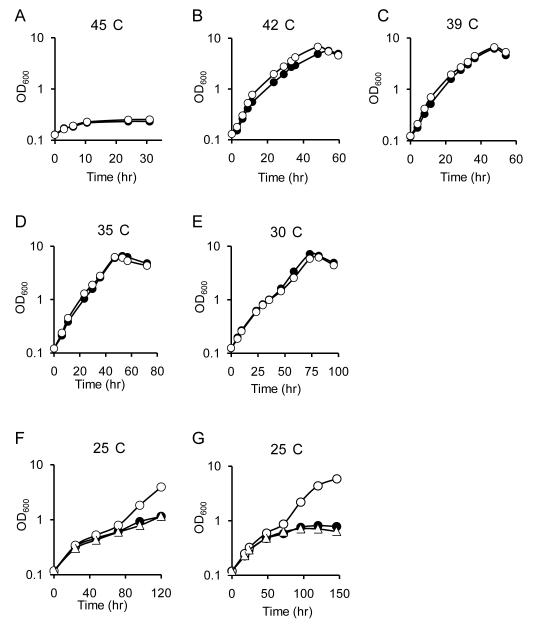
Since the σE system is reported to influence bacterial growth at different temperatures (Chi & Bartlett, 1995; Miticka et al., 2003), the effect of rseA mutation on B. pertussis growth at a range of temperatures was examined (Fig. 3). The optimal temperature for growth of B. pertussis is 35°C - 37°C. Both the wild-type and rseA mutant strains grew equally well at 30°C, 35°C, 39°C and 42°C and both demonstrated similarly poor growth at 45°C. As expected, at 25°C, both the wild-type strain and rseA mutant PM18 exhibited a slow growth rate. However, later during the culture period, PM18 consistently exhibited an increased growth rate compared with the parent strain, with a significantly higher final growth yield. Using a plasmid-borne rseA gene (pRK59), PM18 was genetically complemented, restoring the lower wild-type growth level (Fig. 3F). Replacement of the PM18 chromosomal ΔrseA gene with a copy of wild-type rseA via allelic exchange (“knock-in” strain PMK22) also restored the lower, wild-type growth level (Fig. 3G). These results indicate that mutation of rseA did not affect B. pertussis growth at higher temperatures but rather, it suppressed the slow growth phenotype observed in wild type cells at 25°C.
Figure 3. Influence of temperature on growth of B. pertussis strains.
Strains were grown in SS medium at the indicated temperature and the OD600 values of the cultures were measured over time. Panels A – E: wild-type UT25Sm1 (closed circles), rseA mutant PM18 (open circles). Panel F, wild-type UT25Sm1 (closed circles), PM18 (open circles), PM18(pRK59)(RseA+) (open triangles). Panel G, wild-type UT25Sm1 (closed circles), PM18 (open circles), PMK22 (RseA+) (open triangles). The result of a representative experiment that was repeated independently three times is shown.
Induction of transcription by envelope stress and the effect of RseA
In E. coli and other bacteria, σE recognizes promoters with the conserved -35/-10 sequence motif [GAAC[A/T][A/T]X(16,17)TCXXA] (Lane & Darst, 2006; Rhodius & Mutalik, 2010). An in silico analysis of the B. pertussis genome revealed several candidate σE–dependent promoter sequences upstream of the genes of unknown function, BP0893 and BP3218, as well as BP2014 (annotated as acnA, encoding a putative aconitase A) and BP3748, an ortholog of rpoH. In characterized bacterial systems, rpoH expression is regulated by σE (Raivio & Silhavy, 2001). Since RpoH is known to be involved in bacterial heat shock and stress responses and the recent B. bronchiseptica σE analysis demonstrated its role in rpoH transcription (Barchinger et al., 2012), we examined whether the B. pertussis rpoH gene was also subject to RseA/σE -mediated regulation.
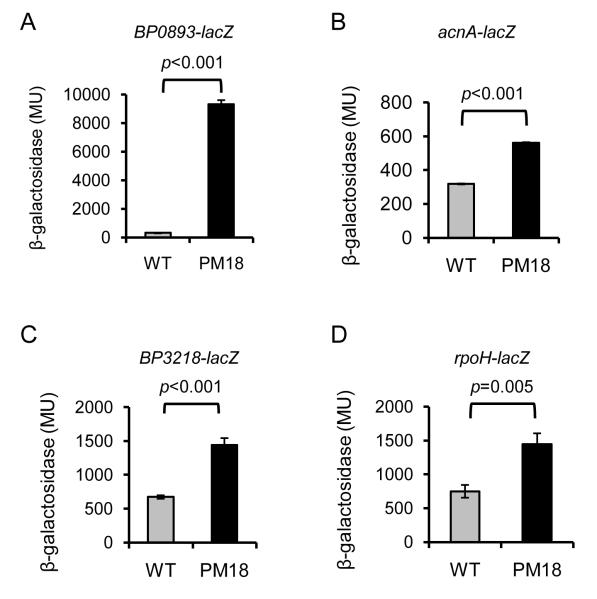
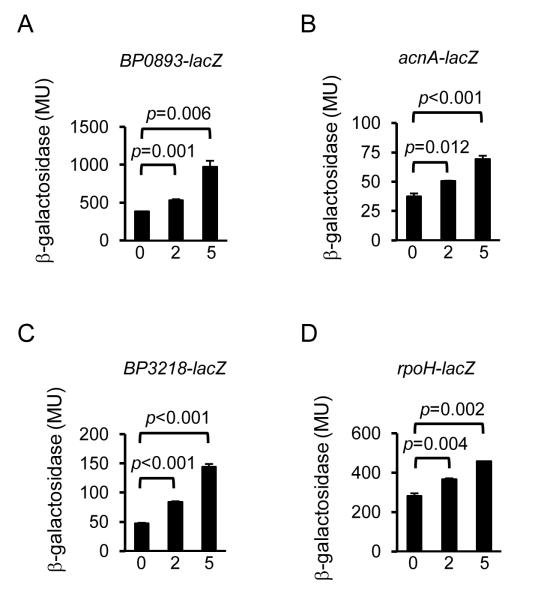
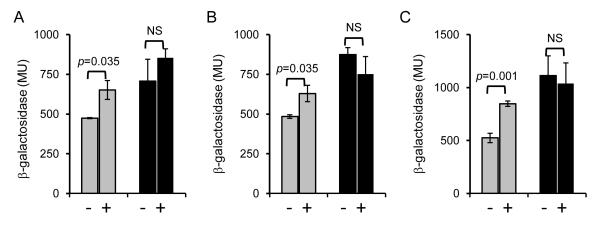
To analyze the putative promoter activities in B. pertussis, lacZ transcriptional fusions were constructed and the ß-galactosidase activity of wild type and rseA mutant strains carrying the fusion plasmids was measured (Fig. 4 A-D). In the wild type strain UT25Sm1, transcription of all four fusions was modest. In contrast, in the rseA mutant PM18, significantly higher levels of LacZ activity were measured, especially for the BP0893 transcriptional fusion (Fig. 4 A). When the same plasmid-borne fusions were expressed in E. coli that was subjected to polymyxin B stress, a dose responsive increase in transcriptional activity was observed (Fig. 5 A-D). These results demonstrate that these promoter activities were elevated in the B. pertussis rseA mutant and could also be activated in a heterologous host background under relevant stress conditions. A similar trend was observed when the rpoH-lacZ fusion was expressed in B. pertussis exposed to cell envelope stress (Fig. 6). In the wild type strain, rpoH expression was modestly increased when the cells were treated with hydrogen peroxide, polymyxin B or 0.4 M sucrose. In contrast, rpoH-lacZ transcription in rseA mutant PM18 was high regardless of the presence or absence of any of the stressors. These results indicate that in wild-type B. pertussis, rpoH transcription is responsive to three types of cell envelope stress and in the absence of RseA, there is an increase in rpoH transcription (presumably σE –mediated). Loss of RseA function also appeared to result in constitutive σE activity and a constitutive envelope stress response.
Figure 4. Effect of RseA on the expression of predicted RpoE-regulated genes in B. pertussis.
lacZ transcriptional fusions to predicted RpoE target gene promoter regions were constructed and the plasmids transferred to B. pertussis wild-type strain UT25Sm1 (gray bars) and isogenic rseA mutant strain PM18 (black bars). Strains were grown in SS medium and ß-galactosidase activity was measured as described in Materials and Methods. Gene fusions are indicated above each graph. Results are reported in Miller units; means and standard deviations of a representative of three experiments are shown. The data were analyzed by Student’s t test (p values are shown).
Figure 5. Stress-induced expression of predicted B. pertussis σE-regulated genes in E. coli.
lacZ transcriptional fusion plasmids were transferred to E. coli strain DH5α. Bacteria were grown in M9 medium for 6.5 h, then treated for 30 min. with 0, 2 or 5 μg ml−1 of polymyxin B. ß-galactosidase activity was measured as described in Materials and Methods and the results are indicated in Miller units. Gene fusions are indicated above each graph. Means and standard deviations of a representative of three experiments are shown. The data were analyzed by Student’s t test (p values are shown).
Figure 6. Analysis of rpoH expression in B. pertussis.
Wild-type strain UT25Sm1 (gray bars) and isogenic rseA mutant PM18 (black bars), each carrying the rpoH-lacZ fusion plasmid pM02, were assessed for ß-galactosidase activity after 3h exposure to cell envelope stress (+) or in the absence of stress (-). A, treatment with 20 mM hydrogen peroxide; B, treatment with 2 μg ml−1 ml polymyxin B; C, treatment with 0.4 M sucrose. ß-galactosidase activity was measured as described in Materials and Methods and the results indicated in Miller units. Means and standard deviations of a representative of three experiments are shown. The data were analyzed by Student’s t test (p values are shown; NS, not significant).
Effect of rseA mutation on protein localization
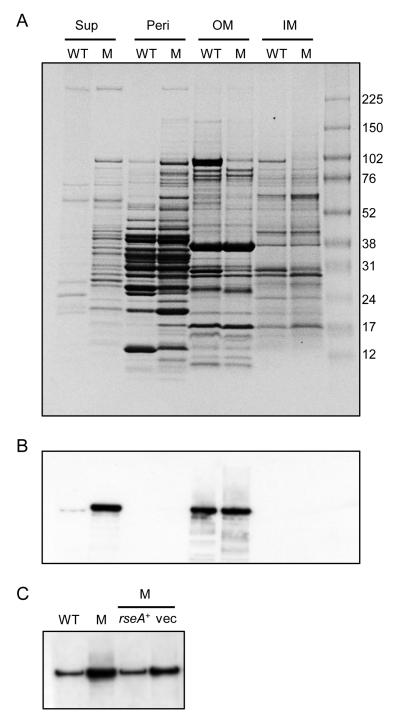
Since cell envelope stress is associated with protein misfolding and mislocalization and with membrane perturbation, the cellular protein profiles of wild-type B. pertussis UT25Sm1 and rseA mutant PM18 were compared. In general, the numbers and relative abundance of proteins in the culture supernatant and those found in the periplasm were increased in mutant PM18, relative to UT25Sm1 (Fig. 7A). In the outer and inner membrane fractions, several proteins of unknown identity detected in the wild-type membrane fractions were less abundant or not visible in the PM18 samples. To determine if the higher abundance of proteins observed in the PM18 culture supernatant could be attributed to PM18 cell lysis during culture, cytoplasmic malate dehydrogenase activity was measured in cell-free culture fluids. No malate dehydrogenase activity was detected in either wild-type or PM18 culture supernatant fluids, compared with positive control cell fractions, indicating that lysis of PM18 cells was not responsible for the increase in proteins in the culture fluids (data not shown). Furthermore, the turbidity and viable counts of the cultures were nearly identical for both the wild type and mutant strains (data not shown).
Figure 7. rseA mutation alters B. pertussis protein localization and CyaA release.
Cell-free culture supernatant (Sup), periplasmic (Peri), outer membrane (OM) and inner membrane fractions (IM) were prepared as described in the Materials and Methods. Panel A: Normalized protein loads from wild-type UT25Sm1 (WT) and rseA mutant PM18 (M) were subjected to SDS-PAGE. Panel B: CyaA was detected in the Bordetella fractions by immunoblotting a replicate gel and using specific monoclonal antibody. Panel C: proteins in culture supernatant fluids were prepared from B. pertussis UT25Sm1 (WT), rseA mutant PM18 (M), and PM18 carrying plasmid pRK59 (rseA+) or the pRK415 plasmid vector control (vec); CyaA was detected by immunoblotting. Protein amounts corresponding to the supernatant of a culture with an OD600 of 1.5 were loaded onto gels. Molecular mass markers in panel A are indicated in kDa to the right. Panels B, C: immunoreactive CyaA migrated with an apparent mass of approximately 200 kDa.
B. pertussis produces and secretes several virulence factors including CyaA, which is considered crucial for pathogenesis (Weiss et al., 1984; Carbonetti, 2010). It was reasoned that RseA and the σE system might affect the localization of CyaA. CyaA is primarily localized to the bacterial cell surface (Glaser et al., 1988), although that which is secreted is the form that primarily intoxicates eukaryotic cells (Gray et al., 2004). The Bordetella protein fractions shown in Fig. 7A were subjected to immunoblotting to detect CyaA (Fig. 7B). Significantly, in this representative immunoblot and in replicate experiments, more CyaA was detected in the culture supernatant of mutant PM18 than that of the parental strain. Complementation of PM18 with the rseA gene supplied in trans appeared to restore the lower wild type levels of CyaA in the culture fluid, compared with PM18 carrying the plasmid vector control (Fig. 7C). This result was further supported by the demonstration of reduced amounts of released CyaA using the B. pertussis PMK22 “knock-in” strain, with rseA in single copy, compared with the ΔrseA PM18 parent derivative (data not shown).
Extracellular CyaA levels are increased by stress
Since the absence of RseA led to an increased amount of CyaA in the culture fluids of the rseA mutant, it was hypothesized that the amount of CyaA in the culture supernatants from the wild type strain would be increased by cell envelope stress. The wild type B. pertussis strain UT25Sm1 was exposed to three concentrations of hydrogen peroxide prior to CyaA detection in the cell-free culture supernatants (Fig. 8). There was no evidence of bacterial cell lysis on exposure to hydrogen peroxide. The levels of CyaA in the culture fluids were increased proportionally to the concentration of hydrogen peroxide used in the culture (Fig. 8A). In addition, the overall amounts of protein in the culture fluids were increased upon exposure of the bacteria to hydrogen peroxide (Fig. 8B). A similar increase in CyaA in the culture fluid following treatment with polymyxin B was also observed (data not shown).
Figure 8. Hydrogen peroxide-mediated stress induces release of CyaA into the culture medium.
Cell-free culture fluids from wild-type B. pertussis UT25Sm1 (WT) were prepared 6 h after addition of hydrogen peroxide, at the indicated mM concentrations, to exponentially growing bacterial cultures. Sample preparation and immunoblotting with anti-CyaA antibody (A) and SDS-PAGE and staining with Coomassie Brilliant Blue (B) were performed as described in the Materials and Methods. Untreated PM18 rseA mutant cell samples are shown in the left lanes. Migration positions of the molecular mass markers are indicated, in kDa.
Analysis of extracellular CyaA
It was possible that the increased amount of extracellular CyaA from the rseA mutant was due to increased cyaA expression. Transcription studies using the wild type and rseA mutant strains carrying a cyaA-lacZ fusion showed that cyaA expression in both strains was similar and could also be decreased using the established method of phenotypic modulation from Bvg+ to Bvg− phase using 40 mM MgSO4 (data not shown) (Lacey, 1960; Melton & Weiss, 1989). Since CyaB is involved in the secretion of CyaA, the rseA mutant and wild type strains were tested and shown to express a cyaB-lacZ fusion at equivalent levels. These results indicate that the increased extracellular level of CyaA in the rseA mutant strain was not due to increased transcription of cyaA or cyaB.
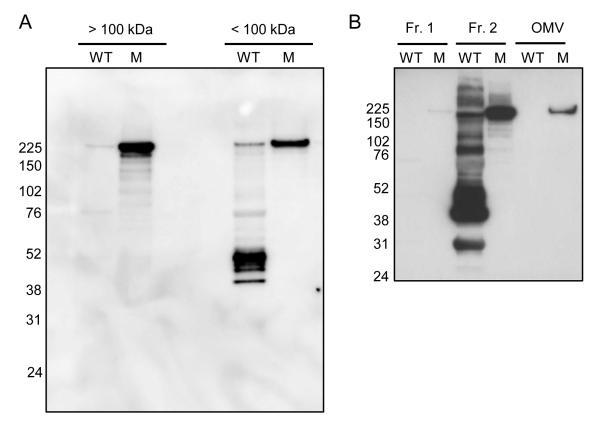
During these studies, it was noted that in highly concentrated protein samples prepared from cell-free culture supernatant fluids, CyaA from the wild type strain appeared to be degraded to lower ~30-50 kDa molecular mass fragments. Interestingly, the rseA mutant samples did not exhibit this degradation. Extracellular proteins from both strains were subjected to ultrafiltration using a 100 kDa molecular mass cutoff filter and examined by immunoblot analysis to detect CyaA (Fig. 9A). In the retentate (> 100 kDa lanes), samples from wild type cells showed little immunoreactive high Mr CyaA, whereas the PM18 rseA mutant sample primarily contained apparently intact ~180 kDa CyaA. The majority of the immunoreactive proteins from wild type cells that passed through the ultrafiltration membrane (< 100 kDa lanes) were of lower molecular mass (38-52 kDa), but a small amount of high Mr CyaA was also observed. The < 100 kDa sample from the rseA mutant contained no obvious CyaA degradation products, and apparently intact CyaA that was in a conformation capable of transiting the membrane. In sum, these results suggested that the extracellular CyaA of wild type cells was more susceptible to degradation, compared with that of the rseA mutant strain.
Figure 9. Enhanced stability of extracellular CyaA from the rseA mutant.
B. pertussis UT25Sm1 (WT) and rseA mutant PM18 (M) were grown in SS medium and cell-free extracellular fluids were obtained, fractionated, and analyzed by SDS-PAGE and immunoblotting to detect CyaA. Panel A: > 100 kDa, protein fractions retained by a 100 kDa molecular weight cutoff filter; < 100 kDa, proteins that passed through the filter. Protein amounts corresponding to the supernatant of a culture with an OD600 of 4.0 for samples were loaded onto gels. Panel B: extracellular proteins were subjected to ultrafiltration using a 50 kDa molecular weight cutoff filter. Fr. 1, proteins that passed through the filter. Proteins retained by the filter were then fractionated by ultracentrifugation: Fr. 2, proteins in the supernatant fluids; OMV, pelleted material enriched in outer membrane vesicles. Protein amounts corresponding to the supernatant of a culture with an OD600 of 6.0 were loaded onto gels.
Since B. pertussis has been reported to produce outer membrane vesicles containing CyaA (Hozbor et al., 1999; Donato et al., 2012), outer membrane vesicles from cell-free culture supernatant fluids were prepared and examined by immunoblotting (Fig. 9B). Analysis of extracellular proteins that passed through the initial 50 kDa Mr cutoff ultrafiltration membrane revealed virtually no immunoreactive proteins in either the wild type or rseA mutant strains (Fig. 9B, Fr. 1). The extracellular protein fractions retained by the membrane were subjected to ultracentrifugation, with the resulting supernatants (Fr. 2) containing soluble proteins, and the pellet material containing crude outer membrane vesicles (OMV). The Fr. 2 soluble protein samples from wild-type cells revealed many immunoreactive bands suggesting proteolytic degradation, while samples from the rseA mutant exhibited what appeared to be intact CyaA. Intact CyaA was detected in the outer membrane vesicle samples from the rseA mutant, but no immunoreactive CyaA was observed in the vesicle samples from the wild type strain.
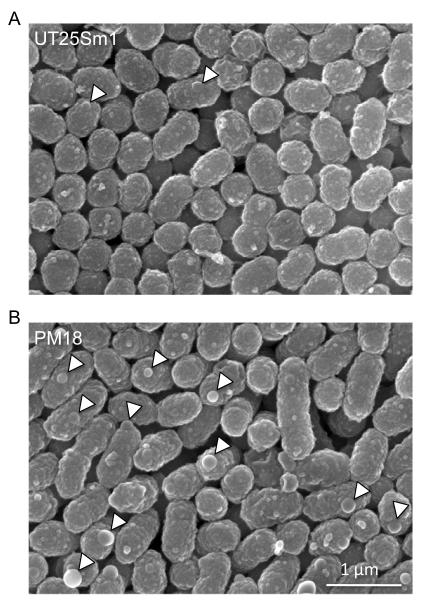
Examination by scanning electron microscopy showed that the rseA mutant cells produced more numerous and larger surface-associated vesicles, compared with the wild-type UT25Sm1 parent cells (Fig. 10). Quantitation of vesicles from four fields of view, showed there were 282 vesicles on the surfaces of 266 wild-type cells, yielding an average of 1.1 vesicles per cell. 646 vesicles were counted on 234 rseA mutant cells, averaging 2.8 vesicles per cell. The vesicles on the rseA cells also had larger diameters than those of wild-type cells: an 82.9 mm average, versus 54.7 mm, respectively. The mutant cells also appeared to be somewhat longer than wild type cells. Overall, these results suggest that the extracellular CyaA of the rseA mutant is more stable than the wild type strain CyaA, which appears to be proteolytically degraded, at least under the conditions used in this study. This apparent stability may also be related to differences in the abundance or content of outer membrane vesicles, which contain CyaA.
Figure 10. Cellular morphology of B. pertussis wild-type strain UT25Sm1 (A) and rseA mutant strain PM18 (B).
Bacterial cells were prepared for scanning electron microscopy as described in Materials and Methods. Arrowheads denote large vesicular structures. Magnification: 20,000 ×.
DISCUSSION
Orthologs of the rpoE gene encoding σE are widely found in bacteria, and σE function is negatively regulated by RseA-type anti-sigma factors (Raivio & Silhavy, 2001). In E. coli, the σE regulon includes essential genes, which may explain why rpoE is essential in that organism (De Las Penas et al., 1997; Hayden & Ades, 2008). A recent report described the construction and characterization of a B. bronchiseptica rpoE mutant and noted that the B. bronchiseptica rpoE gene could complement an E. coli rpoE mutant (Barchinger et al., 2012). Our inability to obtain a B. pertussis rpoE mutant suggests that σE is essential in this human-adapted Bordetella species, which is a possibility that requires further investigation. B. pertussis has the rpoE, rseA, and rseB genes as well as a predicted mucD ortholog. rseA and rseB orthologs have been identified in the rpoE gene clusters of γ- proteobacteria and several ß-proteobacteria. In E. coli and other organisms, RseB is a periplasmic protein that binds to σE and negatively influences its function (Alba & Gross, 2004; Brown & Gulig, 2009). For σE activation, the DegS serine protease requires unassembled outer membrane protein ligands as an activating signal, while RseB function is inhibited by an undefined mechanism; both proteins play a role in sensing signals from the cell envelope. MucD, encoded in the Pseudomonas aeruginosa AlgU (RpoE) genetic system, is a periplasmic serine protease of the HtrA family that is involved in degrading misfolded proteins (Clausen et al., 2002). The B. pertussis rpoE, rseA, rseB and mucD genes are predicted to be cotranscribed from a promoter upstream of fabF. In bacteria, FabF is the 3- oxoacyl-[acyl carrier protein] synthase II involved in fatty acid biosynthesis. Upstream of B. pertussis fabF lies the acpP gene, encoding a predicted acyl carrier protein, and other genes related to fatty acid synthesis including fabH, fabD and fabG as well as a gene similar to maf, annotated as encoding a protein that inhibits septum formation. Other ß –proteobacteria such as Burkholderia spp. also have fab genes adjacent to their rpoE system genes. This organization of the fatty acid biosynthesis genes adjacent to the rpoE stress genes may reflect the involvement of the σE system in responding to cell membrane stress. That our B. pertussis rseA mutant produces excessive numbers of large outer membrane vesicles may also indicate a functional relationship between stress responses and membrane homeostasis. Compared with the wild-type parent, the B. pertussis rseA mutant PM18 exhibited an enhanced ability to grow at 25°C. Interestingly, in the barophilic Photobacterium profundum strain SS9, growth at low temperatures and high pressure is controlled by an σE system (Chi & Bartlett, 1995) and involves FabF-mediated alteration of membrane phospholipids to increase the amounts of unsaturated fatty acids (Allen & Bartlett, 2000).
Many functions of core members of the σE regulons appear to be conserved in bacteria (Miticka et al., 2003; Rhodius et al., 2006; Flannagan & Valvano, 2008; Brown & Gulig, 2009). In general, many of these gene products help to maintain the integrity of the bacterial cell envelope. In the present study, mutation of B. pertussis rseA resulted in a moderate increase in resistance to chemical stress, compared with the wild type strain. These differences in stress susceptibility are similar to the differences observed between B. bronchiseptica wild type and rpoE strains subjected to chemical stressors, where the mutant exhibited modest but significant increases in susceptibility to compounds such as ampicillin and SDS-EDTA (Barchinger et al., 2012). The B. bronchiseptica rpoE mutant was no more sensitive to polymyxin B, hydrogen peroxide or osmotic stress than the wild type parent strain. These results seem to differ from our findings of increased survival of the B. pertussis rseA strain (over the wild type) with either hydrogen peroxide or polymxyin B treatment. However, until parallel studies using a B. pertussis rpoE mutant strain are performed, interpretation of these apparently contrasting results is not possible.
Our studies tested the involvement of RseA on the expression of four candidate RpoE-controlled promoters in B. pertussis and demonstrated that those promoters were stress-activated in both E. coli and B. pertussis. Although expression of the B. pertussis acnA gene encoding aconitase A was influenced by RseA, we could find no reports in the literature of acnA as a member of any σE regulon. The heat shock sigma factor gene, rpoH, is a member of multiple bacterial σE regulons (Raivio & Silhavy, 2001; Alba & Gross, 2004), and the report from Barchinger et al. (2012) and the present analysis indicate that this is also true for B. bronchiseptica and B. pertussis, respectively. The Bordetella rpoH promoter region contains a region bearing high similarity with the E. coli σE consensus recognition sequence, and the B. bronchiseptica rpoE gene was shown to function in E. coli to activate transcription of an E. coli rpoH gene fusion (Barchinger et al., 2012). In the present study, the presumed activation of σE by mutation of rseA in B. pertussis resulted in constitutive rpoH expression.
Our results showed that in the rseA mutant, proteins were found at increased levels in culture supernatants and at decreased levels in the outer membrane fraction. Notably, the amount of CyaA in cell-free culture supernatants was increased by both rseA mutation and by envelope stress. CyaA is an important virulence factor for B. pertussis and has both hemolytic and cytolytic activities (Hewlett et al., 1976; Glaser et al., 1988; Mattoo & Cherry, 2005). Upon translocation of the catalytic domain into a eukaryotic cell, host calmodulin activates the toxin, allowing it to catalyze production of supraphysiologic levels of cAMP, leading to cytotoxicity and inhibition of cellular functions such as phagocyte chemotaxis. CyaA is secreted to the extracellular environment via a Type I secretion apparatus formed by the CyaB, CyaD and CyaE proteins (Glaser et al., 1988). Bordetella CyaA can be found associated with the cell surface (Hewlett et al., 1976), but Gray et al. reported that it was the soluble, newly secreted form of CyaA that intoxicates eukaryotic target cells (Gray et al., 2004). A recent report noted that B. pertussis produces outer membrane vesicles in humans during infection, and experiments showed that B. pertussis outer membrane vesicles contain CyaA (Donato et al., 2012). These investigators also reported that the CyaA within vesicles represents about 1.2 % of the total amount of CyaA released into B. pertussis culture supernatant fluids. In that report, as well as another (Hozbor et al., 1999) B. pertussis cells were noted to naturally produce rather low levels of outer membrane vesicles. Additionally in those studies, suspensions of B. pertussis cells were sonicated to artificially generate greater numbers of vesicles that were shown to contain CyaA; sonically generated vesicles also contain pertussis toxin and other proteins (Hozbor et al., 1999). Our findings confirmed that, compared with the rseA mutant, wild type B. pertussis naturally produces low numbers of outer membrane vesicles (data not shown) which contained little CyaA (Fig. 9B). Scanning electron microscopy revealed that the rseA mutant not only produced more numerous cell surface-associated vesicles than the wild type parent, but those vesicles were larger in size. In unfractionated extracellular culture fluids, a large proportion of wild type CyaA appeared to be degraded to 38-50 kDa fragments, which has also been previously described in the literature (Ladant et al., 1986). If the vesicle-associated CyaA is only a small fraction of the total found in cell-free supernatants, as reported by Donato et al. (2012) and supported by our findings (Fig. 9B), then the majority of it that is in the culture supernatant may susceptible to proteolysis. However, this hypothesized proteolysis was not observed for the extracellular CyaA of the B. pertussis rseA mutant. The CyaA in sonically manufactured B. pertussis vesicles was shown to be sensitive to exogenously added trypsin, indicating surface exposure (Donato et al., 2012). Our results suggest that the rseA mutant extracellular CyaA, whether associated with naturally produced vesicles or free in the culture supernatant, is either sequestered within the vesicles or is otherwise resistant to proteolytic attack. It is also possible that the loss of RseA function interferes with the production or activity of one or more proteases that may act on CyaA.
Outer membrane vesicle formation has been reported as a σE –mediated stress response in Gram negative bacteria (McBroom & Kuehn, 2007). Vesicle formation may be one way for cells to rid the periplasm of excess protein that may induce envelope stress. During growth on the ciliated respiratory epithelial surface, B. pertussis may be subjected to changes in osmolarity and pH, or the presence of complement components and cationic antimicrobial peptides. B. pertussis would also be exposed to significant oxidative stress upon phagocytosis. The Bordetella σE system may play a role in the deflection of, and recovery from, such stresses and may also control the release of virulence factors such as CyaA, which is known to dysregulate phagocytic cell function.
ACKNOWLEDGMENTS
We thank Melanie Straub (Ludwig Maximilian University of Munich, Munich, Germany) and Sophie Failer (Philipps-University Marburg, Germany) for constructing plasmids. We are grateful to Matsubara and Koroishi (Division of Microscopic Anatomy, Kyorin University) for their kind help in the electron microscope analysis. In addition, we thank Tomoko Nozaki (Kyorin University School of Medicine) for technical assistance. We thank Timothy J. Brickman for critical reading of the manuscript and for assistance with figures.
This research was supported by Grants-in-Aid for Scientific Research (23590517) from the Japan Society for the Promotion of Science and by grant AI31088 (to S.K.A.) from the U.S. National Institute of Allergy and Infectious Diseases.
REFERENCES
- Akiyama Y, Kanehara K, Ito K. RseP (YaeL), an Escherichia coli RIP protease, cleaves transmembrane sequences. EMBO J. 2004;23:4434–4442. doi: 10.1038/sj.emboj.7600449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba BM, Gross CA. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol Microbiol. 2004;52:613–619. doi: 10.1111/j.1365-2958.2003.03982.x. [DOI] [PubMed] [Google Scholar]
- Allen EE, Bartlett DH. FabF is required for piezoregulation of cis-vaccenic acid levels and piezophilic growth of the deep-Sea bacterium Photobacterium profundum strain SS9. J Bacteriol. 2000;182:1264–1271. doi: 10.1128/jb.182.5.1264-1271.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arico B, Miller JF, Roy C, Stibitz S, Monack D, Falkow S, Gross R, Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci U S A. 1989;86:6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchinger SE, Zhang X, Hester SE, Rodriguez ME, Harvill ET, Ades SE. sigE facilitates the adaptation of Bordetella bronchiseptica to stress conditions and lethal infection in immunocompromised mice. BMC Microbiol. 2012;12:179–2180. doi: 10.1186/1471-2180-12-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet J, Gengou O. Le microbe de la coqueluche. Ann Inst Pasteur (Paris) 1906;20:731–741. [Google Scholar]
- Brickman TJ, Armstrong SK. The ornithine decarboxylase gene odc is required for alcaligin siderophore biosynthesis in Bordetella spp.: putrescine is a precursor of alcaligin. J Bacteriol. 1996;178:54–60. doi: 10.1128/jb.178.1.54-60.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman TJ, Ozenberger BA, McIntosh MA. Regulation of divergent transcription from the iron-responsive fepB-entC promoter-operator regions in Escherichia coli. J Mol Biol. 1990;212:669–682. doi: 10.1016/0022-2836(90)90229-F. [DOI] [PubMed] [Google Scholar]
- Brown RN, Gulig PA. Roles of RseB, sigmaE, and DegP in virulence and phase variation of colony morphotype of Vibrio vulnificus. Infect Immun. 2009;77:3768–3781. doi: 10.1128/IAI.00205-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetti NH. Pertussis toxin and adenylate cyclase toxin: key virulence factors of Bordetella pertussis and cell biology tools. Future Microbiol. 2010;5:455–469. doi: 10.2217/fmb.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaba R, Grigorova IL, Flynn JM, Baker TA, Gross CA. Design principles of the proteolytic cascade governing the sigmaE-mediated envelope stress response in Escherichia coli: keys to graded, buffered, and rapid signal transduction. Genes Dev. 2007;21:124–136. doi: 10.1101/gad.1496707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi E, Bartlett DH. An rpoE-like locus controls outer membrane protein synthesis and growth at cold temperatures and high pressures in the deep-sea bacterium Photobacterium sp. strain SS9. Mol Microbiol. 1995;17:713–726. doi: 10.1111/j.1365-2958.1995.mmi_17040713.x. [DOI] [PubMed] [Google Scholar]
- Clausen T, Southan C, Ehrmann M. The HtrA family of proteases: implications for protein composition and cell fate. Mol Cell. 2002;10:443–455. doi: 10.1016/s1097-2765(02)00658-5. [DOI] [PubMed] [Google Scholar]
- Cummings CA, Bootsma HJ, Relman DA, Miller JF. Species- and strain-specific control of a complex, flexible regulon by Bordetella BvgAS. J Bacteriol. 2006;188:1775–1785. doi: 10.1128/JB.188.5.1775-1785.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Las Penas A, Connolly L, Gross CA. SigmaE is an essential sigma factor in Escherichia coli. J Bacteriol. 1997;179:6862–6864. doi: 10.1128/jb.179.21.6862-6864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato GM, Goldsmith CS, Paddock CD, Eby JC, Gray MC, Hewlett EL. Delivery of Bordetella pertussis adenylate cyclase toxin to target cells via outer membrane vesicles. FEBS Lett. 2012;586:459–465. doi: 10.1016/j.febslet.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field LH, Parker CD. Differences observed between fresh isolates of Bordetella pertussis and their laboratory passaged derivatives. In: Manclark CR, Hill JC, editors. International Symposium on Pertussis. U. S. Department of Health, Education, and Welfare; Washington, D. C. 1978. pp. 124–32. [Google Scholar]
- Figurski DH, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannagan RS, Valvano MA. Burkholderia cenocepacia requires RpoE for growth under stress conditions and delay of phagolysosomal fusion in macrophages. Microbiology. 2008;154:643–653. doi: 10.1099/mic.0.2007/013714-0. [DOI] [PubMed] [Google Scholar]
- Flynn JM, Levchenko I, Sauer RT, Baker TA. Modulating substrate choice: the SspB adaptor delivers a regulator of the extracytoplasmic-stress response to the AAA+ protease ClpXP for degradation. Genes Dev. 2004;18:2292–2301. doi: 10.1101/gad.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P, Sakamoto H, Bellalou J, Ullmann A, Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988;7:3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MC, Donato GM, Jones FR, Kim T, Hewlett EL. Newly secreted adenylate cyclase toxin is responsible for intoxication of target cells by Bordetella pertussis. Mol Microbiol. 2004;53:1709–1719. doi: 10.1111/j.1365-2958.2004.04227.x. [DOI] [PubMed] [Google Scholar]
- Hayden JD, Ades SE. The extracytoplasmic stress factor, sigmaE, is required to maintain cell envelope integrity in Escherichia coli. PLoS One. 2008;3:e1573. doi: 10.1371/journal.pone.0001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig SM, Hazenbos WL, van de Winkel JG, Mooi FR. Evidence for an intracellular niche for Bordetella pertussis in broncho-alveolar lavage cells of mice. FEMS Immunol Med Microbiol. 1999;26:203–207. doi: 10.1111/j.1574-695X.1999.tb01391.x. [DOI] [PubMed] [Google Scholar]
- Hewlett EL, Edwards KM. Clinical practice. Pertussis--not just for kids. N Engl J Med. 2005;352:1215–1222. doi: 10.1056/NEJMcp041025. [DOI] [PubMed] [Google Scholar]
- Hewlett EL, Urban MA, Manclark CR, Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci U S A. 1976;73:1926–1930. doi: 10.1073/pnas.73.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Hozbor D, Rodriguez ME, Fernandez J, Lagares A, Guiso N, Yantorno O. Release of outer membrane vesicles from Bordetella pertussis. Curr Microbiol. 1999;38:273–278. doi: 10.1007/pl00006801. [DOI] [PubMed] [Google Scholar]
- Humphreys S, Stevenson A, Bacon A, Weinhardt AB, Roberts M. The alternative sigma factor, sigmaE, is critically important for the virulence of Salmonella typhimurium. Infect Immun. 1999;67:1560–1568. doi: 10.1128/iai.67.4.1560-1568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi A, Suzuki Y, Ono S, Sato H, Sato Y. Heptakis(2,6-O-dimethyl)beta-cyclodextrin: a novel growth stimulant for Bordetella pertussis phase I. J Clin Microbiol. 1983;17:781–786. doi: 10.1128/jcm.17.5.781-786.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K, Charles I, Dougan G, Pickard D, O’Gaora P, Costa G, Ali T, Miller I, Hormaeche C. The role of a stress-response protein in Salmonella typhimurium virulence. Mol Microbiol. 1991;5:401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x. [DOI] [PubMed] [Google Scholar]
- Keen NT, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Korbsrisate S, Vanaporn M, Kerdsuk P, Kespichayawattana W, Vattanaviboon P, Kiatpapan P, Lertmemongkolchai G. The Burkholderia pseudomallei RpoE (AlgU) operon is involved in environmental stress tolerance and biofilm formation. FEMS Microbiol Lett. 2005;252:243–249. doi: 10.1016/j.femsle.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Kovacikova G, Skorupski K. The alternative sigma factor σE plays an important role in intestinal survival and virulence in Vibrio cholerae. Infect Immun. 2002;70:5355–5362. doi: 10.1128/IAI.70.10.5355-5362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey BW. Antigenic modulation of Bordetella pertussis. J Hyg (Lond) 1960;58:57–93. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladant D, Brezin C, Alonso JM, Crenon I, Guiso N. Bordetella pertussis adenylate cyclase. Purification, characterization, and radioimmunoassay. J Biol Chem. 1986;261:16264–16269. [PubMed] [Google Scholar]
- Laemmli UK, Beguin F, Gujer-Kellenberger G. A factor preventing the major head protein of bacteriophage T4 from random aggregation. J Mol Biol. 1970;47:69–85. doi: 10.1016/0022-2836(70)90402-x. [DOI] [PubMed] [Google Scholar]
- Lane WJ, Darst SA. The structural basis for promoter -35 element recognition by the group IV sigma factors. PLoS Biol. 2006;4:e269. doi: 10.1371/journal.pbio.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C, Antoine R, Jacob-Dubuisson F. Bordetella pertussis, molecular pathogenesis under multiple aspects. Curr Opin Microbiol. 2001;4:82–89. doi: 10.1016/s1369-5274(00)00169-7. [DOI] [PubMed] [Google Scholar]
- Mathur J, Davis BM, Waldor MK. Antimicrobial peptides activate the Vibrio cholerae sigmaE regulon through an OmpU-dependent signalling pathway. Mol Microbiol. 2007;63:848–858. doi: 10.1111/j.1365-2958.2006.05544.x. [DOI] [PubMed] [Google Scholar]
- Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBroom AJ, Kuehn MJ. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol Microbiol. 2007;63:545–558. doi: 10.1111/j.1365-2958.2006.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton AR, Weiss AA. Environmental regulation of expression of virulence determinants in Bordetella pertussis. J Bacteriol. 1989;171:6206–6212. doi: 10.1128/jb.171.11.6206-6212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in molecular genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y.: 1972. [Google Scholar]
- Miticka H, Rowley G, Rezuchova B, Homerova D, Humphreys S, Farn J, Roberts M, Kormanec J. Transcriptional analysis of the rpoE gene encoding extracytoplasmic stress response sigma factor sigmaE in Salmonella enterica serovar Typhimurium. FEMS Microbiol Lett. 2003;226:307–314. doi: 10.1016/S0378-1097(03)00600-1. [DOI] [PubMed] [Google Scholar]
- Parkhill J, Sebaihia M, Preston A, et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet. 2003;35:32–40. doi: 10.1038/ng1227. [DOI] [PubMed] [Google Scholar]
- Pedersen LL, Radulic M, Doric M, Abu Kwaik Y. HtrA homologue of Legionella pneumophila: an indispensable element for intracellular infection of mammalian but not protozoan cells. Infect Immun. 2001;69:2569–2579. doi: 10.1128/IAI.69.4.2569-2579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivio TL, Silhavy TJ. Periplasmic stress and ECF sigma factors. Annu Rev Microbiol. 2001;55:591–624. doi: 10.1146/annurev.micro.55.1.591. [DOI] [PubMed] [Google Scholar]
- Rhodius VA, Mutalik VK. Predicting strength and function for promoters of the Escherichia coli alternative sigma factor, sigmaE. Proc Natl Acad Sci U S A. 2010;107:2854–2859. doi: 10.1073/pnas.0915066107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodius VA, Suh WC, Nonaka G, West J, Gross CA. Conserved and variable functions of the sigmaE stress response in related genomes. PLoS Biol. 2006;4:e2. doi: 10.1371/journal.pbio.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y.: 1989. [Google Scholar]
- Schneider DR, Parker CD. Effect of pyridines on phenotypic properties of Bordetella pertussis. Infect Immun. 1982;38:548–553. doi: 10.1128/iai.38.2.548-553.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaink HP, Okker RJ, Wijffelman CA, Pees E, Lugtenberg BJ. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol Biol. 1987;9:27–39. doi: 10.1007/BF00017984. [DOI] [PubMed] [Google Scholar]
- Stainer DW, Scholte MJ. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Stibitz S. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol. 1994;235:458–465. doi: 10.1016/0076-6879(94)35161-9. [DOI] [PubMed] [Google Scholar]
- Testerman TL, Vazquez-Torres A, Xu Y, Jones-Carson J, Libby SJ, Fang FC. The alternative sigma factor sigmaE controls antioxidant defences required for Salmonella virulence and stationary-phase survival. Mol Microbiol. 2002;43:771–782. doi: 10.1046/j.1365-2958.2002.02787.x. [DOI] [PubMed] [Google Scholar]
- von Konig CH, Halperin S, Riffelmann M, Guiso N. Pertussis of adults and infants. Lancet Infect Dis. 2002;2:744–750. doi: 10.1016/s1473-3099(02)00452-8. [DOI] [PubMed] [Google Scholar]
- Weiss AA, Hewlett EL, Myers GA, Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984;150:219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]
- Weiss AA, Hewlett EL, Myers GA, Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983;42:33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Hanawa T, Ogata S, Kamiya S. Identification and characterization of the Yersinia enterocolitica gsrA gene, which protectively responds to intracellular stress induced by macrophage phagocytosis and to extracellular environmental stress. Infect Immun. 1996;64:2980–2987. doi: 10.1128/iai.64.8.2980-2987.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, McDermott JE, Porwollik S, McClelland M, Heffron F. Coordinated regulation of virulence during systemic infection of Salmonella enterica serovar Typhimurium. PLoS Pathog. 2009;5:e1000306. doi: 10.1371/journal.ppat.1000306. [DOI] [PMC free article] [PubMed] [Google Scholar]