Introduction
Atrial fibrillation (AF) is the most common human arrhythmia in the world and depending on individual demographics afflicts from 0.1% to 4% of the general population1. With the steady rise of cardiovascular disease in the industrialized world, it is no surprise the incidence of atrial fibrillation is also increasing2,3. However, despite its ubiquitous presence in adult medicine AF remains an enigmatic disease whose pathophysiology is very complex and incompletely understood. Unlike traditional macroreentrant arrhythmias that arise from better understood electrophysiologic substrates, AF is a more complex disease arising from multiple, mutually interacting mechanisms. Due in part to our incomplete understanding of the mechanisms underlying AF, therapeutic success rates in patients treated with either anti-arrhythmic drug therapy, catheter-based ablation or both remain highly variable4.
Emerging data from the basic science laboratory is starting to shed some light on the mechanistic basis of this disease, which may lead to the development of long-term therapies for patients with AF. One of the ideas that has persisted for several decades about AF, and is fast gaining traction, is there may be a strong contribution from the autonomic nervous system (ANS) in both the initiation and maintenance of this disease5. The structural basis for this theory stems from the discovery of rich autonomic innervation and ganglionated plexi (GP) associated with the pulmonary veins and sites in the atria from which AF triggers often originate6. While the exact causal relationship between these autonomic connections and the initiation of AF is not well understood, anecdotal clinical and laboratory findings suggest such a link is plausible. In this review we will explore our current understanding of the cardiac nervous system and its role in the pathogenesis of atrial fibrillation, with an eye towards future therapeutic strategies that could exploit this relationship.
Anatomic Considerations
The cardiac nervous system broadly encompasses all segments of the central and peripheral nervous system that innervate the heart. While this definition is broad enough to include non-autonomic nerves, such as somatosensory nerves innervating the pericardium, in this review we will focus on the autonomic component of the cardiac nervous system. In keeping with this theme, the majority of neuronal structures in the cardiovascular system are components of either the sympathetic and parasympathetic autonomic nervous system.
Anatomically, the cardiac nervous system can be subdivided into an Intrinsic Cardiac Nervous System (ICNS) and an Extrinsic Cardiac Nervous System (ECNS)7. The ECNS comprises fibers that mediate connections between the heart and the nervous system, whereas the ICNS consists of primarily autonomic nerve fibers once they enter the pericardial sac.
The sympathetic input to the ECNS is derived from two major autonomic ganglia located on the great vessels: the superior cervical ganglion (SCG) and the stellate ganglion (cervicothoracic ganglion). Both of these ganglia house the cell bodies of most post-ganglionic sympathetic neurons whose axons terminate on the surface of the heart. These axons form heterogeneous tracts, organizing themselves into superior, middle and inferior cardiac nerves as they travel from the cervical sympathetic trunk to innervate the heart8. Moreover, the left-to-right distribution of these nerve pairs is not symmetric, with at least three major left-sided cardiac nerves and four right-sided nerves that often possess significant inter-individual variability. Remarkably, the pattern of target innervation by these cardiac nerves gives rise to several fascinating electrophysiological properties. For example, differences in the spatial distribution of refractoriness are observed in the anterior vs. posterior ventricular myocardium based on whether sympathetic stimulation is achieved via the right vs. left cardiac nerves9-11. However, due to individual variability in the innervation pattern of the right and left cardiac nerves, the outcome of sympathetic stimulation from these cardiac nerves is not uniformly predictable in a simple left/right to anterior/posterior paradigm. In addition to the unpredictable innervation pattern of the major cardiac nerves, there is also wide variation in how other areas of the heart are innervated by different nerves of the ECNS. These nuances pose some hurdles to employing generalized nerve stimulation as a treatment strategy for various cardiac arrhythmias.
In contrast to the sympathetic nervous system, the parasympathetic component of the ECNS originates predominantly in the nucleus ambiguus and to a lesser extent in the dorsal motor nucleus of the medulla. The parasympathetic preganglionic fibers are carried almost entirely within the vagus nerve and descend to the heart. Unlike their sympathetic counterparts that form extra-cardiac synapses in cervical and thoracic ganglia, the efferent fibers of the cardiac parasympathetic system form synapses on the surface of the atria and send short postganglionic fibers into the sinus node, atrioventricular (AV) node and the atrial myocardium10.
In stark contrast to the relatively straightforward anatomy of the ECNS, the ICNS is more complex and its precise anatomy is still not completely known (Figure 1). Upon entering the pericardial space, the majority of sympathetic afferents either directly innervates the myocardium or form synapses with neurons that constitute the intrinsic cardiac ganglia. Parasympathetic fibers, in contrast, almost entirely synapse with neurons within the cardiac ganglia located in a number of locations throughout the heart. Of note, each cardiac ganglion may contain anywhere from 200-1000 neurons12,13, and the vast majority of these ganglia are organized into GPs on the surface of the atria and ventricles13. Some of these atrial plexi have been mapped to the superior right atrium (adjacent to the SA node), superior left atrium and posterior right atrium (adjacent to the AV node), but a large number of formally unmapped smaller atrial plexi also exist14. The pulmonary vein-left atrial (PV-LA) junction in particular has a confluence of GPs and receives innervation from both limbs of the autonomic nervous system. There is a fair amount of data that suggests AF can be induced by directly stimulating the GPs at the PV-LA junction15,16. Conversely, several studies in which the major GPs in the PV-LA junction and the PV ostia were ablated have achieved attenuation or extinction of pre-existing AF17-20. Some investigators, based on experimental evidence of this nature, have suggested that the atrial GPs serve as “integration units” which act cooperatively with components of the ECNS to manage crosstalk between the heart and the autonomic nervous system (ANS). Atrial GPs also retain the ability to act independently and modulate numerous cardiac functions, such as pacemaker automaticity, myocardial contractility and refractoriness. This body of growing evidence is helping to establish that GPs play an important role in the genesis and propagation of AF.
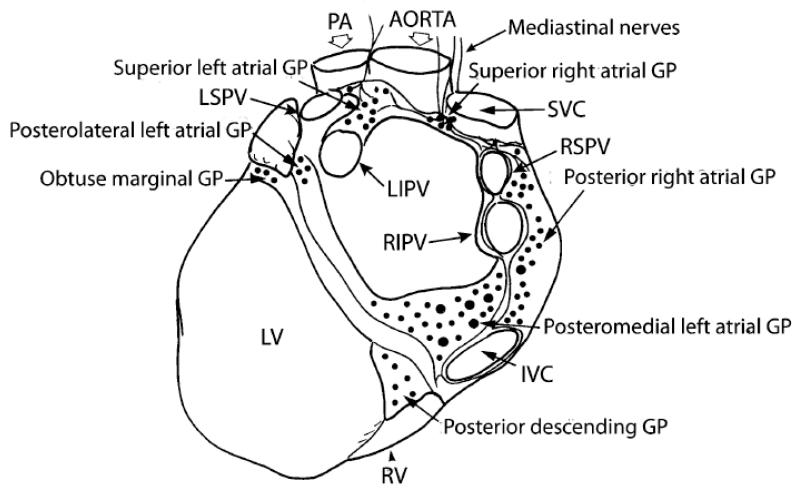
Figure 1. Ganglionated plexi in the human heart.
Shown is an illustration of the posterior aspect of the human heart and major vessels that shows the locations of the posterior atrial and ventricular ganglionated plexi. Note that the mediastinal nerves run adjacent to the aortic root and join the two superior atrial ganglionated plexi. The positions of the superior vena cava (SVC), inferior vena cava (IVC), right ventricle (RV), left ventricle (LV), right superior pulmonary vein (LSPV) are shown. Adopted with permission from Armour et al, Anat. Rec. 1997;247:289-298.
At the cellular level, modulation of afferent autonomic function occurs through a myriad of receptors including mechanoreceptors, baroreceptors and chemoreceptors located on the surface of the heart and great vessels. The afferents from these receptors communicate with the central nervous system, whose efferent output is received by the heart via adrenergic and muscarinic receptors in the myocardium. The interplay of this cast of receptors in the nervous system and heart integrate and facilitate the hemodynamic response to various cardiac stressors in a physiologically meaningful manner. For instance, the discharge pattern of atrial mechanosensors is a function of cardiac and respiratory cycles, with discharge rates increasing commensurate with rising wall tension that induces tachycardia and diuresis. This differs from ventricular mechanosensors, whose response to wall stress is one that results in bradycardia and hypotension.
Pulmonary Vein Isolation
The evolution of catheter-based ablation as an effective strategy for treating atrial fibrillation is rooted in our progressive understanding of its pathogenesis. Early work in this area suggested AF was initiated by multiple wavelets generated within the atria, and sustained by wavebreaks triggered by heterogeneous dispersion in repolarization21. In 1972, several independent investigators provided evidence contrary to this view, contending that the thoracic veins were crucial to the genesis of AF. In fact, these authors were the first to notice rich vagal innervation entering the myocardial sleeve of atrial tissue that extended into the thoracic veins22-24. However, one of the most important discoveries in the field of AF occurred in 1998 when Haïssaguerre and colleagues proposed that focal activity in the pulmonary veins was critical for the initiation and maintenance of AF in humans6. Indeed, this seminal work launched the technique of targeting pulmonary vein triggers as a therapeutic strategy for AF and provided the framework for the technique of pulmonary vein isolation (PVI) that is widely employed to treat AF today.
Of note, focal firing of the PV appears to require autonomic influences to convert the focal firing into AF25, and directly targeting focal sites within the PVs with radiofrequency (RF) energy was not effective as this approach was plagued by recurrent AF and PV stenosis26. The current approach of applying RF lesions outside the PV ostia was then adopted and has proven to be far more efficacious. This strategy employs circumferential RF lesions delivered between the focal source of PV firing and the atria in an attempt to electrically “isolate” the AF triggers to the PV from the substrate of AF propagation in the atria. While this approach works reasonably well it has also produced some interesting observations and insights into AF mechanisms. For example it was realized early on that complete PV isolation (PVI) was not crucial for successfully eliminating AF. In some cases delivering RF lesions partially across the PV ostia was adequate to abolish AF27,28. Moreover, firing from focal sites within the PV was seen to abruptly terminate after PVI suggesting that the atrium contributed to the maintenance of PV firing29. Electrophysiologists also observed that PVI had some unusual effects on heart rate variability, where delivery of RF energy sometimes induced transient bradycardia30. These observations, coupled with our prior knowledge about the connection of the ANS with AF initiation and propagation, implicated the cardiac nervous system in the genesis of AF.
The Cardiac Nervous System in Atrial Fibrillation
More than three decades ago, Coumel et al. published their classic account of the cardiac nervous system and first proposed that AF may have a nervous system trigger5. Coumel’s neural hypothesis was supported by two key clinical observations. First, a distinct subtype of atrial fibrillation that developed with bradycardia was identified and termed vagotonic AF or nocturnal AF. A second group of patients was also described whose AF triggers were the exact opposite to those with vagal AF. In this latter group, arrhythmogenesis was driven by high sympathetic tone induced by stressors such as emotional excess and exercise. Patients with vagotonic AF are often young, healthy individuals with no overt cardiac pathology, whereas patients with sympathetic AF were usually older, with multiple co-morbidities and structural heart disease31. However, these two patient populations are often difficult to differentiate from each other just based on clinical characteristics, since there is often significant overlap between those with vagally-mediated AF and adrenergically-driven AF. In subsequent investigations the question of whether AF was triggered by autonomic stimuli was intensely explored; and numerous reports and retrospective studies clearly supported Coumel’s theory32,33. In fact, the clinical literature is full of case reports of patients whose AF was triggered by vagal events, adrenergic triggers or a combination of both34-39. The major shortcoming in most of these accounts, however, was the absence of a strong causal relationship between the arrhythmia and autonomic dysregulation. In many of these reported cases, autonomic tone was established by reporting heart rate variability which is notoriously unreliable as the patient ages due to the increasing prevalence of co-morbidities and structural heart disease34. This is particularly true in patients with concomitant heart failure, in whom heart rate variability due to altered autonomic tone could be misinterpreted due to underlying sinus node dysfunction or the compensatory effects of heart failure itself40.
Large Animal Studies
Due to the aforementioned problems with studying the contribution of the autonomic system to AF triggers in patients, some of the most compelling data supporting this theory comes from the large animal laboratory where data can be obtained in a precisely controlled setting. Sharifov and colleagues demonstrated that infusing the parasympathetic neurotransmitter acetylcholine into the sinoatrial nodal artery was associated with induction of AF in a dog model41. Interestingly, these authors also found that co-infusion of the beta-adrenergic agonist isoproterenol decreases the threshold for acetylcholine induced AF in this model. This result suggests that not only was AF easily induced by simultaneous sympathovagal stimulation, but that the natural trigger was perhaps a balance between both limbs of the autonomic system. Indeed, experiments by Tan and colleagues with long-term implantable pacemakers and nerve recorders in dogs showed that simultaneous sympathovagal activity was the most frequent trigger of atrial ectopy42. Similarly, cryoablation of bilateral stellate ganglia and cardiac branches of the vagus nerve (eliminating sympathovagal stimulation altogether) eliminated paroxysmal AF induced by rapid atrial pacing43. Taking these experiments a step further, Shen et al, demonstrated that the pre-existing autonomic mileu dictates the propensity for AF induction in a large animal model, by demonstrating that a distinct baseline spatiotemporal pattern of sympathovagal activity is present in dogs that determines the vulnerability to AF induction by rapid atrial pacing. Specifically, these investigators showed that dogs had either a nonlinear (L-shaped) or linear sympathovagal correlation based on a scatter plot of activity of the stellate ganglion versus the vagus nerve. Dogs that possessed a linear sympathovagal correlation consistently had higher baseline vagal tone and a greater propensity for the onset of AF44.
Wijfels et al. and Jayachandran et al. expanded on the idea that pre-existing autonomic tone dictates susceptibility to AF and asked if the converse may also be true. In other words, can the presence of sustained AF invoke changes in the autonomic environment? To address this question, they utilized rapid atrial pacing to induce AF and study changes in the electrophysiologic and neuronal milieu in goats and dogs45,46. Over time, they noted that rapid atrial pacing induced sustained electrical and neural changes in the atria. Electrophysiologic changes in the atria were mostly due to abnormal calcium handling and an abnormally shortened atrial action potential. Neural changes induced by rapid atrial pacing, which were also striking, involved sprouting of new sympathetic axons associated with the PV and atria. Increased sympathetic innervation, in turn, was likely to increase autonomic stimulation with further electrical and neural remodeling of the atrium (Figure 2). Taken together, this cyclical setup suggested a fascinating mechanism for sustained AF progression and propagation over time, leading to the playful aphorism “Afib begets Afib”45.
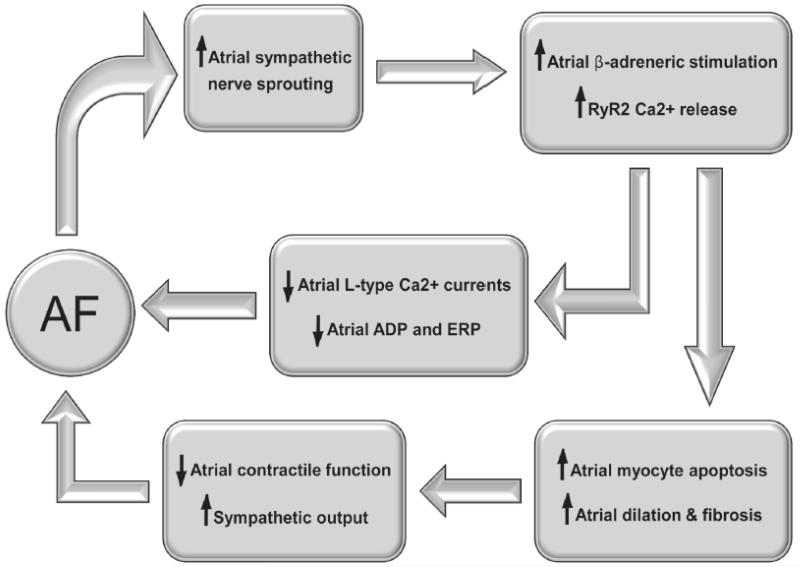
Figure 2. Cyclical Promotion of Atrial Fibrillation through Autonomic Stimulation.
Once the atrium begins to fibrillate (AF), rapid atrial rates promote increased sympathetic nerve sprouting which augments β–adrenergic tone leading to increased protein-kinase A (PKA) mediated hyperphosphorylation of atrial ryanodine receptors (RyR2). Increased RyR2 phosphorylation promotes sarcoplasmic reticular calcium release that induces calcium-mediated inhibition of voltage-dependent L-type calcium currents, leading to shortening of atrial myocyte action potential duration and effective refractory periods. This electrical remodeling promotes further AF and increased sympathetic nerve sprouting. In addition, increased atrial myocyte RyR2 calcium release induces myocyte apoptosis and fibrosis, which leads to atrial dilatation and structural remodeling that further increases the propensity for AF.
Despite compelling data that supports a role for the cardiac nervous system in the initiation and propagation of AF, it remains unclear which of the cardiac nervous system subdivisions may be involved in this process. For instance, it is not clear if both ECNS and ICNS activity is required for AF initiation, or if the ICNS functions independently of the ECNS to initiate AF. Choi and colleagues sought to address these questions by directly recording the electrical activity of ECNS and ICNS neurons in dogs with induced AF. Intermittent rapid atrial pacing reliably induced AF in these animals and the majority of episodes of AF were centrally mediated with associated bursts of activity in ECNS nerves (stellate ganglion, thoracic vagal nerve). Surprisingly, they also noted episodes of AF that were induced by isolated activity in the GPs (and ligament of Marshall) without concomitant activity in the ECNS47. These results suggest that although activity of the ECNS is required to induce most cases of AF, the arrhythmia could also be triggered autonomously from GPs of the ICNS without any higher input.
The possibility that autonomously firing GPs could trigger AF is exciting because it suggests a mechanism for increasing frequency of AF with advancing age. It is well demonstrated that increasing age drastically changes autonomic tone, perhaps in part due to the loss of autonomic neurons, reduced responsiveness to autonomic stimuli or a combination of the two. Smith et al. demonstrated that even weeks after denervation of the ECNS, GP neurons remain fully viable and retain their ability to increase neurotransmitter release48. Thus, it is conceivable that as a person ages there may be an increased proclivity for AF owing to autonomously functioning GPs in the absence of strong autonomic tone. Lo and colleagues tested this hypothesis by ablating the nexus point between the ECNS and ICNS in dogs. Over a period of 10 weeks the denervated dogs were monitored for the development of AF and there was a gradual rise in the AF burden of ablated dogs compared to sham-operated dogs. In fact, in dogs without denervation there was virtually no AF49. In human subjects, similar trends are seen in patients after cardiac transplantation. Noheria and colleagues showed post-cardiac transplant patients have substantially lower rates of post-operative AF compared to native heart patients who undergo surgical maze. While both these groups have pulmonary vein isolation in common, only post-transplant candidates had additional autonomic denervation of the heart50.
Cellular Substrates
Ganglionated plexi that innervate the atrium have pleiotropic effects both on the myocardium, and in turn, on the cardiac neurons themselves. But how the axonal termini in GP neurons communicate with cardiac tissue is not entirely known. Furthermore, it is not clear if there are specialized neurotransmitter receptors on cardiomyocytes, or if there are subpopulations of cells in the atria that either directly communicate with or modulate contact between these two cell types.
To address these questions let’s consider the cellular substrates that contribute to atrial fibrillation. The cellular substrates of atrial fibrillation have been an area of intense investigation in basic science. The earliest studies sought to better characterize the properties of the most obvious cellular target of the cardiac nervous system – the cardiomyocyte. The observation that β-adrenergic receptors (sympathetic) and M2-type muscarinic receptors (parasympathetic) are enriched in atrial and PV myocytes suggested these cells may directly communicate with the ICNS51,52. While these observations suggest cardiomyocytes interact with the ICNS, the actual role of cardiomyocytes in the genesis of AF is probably more complex than as just simple neurotransmitter receptor docks for the autonomic nervous system. Depending on the age and environment of the host, cardiomyocytes can respond in a myriad of different ways to the same neural stimulus53. Part of this complexity at the cellular level may be the result of atrial remodeling that leads to atrial fibrosis and changes in cell signaling, such as calcium handling. For example abnormal calcium release from the sarcoplasmic reticulum through the major calcium-release channel known as the ryanodine receptor, results in the phenomenon of calcium alternans which is a pathologic state where there is beat-to-beat variation in calcium currents54. Calcium alternans is a highly arrhythmogenic state that is mediated by loss of functional integrity of the ryanodine receptor and/or the voltage-dependent L-type calcium channel in the cell membrane55. In addition, there is mounting evidence that G-protein coupled receptors, particularly G-stimulatory (Gs)-proteins, can induce phosphorylation of the ryanodine receptor through protein-kinase A (PKA) that results in permanent calcium mishandling and alternans56. Not surprisingly, one of the most potent stimulators of Gs-proteins is the β-adrenergic receptor that is directly under the influence of the sympathetic nervous system. In pathologic states, such as heart failure, not only is sympathetic tone elevated but PKA-dependent hyperphosphorylation of the ryanodine receptor may also exist. Continued, excessive phosphorylation of ryanodine receptors is probably one mechanism by which patients with longstanding heart failure are more prone to developing AF57.
Despite substantial evidence that suggests cardiomyocytes contribute to the origin of AF in response to altered autonomic tone, these cells are not likely to be the only ones involved in the genesis of AF. The possibility that AF is mediated by subsets of specialized cells is gaining popularity and most of the research that connects a specific cell population with AF comes from animal studies. However, the existence of analogous cell populations in similar anatomic distributions in the human heart provides a rational basis for placing these discoveries in a clinically relevant context.
A few years after Cheung et al. described that isolated PV tissue possessed pacemaker activity58, Fumiaki Masani described the first sub-population of specialized cells in the human atrium, PV-LA junction and PV tissue that could explain this autonomous activity. Ultrastructurally, these cells were nearly identical to sinus node cells and possessed many of the histological characteristics of cardiac pacemaker cells. Furthermore, each of these nodal-like cells was found to be juxtaposed with neurons from the ICNS59. Chou and colleagues further advanced the characterization of these cells by invoking voltage-independent triggered ectopy in Langendorf-perfused canine hearts in the presence of ryanodine and isoproterenol. As atrial arrhythmias were provoked, these authors identified nodal-like clear cells clustered in the endocardium adjacent to sites from which frequent focal discharges were mapped. In addition, histological analysis of these cells revealed they were enriched with glycogen that robustly labeled with periodic acid-Shiff (PAS) stain, similar to Purkinje cells in the cardiac conduction system60. Perez-Lugones et al. found a compelling association between the presence of these PAS-positive cells in the PV tissue and the incidence of AF by clinical histories associated with human autopsy specimens. Samples from four patients with a history of AF had the classic PAS-positive sinus node-like cells in their PV tissue. By comparison, no such cells were discovered in the PVs of five transplanted hearts from patients without a history of AF61.
Recent work has uncovered an additional population of atrial cells that resemble the pacemaker cells of the gastrointestinal tract. Interstitial Cajal Cells (ICCs) are the primary pacemaker cells that mediate peristalsis and motility of the alimentary tract in response to autonomic input. These cells have a unique property of generating and propagating slow waves of electrical activity that drives smooth muscle contraction in the gastrointestinal tract. Interestingly, a peculiar sub-population of Interstitial Cajal-Like Cells (ICLCs) localized to the PV-LA junction and PV that is histologically similar to ICCs has been identified. Morel et al. described a correlation between the presence of ICLCs in the PV and clinical AF62. A report by Gherghiceanu et al. further strengthens the association between AF and ICLCs without any discrete evidence for whether these cells contribute to atrial arrhythmias63. While evidence that ICLCs directly contribute to AF is lacking, the fact that ICCs are well characterized in the gastrointestinal system and known to possess rhythmic pacemaker activity makes it reasonable to infer that ICLCs serve a similar role in the atrium. However, ICCs in the gut and ICLCs differ in one very important electrophysiologic feature, which is their response time. This distinction is important since the induction of AF requires response times that are several orders of magnitude faster than the response time of ICCs. Nevertheless, whether ICLCs possess electrophysiologic properties that differ from their gastrointestinal brethren still remains to be determined. As new data emerges insight into the role of these cells in the cardiovascular system, and their connections to the autonomic nervous system will likely improve.
Perhaps the newest addition to the growing family of specialized atrial cells comes from our own laboratory. We have described a unique pool of melanocyte-like cells localized in atrial tissue, the PV-LA junctions and the PVs which are sites from which AF triggers commonly originate. Like ICLCs and PAS-positive nodal cells, melanocyte-like cells are electrically excitable and appear to be connected to the ICNS. However, more importantly these cells can generate action potentials with the time course and speed sufficient to trigger AF. Immunohistochemically, melanocyte-like cells in the heart are notable for their expression of dopachrome tautomerase (DCT), a melanin synthesis enzyme that is involved in the regulation of intracellular calcium and scavenging of reactive oxygen species including melanin-synthesis byproducts. Due to the fact these cells express melanin synthesis enzymes and morphologically resemble dermal melanocytes, we called this novel cell population cardiac melanocytes. Interestingly, cardiac melanocytes express both adrenergic and muscarinic receptors and are located in close apposition to autonomic nerve terminals of the ICNS (Figure 3). Mice harboring germline deletion of Dct (Dct−/−) are more susceptible to atrial arrhythmias and we showed that this property was cell autonomous. Mice with mutations of the tyrosine kinase receptor c-kit have migratory defects in melanocytes and c-kit mutant mice actually lack cardiac melanocytes within their atria. Remarkably, Dct−/− mice bred on a c-kit mutant background were resistant to atrial arrhythmias suggesting that it was the cardiac melanocyte itself, and not just the lack of DCT that was responsible for atrial arrhythmogenesis64. This finding opens up a number of interesting questions, some of which include delineating how the ICNS influences the electrical properties of these cells and how cardiac melanocytes communicate with atrial myocytes. Whether this unique melanocyte-like population works independently, or in concert with other subpopulations of specialized conduction tissues also remains to be understood.
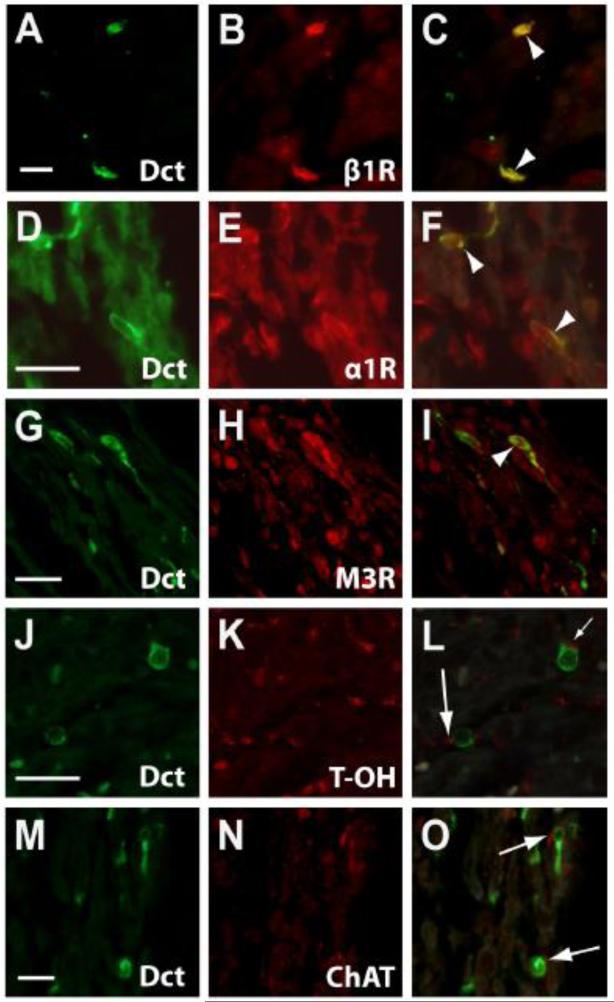
Figure 3. Dct-expressing Cells in the Atrium Co-express Autonomic Receptors and are Near Autonomic Nerve Terminals.
Immunohistochemistry within the adult mouse atrium using an antibody to Dct demonstrates Dct-positive cells with characteristic morphology (A, D, G, J, and M). Sections were co-stained with antibodies to β1-adrenergic receptor (β1R; B), α1-adrenergic receptor (α1R; E), muscarinic receptor subtype 3 (M3R; H), tyrosine hydroxylase (T-OH; K), and choline acetyltransferase (ChAT; N). Merged images of Dct and each respective antibody are shown, demonstrating co-expression of Dct with β1R (C), α1R (F), and M3R (I) receptors (arrowheads). In addition, presumptive sympathetic nerve terminals that express tyrosine hydroxylase (L) and parasympathetic nerve terminals that express choline acetyltransferase (O) were seen in close proximity to Dct-expressing cells (arrows). Scale bars: 20 μm. Reproduced with permission from Levin et al. J Clin Invest. 119, 3420-3436 (2009).
Therapeutic Strategies
Since the first description of PV isolation as a feasible therapeutic strategy for drug-refractory AF, there has been considerable interest in adjunctive therapies that may help enhance the success of this procedure. In addition to the circumferential lesions delivered during a standard PVI, linear lesions that traverse areas with high GP density have also been placed to improve procedural success rates65. The natural inference from this association is that the success of PVI may be indirectly due to coincidental modification of nervous inputs to the atrium19,66. Based on this provocative idea, a number of investigators have proposed that autonomic denervation might be a suitable strategy for AF ablation either in isolation, or in concert with standard PVI67-70. Yet, investigations into this approach have yielded mixed results. Platt and colleagues were the first to attempt selective GP ablation as a therapeutic strategy for AF. These investigators delivered high frequency stimuli to nerve clusters at the PV-LA junction and showed a marked slowing of the ventricular response in patients with AF. Complete ablation of these GPs terminated AF in nearly 90% of patients with persistent AF and resulted in 96% freedom from AF during a 6-month follow-up period71. Scherlag et al. similarly showed that ablation of GPs in addition to PVI increases ablation success from 70% to 91% in patients with paroxysmal or persistent AF up to 1 year later70. However, other studies have been less optimistic regarding this approach. Lemery et al. found that only 50% of patients who underwent combined GP ablation and PVI were free from AF during an 8-month follow-up period72. These conflicting reports with regards to the success rate of GP ablation, with or without PVI, imply that interactions between the ICNS and AF are very complex. An obvious explanation for this variability is technical differences amongst the studies, or the heterogeneity of AF substrates themselves. For example, Scanavacca and colleagues used high-frequency stimulation induced vagal reflexes to identify atrial sites that may be amenable to GP ablation. Out of ten patients with presumed vagal AF, vagal responses were evoked in only seven patients after high-frequency stimulation, and of these only two remained free from AF after GP ablation73. These findings highlight the complexity inherent in the genesis of AF amongst different patients and the difficulty in using a uniform strategy such as high-frequency stimulation to identify and treat even a single entity like vagal AF. In addition, there may be other technical differences such the method by which GPs were identified, either by RF stimulation or anatomic evaluation which could affect the outcome; as does the completeness of GP ablation since plexi close to the phrenic nerve, for example, were not ablated in most studies74-76.
An alternative approach to GP ablation is complete denervation by severing ECNS input to the heart. In large animal studies, partial vagal denervation of the high right atrium decreased AF inducibility75, and sympathetectomy via denervation of the stellate ganglion was shown to reduce atrial tachyarrhythmias42. Based on these and other studies, autonomic denervation has the potential to be a more effective strategy for treating AF than GP ablation, which can be challenging due to poor anatomic localization of the ICNS. Despite its promising outlook, autonomic denervation is neither simple nor uniformly sustainable. The challenges inherent in this approach are primarily due to adaptive mechanisms of the nervous system that counteract most attempts at inducing denervation. Most of the autonomic nerves that innervate the heart are highly resilient peripheral nerves that can rapidly regrow in response to denervation. In vivo these nerves do so under the influence of target-derived trophic signals such as nerve growth factor (NGF), which when over-expressed leads to sustained survival and rapid axonal sprouting of sympathetic and sensory neurons 77,78. It has been shown that RF ablation in the vicinity of the GPs can stimulate massive neuronal hyperinnervation; and building upon this observation Kangavari et al. demonstrated there is a concomitant elevation in the expression of NGF in the peripheral veins after RF ablation79. Thus, in this situation denervation is only a temporary solution with rapid hyperinnervation leading to stronger autonomic neurotransmitter release that promotes atrial arrhythmogenesis. In fact, vagal tone can be restored to near normal range within just four weeks of ablation80.
As an alternative therapy for PV isolation, targeting sites with Complex Fractionated Atrial Electrograms (CFAE)s was first suggested by Nademanee and colleagues81. CFAEs are regions of complex atrial electrical activity that are thought to correspond to areas where pivot points for reentrant wavelets or areas of transient conduction block occur. Based on observational experimental data, these complex regions are assumed to represent AF substrate82. Verma et al. described an algorithm for automated CFAE identification and showed that ablating CFAEs alone terminated AF in 55% of patients and that using CFAE ablation as an adjunctive, rather than alternative therapy to PVI, provides an incremental increase in procedural success rates83. Other studies have further provided proof for this hypothesis with increasing frequency84,85. Still, the mechanisms underlying the origin of CFAEs and what role they play in the genesis of AF is not precisely known. One compelling thought is that CFAEs occur in the vicinity of anatomic GPs, and that the neural communication from the autonomic nervous system directly impacts the electrical activity of the atria17,86. Work by Katritsis and colleagues have lent credence to this hypothesis. Not only do anatomic GP sites cluster at locations where CFAEs occur with increased frequency, but also patients in whom CFAEs were undetectable at GP sites did not generate CFAEs elsewhere in the atria86. In addition to the data supporting CFAEs localizing to regions of atrial innervation, there are other elegant electrophysiologic strategies to identify anatomic substrates of AF. Pachon et al. used Fast Fourier Transform (FFT) to identify two distinct types of atrial myocardium based on frequency-domain analysis of atrial potentials87. These authors identified a compact myocardium that consisted of normal in-phase conduction, suggesting highly compact cellular connections, and fibrillar myocardium consisting of anisotropic out-of-phase conduction inferring a mixture of muscle and “fibrillar” tissue including neural, vascular and pathological tissue. Interestingly, during atrial fibrillation episodes there are clusters of fibrillar myocardium that form “AF nests” which have higher frequencies than the surrounding atrium tissue. Hypothesizing that targeting these “AF nests” in the fibrillar myocardium with RF ablation could terminate AF, these authors treated 35 patients and eliminated AF in all but two of them over a follow-up period of 9 months. While these findings are interesting, the actual data that links CFAEs or the “fibrillar myocardium” to nervous system activity and the autonomic basis of AF is still sparse.
Novel Therapies
New therapeutic strategies that may exploit some of the aforementioned principles of AF genesis have generated considerable excitement in electrophysiology. However, these strategies are still limited to the basic science laboratory and have not been studied in humans in any meaningful fashion. Foremost among these is the potential use of vagal nerve stimulators to control AF. The origin of this idea comes from observations that low-level vagal activation suppresses ectopy within the PV and LA and thereby inhibits AF initiation. Li and colleagues explored this phenomenon in anesthetized dogs and discovered that low-level vagosympathetic nerve stimulation at a voltage only 10% below the threshold for inducing bradycardia could efficiently suppress atrial fibrillation induced by rapid atrial pacing88. Mechanistically, a gradual prolongation of the atrial effective refractory period is purported to induce this phenomenon since rapid atrial pacing without concurrent vagosympathetic stimulation causes a progressive shortening of the atrial refractory period with an increase in AF susceptibility20,89. Furthermore, these findings have been extended to demonstrate that nearly equivalent results could be achieved by placing the stimulating catheter in the superior vena cava without surgically exposing the nerve trunks90. This strategy, notwithstanding its potential elegance, has not been validated beyond animal models but does demonstrate a novel non-pharmacologic, non-ablative therapy for AF that may be clinically useful someday.
The idea that implantable nerve stimulators could be the future of refractory AF treatment may seem far-fetched today, however pharmacologic therapies that impinge upon the nexus between the nervous system and the cardiovascular system is likely to be adopted with less resistance. Among these drugs is botulinum toxin, which, like a myriad of other medical specialties may finally have found a niche in cardiology. The idea that local botulinum toxin injection can cause denervation is certainly not novel. Oh et al. performed local injection of botulinum-toxin into the epicardial fat pads in an attempt to mitigate rapid atrial pacing induced AF in anesthetized dogs. In doing so they were able to achieve transient autonomic denervation, thereby raising the threshold for AF induction91,92. While this approach may sound intriguing, the problem is that nerve termini denervated by botulinum toxin often rapidly re-grow over time, which would necessitate frequent drug injections to attain any meaningful therapeutic effect.
Similarly, the cardiac peptide molecule vasostatin-1 has garnered interest as a candidate molecule for AF suppression by inducing chemical denervation. Vasostatin-1 is released by autonomic nerve stimulation and has potent anti-adrenergic effects93,94. Preliminary experiments by Stavrakis et al. showed vasostatin injection into the cardiac fat pads successfully diminished AF susceptibility95. Further experiments are clearly needed before this molecule is ready for clinical studies, but the ability of this single peptide to achieve such a level of efficacy raises interesting questions about its mechanism of action. Whether vasostatin reduces AF by lengthening the atrial refractory period, suppressing early afterdepolarizations or both is currently unknown.
Lastly, there are a number of other potential therapeutic targets of AF which include the use of peptides that modulate signal transduction via adrenergic and muscarinic receptors. For instance, Aistrup et al. demonstrated that attenuation of vagosympathetic signaling and AF suppression could be achieved by disrupting G-protein coupled receptor signaling between the ICNS and the atrial myocardium. To show this these investigators experimentally injected an inhibitory G-protein alpha (Gαi) C-terminal peptide (believed to disrupt the interaction between cardiac M2-muscarinic receptors and activation of its downstream G-protein Gαi) into the LA in a targeted fashion. This approach not only reproducibly diminished vagal responsiveness throughout the left atrium, but also achieved sustained inhibition of vagotonic AF96,97.
Conclusion
In this review, we have sought to emphasize the mechanistic underpinnings of atrial fibrillation with respect to the autonomic nervous system and local innervation of the heart. A number of studies from both the basic science laboratory and clinical investigations have provided compelling evidence that implicate abnormal autonomic reactivity in the genesis and propagation of AF. Indeed, as highlighted in this article, local innervation-induced changes in the electrophysiological milieu of the atrial myocardium can itself act as an impetus for further propagation of sustained AF. While the exact mechanism of this interaction is not known, emerging data suggests neural communication with the cardiovascular system may be achieved via the autonomic nervous system. These effects may be induced by directly stimulating cardiac myocytes or key non-cardiomyocyte intermediaries such as cardiac melanocytes, which in turn induce changes in signal transduction and calcium handling in cardiomyocytes. The disruption of neural regulation of myocardial arrhythmogenesis may prove to be a compelling mechanism for treatment of refractory AF. A number of strategies are actively being explored at this time which includes direct ablation of ganglionated plexi, pharmacologic and surgical autonomic denervation, implantable stimulators and small molecule inhibitors. If any of these strategies will successfully transition from the laboratory to the bedside as clinically useful therapies for the treatment of refractory AF remains to be seen.
Acknowledgments
We apologize to the many investigators whose work in this area we could not mention due to space limitations.
Funding Sources: Investigations in Dr. Patel’s laboratory are supported by grants from the National Institutes of Health (HL105734, HL114110). Dr. Patel is also an Innovative Researcher of the American Heart Association supported by award 11IRG4930008.
Footnotes
Journal Subject Codes: Etiology:[5] Arrhythmias, clinical electrophysiology, drugs, Basic science research:[130] Animal models of human disease, Basic science research:[132] Arrhythmias - basic studies
Conflict of Interest Disclosures: None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lip GYH, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142:1489–1498. doi: 10.1378/chest.11-2888. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Chen P-S, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd-Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TSM, Van Wagoner DR, Waldo AL, Wyse DG. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop; 2009; pp. 606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am. J. Cardiol. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Heist EK, Chalhoub F, Barrett C, Danik S, Ruskin JN, Mansour M. Predictors of atrial fibrillation termination and clinical success of catheter ablation of persistent atrial fibrillation. Am. J. Cardiol. 2012;110:545–551. doi: 10.1016/j.amjcard.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Coumel P, Attuel P, Lavallée J, Flammang D, Leclercq JF, Slama R. [The atrial arrhythmia syndrome of vagal origin] Arch Mal Coeur Vaiss. 1978;71:645–656. [PubMed] [Google Scholar]
- 6.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 7.Armour JA. Cardiac neuronal hierarchy in health and disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R262–71. doi: 10.1152/ajpregu.00183.2004. [DOI] [PubMed] [Google Scholar]
- 8.Kapa S, Venkatachalam KL, Asirvatham SJ. The autonomic nervous system in cardiac electrophysiology: an elegant interaction and emerging concepts. Cardiol Rev. 2010;18:275–284. doi: 10.1097/CRD.0b013e3181ebb152. [DOI] [PubMed] [Google Scholar]
- 9.Smith DC. Synaptic sites in sympathetic and vagal cardioaccelerator nerves of the dog. Am. J. Physiol. 1970;218:1618–1623. doi: 10.1152/ajplegacy.1970.218.6.1618. [DOI] [PubMed] [Google Scholar]
- 10.Van Stee EW. Autonomic innervation of the heart. Environ. Health Perspect. 1978;26:151–158. doi: 10.1289/ehp.7826151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randall WC, Armour JA, Geis WP, Lippincott DB. Regional cardiac distribution of the sympathetic nerves. Fed. Proc. 1972;31:1199–1208. [PubMed] [Google Scholar]
- 12.Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat. Rec. 2000;259:353–382. doi: 10.1002/1097-0185(20000801)259:4<353::AID-AR10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 13.Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat. Rec. 1997;247:289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Johnson PI, Lee RE, Orfei E, Lonchyna VA, Sullivan HJ, Montoya A, Tran H, Wehrmacher WH, Wurster RD. Topography of cardiac ganglia in the adult human heart. J. Thorac. Cardiovasc. Surg. 1996;112:943–953. doi: 10.1016/S0022-5223(96)70094-6. [DOI] [PubMed] [Google Scholar]
- 15.Lim PB, Malcolme-Lawes LC, Stuber T, Wright I, Francis DP, Davies DW, Peters NS, Kanagaratnam P. Intrinsic cardiac autonomic stimulation induces pulmonary vein ectopy and triggers atrial fibrillation in humans. J. Cardiovasc. Electrophysiol. 2011;22:638–646. doi: 10.1111/j.1540-8167.2010.01992.x. [DOI] [PubMed] [Google Scholar]
- 16.Lim PB, Malcolme-Lawes LC, Stuber T, Kojodjojo P, Wright IJ, Francis DP, Wyn Davies D, Peters NS, Kanagaratnam P. Stimulation of the intrinsic cardiac autonomic nervous system results in a gradient of fibrillatory cycle length shortening across the atria during atrial fibrillation in humans. J. Cardiovasc. Electrophysiol. 2011;22:1224–1231. doi: 10.1111/j.1540-8167.2011.02097.x. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Scherlag BJ, Zhou J, Lu Z, Patterson E, Jackman WM, Lazzara R, Po SS. Autonomic mechanism to explain complex fractionated atrial electrograms (CFAE) J. Cardiovasc. Electrophysiol. 2007;18:1197–1205. doi: 10.1111/j.1540-8167.2007.00976.x. [DOI] [PubMed] [Google Scholar]
- 18.Lin J, Scherlag BJ, Lu Z, Zhang Y, Liu S, Patterson E, Jackman WM, Lazzara R, Po SS. Inducibility of atrial and ventricular arrhythmias along the ligament of marshall: role of autonomic factors. J. Cardiovasc. Electrophysiol. 2008;19:955–962. doi: 10.1111/j.1540-8167.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- 19.Lemola K, Chartier D, Yeh Y-H, Dubuc M, Cartier R, Armour A, Ting M, Sakabe M, Shiroshita-Takeshita A, Comtois P, Nattel S. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation. 2008;117:470–477. doi: 10.1161/CIRCULATIONAHA.107.737023. [DOI] [PubMed] [Google Scholar]
- 20.Lu Z, Scherlag BJ, Lin J, Niu G, Fung K-M, Zhao L, Ghias M, Jackman WM, Lazzara R, Jiang H, Po SS. Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ Arrhythm Electrophysiol. 2008;1:184–192. doi: 10.1161/CIRCEP.108.784272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moe GK. A conceptual model of atrial fibrillation. J Electrocardiol. 1968;1:145–146. doi: 10.1016/s0022-0736(68)80020-2. [DOI] [PubMed] [Google Scholar]
- 22.Zipes DP, Knope RF. Electrical properties of the thoracic veins. Am. J. Cardiol. 1972;29:372–376. doi: 10.1016/0002-9149(72)90533-4. [DOI] [PubMed] [Google Scholar]
- 23.Scherlag BJ, Yeh BK, Robinson MJ. Inferior interatrial pathway in the dog. Circ. Res. 1972;31:18–35. doi: 10.1161/01.res.31.1.18. [DOI] [PubMed] [Google Scholar]
- 24.Spach MS, Barr RC, Jewett PH. Spread of excitation from the atrium into thoracic veins in human beings and dogs. Am. J. Cardiol. 1972;30:844–854. doi: 10.1016/0002-9149(72)90009-4. [DOI] [PubMed] [Google Scholar]
- 25.Scherlag BJ, Yamanashi W, Patel U, Lazzara R, Jackman WM. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. Journal of the American College of Cardiology. 2005;45:1878–1886. doi: 10.1016/j.jacc.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 26.Robbins IM, Colvin EV, Doyle TP, Kemp WE, Loyd JE, McMahon WS, Kay GN. Pulmonary vein stenosis after catheter ablation of atrial fibrillation. Circulation. 1998;98:1769–1775. doi: 10.1161/01.cir.98.17.1769. [DOI] [PubMed] [Google Scholar]
- 27.Stabile G, Turco P, La Rocca V, Nocerino P, Stabile E, De Simone A. Is pulmonary vein isolation necessary for curing atrial fibrillation? Circulation. 2003;108:657–660. doi: 10.1161/01.CIR.0000086980.42626.34. [DOI] [PubMed] [Google Scholar]
- 28.Lemola K, Oral H, Chugh A, Hall B, Cheung P, Han J, Tamirisa K, Good E, Bogun F, Pelosi F, Morady F. Pulmonary vein isolation as an end point for left atrial circumferential ablation of atrial fibrillation. Journal of the American College of Cardiology. 2005;46:1060–1066. doi: 10.1016/j.jacc.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 29.Oral H, Ozaydin M, Tada H, Chugh A, Scharf C, Hassan S, Lai S, Greenstein R, Pelosi F, Knight BP, Strickberger SA, Morady F. Mechanistic significance of intermittent pulmonary vein tachycardia in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2002;13:645–650. doi: 10.1046/j.1540-8167.2002.00645.x. [DOI] [PubMed] [Google Scholar]
- 30.Tsai CF, Chen SA, Tai CT, Chiou CW, Prakash VS, Yu WC, Hsieh MH, Ding YA, Chang MS. Bezold-Jarisch-like reflex during radiofrequency ablation of the pulmonary vein tissues in patients with paroxysmal focal atrial fibrillation. J. Cardiovasc. Electrophysiol. 1999;10:27–35. doi: 10.1111/j.1540-8167.1999.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 31.Coumel P. Autonomic influences in atrial tachyarrhythmias. J. Cardiovasc. Electrophysiol. 1996;7:999–1007. doi: 10.1111/j.1540-8167.1996.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 32.Zimmermann M, Kalusche D. Fluctuation in autonomic tone is a major determinant of sustained atrial arrhythmias in patients with focal ectopy originating from the pulmonary veins. J. Cardiovasc. Electrophysiol. 2001;12:285–291. doi: 10.1046/j.1540-8167.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- 33.Dimmer C, Tavernier R, Gjorgov N, Van Nooten G, Clement DL, Jordaens L. Variations of autonomic tone preceding onset of atrial fibrillation after coronary artery bypass grafting. Am. J. Cardiol. 1998;82:22–25. doi: 10.1016/s0002-9149(98)00231-8. [DOI] [PubMed] [Google Scholar]
- 34.Fioranelli M, Piccoli M, Mileto GM, Sgreccia F, Azzolini P, Risa MP, Francardelli RL, Venturini E, Puglisi A. Analysis of heart rate variability five minutes before the onset of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 1999;22:743–749. doi: 10.1111/j.1540-8159.1999.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 35.Tomita T, Takei M, Saikawa Y, Hanaoka T, Uchikawa S-I, Tsutsui H, Aruga M, Miyashita T, Yazaki Y, Imamura H, Kinoshita O, Owa M, Kubo K. Role of autonomic tone in the initiation and termination of paroxysmal atrial fibrillation in patients without structural heart disease. J. Cardiovasc. Electrophysiol. 2003;14:559–564. doi: 10.1046/j.1540-8167.2003.02462.x. [DOI] [PubMed] [Google Scholar]
- 36.Coccagna G, Capucci A, Bauleo S, Boriani G, Santarelli A. Paroxysmal atrial fibrillation in sleep. Sleep. 1997;20:396–398. doi: 10.1093/sleep/20.6.396. [DOI] [PubMed] [Google Scholar]
- 37.Herweg B, Dalal P, Nagy B, Schweitzer P. Power spectral analysis of heart period variability of preceding sinus rhythm before initiation of paroxysmal atrial fibrillation. Am. J. Cardiol. 1998;82:869–874. doi: 10.1016/s0002-9149(98)00494-9. [DOI] [PubMed] [Google Scholar]
- 38.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753–2759. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- 39.Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. Journal of the American College of Cardiology. 2003;42:1262–1268. doi: 10.1016/s0735-1097(03)00955-0. [DOI] [PubMed] [Google Scholar]
- 40.Piccirillo G, Ogawa M, Song J, Chong VJ, Joung B, Han S, Magrì D, Chen LS, Lin S-F, Chen P-S. Power spectral analysis of heart rate variability and autonomic nervous system activity measured directly in healthy dogs and dogs with tachycardia-induced heart failure. Heart Rhythm. 2009;6:546–552. doi: 10.1016/j.hrthm.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. Journal of the American College of Cardiology. 2004;43:483–490. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 42.Tan AY, Zhou S, Ogawa M, Song J, Chu M, Li H, Fishbein MC, Lin S-F, Chen LS, Chen P-S. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogawa M, Tan AY, Song J, Kobayashi K, Fishbein MC, Lin S-F, Chen LS, Chen P-S. Cryoablation of stellate ganglia and atrial arrhythmia in ambulatory dogs with pacing-induced heart failure. Heart Rhythm. 2009;6:1772–1779. doi: 10.1016/j.hrthm.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen MJ, Choi E-K, Tan AY, Han S, Shinohara T, Maruyama M, Chen LS, Shen C, Hwang C, Lin S-F, Chen P-S. Patterns of baseline autonomic nerve activity and the development of pacing-induced sustained atrial fibrillation. Heart Rhythm. 2011;8:583–589. doi: 10.1016/j.hrthm.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 46.Jayachandran JV, Sih HJ, Winkle W, Zipes DP, Hutchins GD, Olgin JE. Atrial fibrillation produced by prolonged rapid atrial pacing is associated with heterogeneous changes in atrial sympathetic innervation. Circulation. 2000;101:1185–1191. doi: 10.1161/01.cir.101.10.1185. [DOI] [PubMed] [Google Scholar]
- 47.Choi E-K, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, Piccirillo G, Frick K, Fishbein MC, Hwang C, Lin S-F, Chen P-S. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–2623. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith FM, McGuirt AS, Hoover DB, Armour JA, Ardell JL. Chronic decentralization of the heart differentially remodels canine intrinsic cardiac neuron muscarinic receptors. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H1919–30. doi: 10.1152/ajpheart.2001.281.5.H1919. [DOI] [PubMed] [Google Scholar]
- 49.Lo L-W, Scherlag BJ, Chang H-Y, Lin Y-J, Chen S-A, Po SS. Paradoxical Long-Term Proarrhythmic Effects after Ablating the “Head Station” Ganglionated Plexi of the Vagal Innervation to the Heart. Heart Rhythm. 2013;10:751–757. doi: 10.1016/j.hrthm.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 50.Noheria A, Patel SM, Mirzoyev S, Madhavan M, Friedman PA, Packer DL, Daly RC, Kushwaha SS, Edwards BS, Asirvatham SJ. Decreased postoperative atrial fibrillation following cardiac transplantation: the significance of autonomic denervation. Pacing Clin Electrophysiol. 2013;36:741–747. doi: 10.1111/pace.12102. [DOI] [PubMed] [Google Scholar]
- 51.Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) 3rd edition. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Löffelholz K, Pappano AJ. The parasympathetic neuroeffector junction of the heart. Pharmacol. Rev. 1985;37:1–24. [PubMed] [Google Scholar]
- 53.Boknik P, Grote-Wessels S, Barteska G, Jiang M, Müller FU, Schmitz W, Neumann J, Birnbaumer L. Genetic disruption of G proteins, G(i2)alpha or G(o)alpha, does not abolish inotropic and chronotropic effects of stimulating muscarinic cholinoceptors in atrium. Br. J. Pharmacol. 2009;158:1557–1564. doi: 10.1111/j.1476-5381.2009.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Díaz ME, O’Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ. Res. 2004;94:650–656. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- 55.Hove-Madsen L, Llach A, Bayes-Genís A, Roura S, Rodriguez Font E, Arís A, Cinca J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110:1358–1363. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- 56.Vest JA, Wehrens XHT, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR, Marks AR. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- 57.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 58.Cheung DW. Electrical activity of the pulmonary vein and its interaction with the right atrium in the guinea-pig. J. Physiol. (Lond.) 1981;314:445–456. doi: 10.1113/jphysiol.1981.sp013718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masani F. Node-like cells in the myocardial layer of the pulmonary vein of rats: an ultrastructural study. J. Anat. 1986;145:133–142. [PMC free article] [PubMed] [Google Scholar]
- 60.Chou C-C, Nihei M, Zhou S, Tan A, Kawase A, Macias ES, Fishbein MC, Lin S-F, Chen P-S. Intracellular calcium dynamics and anisotropic reentry in isolated canine pulmonary veins and left atrium. Circulation. 2005;111:2889–2897. doi: 10.1161/CIRCULATIONAHA.104.498758. [DOI] [PubMed] [Google Scholar]
- 61.Perez-Lugones A, McMahon JT, Ratliff NB, Saliba WI, Schweikert RA, Marrouche NF, Saad EB, Navia JL, McCarthy PM, Tchou P, Gillinov AM, Natale A. Evidence of specialized conduction cells in human pulmonary veins of patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2003;14:803–809. doi: 10.1046/j.1540-8167.2003.03075.x. [DOI] [PubMed] [Google Scholar]
- 62.Morel E, Meyronet D, Thivolet-Bejuy F, Chevalier P. Identification and distribution of interstitial Cajal cells in human pulmonary veins. Heart Rhythm. 2008;5:1063–1067. doi: 10.1016/j.hrthm.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 63.Gherghiceanu M, Hinescu ME, Andrei F, Mandache E, Macarie CE, Faussone-Pellegrini M-S, Popescu LM. Interstitial Cajal-like cells (ICLC) in myocardial sleeves of human pulmonary veins. J. Cell. Mol. Med. 2008;12:1777–1781. doi: 10.1111/j.1582-4934.2008.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levin MD, Lu MM, Petrenko NB, Hawkins BJ, Gupta TH, Lang D, Buckley PT, Jochems J, Liu F, Spurney CF, Yuan LJ, Jacobson JT, Brown CB, Huang L, Beermann F, Margulies KB, Madesh M, Eberwine JH, Epstein JA, Patel VV. Melanocyte-like cells in the heart and pulmonary veins contribute to atrial arrhythmia triggers. J. Clin. Invest. 2009;119:3420–3436. doi: 10.1172/JCI39109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bauer A, Deisenhofer I, Schneider R, Zrenner B, Barthel P, Karch M, Wagenpfeil S, Schmitt C, Schmidt G. Effects of circumferential or segmental pulmonary vein ablation for paroxysmal atrial fibrillation on cardiac autonomic function. Heart Rhythm. 2006;3:1428–1435. doi: 10.1016/j.hrthm.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 66.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 67.Pokushalov E, Romanov A, Shugayev P, Artyomenko S, Shirokova N, Turov A, Katritsis DG. Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2009;6:1257–1264. doi: 10.1016/j.hrthm.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 68.Katritsis D, Giazitzoglou E, Sougiannis D, Goumas N, Paxinos G, Camm AJ. Anatomic approach for ganglionic plexi ablation in patients with paroxysmal atrial fibrillation. Am. J. Cardiol. 2008;102:330–334. doi: 10.1016/j.amjcard.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 69.Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm. 2011;8:672–678. doi: 10.1016/j.hrthm.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 70.Scherlag BJ, Nakagawa H, Jackman WM, Yamanashi WS, Patterson E, Po S, Lazzara R. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13(Suppl 1):37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 71.Abstract session 6: catheter ablation II: novel approaches to atrial fibrillation ablation. Heart Rhythm. 2004;1:S10–S12. [Google Scholar]
- 72.Lemery R. Catheter ablation of paroxysmal atrial fibrillation: long-term follow-up and the inevitability to fibrillate. Europace. 2011;13:301–303. doi: 10.1093/europace/euq458. [DOI] [PubMed] [Google Scholar]
- 73.Scanavacca M, Pisani CF, Hachul D, Lara S, Hardy C, Darrieux F, Trombetta I, Negrão CE, Sosa E. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006;114:876–885. doi: 10.1161/CIRCULATIONAHA.106.633560. [DOI] [PubMed] [Google Scholar]
- 74.Scanavacca M, Sosa E. Catheter ablation techniques for selective cardiac autonomic denervation to treat patients with paroxysmal atrial fibrillation. Heart Rhythm. 2009;6:1265–1266. doi: 10.1016/j.hrthm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 75.Hirose M, Leatmanoratn Z, Laurita KR, Carlson MD. Partial vagal denervation increases vulnerability to vagally induced atrial fibrillation. J. Cardiovasc. Electrophysiol. 2002;13:1272–1279. doi: 10.1046/j.1540-8167.2002.01272.x. [DOI] [PubMed] [Google Scholar]
- 76.Oh S, Zhang Y, Bibevski S, Marrouche NF, Natale A, Mazgalev TN. Vagal denervation and atrial fibrillation inducibility: epicardial fat pad ablation does not have long-term effects. Heart Rhythm. 2006;3:701–708. doi: 10.1016/j.hrthm.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 77.Patel TD, Jackman A, Rice FL, Kucera J, Snider WD. Development of Sensory Neurons in the Absence of NGF/TrkA Signaling In Vivo. Neuron. 2000;25:345–357. doi: 10.1016/s0896-6273(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 78.Wickramasinghe SR, Alvania RS, Ramanan N, Wood JN, Mandai K, Ginty DD. Serum response factor mediates NGF-dependent target innervation by embryonic DRG sensory neurons. Neuron. 2008;58:532–545. doi: 10.1016/j.neuron.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kangavari S, Oh Y-S, Zhou S, Youn H-J, Lee M-Y, Jung W-S, Rho T-H, Hong S-J, Kar S, Kerwin WF, Swerdlow CD, Gang ES, Gallik DM, Goodman JS, Chen Y-DI, Chen P-S. Radiofrequency catheter ablation and nerve growth factor concentration in humans. Heart Rhythm. 2006;3:1150–1155. doi: 10.1016/j.hrthm.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 80.Sakamoto S-I, Schuessler RB, Lee AM, Aziz A, Lall SC, Damiano RJ. Vagal denervation and reinnervation after ablation of ganglionated plexi. J. Thorac. Cardiovasc. Surg. 2010;139:444–452. doi: 10.1016/j.jtcvs.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. Journal of the American College of Cardiology. 2004;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 82.Konings KT, Kirchhof CJ, Smeets JR, Wellens HJ, Penn OC, Allessie MA. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994;89:1665–1680. doi: 10.1161/01.cir.89.4.1665. [DOI] [PubMed] [Google Scholar]
- 83.Verma A, Novak P, Macle L, Whaley B, Beardsall M, Wulffhart Z, Khaykin Y. A prospective, multicenter evaluation of ablating complex fractionated electrograms (CFEs) during atrial fibrillation (AF) identified by an automated mapping algorithm: acute effects on AF and efficacy as an adjuvant strategy. Heart Rhythm. 2008;5:198–205. doi: 10.1016/j.hrthm.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 84.Arruda M, Natale A. Ablation of permanent AF: adjunctive strategies to pulmonary veins isolation: targeting AF NEST in sinus rhythm and CFAE in AF. J Interv Card Electrophysiol. 2008;23:51–57. doi: 10.1007/s10840-008-9252-z. [DOI] [PubMed] [Google Scholar]
- 85.Porter M, Spear W, Akar JG, Helms R, Brysiewicz N, Santucci P, Wilber DJ. Prospective study of atrial fibrillation termination during ablation guided by automated detection of fractionated electrograms. J. Cardiovasc. Electrophysiol. 2008;19:613–620. doi: 10.1111/j.1540-8167.2008.01189.x. [DOI] [PubMed] [Google Scholar]
- 86.Katritsis D, Giazitzoglou E, Sougiannis D, Voridis E, Po SS. Complex fractionated atrial electrograms at anatomic sites of ganglionated plexi in atrial fibrillation. Europace. 2009;11:308–315. doi: 10.1093/europace/eup036. [DOI] [PubMed] [Google Scholar]
- 87.Pachon MJC, Pachon MEI, Pachon MJC, Lobo TJ, Pachon MZ, Vargas RNA, Pachon DQV, Lopez MFJ, Jatene AD. A new treatment for atrial fibrillation based on spectral analysis to guide the catheter RF-ablation. Europace. 2004;6:590–601. doi: 10.1016/j.eupc.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 88.Li S, Scherlag BJ, Yu L, Sheng X, Zhang Y, Ali R, Dong Y, Ghias M, Po SS. Low-level vagosympathetic stimulation: a paradox and potential new modality for the treatment of focal atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:645–651. doi: 10.1161/CIRCEP.109.868331. [DOI] [PubMed] [Google Scholar]
- 89.Sheng X, Scherlag BJ, Yu L, Li S, Ali R, Zhang Y, Fu G, Nakagawa H, Jackman WM, Lazzara R, Po SS. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vagosympathetic nerve stimulation. Journal of the American College of Cardiology. 2011;57:563–571. doi: 10.1016/j.jacc.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 90.Sha Y, Scherlag BJ, Yu L, Sheng X, Jackman WM, Lazzara R, Po SS. Low-level right vagal stimulation: anticholinergic and antiadrenergic effects. J. Cardiovasc. Electrophysiol. 2011;22:1147–1153. doi: 10.1111/j.1540-8167.2011.02070.x. [DOI] [PubMed] [Google Scholar]
- 91.Oh S, Choi E-K, Choi Y-S. Short-term autonomic denervation of the atria using botulinum toxin. Korean Circ J. 2010;40:387–390. doi: 10.4070/kcj.2010.40.8.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oh S, Choi E-K, Zhang Y, Mazgalev TN. Botulinum toxin injection in epicardial autonomic ganglia temporarily suppresses vagally mediated atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:560–565. doi: 10.1161/CIRCEP.111.961854. [DOI] [PubMed] [Google Scholar]
- 93.Corti A, Mannarino C, Mazza R, Angelone T, Longhi R, Tota B. Chromogranin A N-terminal fragments vasostatin-1 and the synthetic CGA 7-57 peptide act as cardiostatins on the isolated working frog heart. Gen. Comp. Endocrinol. 2004;136:217–224. doi: 10.1016/j.ygcen.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 94.Liang F, Dillen L, Zhang XY, Coen EP, Hogue-Angeletti R, Claeys M, De Potter WP. Vasostatins, N-terminal products of chromogranin A, are released from the stimulated calf spleen in vitro. Acta Physiol. Scand. 1995;155:23–30. doi: 10.1111/j.1748-1716.1995.tb09944.x. [DOI] [PubMed] [Google Scholar]
- 95.Stavrakis S, Scherlag BJ, Fan Y, Liu Y, Liu Q, Mao J, Cai H, Lazzara R, Po SS. Antiarrhythmic effects of vasostatin-1 in a canine model of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2012;23:771–777. doi: 10.1111/j.1540-8167.2012.02317.x. [DOI] [PubMed] [Google Scholar]
- 96.Aistrup GL, Villuendas R, Ng J, Gilchrist A, Lynch TW, Gordon D, Cokic I, Mottl S, Zhou R, Dean DA, Wasserstrom JA, Goldberger JJ, Kadish AH, Arora R. Targeted G-protein inhibition as a novel approach to decrease vagal atrial fibrillation by selective parasympathetic attenuation. Cardiovasc. Res. 2009;83:481–492. doi: 10.1093/cvr/cvp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aistrup GL, Cokic I, Ng J, Gordon D, Koduri H, Browne S, Arapi D, Segon Y, Goldstein J, Angulo A, Wasserstrom JA, Goldberger JJ, Kadish AH, Arora R. Targeted nonviral gene-based inhibition of Gα(i/o)-mediated vagal signaling in the posterior left atrium decreases vagal-induced atrial fibrillation. Heart Rhythm. 2011;8:1722–1729. doi: 10.1016/j.hrthm.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]