Abstract
Objective
We studied the expression and function of an mRNA-binding protein, Zinc Finger Protein 36 (ZFP36), in vascular endothelial cells in vivo and in vitro. We tested the hypotheses that ZFP36 regulates inflammation in vascular endothelial cells and that it functions through direct binding to target cytokine mRNAs. We also tested whether ZFP36 inhibits Nuclear Factor-κB (NF-κB) mediated transcriptional responses in vascular endothelial cells.
Approach and Results
ZFP36 was minimally expressed in healthy aorta but was expressed in endothelial cells overlying atherosclerotic lesions in mice and humans. The protein was also expressed in macrophage foam cells of atherosclerosis. ZFP36 was expressed in human aortic endothelial cells in response to bacterial lipopolysaccharide, glucocorticoid, and forskolin but not oxidized low density lipoproteins or angiotensin II. Functional studies demonstrated that ZFP36 reduces the expression of inflammatory cytokines in target cells by two distinct mechanisms: ZFP36 inhibits NF-κB transcriptional activation and also binds to cytokine mRNAs, leading to reduced transcript stability.
Conclusions
ZFP36 is expressed in vascular endothelial cells and macrophage foam cells where it inhibits the expression of pro-inflammatory mRNA transcripts. The anti-inflammatory effects of ZFP36 in endothelial cells occur via both transcriptional and post-transcriptional mechanisms. Our data suggest that enhancing vascular ZFP36 expression might reduce vascular inflammation.
Keywords: Cytokines, Zinc Finger Protein-36 (ZFP36), Endothelial Cell, AU-rich Element, Inflammation, Atherosclerosis
Atherosclerosis is an inflammatory disease and cardiovascular disease remains the leading cause of death in the United States. Initial accumulation of lipoproteins in the subendothelial space leads to an inflammatory response in overlying endothelial cells and subsequent recruitment of monocyte/macrophage cells to the subendothelial compartment.1 Activated endothelial cells secrete and respond to a variety of inflammatory signaling molecules including Monocyte Chemoattractant Protein-1 (MCP-1/CCL2), Interleukin-6 (IL-6), and Interferon-γ (IFNG). The resulting recruitment of immune cells leads to the initiation and establishment of atherosclerotic lesions.2
The chemokine MCP-1 is expressed in vascular endothelial cells, vascular smooth muscle cells, and macrophages of atherosclerotic lesions.3 In mouse models, genetic deletion of Mcp-1 or its receptor Ccr2 results in protection from atherosclerosis.4–6 In humans, inflammation engendered by IL-6 correlates with the risk for coronary artery disease and direct effects of IL-6 on atherogenesis have been demonstrated in mouse models of the disease.7–9
An essential means of regulating cytokine gene expression occurs at the level of mRNA stability.10 Much of this regulation occurs by the binding and stabilizing, or destabilizing, of target mRNAs by proteins that recognize AU-rich Elements (AREs, core sequence 5’-AUUUA) located in the untranslated regions of these transcripts.11 Zinc Finger Protein 36 (ZFP36) is an ARE-binding protein (ARE-BP) that destabilizes target mRNAs leading to message destruction and diminished protein expression.12,13
There are three members of the human ZFP36 family (ZFP36, ZFP36L1, and ZPF36L2) which share tandem zinc finger domains that bind to AREs of target transcripts.12 These proteins are expressed in a variety of cell types including monocytes and macrophages, and their targets include Tumor Necrosis Factor-α (TNFα/TNFA), Granulocyte Macrophage Colony-Stimulating Factor (GM-CSF/CSF2), IL-3, IL-8, Vascular Endothelial Growth Factor (VEGFA), and cyclooxygenase-2 (COX2).12,14 ZFP36 null mice demonstrate cachexia, arthritis, and auto-immunity that is reversible when systemic TNFα is neutralized, indicating that ZFP36 subserves important and non-redundant functions.15,16 Recently, ZFP36 was demonstrated to interact with the p65 subunit of Nuclear factor-κB (NF-κB), leading to decreased nuclear import and diminished transcriptional activation mediated by NF-κB.17,18 These data suggest two important mechanisms by which ZFP36 might suppress inflammatory responses: by regulating NF-κB-mediated transcriptional responses and by regulating cytokine mRNA stability.
While studying the expression of ZFP36 in the fat depots of obese mice19 we observed that ZFP36 is expressed in the vascular endothelium of mice with atherosclerosis but not in the vascular endothelium of normal mice. Little is known about the function of ZFP36 in endothelial cells although a recent report demonstrated that Hypoxia Inducible Factor-1α (HIF1α/HIF1A) is regulated by ZFP36 in an immortalized endothelial cell line.20
In this report, we have studied the expression and function of ZFP36 in vascular endothelial cells in vivo and in vitro. We hypothesized that enhancing vascular endothelial cell expression of ZFP36 would attenuate inflammatory cytokine/chemokine expression in these cells. We reasoned that the anti-inflammatory effects of endothelial cell ZFP36 might identify ZFP36 as a potential therapeutic target for the prevention or treatment of atherosclerosis.
Methods
Materials and Methods are provided in the online-only Supplement.
Results
Characterization of Rabbit Anti-ZFP36 Antiserum
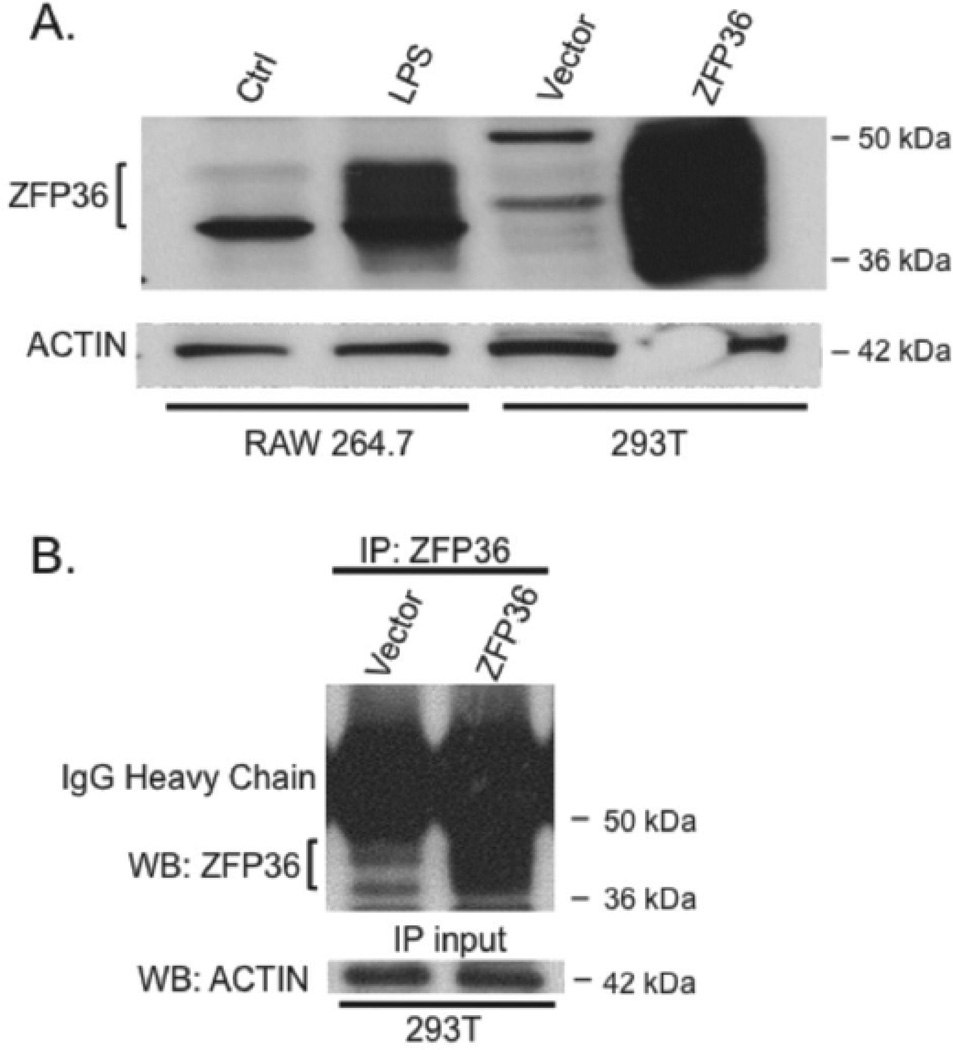
Full length human ZFP36 protein was purified and used to generate rabbit antiserum. Figure 1A shows that the resulting antiserum detected both mouse and human ZFP36 proteins (which share nearly 84% amino acid identity) by Western blot (WB). To test the antiserum for its ability to detect mouse Zfp36 we used murine RAW 264.7 monocyte/macrophage cells, wherein four hours of lipopolysaccharide (LPS) treatment potently induces endogenous ZFP36 expression (Figure 1A).13 Because ZFP36 protein is variably phosphorylated at multiple residues, the protein migrates as a broad-staining band of ~37–44 kDa apparent molecular weight.13,21–23 We engineered human 293T cells transfected with either empty vector or human ZFP36 expression vector to confirm that our antiserum detects human ZFP36 protein (Figure 1A). Comparison of our anti-human ZFP36 antiserum and an anti-mouse ZFP36 antiserum24 is shown in supplementary Figure II. Specificity of our antiserum against ZFP36 was also demonstrated using shRNA-mediated knockdown of ZFP36 expression in HAEC cells (see supplementary Figure III).
Figure 1.
ZFP36 antiserum detects murine and human ZFP36. A) Western blot of murine RAW 264.7 cells with and without LPS stimulation and 293T cells transfected with empty vector or human ZFP36 expression vector. B) Immunoprecipitation (IP) of ZFP36 from ZFP36-expressing 293T cells followed by WB using the same antiserum (with ACTIN loading control from IP-input). Data are representative of experiments performed three times.
Control and ZFP36 over-expressing 293T cell lysates were next subjected to immunoprecipitation (IP) using the anti-human ZFP36 antiserum, followed by WB of the immunoprecipitates using the same antiserum (i.e. IP-Western). Our antiserum effectively immunoprecipitated ZFP36 protein by IP-Western (Figure 1B) confirming the utility of this antiserum in subsequent RIP-PCR studies (see below).
ZFP36 is Expressed in Atherosclerosis
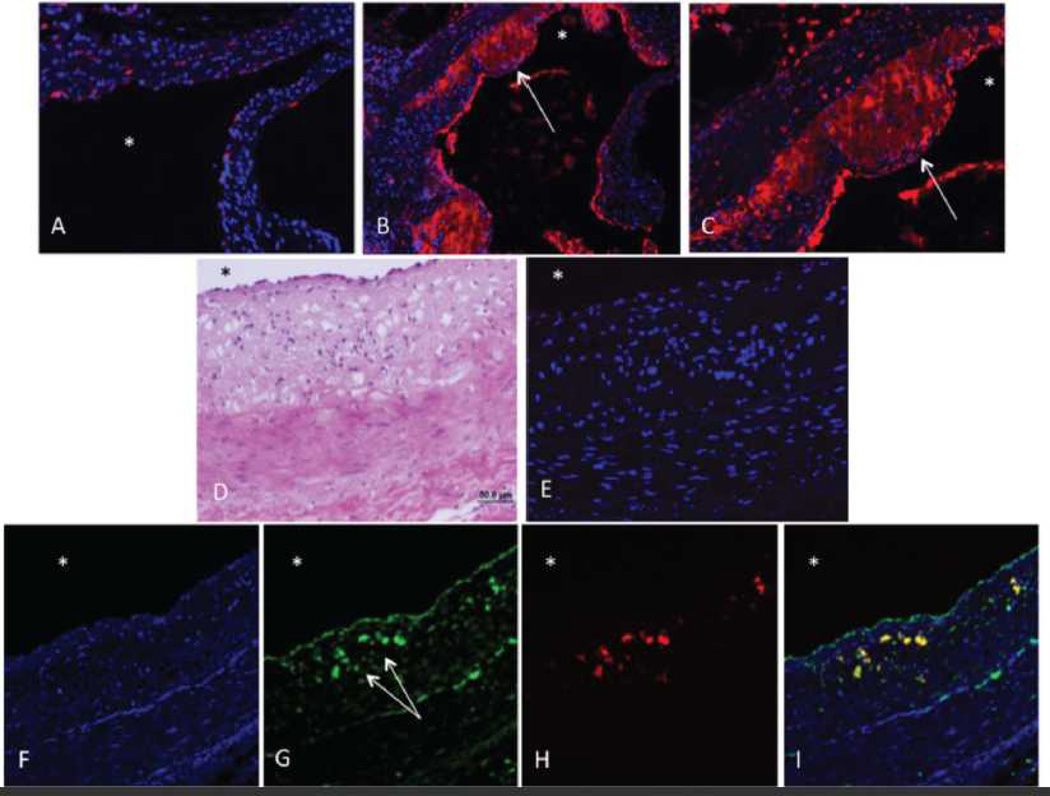
We recently demonstrated the anti-inflammatory effects of ZFP36 in adipocytes19 and we hypothesized that ZFP36 might play a role in reducing inflammation in atherosclerosis. Immunofluorescent microscopy was performed to assess ZFP36 expression patterns in vivo. Wild type C57BL/6 mice fed regular chow did not develop atherosclerosis and demonstrated low ZFP36 expression (red) in the aorta (Figure 2A, with DAPI-stained nuclei in blue). By contrast, C57BL/6 mice on the ApoE−/− background developed substantial atherosclerosis when fed a high calorie/high fat "Western" diet for six weeks: these mice demonstrated significant expression of ZFP36 (red) in the macrophage-laden lesions of the aortic neointima (Figure 2B (10x magnification) and 2C (20x magnification)). Interestingly, ZFP36 expression was also high in the vascular endothelial cells overlying atherosclerotic lesions (Figure 2B–C) but not in healthy endothelium (Figure 2A).
Figure 2.
ZFP36 Expression in Mouse and Human Arteries. A) Wild-type mouse aorta, regular diet (20x), Zfp36 (red, anti-mouse Zfp36 antiserum) and DAPI-nuclei (blue). B) and C) ApoE null mouse aorta using the same antiserum (B is 10x and C is 20x, colors as above). D) – I) Dual label fluorescent microscopy of human coronary artery. D) H&E stained, E) Pre-immune serum (red), F) DAPI-nuclei (blue), G) ZFP36 (green, anti-human ZFP36 antiserum), H) CD68 (red), and I) ZFP36 and CD68, merged (yellow). Asterisks indicate vessel lumen, arrows indicate strong endothelial and neointimal staining.
ZFP36 expression was next assessed in human coronary arteries from subjects with stage 2 atherosclerosis. Hematoxylin and eosin staining revealed neointimal expansion and macrophage foam cell accumulation in the neointima (Figure 2D). Staining of the same lesion with pre-immune serum revealed minimal background immunofluorescence (Figure 2E, red). Using fluorescent microscopy of human coronary artery atherosclerosis with DAPI-stained nuclei (Figure 2F, DAPI alone), we observed strong expression of ZFP36 in neointimal cells and endothelial cells (Figure 2G, green). Using dual immunofluorescence confocal microscopy (Figure 2G–I) we confirmed co-localization of ZFP36 and CD68 expression in the monocyte/macrophage cells of the neointima but not in ZFP36 expressing vascular endothelial cells. Similar findings were observed when staining with an anti-human ZFP36 peptide antibody that was obtained commercially (Sigma-T5242, see Supplementary Figure IV). These data demonstrate enhanced ZFP36 expression in activated macrophages and endothelial cells of atherosclerotic lesions in mice and humans.
ZFP36 is Expressed in Primary Human Aortic Endothelial Cells (HAEC)
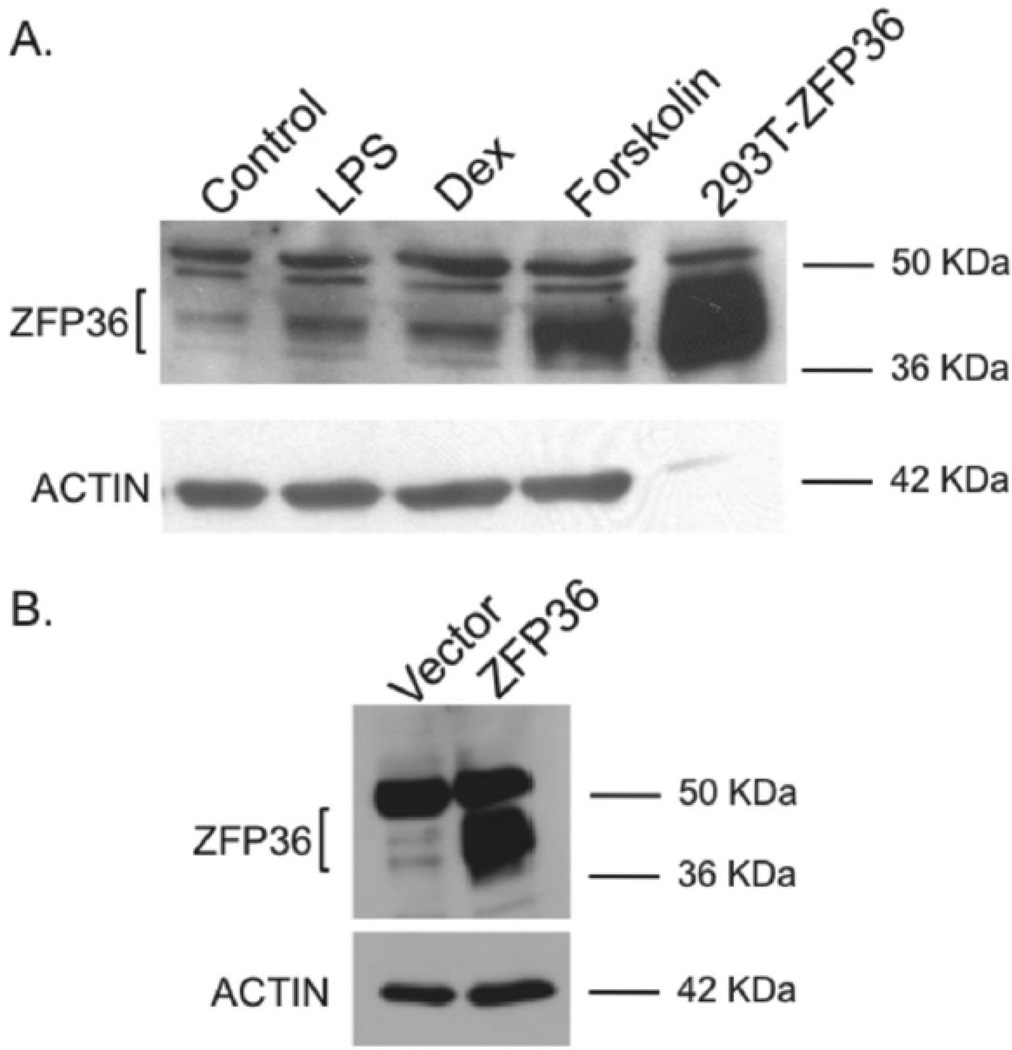
In order to study the functions of ZFP36 in vascular cells, we first tested for endogenous ZFP36 expression in primary HAEC cells. ZFP36 expression was low in un-stimulated HAEC cells, consistent with its role as an acute phase reactant in other cell types (Figure 3A). As previously observed in adipocytes19, treatment of HAEC cells with bacterial lipopolysaccharide (LPS, 500 ng/ml) for 4h resulted in enhanced expression of ZFP36 in HAEC cells (Figure 3A). The glucocorticoid dexamethasonse (1 µM) and the activator of adenylate cyclase, forskolin (10 µM), which generates intracellular cyclic adenosine monophosphate (cAMP), both enhanced ZFP36 expression in primary HAEC cells (Figure 3A). Positive control 293T cells engineered to over-express ZFP36 are also shown (Figure 3A).
Figure 3.
A. ZFP36 expression in primary HAEC cells. HAEC cells were stimulated for four hours with LPS, dexamethasone (DEX), and forskolin (all 50 ug protein/lane). Also shown is positive control 293T cells transfected with ZFP36 expressing plasmid (5 ug protein/lane). B. Lentiviral expression of ZFP36 in HAEC cells compared to vector control cells. Data representative of three independent experiments.
These data confirmed vascular endothelial cell expression of ZFP36 in response to inflammatory and endocrine stimuli previously demonstrated to enhance ZFP36 expression in other cell systems.13,19 These treatments in our model system serve to recapitulate classic inflammatory (LPS) and anti-inflammatory (Dex, cAMP) stimuli relevant to the pathogenesis of atherosclerosis (see discussion). Recent data indicate that the glucocorticoid receptor directly regulates ZFP36 expression in pulmonary epithelial cells.25
We tested oxidized low density lipoproteins (Ox-LDL) for their well recognized ability to trigger atherosclerosis in part by activating Toll-Like Receptor 4 (TLR4) receptors that are targeted by LPS. We did not observe any effects of Ox-LDL on ZFP36 expression in HAEC cells (Supplementary Figure VI). Similarly, while Angiotensin II (AngII) is recognized as a pro-inflammatory molecule important to the pathogenesis of atherosclerosis, we did not observe an effect of AngII treatment on ZFP36 expression in HAEC cells (Supplementary Figure VI)26.
Because our lab has observed cell-type specific stimuli regulating ZFP36 in adipocytes compared with macrophages19, we also tested a macrophage-like cell line (THP-1) for responsiveness to Ox-LDL. THP-1 macrophages accumulate Ox-LDLs in a process that recapitulates in vivo macrophage foam cell behavior.27 As observed for primary HAEC cells, LPS was a potent stimulator of ZFP36 expression in THP-1 macrophages whereas Ox-LDL did not regulate ZFP36 in these cells under our culture conditions (Supplementary Figure VI).
The observation that LPS, dexamethasone, and intracellular cAMP each enhances ZFP36 expression in HAEC cells indicates that several distinct pathways and second messenger systems are conserved in diverse cell types to regulate the expression of ZFP36.13,19 These data supported the use of isolated primary HAEC cells for further studies regarding ZFP36 function.
ZFP36 Reduces Expression of Inflammatory Transcripts in HAEC Cells
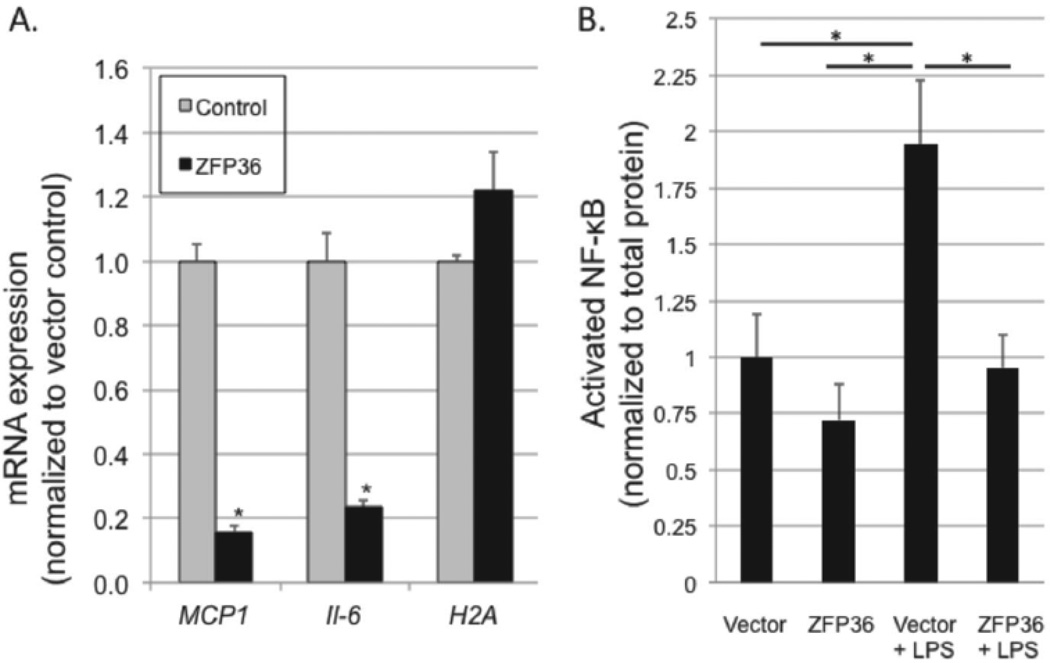
We recently demonstrated that the net effect of ZFP36 in adipocytes is anti-inflammatory.19 This observation is consistent with the phenotype of Zfp36 null mice which are chronically inflamed and cachectic.15,16 We tested whether elevating expression of ZFP36 in primary HAEC cells would reduce expression of mRNAs for inflammatory factors. First, we confirmed that lentiviral expression systems could be used to over-express ZFP36 in primary HAEC cells (Figure 3B). Next, using empty vector or ZFP36-expressing lentiviral particles, we generated low- and high-ZFP36 expressing HAEC cell lines, respectively, and measured mRNA levels of MCP-1 and IL-6. High expression of ZFP36 potently repressed mRNA expression of MCP-1 and IL-6 in primary HAEC cells (Figure 4A). By contrast, ZFP36 over-expression had no significant effect on expression of H2A mRNAs which lack AU-rich elements. These data indicate that ZFP36 represses the expression of MCP-1 and IL-6 transcripts in primary human endothelial cells.
Figure 4.
A. ZFP36 reduces mRNA expression of inflammatory factors in primary HAEC cells. HAEC cells were transduced with LV control virus (gray) or ZFP36-expressing LV particles (black). Q-RT-PCR was performed and normalized to vector control for each mRNA target. Values represent the means of three independent experiments with SEM, *P < 0.01 relative to vector control. B. ZFP36 inhibits transcriptional activation of nuclear NF-κB. HAECs were transduced with empty vector-containing lentiviral particles (vector) or human ZFP36-expressing lentiviral particles (ZFP36) with and without LPS stimulation for 30 minutes. NF-κB activation assays were performed from nuclear lysates and normalized to total nuclear protein for each sample, shown relative to vector control. Experiments were performed in duplicate, and mean values with SEM are shown for five independent experiments. *P < 0.05.
ZFP36 Reduces Expression of Inflammatory Proteins in HAEC Cells
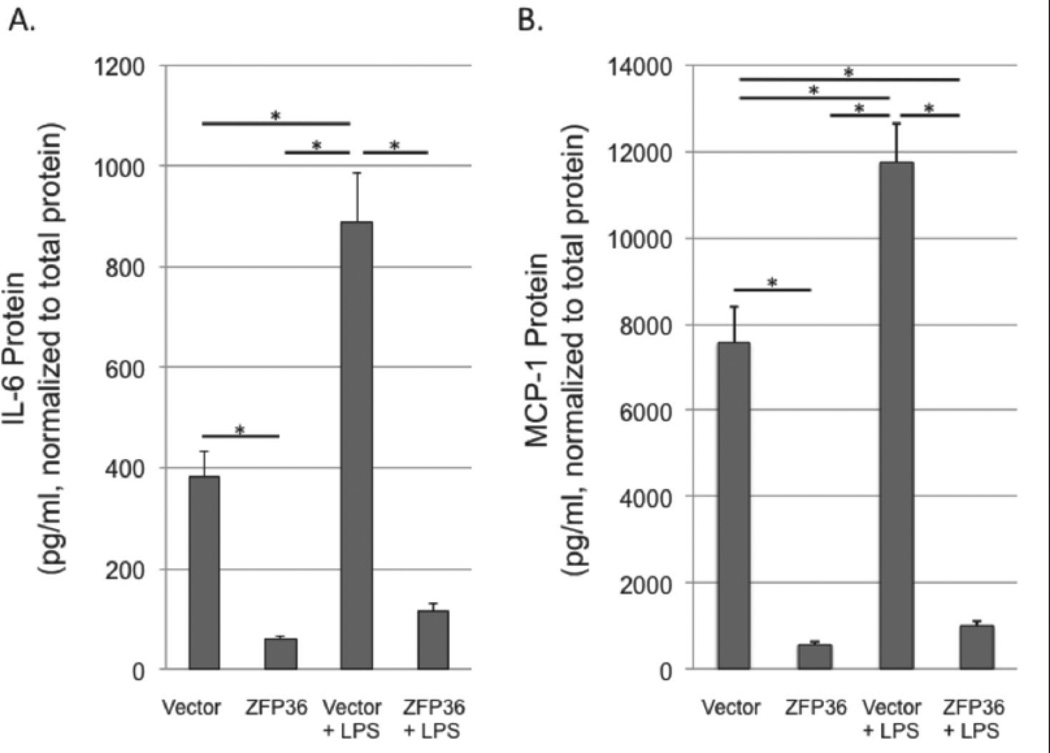
We next tested whether ZFP36-dependent mRNA expression changes correlated with protein expression changes for inflammatory factors. We measured the levels of MCP-1 and IL-6 secreted into the media of low- and high-ZFP36 expressing HAEC cells generated using lentiviral particles (above). Cells were placed in serum-free culture medium for 24 hours prior to treatment with vehicle control or LPS (500 ng/ml) for an additional 24 hours. Conditioned media was then tested by ELISA and data were normalized to total protein in each well. High ZFP36 expression significantly reduced the expression of MCP-1 and IL-6 proteins in both basal and LPS-stimulated cells (Figure 5).
Figure 5.
ZFP36 represses IL-6 and MCP-1 protein expression in basal and LPS-stimulated HAEC cells. Cells were transduced with empty vector-containing lentiviral particles (vector) or human ZFP36-expressing lentiviral particles (ZFP36) with and without LPS stimulation for 24h. Culture medium was collected and IL-6 (A) or MCP-1 (B) proteins were quantitated using ELISA. Data were normalized to total protein in each well. Shown are means with SEM of seven (MCP-1) and eight (IL-6) independent experiments. *P < 0.05.
ZFP36 Reduces Activation of Nuclear NF-κB in HAEC Cells
ZFP36 represses mRNA expression of inflammatory factors by at least two mechanisms: direct binding to AREs in target mRNAs leading to transcript destruction12 and/or binding to NF-κB leading to decreased nuclear import and diminished NF-κB-mediated transcriptional activation of cytokine/chemokine genes.17,18 Interestingly, both transcriptional and post-transcriptional activities of ZFP36 have been shown to modulate the expression of TNFα and Interleukin-8.17,28, 29
The mechanisms by which ZFP36 regulates IL-6 and MCP-1 mRNA expression are not known. While IL-6 transcripts have six minimal pentamer ARE elements (AUUUA) in the 3'-untranslated region of their mRNAs, MCP-1 transcripts have only two AREs. Both genes are known transcriptional targets of NF-κB.30,31 We tested low and high ZFP36 expressing HAEC cells for activation of nuclear NF-κB after 30 minutes stimulation with LPS. While LPS produced nearly twofold activation of NF-κB in low ZFP36 expressing cells, this effect was blocked by over-expression of ZFP36 (Figure 4B). These data indicate that the anti-inflammatory effects of ZFP36 in HAEC cells occur partly by inhibiting NF-κB mediated transcriptional responses.
ZFP36 Binds IL-6 and MCP-1 Transcripts
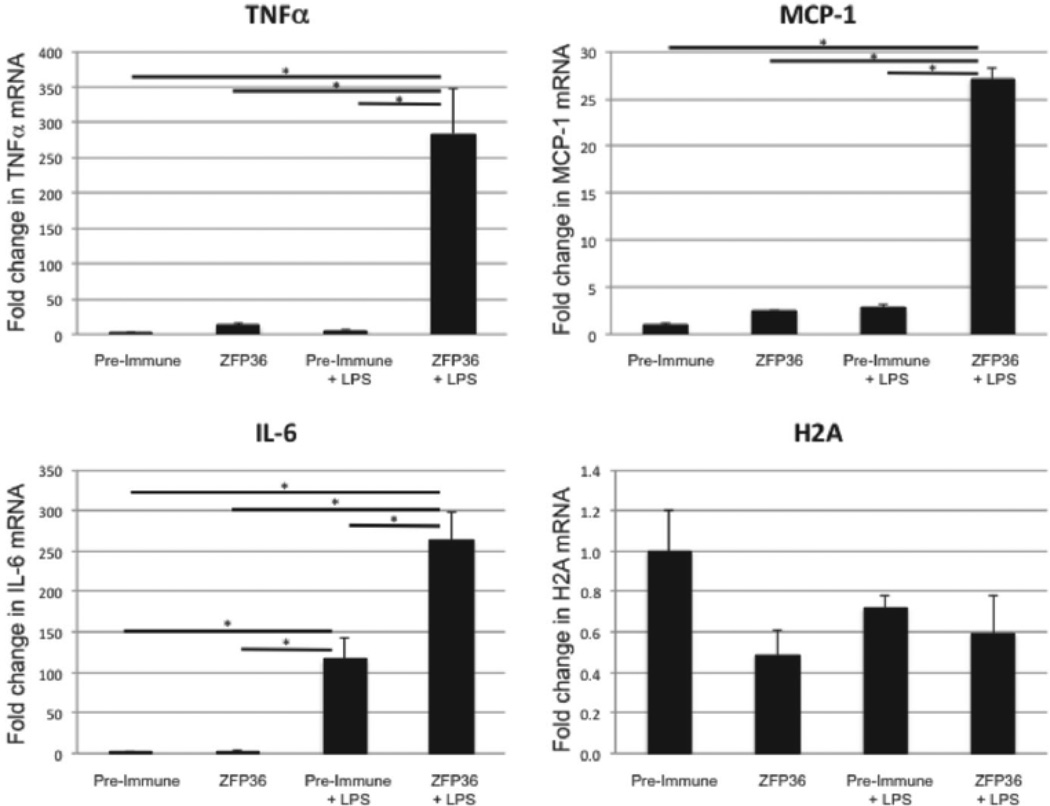
We next tested the extent to which ZFP36 binds to target transcripts in order to regulate mRNA expression. We used RAW 264.7 cells because these cells propagate robustly and maintain their phenotype even after many passages whereas primary HAEC cells lose their phenotype after several passages. Further, RAW 264.7 cells express high levels of ZFP36 and high levels of inflammatory factors when stimulated with LPS, facilitating their use in RIP-PCR assays.
We first confirmed that the RIP-PCR assay is able to detect the previously established interaction of ZFP36 with TNFα transcripts (Figure 6). We then demonstrated that ZFP36 binds directly to IL-6, and MCP-1 transcripts, but not to H2A transcripts which lack ARE elements (Figure 6). In the basal state, where ZFP36 and cytokine expression remain low, there was a non-significant trend towards enrichment of TNFα and MCP-1 transcripts bound to ZFP36 compared to control (pre-immune serum). In LPS-stimulated cells, which express high levels of inflammatory mRNAs and high levels of endogenous ZFP36, there was significant enrichment of TNFα, IL-6, and MCP-1 transcripts bound to ZFP36. We observed no enrichment for H2A mRNA using ZFP36 antiserum in basal or LPS-stimulated cells, consistent with the fact that H2A transcripts lack binding sites for ZFP36 (Figure 6). These data establish for the first time that ZFP36 interacts with IL-6 and MCP-1 mRNA.
Figure 6.
ZFP36 directly binds to inflammatory mRNA transcripts. RAW cells ± LPS stimulation were subjected to ribonucleoprotein IP (using pre-immune or ZFP36 antiserum) followed by Q-RT-PCR. ZFP36 bound directly to Tnfα, IL-6, and Mcp-1 (but not H2A) transcripts. Means with SEM for three independent experiments normalized to pre-immune control. *P < 0.05.
Discussion
Healthy arterial endothelium plays important roles in maintaining vessel wall function by regulating vessel tone and limiting leukocyte adhesion, thrombus formation, and proliferation of vascular smooth muscle cells. Established triggers of atherosclerosis include the accumulation of oxidized low density lipoproteins (Ox-LDL) in the vascular intima and insult by bacterial pathogens or tissue injury, each leading to endothelial cell dysfunction and activation.32 Responding to innate immune system signals, endothelial cells then produce cell surface adhesion molecules, inflammatory cytokines, and chemokines which contribute to the initiation and progression of atherosclerotic lesions.33
This paper reports ZFP36 expression in vascular endothelial cells of atherosclerotic lesions in vivo and in primary HAEC cells in vitro. Our interest in developing new targets with which to decrease the inflammation associated with obesity prompted our studies of ZFP36 in adipose tissues19, leading to our observation that ZFP36 is also expressed in atherosclerosis. Interestingly, both obesity and atherosclerosis share the pathogenic processes of lipid accumulation, inflammatory cell infiltration, cytokine production, and disruption of vascular endothelial cell functions.
ZFP36 regulates mRNA expression both transcriptionally17,18 and post-transcriptionally.12 We studied the effects of enhanced expression of ZFP36 on IL-6 and MCP-1 production in cultured aortic endothelial cells. Enhanced expression of ZFP36 reduced the mRNA and protein expression of IL-6 and MCP-1 (Figures 4 and 5). We went on to demonstrate that ZFP36 reduces activated NF-κB activity in HAEC cells, indicating an indirect transcriptional response to ZFP36 expression (Figure 4B). Our gene expression data combined with the RIP-PCR data demonstrated that ZFP36 also regulates MCP-1 and IL-6 mRNA stability via direct binding to these transcripts (Figures 4A and 6). Our positive control for these assays confirmed TNFα transcripts to be direct targets of ZFP36 (Figure 6).21 Together, our data indicate that ZFP36 reduces inflammatory gene expression in HAEC cells both transcriptionally and post-transcriptionally. Studies are underway to quantify the relative contribution of transcriptional vs. post-transcriptional effects of ZFP36 with respect to cytokine expression in HAEC cells. Because cytokine and non-cytokine mRNAs may be subject to ARE-BP mediated regulation, unbiased approaches are underway to comprehensively determine the global gene expression changes engendered by ZFP36 in endothelial cells.
Human and mouse MCP-1 transcripts have one and two core ARE elements, respectively. Because AREs often occur in multiple copies in transcripts that are regulated by ARE-BPs, the low copy number of AREs in the MCP-1 transcripts were suspected to be insufficient for ZFP36 binding.17 Our data indicate that ZPF36 binds MCP-1 mRNA and that exogenous ZFP36 represses MCP-1 protein expression (Figures 4 and 6). These data are consistent with a recent report in which an alternative ARE-BP, HuR, was shown to bind to MCP-1 transcripts in human airway epithelial cells.34 Thus, it is likely that MCP-1 expression is regulated, at least in part, by ARE-BPs functioning at the post-transcriptional level in a variety of cell types. Whether protein intermediaries facilitate ZFP36 binding to MCP-1 transcripts, and whether atypical ARE sequences reside in MCP-1 transcripts, remains to be explored.
We have identified pro-inflammatory (LPS) and anti-inflammatory (dexamethasone, cAMP) factors that enhance ZFP36 expression in primary HAEC cells (Figure 3). Prior work has identified several transcription factors that regulate ZFP36 gene expression in diverse cell types: activator protein 235, NF-κB36 glucocorticoid receptor, EGR1, Sp1, TPE1, and STAT3.37 Given the data implicating NF-κB (which is activated by cAMP via PKA and also via LPS-stimulated TLR438) and GR in the transcriptional regulation of the ZFP36 promoter, our data demonstrating ZFP36 responsiveness to LPS, cAMP, and Dex exposure are consistent with these pathways regulating ZFP36 expression in HAEC cells. The magnitude of the ZFP36 response to each ligand was not uniform and suggests complex metabolic or gene regulatory pathways that remain to be studied in HAEC cells (including ligand receptor levels, co-regulatory protein levels, and counter-regulatory pathways).
There is an emerging appreciation that cAMP produces anti-inflammatory effects in vascular endothelial cells in response to inflammatory stimuli including VEGF, adrenomedullin, prostacyclin, prostaglandin E2 (PGE2), β-adrenergic agonists39 and shear stress. cAMP acts via multiple signaling pathways including PKA and may play a role in the pathogenesis of atherosclerosis.40–42 To this end, ours is the first report implicating the mRNA binding protein ZFP36 in the anti-inflammatory response to cAMP in vascular endothelial cells. In addition to enhancing ZFP36 gene expression in HAEC cells, it is noteworthy that ZFP36 is also a target of PKA kinase activity.43 More than six kinases appear to phosphorylate at least 12 Ser/Thr residues of ZFP36, with ongoing studies addressing the net functional effects of ZFP36 phosphorylation (effects which may modulate ZFP36 localization, half-life, and protein:protein interactions).44
Endotoxemia (elevated serum LPS) has recently been linked to high fat diets and has been associated with increased risk of diabetes and atherosclerosis45–50. The receptors for LPS include innate immune TLR4 receptors for which there is considerable data that both endotoxin (i.e. LPS) as well as modified LDLs may serve as activators which promote atherogenesis in the setting of high-fat diet, bacterial infection, periodontal disease, alcohol consumption, and cigarettes (recently reviewed in51) and which may be mitigated in TLR4 deficient mice as well as mice treated with TLR4 antagonists.52 We observed that acute (4hr) exposure of HAEC cells to Ox-LDL and AngII, traditional molecules associated with atherogenesis, did not modulate ZFP36 expression in these cells. Meanwhile, acute exposure to LPS promoted ZFP36 expression in these cells, consistent with the proposed role of chronic infection as a stimulus for the development of atherosclerosis in some settings (and consistent with our hypothesis that ZFP36 acts to repress inflammation engendered by some inflammatory stimuli, see below).32 Additional studies will be required to test which atherogenic molecules regulate ZFP36 expression in vivo.
This work establishes that ZPF36 is expressed in activated macrophages and endothelial cells of atherosclerotic lesions in mice and humans (Figure 2). The authors surveyed ApoE null mouse aortas with modest and moderate amounts of atherosclerosis and observed no significant differences when comparing ZFP36 staining of the athero-prone inner arch to the athero-resistant outer arch (not shown). These data indicate that ZFP36 expression is probably not regulated by hemodynamics and is not expressed prior to the development of systemic inflammation.
Our observations are consistent with a model in which inflammation is tightly regulated by compensatory increases in anti-inflammatory proteins such as ZFP36.21,53 A negative feedback loop in which inflammatory factors enhance expression of the anti-inflammatory ZFP36 is analogous to regulation observed in other immune processes: Toll-like receptor (TLR) signaling in macrophages is followed by inactivation of MyD88 leading to inhibition of further TLR signaling54, T cell receptor signaling leads to activation of proteins (i.e. NFKBIB and the RCAN family) which ultimately terminate the T cell response55, and many inflammatory stimuli also induce expression of suppressor of cytokine signaling (SOCS) proteins which feedback to inhibit additional cytokine signaling.56 Future studies will identify in vivo (and possibly cell-type specific) regulators of endothelial cell ZFP36 expression with a view to developing new therapies for the prevention of atherosclerosis.
Our data suggest a role for ZFP36 in responding to inflammatory stimuli to reduce cytokine/chemokine expression in both adipocytes and vascular endothelial cells. Interestingly, recent SNP analysis revealed ZFP36 to be a candidate gene for influencing the metabolic syndrome in humans.57 Additional work suggested that high ZFP36 expression in adipose tissue may offer protection against the development of insulin resistance and the metabolic syndrome.58 In light of these observational studies linking ZFP36 to the metabolic syndrome, which is a risk factor for atherosclerosis, a broader role for adipocyte- and endothelial cell-expressed ZFP36 in modulating inflammatory responses warrants investigation.
In this report, we have shown that forced expression of ZFP36 reduces basal and LPS-stimulated IL-6 and MCP-1 expression in vascular endothelial cells. Further, we have demonstrated two mechanisms of ZFP36-mediated inhibition of these transcripts: transcriptional and post-transcriptional. Studies to characterize additional cytokine and non-cytokine mRNA targets of vascular endothelial cell ZFP36 are ongoing. The targeted manipulation of mRNA-binding proteins such as ZFP36 represents a novel candidate approach to reducing inflammation and may prove beneficial in preventing or treating inflammatory disorders such as atherosclerosis.
Supplementary Material
Significance.
Atherosclerosis is an inflammatory disease. Zinc Finger Protein 36 (ZFP36) is an mRNA-binding protein that destabilizes inflammatory mRNAs leading to message destruction and diminished protein expression. Our work demonstrates that ZFP36 is expressed in vascular endothelial cells and macrophage foam cells, in vivo, in atherosclerotic tissues. We have shown that ZFP36 inhibits the expression of pro-inflammatory mRNA transcripts in primary vascular endothelial cells and prior work indicates similar functions for ZFP36 in immune cells. We demonstrated that the anti-inflammatory effects of ZFP36 in endothelial cells occur via both transcriptional and post-transcriptional mechanisms. Our data suggest that early or enhanced vascular ZFP36 expression might reduce vascular inflammation and protect against the development of atherosclerosis. The pharmacologic targeting of an mRNA-binding protein for therapeutic benefit represents a potentially promising and mechanistically unexploited approach to preventing or treating atherosclerosis.
Acknowledgements
The authors are grateful to Dr. Joshua R. Friedman for scientific discussions, to Dr. Kerry Ressler (and grant NS055077) for assistance in developing the lentiviral expression systems.
Sources of Funding
This work was supported by National Institutes of Health (NIH) grants R01-HD055379 and R01-CA129424 (N.S.), 5R01HD059909 (E.S.), 5R01HL090584 and 5P01HL095070 and 5R01HL070531 (W.R.T.), Reproductive Scientist Development Program (RSDP)/UCSF-K12-HD000849 and NIH-5R01DK091841 (C.B.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no conflicts of interest.
References
- 1.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 3.Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 5.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 7.Schuett H, Oestreich R, Waetzig GH, et al. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:281–290. doi: 10.1161/ATVBAHA.111.229435. [DOI] [PubMed] [Google Scholar]
- 8.Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86:211–218. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 10.Cheadle C, Fan J, Cho-Chung YS, Werner T, Ray J, Do L, Gorospe M, Becker KG. Stability regulation of mRNA and the control of gene expression. Ann N Y Acad Sci. 2005;1058:196–204. doi: 10.1196/annals.1359.026. [DOI] [PubMed] [Google Scholar]
- 11.Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol. 2007;8:113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- 12.Blackshear PJ. Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem Soc Trans. 2002;30:945–952. doi: 10.1042/bst0300945. [DOI] [PubMed] [Google Scholar]
- 13.Cao H, Tuttle JS, Blackshear PJ. Immunological characterization of tristetraprolin as a low abundance, inducible, stable cytosolic protein. J Biol Chem. 2004;279:21489–21499. doi: 10.1074/jbc.M400900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patino WD, Kang JG, Matoba S, Mian OY, Gochuico BR, Hwang PM. Atherosclerotic plaque macrophage transcriptional regulators are expressed in blood and modulated by tristetraprolin. Circ Res. 2006;98:1282–1289. doi: 10.1161/01.RES.0000222284.48288.28. [DOI] [PubMed] [Google Scholar]
- 15.Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, Blackshear PJ. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4:445–454. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- 16.Carballo E, Blackshear PJ. Roles of tumor necrosis factor-alpha receptor subtypes in the pathogenesis of the tristetraprolin-deficiency syndrome. Blood. 2001;98:2389–2395. doi: 10.1182/blood.v98.8.2389. [DOI] [PubMed] [Google Scholar]
- 17.Schichl YM, Resch U, Hofer-Warbinek R, de Martin R. Tristetraprolin impairs NF-kappaB/p65 nuclear translocation. J Biol Chem. 2009;284:29571–29581. doi: 10.1074/jbc.M109.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang J, Lei T, Song Y, Yanes N, Qi Y, Fu M. RNA-destabilizing factor tristetraprolin negatively regulates NF-kappaB signaling. J Biol Chem. 2009;284:29383–29390. doi: 10.1074/jbc.M109.024745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brahma PK, Zhang H, Murray BS, Shu FJ, Sidell N, Seli E, Kallen CB. The mRNA-binding protein Zfp36 is upregulated by beta-adrenergic stimulation and represses IL-6 production in 3T3-L1 adipocytes. Obesity (Silver Spring) 2012;20:40–47. doi: 10.1038/oby.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamboredon S, Ciais D, Desroches-Castan A, Savi P, Bono F, Feige JJ, Cherradi N. Hypoxia-inducible factor-1alpha mRNA: a new target for destabilization by tristetraprolin in endothelial cells. Mol Biol Cell. 2011;22:3366–3378. doi: 10.1091/mbc.E10-07-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 22.Taylor GA, Thompson MJ, Lai WS, Blackshear PJ. Phosphorylation of tristetraprolin, a potential zinc finger transcription factor, by mitogen stimulation in intact cells and by mitogen-activated protein kinase in vitro. J Biol Chem. 1995;270:13341–13347. doi: 10.1074/jbc.270.22.13341. [DOI] [PubMed] [Google Scholar]
- 23.Cao H, Deterding LJ, Venable JD, Kennington EA, Yates JR, 3rd, Tomer KB, Blackshear PJ. Identification of the anti-inflammatory protein tristetraprolin as a hyperphosphorylated protein by mass spectrometry and site-directed mutagenesis. Biochem J. 2006;394:285–297. doi: 10.1042/BJ20051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai WS, Carballo E, Strum JR, Kennington EA, Phillips RS, Blackshear PJ. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol Cell Biol. 1999;19:4311–4323. doi: 10.1128/mcb.19.6.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smoak K, Cidlowski JA. Glucocorticoids regulate tristetraprolin synthesis and posttranscriptionally regulate tumor necrosis factor alpha inflammatory signaling. Mol Cell Biol. 2006;26:9126–9135. doi: 10.1128/MCB.00679-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss D, Sorescu D, Taylor WR. Angiotensin II and atherosclerosis. Am J Cardiol. 2001;87:25C–32C. doi: 10.1016/s0002-9149(01)01539-9. [DOI] [PubMed] [Google Scholar]
- 27.Fu Y, Luo N, Lopes-Virella MF, Garvey WT. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis. 2002;165:259–269. doi: 10.1016/s0021-9150(02)00305-2. [DOI] [PubMed] [Google Scholar]
- 28.Zhu W, Brauchle MA, Di Padova F, Gram H, New L, Ono K, Downey JS, Han J. Gene suppression by tristetraprolin and release by the p38 pathway. Am J Physiol Lung Cell Mol Physiol. 2001;281:L499–L508. doi: 10.1152/ajplung.2001.281.2.L499. [DOI] [PubMed] [Google Scholar]
- 29.Suswam E, Li Y, Zhang X, Gillespie GY, Li X, Shacka JJ, Lu L, Zheng L, King PH. Tristetraprolin down-regulates interleukin-8 and vascular endothelial growth factor in malignant glioma cells. Cancer Res. 2008;68:674–682. doi: 10.1158/0008-5472.CAN-07-2751. [DOI] [PubMed] [Google Scholar]
- 30.Ciesielski CJ, Andreakos E, Foxwell BM, Feldmann M. TNFalpha-induced macrophage chemokine secretion is more dependent on NF-kappaB expression than lipopolysaccharides-induced macrophage chemokine secretion. Eur J Immunol. 2002;32:2037–2045. doi: 10.1002/1521-4141(200207)32:7<2037::AID-IMMU2037>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 31.Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, Cheshire N, Paleolog E, Feldmann M. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci U S A. 2004;101:5634–5639. doi: 10.1073/pnas.0401060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 33.Sikorski K, Czerwoniec A, Bujnicki JM, Wesoly J, Bluyssen HA. STAT1 as a novel therapeutical target in pro-atherogenic signal integration of IFNgamma, TLR4 and IL-6 in vascular disease. Cytokine Growth Factor Rev. 2011;22:211–219. doi: 10.1016/j.cytogfr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Fan J, Ishmael FT, Fang X, Myers A, Cheadle C, Huang SK, Atasoy U, Gorospe M, Stellato C. Chemokine transcripts as targets of the RNA-binding protein HuR in human airway epithelium. J Immunol. 2011;186:2482–2494. doi: 10.4049/jimmunol.0903634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jalonen U, Paukkeri EL, Moilanen E. Compounds that increase or mimic cyclic adenosine monophosphate enhance tristetraprolin degradation in lipopolysaccharide-treated murine j774 macrophages. J Pharmacol Exp Ther. 2008;326:514–522. doi: 10.1124/jpet.107.133702. [DOI] [PubMed] [Google Scholar]
- 36.King EM, Kaur M, Gong W, Rider CF, Holden NS, Newton R. Regulation of tristetraprolin expression by interleukin-1 beta and dexamethasone in human pulmonary epithelial cells: roles for nuclear factor-kappa B and p38 mitogen-activated protein kinase. J Pharmacol Exp Ther. 2009;330:575–585. doi: 10.1124/jpet.109.151423. [DOI] [PubMed] [Google Scholar]
- 37.Lai WS, Thompson MJ, Taylor GA, Liu Y, Blackshear PJ. Promoter analysis of Zfp-36, the mitogen-inducible gene encoding the zinc finger protein tristetraprolin. J Biol Chem. 1995;270:25266–25272. doi: 10.1074/jbc.270.42.25266. [DOI] [PubMed] [Google Scholar]
- 38.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 39.Yamamizu K, Yamashita JK. Roles of cyclic adenosine monophosphate signaling in endothelial cell differentiation and arterial-venous specification during vascular development. Circ J. 2011;75:253–260. doi: 10.1253/circj.cj-10-0915. [DOI] [PubMed] [Google Scholar]
- 40.Fantidis P. The role of intracellular 3'5'-cyclic adenosine monophosphate (cAMP) in atherosclerosis. Curr Vasc Pharmacol. 2010;8:464–472. doi: 10.2174/157016110791330843. [DOI] [PubMed] [Google Scholar]
- 41.Parnell E, Smith BO, Palmer TM, Terrin A, Zaccolo M, Yarwood SJ. Regulation of the inflammatory response of vascular endothelial cells by EPAC1. Br J Pharmacol. 2012;166:434–446. doi: 10.1111/j.1476-5381.2011.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maurice DH. Subcellular signaling in the endothelium: cyclic nucleotides take their place. Curr Opin Pharmacol. 2011;11:656–664. doi: 10.1016/j.coph.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Cao H, Lin R. Phosphorylation of recombinant tristetraprolin in vitro. Protein J. 2008;27:163–169. doi: 10.1007/s10930-007-9119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brook M, Tchen CR, Santalucia T, McIlrath J, Arthur JS, Saklatvala J, Clark AR. Posttranslational regulation of tristetraprolin subcellular localization and protein stability by p38 mitogen-activated protein kinase and extracellular signal-regulated kinase pathways. Mol Cell Biol. 2006;26:2408–2418. doi: 10.1128/MCB.26.6.2408-2418.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiesner P, Choi SH, Almazan F, Benner C, Huang W, Diehl CJ, Gonen A, Butler S, Witztum JL, Glass CK, Miller YI. Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor kappa B and activator protein-1: possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ Res. 2010;107:56–65. doi: 10.1161/CIRCRESAHA.110.218420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 47.Harte AL, Varma MC, Tripathi G, McGee KC, Al-Daghri NM, Al-Attas OS, Sabico S, O'Hare JP, Ceriello A, Saravanan P, Kumar S, McTernan PG. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care. 2012;35:375–382. doi: 10.2337/dc11-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller MA, McTernan PG, Harte AL, Silva NF, Strazzullo P, Alberti KG, Kumar S, Cappuccio FP. Ethnic and sex differences in circulating endotoxin levels: A novel marker of atherosclerotic and cardiovascular risk in a British multi-ethnic population. Atherosclerosis. 2009;203:494–502. doi: 10.1016/j.atherosclerosis.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 49.Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J Am Coll Cardiol. 1999;34:1975–1981. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 50.Lehr HA, Sagban TA, Ihling C, Zahringer U, Hungerer KD, Blumrich M, Reifenberg K, Bhakdi S. Immunopathogenesis of atherosclerosis: endotoxin accelerates atherosclerosis in rabbits on hypercholesterolemic diet. Circulation. 2001;104:914–920. doi: 10.1161/hc3401.093153. [DOI] [PubMed] [Google Scholar]
- 51.Glaros TG, Chang S, Gilliam EA, Maitra U, Deng H, Li L. Causes and consequences of low grade endotoxemia and inflammatory diseases. Front Biosci (Schol Ed) 2013;5:754–765. doi: 10.2741/s405. [DOI] [PubMed] [Google Scholar]
- 52.Michelsen KS, Arditi M. Toll-like receptor signaling and atherosclerosis. Curr Opin Hematol. 2006;13:163–168. doi: 10.1097/01.moh.0000219662.88409.7c. [DOI] [PubMed] [Google Scholar]
- 53.Sauer I, Schaljo B, Vogl C, Gattermeier I, Kolbe T, Muller M, Blackshear PJ, Kovarik P. Interferons limit inflammatory responses by induction of tristetraprolin. Blood. 2006;107:4790–4797. doi: 10.1182/blood-2005-07-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol. 2010;11:734–742. doi: 10.1038/ni.1908. [DOI] [PubMed] [Google Scholar]
- 55.Liu JO. The yins of T cell activation. Sci STKE. 2005;2005 doi: 10.1126/stke.2652005re1. re1. [DOI] [PubMed] [Google Scholar]
- 56.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 57.Bouchard L, Tchernof A, Deshaies Y, Marceau S, Lescelleur O, Biron S, Vohl MC. ZFP36: a promising candidate gene for obesity-related metabolic complications identified by converging genomics. Obes Surg. 2007;17:372–382. doi: 10.1007/s11695-007-9067-5. [DOI] [PubMed] [Google Scholar]
- 58.Bouchard L, Vohl MC, Deshaies Y, Rheaume C, Daris M, Tchernof A. Visceral adipose tissue zinc finger protein 36 mRNA levels are correlated with insulin, insulin resistance index, and adiponectinemia in women. Eur J Endocrinol. 2007;157:451–457. doi: 10.1530/EJE-07-0073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.