Abstract
Background
We previously demonstrated that propofol interacted with the leukocyte adhesion molecule leukocyte function–associated antigen-1 (LFA-1) and inhibited the production of interleukin-2 via LFA-1 in a dependent manner. However, the binding site(s) of propofol on LFA-1 remains unknown.
Methods
First, the inhibition of LFA-1's ligand binding by propofol was confirmed in an ELISA-type assay. The binding site of propofol on LFA-1 was probed with a photolabeling experiment using a photoactivatable propofol analog called azi-propofol-m. The adducted residues of LFA-1 by this compound were determined using liquid chromatography–mass spectrometry. In addition, the binding of propofol to the ligand-binding domain of LFA-1 was examined using 1-aminoanthracene (1-AMA) displacement assay. Furthermore, the binding site(s) of 1-AMA and propofol on LFA-1 was studied using the docking program GLIDE.
Results
We demonstrated that propofol impaired the binding of LFA-1 to its ligand intercellular adhesion molecule-1. The photolabeling experiment demonstrated that the adducted residues were localized in the allosteric cavity of the ligand-binding domain of LFA-1 called “lovastatin site. ” The shift of fluorescence spectra was observed when 1-AMA was coincubated with the low-affinity conformer of LFA-1 ligand-binding domain (wild-type [WT] αL I domain), not with the high-affinity conformer, suggesting that 1-AMA bound only to WT αL I domain. In the 1-AMA displacement assay, propofol decreased 1-AMA fluorescence signal (at 520 nm), suggesting that propofol competed with 1-AMA and bound to the WT αL I domain. The docking simulation demonstrated that both 1-AMA and propofol bound to the lovastatin site, which agreed with the photolabeling experiment.
Conclusions
We demonstrated that propofol bound to the lovastatin site in LFA-1. Previously we showed that the volatile anesthetics isoflurane and sevoflurane bound to this site. Taken together, the lovastatin site is an example of the common binding sites for anesthetics currently used clinically.
There is a growing awareness that anesthetics may affect our immune system.1–3 Although there is no large clinical study to delineate the clinical implication of anesthetic-induced immunomodulation, small case series have suggested that the choice of anesthetic might impact patient outcomes.4,5 For example, a number of patients presenting for surgical procedures are immunocompromised preoperatively. Any iatrogenic reduction of immune responses may further endanger this population. On the contrary, some surgical procedures (such as cardiac surgery) provoke a significant systemic and perhaps harmful inflammatory response.6,7 The attenuation of exaggerated immune responses by anesthetics may be beneficial in these cases, and in cases where there are ongoing, chronic inflammatory conditions. Thus, it is reasonable to speculate that anesthetics can be beneficial in some situations and detrimental in others.
After approximately 150 years of clinical use, the mechanism of anesthetics' primary effect, hypnosis, is still the target of investigation. Therefore, it is not surprising that an understanding of an off-pathway effect, such as how anesthetics modulate the immune system, is incomplete. The current consensus for most effects caused by anesthetics, however, is that they modulate the function of protein(s) through direct binding interactions. Therefore, while inducing hypnosis by binding to target proteins in the central nervous system (CNS), anesthetics might also modulate immune cells by interacting with other specific proteins on leukocytes and/or a subset of the CNS targets that are also expressed on leukocytes. We have previously demonstrated that the commonly used anesthetics isoflurane, sevoflurane, and propofol inhibit native ligand binding of leukocyte function–associated antigen-1 (LFA-1),8–10 suggesting that LFA-1 is one of these alternative leukocyte targets. On the contrary, chloroform, midazolam, and dexmedetomidine did not inhibit native ligand binding,8,10 indicating a degree of specificity. Belonging to the family of adhesion molecules, integrin LFA-1 is a critical adhesion molecule ubiquitously expressed on leukocytes. It is required in a wide variety of intracellular functions including leukocyte arrest on the endothelium,11 T-cell interactions with antigen-presenting cells and B cells, costimulation of T-cell responses such as interleukin-2 (IL-2) production,12 and natural killer cell cytotoxicity.13 We previously demonstrated that propofol inhibited IL-2 production in peripheral blood mononuclear cells in an LFA-1 dependent fashion.8 Understanding how anesthetics alter the function of this ubiquitously expressed leukocyte adhesion molecule at the structural level will be important to redesign anesthetics in the future.
LFA-1 consists of α and β subunits with the native ligand-binding domain at the top of the α subunit called “the α I domain ” (Fig. 1A) The native ligand-binding site on the α I domain is at the metal ion–dependent adhesion site (Fig. 1, A and B). We previously demonstrated that isoflurane and sevoflurane bind to the allosteric pocket of the α I domain located underneath the α7 helix away from the metal ion–dependent adhesion site (Fig. 1B).9,10,17,18 This pocket binds lovastatin and is hence termed the “lovastatin site. ” When unoccupied, it is considered to play a critical role in the transition of LFA-1 from a low- to high-affinity state.19 Because propofol inhibited LFA-1 as did isoflurane and sevoflurane, we hypothesized that it would bind LFA-1 in this same allosteric cavity. To test this hypothesis, we explored the binding site(s) of propofol on LFA-1 using a novel, photoactivatable propofol analog.20 This compound, azi-propofol-m (azi-Pm) is particularly useful in the case of LFA-1, whose full ectodomain X-ray crystallographic structure is not available, because it allows us to probe the binding site(s) of propofol on full LFA-1 structure, not just the α I domain.
Figure 1.
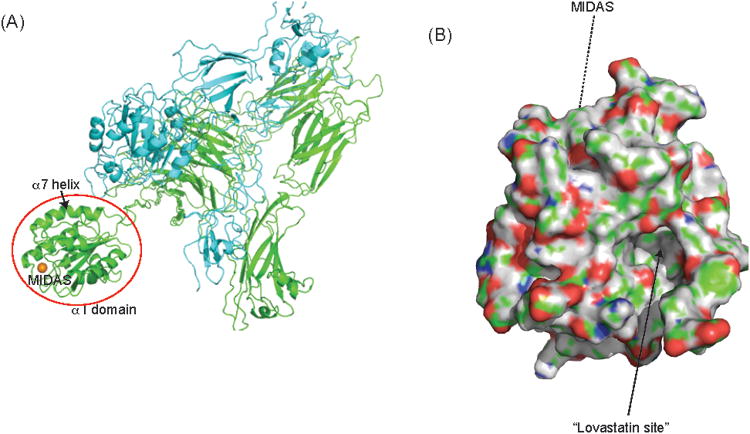
Structure of leukocyte integrin. A, Global structure of leukocyte integrin. The structure shown is from protein data bank (PDB) 3K6S of other leukocyte integrin αXβ2.14 Integrin molecule leukocyte function–associated antigen-1 (LFA-1) (αLβ2) and αXβ2 share the β subunit in common and their α subunits (αL and αX) are highly homologous.15 Both bind to the intercellular adhesion molecule-1 (ICAM-1). The α subunit is shown in blue, and the β subunit is in green. The ligand-binding domain called the α I domain is shown in the red circle. The metal ion–dependent adhesion site (MIDAS) is shown in the gold sphere. ICAM-1 binds to the MIDAS. B, The surface of the α I domain of the LFA-1 is shown (PDB, 1ZOO).16 The allosteric pocket underneath the α7 helix called the “lovastatin site ” is shown in an arrow. The MIDAS is not seen in this figure.
Methods
Protein Expression and Purification
Soluble full ectodomain LFA-1 protein was expressed and purified as previously described from Chinese Hamster Ovary Lec 3.2.8.1 cells stably transfected with αL and β2 plasmids.21 The wild-type (WT) αL I domain (residues 128–307 of the integrin αL subunit) (low-affinity conformer) and high-affinity mutant (HA, residues 127–300 of the integrin αL subunit with K287C/K294C mutation) of LFA-1 were expressed in BL21 (DE3).22 The α I domains were expressed in inclusion bodies, solubilized and refolded as previously described.22
Intercellular Adhesion Molecule-1 Binding Assay
Intercellular adhesion molecule-1 (ICAM-1) is the major ligand for LFA-1. The ICAM-1 binding assay was performed as previously described.8 Briefly, soluble full ectodomain LFA-1 (5 μg/mL) was immobilized indirectly on anti–LFA-1 capturing antibody CBR LFA1/2 (Immune Disease Institute, Boston, MA) on ELISA plates. Nonspecific binding was blocked with HEPES-buffered saline (HBS)/2% bovine serum albumin (Sigma, St. Louis, MO). Then, human ICAM-1–Fcα fusion protein (5 μg/mL) was added to wells with HBS containing 1 mM MnCl2 and di-isopropylphenol (propofol) (Sigma) or 2,6-di-tert-butylphenol (Sigma) at various concentrations. All experiments included mock-treated samples. After incubation for 1 hour at room temperature, unbound ICAM-1 was washed off. Bound ICAM-1–Fcα was detected by peroxidase-labeled goat anti–human immunoglobulin A (IgA) (KPL, Inc., Gaithersburg, MD) and substrate (BD, Franklin Lakes, NJ). After 15 minutes, absorbance was measured at 405 nm. ICAM-1 binding % was defined as ([optimal density {OD} of propofol or 2,6-di-tert-butylphenol at various concentrations]/[OD of mock-treated]) × 100 (%).
TS1/22 Competition Assay
Soluble LFA-1 (10 μg/mL) was immobilized directly on ELISA plates overnight at 4°C. Wells were blocked with HBS/2% bovine serum albumin. LFA-1 competitive antagonist TS1/22 antibody (final concentration 5 μg/mL; Immune Disease Institute) and propofol at various concentrations were added to each well and coincubated for 1 hour at room temperature. After wash, bound antibody was detected using peroxidase-labeled goat anti–mouse IgG (Invitrogen, Carlsbad, CA) and substrate. After 15 minutes, absorbance was measured at 405 nm. TS1/22 binding % was defined as (OD of propofol-treated sample/OD of mock-treated sample) × 100 (%).
Photolabeling Experiments
Azi-Pm has been described previously.20 Azi-Pm (final concentration 100 μM) was equilibrated with soluble LFA-1 for 15 minutes. The sample was exposed to 350 nm light (Rayonet RPR-3500 lamp) at approximately 1 cm distance for 20 minutes in 300 μL, 1 mm pathlength quartz cuvettes. The sample was trypsinized and injected into a 10 cm C18 capillary column run at 200 nL/min for 60 minutes with gradient elution. Mass spectrometry (MS) detected peptides that were searched for adducts of the appropriate mass (216 Da) and then further fragmentation patterns were searched using Sequest to determine the adduct attachment site. MS work was performed at the University of Pennsylvania Proteomics Core Facility.
Rigid Docking
The structure of the αL I domain was obtained from the protein data bank (PDB) (e.g., http://www.rcsb.org/pdb/home/home.do), which is the repository for the 3-dimensional structural data of biological macromolecules. The structure 1ZOO was chosen16 because it represents the αL I domain in a low-affinity conformation. The structure of propofol was obtained through PubChem (http://pubchem.ncbi.nlm.nih.gov/).
Docking is a modeling method that attempts to find an optimal steric and energetic complex between 2 or more interacting biological molecules, such as ligand–receptor complex, using computer algorithms. The program GLIDE (Schrodinger, Cambridge, MA) was used to perform rigid molecular docking of propofol with the αL I domain. GLIDE stands for Grid-based Ligand Docking with Energetics.23 The receptor is prepared as “grid, ” which represents the volume of the receptor. This program first evaluates sterics and complementarity of ligand–receptor interactions. The poses that pass this initial screening enter the final stage of the algorithm and are allowed to undergo slight adjustments to minimize the free energy of ligand–receptor interaction. Final scoring, the “glidescore, ” is performed on the resultant energy-minimized poses. The pose with the most negative glidescore is considered to have the highest affinity. A propofol binding position was sought with the grid size of 25 × 25 × 25 Å3 and the centroid grid residue of Tyr-257. No positional constraint was applied. We selected the docked complex with the most negative glidescore.
1-Aminoanthracene Displacement Assay
1-Aminoanthracene (1-AMA) is a small molecule with environment-dependent fluorescence properties and which has been used to explore various protein cavities.24,25 1-AMA has physicochemical properties quite similar to propofol and possess general anesthetic properties.26 The 1-AMA/αL I domain displacement assay was performed as previously described for the 1-AMA/apoferritin displacement assay.27 First, the αL I domain (200 nM) was preequilibrated with 10 μM 1-AMA (Sigma). After addition of 0.1 to 200 μM propofol, samples were equilibrated, excited at 380 nm, and fluorescence data were collected from 400 to 700 nm. The reduction of fluorescence signal at 520 nm was plotted as the displacement of 1-AMA by propofol. Analysis was performed using PRISM 5 software (GraphPad Software, La Jolla, CA).
Binding Cavity Volume Calculation Using VOIDOO
The cavity volume of the “lovastatin site ” was calculated using VOIDOO.28 The structures of the αL I domain (PDB; 1LFA, 1ZOO) were superposed before volume analysis. The cavity volumes were calculated using a probe size of 1.4 Å and primary grid spacing of 0.5 Å. The volumes were refined for 25 cycles or until consecutive volumes converged to <0.05%.
Results
Propofol and TS1/22 Show Minimal Competition
In our previous studies,8 we expressed full ectodomain LFA-1 using the construct containing αL subunit and β2 subunit fused with complementary ACID–BASE coiled-coil peptides without any tags. The fourth amino acids of coiled-coil peptides were mutated into cysteine to form a disulfide bond. Propofol inhibited the binding of ICAM-1 to this LFA-1. In the present experiments, we used a new construct to express full ectodomain LFA-1 as previously described.21 This construct, which also contained ACID–BASE coiled-coil peptides with cysteine point mutations, has a hexahistidine and a Strep II tag at the C-terminus for the ease of purification. Propofol diminished the binding of ICAM-1 to this LFA-1 as well (Fig. 2A). We previously demonstrated that propofol at 10 μM inhibited IL-2 production in human peripheral blood mononuclear cells by about 50% and at 50 μM by about 80% in an LFA-1–dependent manner.8 Our ELISA system showed less inhibition of ICAM-1 binding. The following possibilities may explain these discrepancies. The first explanation could be our ELISA system. We used ICAM-1–IgA, which is a dimer (2 ICAM-1 fused to 1 IgA-Fc region). As long as one of 2 ICAM-1 binds to LFA-1, there will be a positive signal. Therefore, the actual percentage of LFA-1 blocked by propofol may be more than shown in Figure 2A. The other explanation could be made from the biology of LFA-1. LFA-1 differs from typical cell-surface receptors in that it binds its ligand (ICAM-1) with relatively low affinity in a resting state and is usually present at higher concentration on the cell surface. It increases the interaction of affinity to ICAM-1 by increasing affinity and avidity. The ELISA system was primarily used to evaluate the affinity of LFA-1 to ICAM-1. Although the inhibition of affinity may be small, the sum effect on both affinity and avidity may be larger.
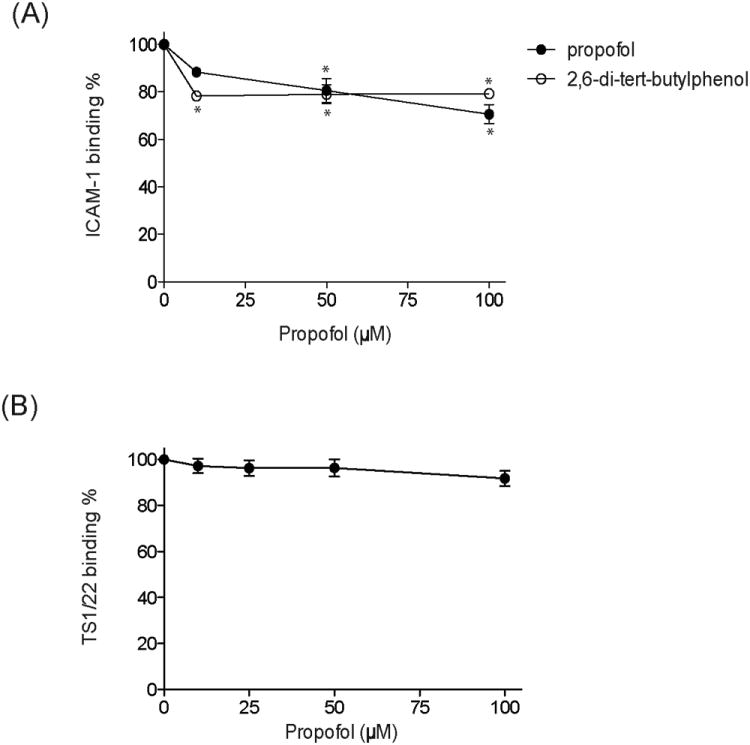
Figure 2.
Propofol inhibits intercellular adhesion molecule-1 (ICAM-1) binding to molecule leukocyte function–associated antigen-1 (LFA-1). A, ELISA-type ICAM-1–LFA-1 binding assay was performed in the presence of propofol or propofol analog (2,6-di-tert-butylphenol [DTBP]) at various concentrations. Both propofol and DTBP inhibited ICAM-1–LFA-1 binding. The data represent mean ± SD of triplicates. Each data point represents a unique replication. Statistical analysis was performed using 1-way analysis of variance with Tukey post hoc pairwise comparisons. *P < 0.01 versus mock-treated sample (no propofol, no DTBP). B, The competition assay of competitive LFA-1 antagonist TS1/22 antibody with propofol at various concentrations was performed. Soluble LFA-1 was used for coating at the concentration of 10 μg/mL, and TS1/22 was 5 μg/mL. The data represent mean ± SD of triplicates. Each data point represents a unique replication. Statistical analysis was performed using 1-way analysis of variance with Tukey post hoc pairwise comparisons. Although TS1/22 demonstrates a trend of some competition with propofol at 100 μM, statistically no difference was observed with narrow 99% 2-sided confidence intervals.
We previously observed that the LFA-1 competitive antagonist TS1/22 antibody competed with propofol, but only at a high concentration (100 μM). We, therefore, suspected that there would be a binding site of propofol near the antibody epitope(s). Using LFA-1 from our new construct, we tested to see whether the TS1/22 antibody competed with various concentrations of propofol. TS1/22 binding was not significantly diminished even in the presence of 100 μM propofol (Fig. 2B). This strongly suggests that propofol may alter LFA-1 function by binding to other region(s) of the intact protein.
Azi-Pm Binds to the αL I Domain
A high-resolution crystal structure of intact LFA-1 has not yet been reported, and this protein is beyond the size accessible by nuclear magnetic resonance (approximately 250 kDa). Therefore, we attempted to determine the propofol binding site(s) on LFA-1 using azi-Pm.20 Analyzing tryptic digests of photolabeled LFA-1 using MS, we were able to detect peptides covering about 49% of the sequence of the α subunit and 66% of the β subunit (Table 1, experiment 1). Ten peptides in the α I domain were found to contain adducted residues in the liquid chromatography/MS. MS/MS approaches revealed the adducted α I domain residues as Ile-254, Tyr-257, Ile-258, Lys-287, Leu-302, and Lys-304. Some of these residues (Ile-254, Tyr-257, and Ile-258) are located in the β5 strand, Lys-287 is in the β6 strand, and Leu-302 and Lys-304 are in the α7 helix (Fig. 3, A and B). All these amino acids are lining residues in the previously identified isoflurane and sevoflurane binding cavity, which is also the binding site of known LFA-1 allosteric inhibitors such as lovastatin.29,30 Taken together with the results in Figure 2, this may suggest that propofol is an LFA-1 allosteric antagonist.
Table 1. The Photolabeled Residues of Leukocyte Function–Associated Antigen-1, by Azi-Propofol-m.
| Sequence coverage | Photolabeled residues | |
|---|---|---|
| Experiment 1 | ||
| α subunit | 49.1% | I254, Y257, I258, K287, L302, K304 |
| β subunit | 66.0% | (−) |
|
| ||
| Experiment 2 | ||
| α subunit | 21.7% | Y257, K263 |
|
| ||
| β subunit | 40.5% | (−) |
Two independent photolabeling experiments were performed in the same experimental condition. Sequence coverage and labeled residues are shown.
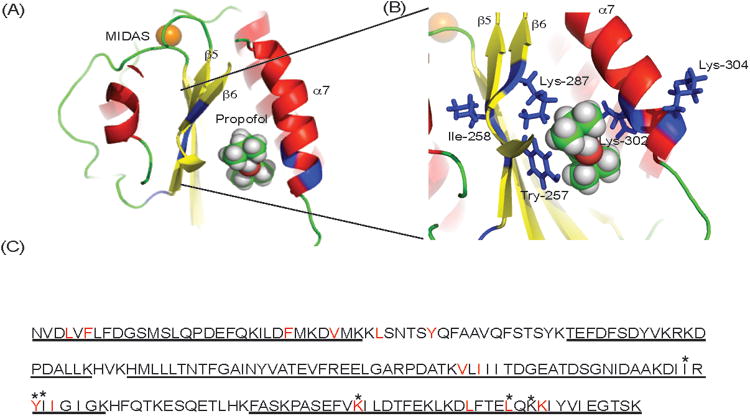
Figure 3.
Propofol binding site(s) on molecule leukocyte function–associated antigen-1 suggested by photolabeling experiment and docking calculation. Azi-propofol-m (azi-Pm) photolabeling experiments were performed twice as shown in Table 1. The sequence coverage was significantly better in experiment 1, and we used experiment 1 as a representative. Notably, the adducted residues by azi-Pm were located at the allosteric pocket underneath the C-terminal α 7 helix of the αL I domain. A and B, The docked propofol on the α I domain (protein data bank 1ZOO) is shown. Residues photolabeled by azi-Pm are shown in blue. The blowup of the docking site is shown in panel B. The metal ion–dependent adhesion site (MIDAS) is shown in the gold sphere. In propofol: red, oxygen; green, carbon. C, Amino acid residues of the α I domain. Sequenced residues by mass spectrometry in experiment 1 are underlined. Adducted residues by azi-Pm are shown in asterisk. Residues with 4 Å from docked propofol are in red.
The epitopes of TS1/22 antibody are located at Gln-266 and Ser-270 of the αL I domain.31 Peptides containing these residues were not detected with MS in either experiment 1 (Fig. 3C) or experiment 2 (data not shown), so we cannot exclude that propofol also binds to this site. Similarly, other allosteric sites with lining residues on peptides were not detected and could not be excluded.
Propofol Docked to the WT αL I Domain
Photolabeling experiments demonstrated that azi-Pm bound to the αL I domain of LFA-1. Because azi-Pm and propofol are slightly different, we performed docking calculations of propofol to the αL I domain using the program GLIDE (Schrodinger, Cambridge, MA). Propofol docked to the allosteric pocket underneath the C-terminal α7 helix of the αL I domain (GLIDE docking score-6.519) (Fig. 3, A and B), the pocket that volatile anesthetics isoflurane and sevoflurane bind to,9,10,17 and the pocket indicated by the photolabeling experiment. The residues surrounding docked propofol matched well with the adducted residues as shown in Figure 3C. In the crystal structure of the isoflurane/αL I domain complex, isoflurane was coordinated in the cavity largely by hydrophobic effects and by weak polar interactions.17 Similarly, propofol is most likely coordinated by hydrophobic interactions and weak van der Waals forces, as suggested by our docking model (Fig. 3, A and B). One of the isopropyl groups packed against the side chain of Leu-132, Val-157, and Leu-161. The other isopropyl group points into the interior of the cavity and can form hydrophobic interactions with Ile-235, Tyr-257, Leu-298, and Leu-302. Those hydrophobic interactions were also found in the isoflurane-α I domain complex.17 In the apoferritin/propofol complex, the hydroxyl of propofol did not form a hydrogen bond in the binding cavity.32 Likewise, a hydrogen bond with the hydroxyl was not observed in our docking of the α I domain/propofol complex.
1-AMA Binds to WT α I Domain but Not HA
1-AMA is a polarity-sensitive hydrophobic probe that has been used previously to investigate different protein cavities.33,34 It fluoresces strongly when excited in a nonpolar environment, such as a hydrophobic protein cavity. Samples were excited at 380 nm. The addition of WT α I domain (low-affinity conformer) to 1-AMA demonstrated a shift and increase in fluorescence yield (Fig. 4A), suggesting that 1-AMA binds to WT α I domain, whereas the addition of the α I domain of LFA-1 fixed (by mutation) in a high-affinity conformation (HA α I domain) did not change the fluorescence spectra of 1-AMA. The biggest cavity in LFA-1, predicted using VOIDOO, is the allosteric lovastatin site. The cavity sizes of the lovastatin site of WT α I domain X-ray crystal structures were 349 Å3 in 1ZOO and 528 Å3 in 1LFA (Table 2). Other than the lovastatin site, there were no cavities that would accommodate 1-AMA (estimated ligand volume approximately 260 Å3). In the structure of HA α I domain (PDB; 1MQ9), there was no longer a lovastatin cavity because of the rearrangement of α7 helix. Taken together, these data indicate that 1-AMA binds at the lovastatin site of WT α I domain. Additionally, 1-AMA docked to the WT αL I domain cavity with reasonable docking scores (−7.300) (Fig. 4B). The typical range of glidescore for known active ligands is from −6 to −14.35 The docking scores of both propofol and 1-AMA with WT α I domain lie within this range.
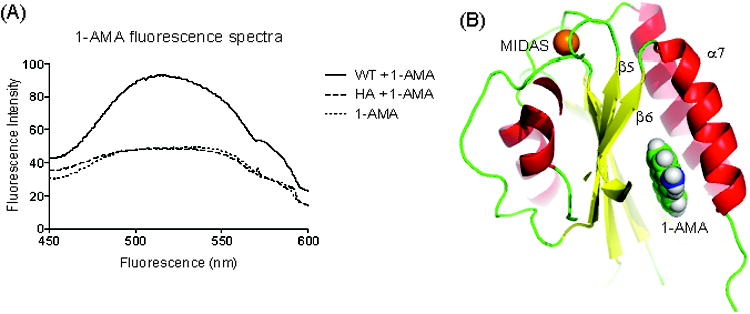
Figure 4.
1-Aminoanthracene (1-AMA) directly interacts with the wild-type α I domain. A, Fluorescence assay measuring the binding of 1-AMA (10 μM) to the wild-type (WT) α I domain and high-affinity mutant (HA; 200 nM). Samples were excited at 380 nm. The representative fluorescence spectra of 2 experiments are shown in the figure. B, The docked 1-AMA on the α I domain (protein data bank [PDB] 1ZOO) is shown. The metal ion–dependent adhesion site (MIDAS) is shown in gold sphere. In 1-AMA: blue, oxygen; green, carbon.
Table 2. The Cavity Size of “Lovastatin Site ” of the Wild-Type α I Domain.
| Protein data bank | Cavity volume (Å3) |
|---|---|
| 1LFA | 528 |
| 1ZOO | 349 |
The cavity size of 2 I domain structures was calculated using VOIDOO as described in the Methods section.
Propofol Displaced 1-AMA from the α I Domain
Because 1-AMA has physicochemical properties quite similar to propofol,26 we expected that propofol would displace 1-AMA from the α I domain binding site. As expected, propofol competed with 1-AMA for the α I domain WT and decreased the 1-AMA fluorescence signal at 520 nm (Fig. 5, A and B). Curve fitting to the Hill equation estimated the half-maximal inhibitory concentration (IC50) of propofol as 90.3 μM. Calculating propofol KD from this value requires the 1-AMA KD value, which is difficult to measure because its limited solubility precludes full occupancy.
Figure 5.
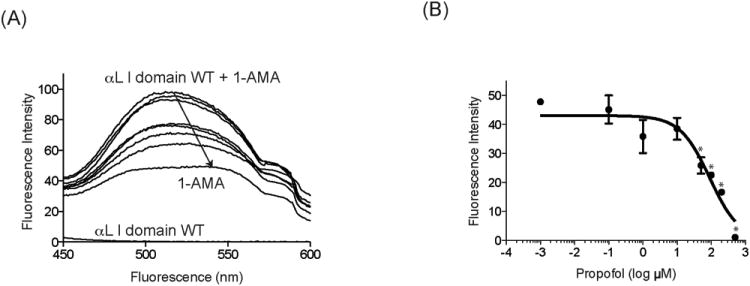
Propofol binds to the wild-type (WT) α I domain. A, The binding of propofol to the α I domain was determined using 1-aminoanthracene (1-AMA) displacement assay. Fluorescence emission spectra after the excitation of samples at 380 nm are shown. Downward arrow indicates that 1-AMA is displaced by propofol titration. The WT αL I domain contributes little to fluorescence at 520 nm. The figure is representative of 3 experiments. B, The shift of fluorescence intensity at 520 nm from 1-AMA alone + the WT α I domain alone is plotted against different concentration of propofol. Data represent mean ± SD of triplicates. Each data point represents a unique replication. Statistical analysis was performed using 1-way analysis of variance using Tukey post hoc pairwise comparisons. * P < 0.01 versus mock-treated sample (no propofol).
Discussion
In this study, we demonstrated that propofol binds the allosteric cavity of the α I domain called the lovastatin site. This cavity is the binding site of known LFA-1 allosteric antagonists as well as volatile anesthetics isoflurane and sevoflurane. Our study also suggested that propofol functions as an allosteric antagonist against LFA-1 activity.
Propofol is thought to produce its desirable hypnotic effects through the γ-aminobutyric acid A (GABAA) and glycine receptors.36–38 However, propofol directly interacts with various proteins not suspected as being involved in anesthetic mechanisms such as apoferritin, protein kinase C,39 and albumin.40 LFA-1 is one more example of a side effect or “off-pathway ” target engaged by propofol. An understanding of the existence of propofol's targets outside the CNS will be important to understand its overall systemic effects. How propofol interacts with its target proteins from the structural level is an important question that will be essential to address if we are to optimize its structure. The characteristics of propofol binding sites from X-ray crystallographic structure are described in apoferritin,32 bacterial Gloebacter violaceus pentameric proton-gated ion channel,41 and albumin.40 Apoferritin is a multimer of 4-helix bundles. Propofol binds to the small cavity at the dimer interface of apoferritin, to which the volatile anesthetics, isoflurane and halothane, and barbiturates also bind.42 The major interaction is the hydrophobic effect and van der Waals forces with no apparent hydrogen bond involvement. Propofol also interacts with Gloebacter violaceus pentameric proton-gated ion channel mainly via van der Waals contacts. The propofol hydroxyl group may form a hydrogen bond in this case41 and in the case of human serum albumin.43 Our docking results suggest that propofol also interacts with LFA-1 primarily via van der Waals forces and the hydrophobic effect. No hydrogen bond was observed. This is slightly different from volatile anesthetics–LFA-1 interactions, which may invoke more amphipathic character.17,41,42 The structural information on the propofol/target protein complexes are helpful to understand the pharmacophore, but, unfortunately, the absence of specific atomic interactions make optimized design difficult.
The other important question is how propofol selects the binding site(s) on target proteins. Understanding the difference between propofol (molecular weight, 178.3) and the propofol analog called di-tert-butyl-phenol (DTBP; molecular weight, 206.3) may provide a clue on this matter. DTBP is a nonhypnotic anesthetic analog. In contrast to propofol, this compound does not alter the function of the GABAA receptor44,45 or cause immobility of tadpoles. However, it alters the function of the α1β glycine receptor46 and LFA-1 as propofol does. One possibility is that the GABAA receptor has a smaller cavity than the glycine receptor or the LFA-1 site. At this point, however, there is no structural information on the nature of the propofol binding pocket on a GABAA receptor. Such information will help us understand the selectivity of these different protein sites for anesthetics.
Propofol is a very common clinical practice drug to provide anesthesia and sedation. A small study suggested that propofol infusion might increase the risk of infection in the intensive care unit setting.47 In vitro, propofol has been shown to decrease the proinflammatory cytokine response of lipopolysaccharide stimulation of microglial cells.48 In the intensive care unit setting, 48 hours of propofol infusion (mean initial loading dose, 1.73 mg/kg; mean infusion rate, 0.98 mg/kg/h) decreased the mean IL-2 level from 1085 to 345 pg/mL (68% reduction).49 The plasma concentration of patients receiving and initial loading dose of 1 to 3 mg/kg and maintenance infusion of 3 mg/kg/h was approximately 2 μg/mL (11.3 μM).50 Therefore, the plasma propofol in this study is expected to be well below 10 μM. IL-2 production is predominantly by activated CD4+ T cells and is inhibited by the anti–LFA-1 antibody.51 We previously demonstrated that propofol even at 10 μM caused about 50% reduction of IL-2 production via an LFA-1–dependent mechanism.8 Taken together, it can be speculated that propofol has an impact on LFA-1 function at clinical concentrations. Understanding the potential mechanism of immunomodulation is predicted to have considerable clinical importance. In this study, we demonstrated that propofol directly interacted with LFA-1, which is unlikely to be a CNS target responsible for hypnosis. However, the mechanisms for propofol's immunomodulatory effects could also be derived from the conventional “on-pathway ” targets. For example, some leukocytes express GABAA and glycine receptors (Table 3). The GABAA receptor/channel is a pentamer made from the combinations of 16 subunits (α1–6, β1–3, γ1–3, δ, ε, ϑ, π). Mutagenesis analysis demonstrated that sites on the β1 subunit (M286), β2 subunit (M286), and β3 subunit (N265) of the transmembrane domains of GABAA receptor are crucial for the hypnotic action of propofol.36,52 The expression of β subunits has been shown in monocyte, macrophage, T cells, and B cells (Table 3). Thus, it is possible that propofol may produce immunomodulatory effects via interactions with these cell types via the GABAA receptor as suggested previously.53 Similarly, the glycine receptor is a pentameric protein composed of α1 to α4 and β subunits. There may be a propofol binding site on the α1 subunit (S267) of this receptor,54 and α1 subunits are expressed on hepatic resident macrophage Kupper cells.55 It is not yet clear how these cys-loop ligand-gated ion channels on immune cells contribute to propofol immunomodulation, or whether LFA-1, GABAA, and glycine receptors will interact each other.
Table 3. Distributions of γ-Aminobutyric Acid A (GABAA) and Glycine Receptors on Leukocytes.
| Cell type | mRNA | Protein |
|---|---|---|
| GABAA receptor | ||
|
| ||
| Neutrophil | ? | α152 |
|
| ||
| Monocyte | β247 | Possibly α1, α4, β2, γ1, δ50 |
|
| ||
| Macrophage | α1, α2, β2, β3, δ50 | α150 |
|
| ||
| T cell | α1, α2, α3, β1, β2, δ (resting)51 | α152 |
| α1, α2, α3, β1, β2, δ (activated) | Possibly α152 | |
| B cell | α1, α3, β252 | |
|
| ||
| Glycine receptor | ||
|
| ||
| Neutrophil | α2, α4, β49 | α249 |
| Monocyte | ? | ? |
| Macrophage (alveolar, splenic) | α2, α4, β49 | α249 |
| Kupper cells | α1, α4, β49 | α149 |
| T cell | ? | ? |
| B cell | ? | ? |
The known mRNA and protein distributions are listed.
In conclusion, we demonstrated that propofol binds to the allosteric lovastatin site of the LFA-1. Occupancy of this site impairs LFA-1 function, but it is not yet clear what effect on integrated immune function this will have in people.
Acknowledgments
We thank Dr. W. P. Dailey (University of Pennsylvania) for providing azi-propofol-m.
Funding: This work is in part supported by National Institute of Health (NIH) grants P01GM55876 (R.G.E.), K08GM101345 (K.Y.) and CHMC Anesthesia Foundation (K.Y.).
Footnotes
The authors declare no conflicts of interest.
Disclosures: Name: Koichi Yuki, MD.
Contribution: This author helped in study design, conduct of study, data analysis, and manuscript preparation.
Attestation: This author approved the final manuscript.
Name: Weiming Bu, PhD.
Contribution: This author helped in study design, conduct of study, data analysis, and manuscript preparation.
Attestation: This author approved the final manuscript.
Name: Jin Xi, MS.
Contribution: This author helped in conduct of study and data analysis.
Attestation: This author approved the final manuscript.
Name: Motomu Shimaoka, MD, PhD.
Contribution: This author helped in study design, data analysis, and manuscript preparation.
Attestation: This author approved the final manuscript.
Name: Roderic Eckenhoff, MD.
Contribution: This author helped in study design, data analysis, and manuscript preparation.
Attestation: This author approved the final manuscript.
This manuscript was handled by: Marcel E. Durieux, MD, PhD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Koichi Yuki, Department of Anesthesiology, Perioperative and Pain Medicine, Boston Children's Hospital, Boston, Massachusetts; Program in Cellular and Molecular Medicine, Boston Children's Hospital, Boston, Massachusetts; Department of Anaesthesia, Harvard Medical School, Boston, Massachusetts.
Weiming Bu, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Jin Xi, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Motomu Shimaoka, Department of Anesthesiology, Perioperative and Pain Medicine, Boston Children's Hospital, Boston, Massachusetts; Program in Cellular and Molecular Medicine, Boston Children's Hospital, Boston, Massachusetts; Department of Anaesthesia, Harvard Medical School, Boston, Massachusetts; Department of Molecular Pathobiology and Cell Adhesion Biology, Mie University, Graduate School of Medicine, Tsu, Mie, Japan.
Roderic Eckenhoff, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
References
- 1.Sanders RD, Hussell T, Maze M. Sedation & immunomodulation. Crit Care Clin. 2009;25:551–70. ix. doi: 10.1016/j.ccc.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22:263–77. doi: 10.1007/s00540-008-0626-2. [DOI] [PubMed] [Google Scholar]
- 3.Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg. 2010;110:1636–43. doi: 10.1213/ANE.0b013e3181de0ab6. [DOI] [PubMed] [Google Scholar]
- 4.Von Dossow V, Baur S, Sander M, Tønnesen H, Marks C, Paschen C, Berger G, Spies CD. Propofol increased the interleukin-6 to interleukin-10 ratio more than isoflurane after surgery in long-term alcoholic patients. J Int Med Res. 2007;35:395–405. doi: 10.1177/147323000703500315. [DOI] [PubMed] [Google Scholar]
- 5.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–4. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall RI, Smith MS, Rocker G. The systemic inflammatory response to cardiopulmonary bypass: pathophysiological, therapeutic, and pharmacological considerations. Anesth Analg. 1997;85:766–82. doi: 10.1097/00000539-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112:676–92. doi: 10.1378/chest.112.3.676. [DOI] [PubMed] [Google Scholar]
- 8.Yuki K, Soriano SG, Shimaoka M. Sedative drug modulates T-cell and lymphocyte function-associated antigen-1 function. Anesth Analg. 2011;112:830–8. doi: 10.1213/ANE.0b013e31820dcabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuki K, Astrof NS, Bracken C, Yoo R, Silkworth W, Soriano SG, Shimaoka M. The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. FASEB J. 2008;22:4109–16. doi: 10.1096/fj.08-113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuki K, Astrof NS, Bracken C, Soriano SG, Shimaoka M. Sevoflurane binds and allosterically blocks integrin lymphocyte function-associated antigen-1. Anesthesiology. 2010;113:600–9. doi: 10.1097/ALN.0b013e3181e89a77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shamri R, Grabovsky V, Gauguet JM, Feigelson S, Manevich E, Kolanus W, Robinson MK, Staunton DE, von Andrian UH, Alon R. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol. 2005;6:497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- 12.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–62. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barber DF, Faure M, Long EO. LFA-1 contributes an early signal for NK cell cytotoxicity. J Immunol. 2004;173:3653–9. doi: 10.4049/jimmunol.173.6.3653. [DOI] [PubMed] [Google Scholar]
- 14.Xie C, Zhu J, Chen X, Mi L, Nishida N, Springer TA. Structure of an integrin with an alphaI domain, complement receptor type 4. EMBO J. 2010;29:666–79. doi: 10.1038/emboj.2009.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnaout MA. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–50. [PubMed] [Google Scholar]
- 16.Qu A, Leahy DJ. The role of the divalent cation in the structure of the I domain from the CD11a/CD18 integrin. Structure. 1996;4:931–42. doi: 10.1016/s0969-2126(96)00100-1. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Astrof NS, Liu JH, Wang JH, Shimaoka M. Crystal structure of isoflurane bound to integrin LFA-1 supports a unified mechanism of volatile anesthetic action in the immune and central nervous systems. FASEB J. 2009;23:2735–40. doi: 10.1096/fj.09-129908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuki K, Bu W, Xi J, Sen M, Shimaoka M, Eckenhoff RG. Isoflurane binds and stabilizes a closed conformation of the leukocyte function-associated antigen-1. FASEB J. 2012;26:4408–17. doi: 10.1096/fj.12-212746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimaoka M, Springer TA. Therapeutic antagonists and conformational regulation of integrin function. Nat Rev Drug Discov. 2003;2:703–16. doi: 10.1038/nrd1174. [DOI] [PubMed] [Google Scholar]
- 20.Hall MA, Xi J, Lor C, Dai S, Pearce R, Dailey WP, Eckenhoff RG. m-Azipropofol (AziPm) a photoactive analogue of the intravenous general anesthetic propofol. J Med Chem. 2010;53:5667–75. doi: 10.1021/jm1004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishida N, Xie C, Shimaoka M, Cheng Y, Walz T, Springer TA. Activation of leukocyte beta2 integrins by conversion from bent to extended conformations. Immunity. 2006;25:583–94. doi: 10.1016/j.immuni.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Shimaoka M, Xiao T, Liu JH, Yang Y, Dong Y, Jun CD, McCormack A, Zhang R, Joachimiak A, Takagi J, Wang JH, Springer TA. Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell. 2003;112:99–111. doi: 10.1016/s0092-8674(02)01257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–49. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 24.Vincent F, Ramoni R, Spinelli S, Grolli S, Tegoni M, Cambillau C. Crystal structures of bovine odorant-binding protein in complex with odorant molecules. Eur J Biochem. 2004;271:3832–42. doi: 10.1111/j.1432-1033.2004.04315.x. [DOI] [PubMed] [Google Scholar]
- 25.Paliwal A, De PK. Purification, cloning and regulation of a novel acid-lipase-like protein of hamster expressed in lacrimal glands and tears during lactation. Biochim Biophys Acta. 2007;1771:55–65. doi: 10.1016/j.bbalip.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Butts CA, Xi J, Brannigan G, Saad AA, Venkatachalan SP, Pearce RA, Klein ML, Eckenhoff RG, Dmochowski IJ. Identification of a fluorescent general anesthetic, 1-aminoanthracene. Proc Natl Acad Sci U S A. 2009;106:6501–6. doi: 10.1073/pnas.0810590106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lea WA, Xi J, Jadhav A, Lu L, Austin CP, Simeonov A, Eckenhoff RG. A high-throughput approach for identification of novel general anesthetics. PLoS One. 2009;4:e7150. doi: 10.1371/journal.pone.0007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleywegt GJ, Jones TA. Detection, delineation, measurement and display of cavities in macromolecular structures. Acta Crystallogr D Biol Crystallogr. 1994;50:178–85. doi: 10.1107/S0907444993011333. [DOI] [PubMed] [Google Scholar]
- 29.Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–92. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 30.Kallen J, Welzenbach K, Ramage P, Geyl D, Kriwacki R, Legge G, Cottens S, Weitz-Schmidt G, Hommel U. Structural basis for LFA-1 inhibition upon lovastatin binding to the CD11a I-domain. J Mol Biol. 1999;292:1–9. doi: 10.1006/jmbi.1999.3047. [DOI] [PubMed] [Google Scholar]
- 31.Lu C, Shimaoka M, Salas A, Springer TA. The binding sites for competitive antagonistic, allosteric antagonistic, and agonistic antibodies to the I domain of integrin LFA-1. J Immunol. 2004;173:3972–8. doi: 10.4049/jimmunol.173.6.3972. [DOI] [PubMed] [Google Scholar]
- 32.Vedula LS, Brannigan G, Economou NJ, Xi J, Hall MA, Liu R, Rossi MJ, Dailey WP, Grasty KC, Klein ML, Eckenhoff RG, Loll PJ. A unitary anesthetic binding site at high resolution. J Biol Chem. 2009;284:24176–84. doi: 10.1074/jbc.M109.017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson JS, Manderson GA, Ramoni R, Grolli S, Eckenhoff RG. Binding of the volatile general anesthetics halothane and isoflurane to a mammalian beta-barrel protein. FEBS J. 2005;272:573–81. doi: 10.1111/j.1742-4658.2004.04500.x. [DOI] [PubMed] [Google Scholar]
- 34.Nespoulous C, Briand L, Delage MM, Tran V, Pernollet JC. Odorant binding and conformational changes of a rat odorant-binding protein. Chem Senses. 2004;29:189–98. doi: 10.1093/chemse/bjh017. [DOI] [PubMed] [Google Scholar]
- 35.Repasky MP, Shelley M, Friesner RA. Flexible ligand docking with Glide. Curr Protoc Bioinformatics. 2007;Chapter 8:Unit 8.12. doi: 10.1002/0471250953.bi0812s18. [DOI] [PubMed] [Google Scholar]
- 36.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–2. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 37.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–20. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 38.Grasshoff C, Antkowiak B. Propofol and sevoflurane depress spinal neurons in vitro via different molecular targets. Anesthesiology. 2004;101:1167–76. doi: 10.1097/00000542-200411000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Wickley PJ, Yuge R, Martin BA, Meyer JS, Damron DS. Propofol activates and allosterically modulates recombinant protein kinase C epsilon. Anesthesiology. 2009;111:36–43. doi: 10.1097/ALN.0b013e3181a3274b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhattacharya AA, Curry S, Franks NP. Binding of the general anesthetics propofol and halothane to human serum albumin. High resolution crystal structures. J Biol Chem. 2000;275:38731–8. doi: 10.1074/jbc.M005460200. [DOI] [PubMed] [Google Scholar]
- 41.Nury H, Van Renterghem C, Weng Y, Tran A, Baaden M, Dufresne V, Changeux JP, Sonner JM, Delarue M, Corringer PJ. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature. 2011;469:428–31. doi: 10.1038/nature09647. [DOI] [PubMed] [Google Scholar]
- 42.Liu R, Loll PJ, Eckenhoff RG. Structural basis for high-affinity volatile anesthetic binding in a natural 4-helix bundle protein. FASEB J. 2005;19:567–76. doi: 10.1096/fj.04-3171com. [DOI] [PubMed] [Google Scholar]
- 43.Liu R, Meng Q, Xi J, Yang J, Ha CE, Bhagavan NV, Eckenhoff RG. Comparative binding character of two general anaesthetics for sites on human serum albumin. Biochem J. 2004;380:147–52. doi: 10.1042/BJ20031652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krasowski MD, Jenkins A, Flood P, Kung AY, Hopfinger AJ, Harrison NL. General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of gamma-aminobutyric acid (GABA) current at the GABA(A) receptor but not with lipid solubility. J Pharmacol Exp Ther. 2001;297:338–51. [PubMed] [Google Scholar]
- 45.Ahrens J, Leuwer M, de la Roche J, Foadi N, Krampfl K, Haeseler G. The non-anaesthetic propofol analogue 2,6-di-tert-butylphenol fails to modulate GABA(A) receptor function. Pharmacology. 2009;83:95–8. doi: 10.1159/000180125. [DOI] [PubMed] [Google Scholar]
- 46.Ahrens J, Haeseler G, Leuwer M, Mohammadi B, Krampfl K, Dengler R, Bufler J. 2,6 di-tert-butylphenol, a nonanesthetic propofol analog, modulates alpha1beta glycine receptor function in a manner distinct from propofol. Anesth Analg. 2004;99:91–6. doi: 10.1213/01.ANE.0000120083.10269.54. [DOI] [PubMed] [Google Scholar]
- 47.Haddad S, Tamim H, Memish ZA, Arabi Y. Association of preservative-free propofol use and outcome in critically ill patients. Am J Infect Control. 2011;39:141–7. doi: 10.1016/j.ajic.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 48.Ye X, Lian Q, Eckenhoff MF, Eckenhoff RG, Pan JZ. Differential general anesthetic effects on microglial cytokine expression. PLoS One. 2013;8:e52887. doi: 10.1371/journal.pone.0052887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helmy SA, Al-Attiyah RJ. The immunomodulatory effects of prolonged intravenous infusion of propofol versus midazolam in critically ill surgical patients. Anaesthesia. 2001;56:4–8. doi: 10.1046/j.1365-2044.2001.01713.x. [DOI] [PubMed] [Google Scholar]
- 50.Albanese J, Martin C, Lacarelle B, Saux P, Durand A, Gouin F. Pharmacokinetics of long-term propofol infusion used for sedation in ICU patients. Anesthesiology. 1990;73:214–7. doi: 10.1097/00000542-199008000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Bachmann MF, Oxenius A. Interleukin 2: from immunostimulation to immunoregulation and back again. EMBO Rep. 2007;8:1142–8. doi: 10.1038/sj.embor.7401099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krasowski MD, Nishikawa K, Nikolaeva N, Lin A, Harrison NL. Methionine 286 in transmembrane domain 3 of the GABAA receptor beta subunit controls a binding cavity for propofol and other alkylphenol general anesthetics. Neuropharmacology. 2001;41:952–64. doi: 10.1016/s0028-3908(01)00141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wheeler DW, Thompson AJ, Corletto F, Reckless J, Loke JC, Lapaque N, Grant AJ, Mastroeni P, Grainger DJ, Padgett CL, O'Brien JA, Miller NG, Trowsdale J, Lummis SC, Menon DK, Beech JS. Anaesthetic impairment of immune function is mediated via GABA(A) receptors. PLoS One. 2011;6:e17152. doi: 10.1371/journal.pone.0017152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahrens J, Leuwer M, Stachura S, Krampfl K, Belelli D, Lambert JJ, Haeseler G. A transmembrane residue influences the interaction of propofol with the strychnine-sensitive glycine alpha1 and alpha1beta receptor. Anesth Analg. 2008;107:1875–83. doi: 10.1213/ane.0b013e3181875a31. [DOI] [PubMed] [Google Scholar]
- 55.Froh M, Thurman RG, Wheeler MD. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am J Physiol Gastrointest Liver Physiol. 2002;283:G856–63. doi: 10.1152/ajpgi.00503.2001. [DOI] [PubMed] [Google Scholar]


