Abstract
Recently, α-synuclein (α-syn) and DJ-1, two proteins critically involved in Parkinson’s disease (PD), have been shown to be present in saliva, suggesting their potential utility as biomarkers of PD. However, the origin and influence of demographic characteristics (e.g., age or gender) on these proteins are unknown. We identified cheek epithelium, which forms the majority of the cellular component of saliva and is readily accessible clinically, as one of several potential sources of salivary α-syn and DJ-1. However, no PD-related trend in the cellular component was present. In the supernatant collected from 198 healthy subjects, no correlation was seen between salivary DJ-1 or α-syn with age. When male and female subjects were analyzed separately, a weak age-dependent increase in DJ-1 level was present in male subjects, along with slightly increased α-syn in female subjects. These results, though largely negative, provide critical information for understanding the salivary gland pathology and saliva as a PD biomarker source, and must be considered in future investigations of salivary changes in PD.
Keywords: Parkinson’s disease, Neurodegeneration, Movement disorder, α-synuclein, DJ-1, Saliva, Biomarker
1. Introduction
Development of therapeutic treatments for Parkinson’s disease (PD) is hindered by the lack of robust, non-invasive biomarkers, especially for early disease stages. Recent work demonstrating Lewy body pathology in the salivary glands (Beach et al., 2010, Cersosimo et al., 2010, Del Tredici et al., 2010) suggests that saliva may yield readily accessible biomarkers for PD. We previously identified two PD-related proteins, DJ-1 and α-synuclein (α-syn), in human saliva (Devic et al., 2011). Further, we observed a trend toward increasing DJ-1 and decreasing α-syn in PD patients compared to controls, supporting the potential for identification of salivary biomarkers for PD. In order to characterize the variables contributing to salivary distribution of DJ-1 and α-syn in saliva, this study investigated potential sites of their origin, tested whether their levels in the cellular saliva component distinguish between control and PD subjects, and examined demographic effects on their concentration in a cohort of healthy subjects.
2. Methods
Immunohistochemical staining was performed to visualize DJ-1 and α-syn, along with neurofilament, in cheek and submandibular glands obtained at autopsies of three subjects without neurological diseases. Whole saliva was collected from healthy subjects across a wide age range, as well as PD patients and age-matched controls, as previously described (Devic et al., 2011). DJ-1 and α-syn were measured in lysate obtained from the cellular component of saliva from PD patients and controls as well as in the acellular component of saliva from healthy subjects. Analyses were performed using PASW Statistics 18.0 (SPSS, Inc, Chicago, IL). Non-parametric tests (Mann-Whitney) were used to analyze differences in the distribution by sex of DJ-1 and α-syn in saliva supernatants and pellets. Linear regression was used to examine the relationships between age and analytes. Power analysis was carried out using R v. 2.15.2. See supplemental Methods for further details.
3. Core Results
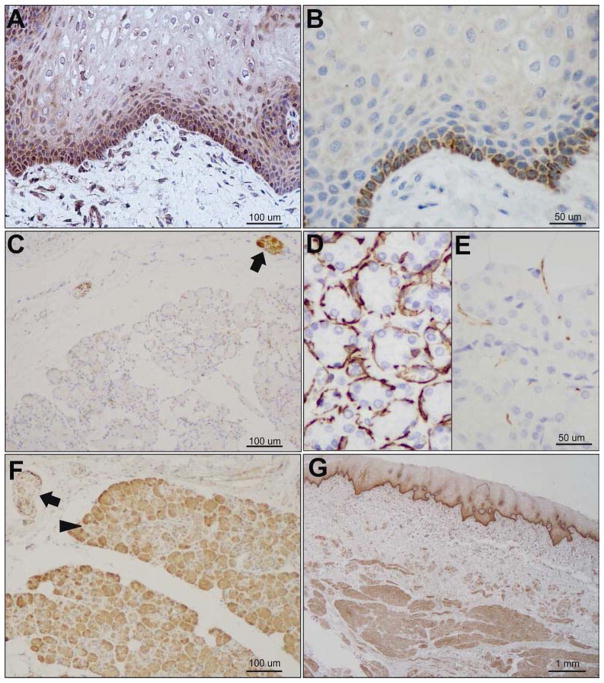
DJ-1 was clearly present in cheek squamous epithelium (Fig 1A), as well as underlying muscle, nerve, and vascular structures (Fig 1F). In contrast, the basal cheek epithelial layer stained for α-syn (Fig 1B), but superficial layers and underlying tissue did not (not shown). We further examined the distribution of both proteins in submandibular glands of subjects without neurodegenerative disease. We observed DJ-1 staining throughout the gland and innervating nerve fibers (Fig 1E), and α-syn in the nerve but not the gland (Fig 1D), similar to the distribution of neurofilament (Fig 1C).
Figure 1.
Expression of DJ-1 and α-syn in cheek and submandibular gland (SMG). A) DJ-1 and B) α-syn in cheek epithelium. C) Neurofilament in nerve in SMG. D) α-syn in SMG is localized to nerve. E) DJ-1 is expressed throughout the SMG, including gland (arrowhead) and nerve fibers (arrow). F) DJ-1 expression throughout cheek epithelium, nerve, muscle and vasculature in cheek biopsy. All samples collected at autopsy from subjects without neurological disease.
DJ-1 and α-syn are detectable in both cellular (pellet) and acellular (supernatant) components of saliva. Because multiple potential sources may contribute to DJ-1 and α-syn in saliva, we chose to determine whether the most proximal (i.e., within the mouth) and clinically accessible source, the cheek epithelial cells, can differentiate between PD patients and control subjects. With optimized assays, we measured DJ-1 and α-syn in lysate from cellular pellets obtained from the same subjects described in our previous study (Devic et al., 2011), and found that, while both proteins were easily detectable in cell pellet lysate, no significant alterations in either analyte were observed (Suppl Fig 1). Power analysis suggested that this sample size was sufficient to detect a moderate (0.5) effect size with 63% power at 0.05 significance level.
Because these results suggest that the previously observed trends are largely driven by protein in the acellular component, we investigated the effects of demographic characteristics on DJ-1 and α-syn in saliva supernatants obtained from 198 healthy subjects (Table 1). Although female subjects had higher average values of both analytes, the difference was only significant for α-syn (Suppl Fig 2). A weak association with age was observed for DJ-1 in males, but not females or the combined group. No association with age was observed for α-syn (Suppl Fig 3). Tests were sufficiently powered to detect gender differences (effect size 0.5) and/or correlations with age (R=0.2) with at least 80% power at a 0.05 significance level.
Table 1.
Demographic distribution and DJ-1/α-syn levels of subjects. Analytes reported as pg/μg total protein ± (standard deviation).
| N | Age, average (range) | DJ-1 (pg/μg total protein ± SEM) | α-syn (pg/μg total protein ± SEM) | |
|---|---|---|---|---|
| Total | 198 | 54.9 (24–88) | 184.4 ± 10.2 | 0.37 ± 0.02 |
| Male | 137 | 56.9 (24–88) | 179.8 ± 11.8 | 0.34 ± 0.02 |
| Female | 61 | 50.6 (32–67) | 194.8 ± 19.7 | 0.45 ± 0.05 |
4. Discussion
We demonstrated the presence of DJ-1 and α-syn in cheek epithelial cells; however, no differences were found in comparing the saliva cellular components of PD patients and control subjects. This study, powered to detect moderate to large effects, cannot exclude subtle alterations, which might be revisited if larger cohorts become available. Additionally, while these negative results may eliminate the possibility of cheek cells as a potential sample source for PD diagnosis, at least for total DJ-1 and α-syn, variant forms of these proteins, such as phosphorylated α-syn (e.g., pS129, the form in SMG found to be useful in detecting PD by Beach and colleagues) or secreted protein (Devic et al., 2011) that might arise from the innervating nerve fibers in the gland, may still prove to be excellent PD biomarkers. While the roles of both proteins in PD pathogenesis have been extensively investigated, their function in peripheral tissues, especially in non-neuronal tissues, as well as the effects of their aggregation in peripheral systems, warrants further investigation. Moreover, given the high expression of both proteins in the nerves innervating the gland, secreted forms need to be pursued preferentially in future studies. To this end, in acellular saliva from healthy subjects, we found slightly higher concentrations of both analytes in female subjects, but the difference was significant only for α-syn. We found no correlation between age and either analyte, though a small correlation was observed for DJ-1 if the group was restricted to males only. These correlations, though subtle, need to be considered in future investigations.
Supplementary Material
Acknowledgments
We wish to thank all subjects for the generous donation of their time and the samples used in this study. The research is supported by generous funds from the Michael J Fox Foundation and from the National Institutes of Health (AG033398, ES004696 (subaward 5897), ES007033 (subaward 6364), ES016873, ES019277 (subaward 02S1), NS057567, NS062684 (subaward 6221), NS065070, NS082137 and T32ES015459). We are grateful to the Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona for providing human submandibular gland tissue. The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium), The KS Ervik Donation, Norway, and the Michael J. Fox Foundation for Parkinson’s Research.
Disclosure statement: Dr. David Wong is co-founder of RNAmeTRIX Inc., a molecular diagnostic company which licenses salivary diagnostic technologies from the University of California Regents. The authors report no other conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Selected References
- Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL, III, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo MG, Perandones C, Micheli FE, Raina GB, Beron AM, Nasswetter G, Radrizzani M, Bearroch EE. Alpha-synuclein immunoreactivity in minor salivary gland biopsies of Parkinson’s disease patients. Mov Disord. 2010;26:188–190. doi: 10.1002/mds.23344. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta Neuropathol. 2010;119:703–713. doi: 10.1007/s00401-010-0665-2. [DOI] [PubMed] [Google Scholar]
- Devic I, Hwang H, Edgar JS, Izutsu K, Presland R, Pan C, Goodlett DR, Wang Y, Armaly J, Tumas V, Zabetian CP, Leverenz JB, Shi M, Zhang J. Salivary alpha-synuclein and DJ-1: potential biomarkers for Parkinson’s disease. Brain. 2011;134:e178. doi: 10.1093/brain/awr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.