Abstract
Background
The clinical phenotype of bipolar disorder (BPD) is heterogeneous and the genetic architecture of the disorder is complex and not well understood. Given these complications, it is possible that the identification of intermediate phenotypes (“endophenotypes”) will be useful in elucidating the complex genetic mechanisms that result in the disorder. The examination of unaffected relatives is critical in determining whether a particular trait is genetically-relevant to BPD. However, few dimensional traits related to BPD have been assessed in unaffected relatives of patients.
Methods
We assessed affective temperament and schizotypy in 55 discordant sibling pairs and 113 healthy controls (HCs) using the Temperament Evaluation of Memphis, Pisa, Paris, and San Diego, Auto-questionnaire version (TEMPS-A) to assess affective temperament and the Schizotypal Personality Questionnaire (SPQ) to assess schizotypy.
Results
BPD patients scored significantly higher than HCs on all subscales of the SPQ and on all but one subscale (hyperthymic) of the TEMPS-A (all p <0.01). Siblings demonstrated scores that were significantly intermediate to patients and HCs on the anxious subscale of the TEMPS-A and on the interpersonal deficits and disorganized subscales of the SPQ.
Limitations
We did not investigate the BPD spectrum as most patients were diagnosed with BPD I (n = 47). Most of the patients had experienced psychosis (n = 42) and so we were unable to examine whether psychosis status impacted upon affective temperament or schizotypy in patients or their siblings.
Conclusion
These data suggest that schizotypy and affective temperament represent dimensional traits that are likely to underlie the genetic risk for BPD.
Keywords: Bipolar Disorder, Endophenotype, Temperament, Schizotypy
INTRODUCTION
Proneness to psychopathology can be conceptualized from the perspective of quantitative genetic models, such that variability along dimensional traits contributes to an individual’s risk for developing a clinical disorder. The identification of genetically-relevant dimensional traits is an important step in understanding the functional relevance of risk variants. Bipolar disorder (BPD), in particular, has repeatedly been found to be associated with characteristic variations in personality and temperament (e.g. Savitz and Ramesar, 2006). Less is known, however, about the endophenotypic status of such variations and, specifically, whether unaffected relatives of patients with BPD demonstrate similar alterations in personality and temperament. Moreover, few studies have examined how different aspects of temperament may be related to one another within and across patients, relatives, and healthy participants.
Temperament is comprised of a complex set of traits reflecting, among other things, one’s general activity level, worldview, and interpersonal style. Two important aspects of this construct that have been found to be associated with risk for psychopathology are affective temperament (e.g. Akiskal and Mallya, 1987; Cassano et al., 1992) and psychosis proneness (Claridge et al., 1996; Raine, 1991; Rossi and Daneluzzo, 2002). Decades of research by Akiskal and others have led to the delineation of five main affective temperaments: hyperthymic, cyclothymic, depressive, irritable, and anxious (e.g. Akiskal, 1998; Akiskal and Mallya, 1987; Placidi et al., 1998). When compared with healthy controls, patients with BPD have been found to have higher rates of affective temperaments when viewed as a categorical measure (e.g. Kesebir et al., 2005) and to have higher scores on measures that assess for such temperaments (Aguiar Ferreira et al., 2012; Evans et al., 2005; Gandotra et al., 2011; Mendlowicz et al., 2005; Savitz et al., 2008a).
Several studies support the familial aggregation of affective temperaments among relatives of BPD patients (Akiskal et al., 1985; Evans et al., 2005; Gandotra et al., 2011; Kesebir et al., 2005; Mendlowicz et al., 2005; Savitz et al., 2008b; Vázquez et al., 2008). However, although many studies have reported significantly increased affective temperaments among patients compared to controls, studies involving relatives have yielded less consistent results. One study to date has demonstrated a gradient, consistent with the heritability of risk for bipolar disorders, with BPD patients having the highest rates of cyclothymic temperament, followed by their unaffected relatives, and then by unrelated healthy controls (Mendlowicz et al., 2005). Several other studies have not found any differences in cyclothymic temperament between unaffected relatives and controls (Aguiar Ferreira et al., 2012; Evans et al., 2005) but have reported elevations in patients compared to relatives (Savitz et al., 2008b). Another study of healthy relatives of patients with BPD reported elevations in affective temperaments among the relatives compared to controls for the dysthymic, cyclothymic, irritable, and anxious temperaments but reported null results for the hyperthymic temperament (Vázquez et al., 2008). The directionality of findings for the hyperthymic temperament has also been inconsistent, with some studies finding that patients and relatives have elevated levels of hyperthymia compared to controls (Gandotra et al., 2011; Kesebir et al., 2005), other studies reporting that controls are elevated on this subscale (Evans et al., 2005), and several studies reporting no significant differences in hyperthymia between patients, relatives, and controls (Mendlowicz et al., 2005) or between relatives and controls (Aguiar Ferreira et al., 2012; Vázquez et al., 2008).
One possible reason for the discrepancies between the findings is the inclusion of parents and offspring, in addition to siblings, in the samples of first degree relatives. The inclusion of parents allows for the possibility that some of the included relatives did not contribute hereditary risk for BPD to the proband. The inclusion of offspring may be problematic in that some young adults may themselves go on to develop BPD but may be too young to have developed the full phenotype. As the majority of patients have developed the disorder by age 25, and siblings tend to develop the disorder at around the same age as probands (Bellivier et al., 2003), restricting unaffected first-degree relatives to siblings past the age of 25 and approximately the same age or older than the age of illness onset in their affected sibling may be the most powerful design from which to examine the endophenotypic nature of temperamental traits.
Another temperamental aspect that may be related to risk for BPD is schizotypy, a personality type marked by odd, irritable, socially isolated, and hypersensitive behaviors (e.g. Raine, 1991). There is some evidence that schizotypy ratings are elevated in patients as well as in their healthy relatives (Kendler et al., 1995) compared to controls, indicating a relationship with genetic risk. Schizotypy may also be thought of as psychosis proneness, as several lines of evidence suggest that individuals who rate high on schizotypy are at increased risk for developing psychosis (Claridge et al., 1996). Given that approximately 50%–70% of patients with BPD I exhibit psychotic symptoms during mood episodes (Goodwin and Jamison, 2007), it would be expected that patients with BPD are elevated on this trait. Indeed, limited data suggest that BPD patients score higher than healthy controls on measures of schizotypy (Rossi and Daneluzzo, 2002) although replication of these results is required. Given these data, psychosis proneness may be a candidate endophenotype for BPD. To date, however, there have been few studies of this trait in family members of BPD patients. In one study of unaffected relatives of patients with BPD and schizophrenia, psychosis proneness scores did not differ between the two groups (Schürhoff et al., 2005). Furthermore, psychosis proneness was found to be elevated in relatives of BPD patients with psychosis compared to relatives of BPD patients without psychosis (Schürhoff et al., 2005). In order to examine the endophenotypic status of this trait, however, an examination of patients, relatives, and controls is required.
In the present study, we aimed to further clarify the relationship between affective temperamental traits, psychosis proneness, and the genetic susceptibility for bipolar disorder by examining patients with BPD and their unaffected siblings and comparing them with unrelated healthy controls. We hypothesized that affective temperament and psychosis proneness would demonstrate a gradient, such that levels of each were highest in patients, intermediate in unaffected siblings, and lowest in healthy controls. We also expected that affective temperament and psychosis proneness would be strongly correlated within each of the three groups. Finally, we expected that unaffected siblings of probands with psychosis would have elevated levels of psychosis proneness compared to siblings of probands without psychosis.
Methods
Sample
Fifty-five sibling pairs discordant for Bipolar Disorder participated in the study, along with 113 healthy control participants. Of the 55 patients with BPD who participated in the study, 47 were diagnosed with Bipolar I Disorder, 5 were diagnosed with Bipolar II Disorder, and the remaining 3 patients were diagnosed with Bipolar Disorder NOS as determined using the Structured Clinical Interview for DSM-IV Disorders (SCID) (First et al., 1994). None of the patients were related to one another and all were clinically stable outpatients at the time of the assessment. Patients who expressed an interest in the study were asked to contact their unaffected siblings who might also be interested in participating; these unaffected siblings then contacted the study team. Siblings were at least 25 years of age and were at least two years older than the age of onset in their affected sibling. In addition, unaffected siblings were free from any major Axis I mood or psychotic disorder as determined by the SCID. Eight of the siblings were diagnosed with Depressive Disorder NOS and an additional three subjects in the unaffected sibling group were diagnosed with an anxiety disorder. All healthy volunteers were free from any current or lifetime Axis I diagnoses, as determined by the SCID-NP, and did not have any first degree relatives with any Axis I disorder. A diagnostic consensus conference involving psychologists and psychiatrists reviewed the SCID interview for each participant to confirm the diagnosis of Bipolar Disorder in patients and to screen for Axis I disorders in unaffected siblings and healthy controls. All participants denied substance abuse or dependence in the three months prior to their participation. All procedures were approved by the local IRB and written informed consent was obtained from all participants.
Measures
Temperament was assessed using the Temperament Evaluation of Memphis, Pisa, Paris, and San Diego, Auto-questionnaire version (TEMPS-A) (Akiskal et al., 2005). This self-report questionnaire consists of true/false items relating to how one generally acts and assesses five empirically-derived affective temperaments thought to confer vulnerability to mood disorders: cyclothymic (i.e. tendency to have abrupt changes in mood as well as exaggerated mood states), dysthymic, irritable, hyperthymic (i.e. tendency toward extraversion, expansive mood, cheerfulness, etc.), and anxious.
Psychosis proneness was assessed in all participants using the Schizotypal Personality Questionnaire (Raine, 1991). This self-report questionnaire assesses three main factors associated with schizotypy: (1) the cognitive-perceptual deficits factor is associated with a tendency toward magical thinking and unusual perceptual experiences that approximate the positive symptoms of psychosis; (2) the interpersonal deficits factor is associated with social anxiety, a tendency toward social withdrawal, and a lack of close relationships that resemble aspects of the negative symptoms of psychosis; and (3) the disorganization factor, which assesses one’s tendency toward odd speech and behavior (Raine at el., 1994; Reynolds et al., 2000).
Current symptoms of mania and depression were measured in each participant using the Clinician Administered Rating Scale for Mania (CARS-M (Altman et al., 1994)) and the Hamilton Depression Rating Scale [HDRS (Hamilton, 1960)], respectively. The Wide Range Achievement Test – Third Edition (WRAT 3) was administered to provide an estimate of each participant’s premorbid IQ.
Statistical Analysis
Demographic and clinical differences between the groups were examined using ANOVA for continuous variables and Chi-square tests for discrete variables. Because of the nonparametric nature of the data, for each subscale of the SPQ and TEMPS-A, a Kruskal-Wallis test with subject-type (patients, siblings, and controls) as the between-subjects factor was carried out using SPSS version 20. Post-hoc testing was performed using nonparametric Mann-Whitney U tests with a Bonferroni correction. Relationships between the variables were examined using Spearman correlations. In order to investigate the potential impact of demographic and/or clinical variables that differed between the groups, we performed a parametric MANCOVA analysis.
We also performed an exploratory analysis to examine whether patients with psychosis differed from patients without psychosis, and whether siblings of probands with psychosis differed from siblings of probands without psychosis, on any of the subscales of the TEMPS-A or the SPQ. Such comparisons were performed using the Mann-Whitney U test with Bonferroni corrections.
RESULTS
The demographic and clinical data for the three groups are presented in Table 1. Two of the sibling participants did not complete the TEMPS-A or the SPQ and were subsequently removed from the analysis. The three subject groups did not differ on age, race, or estimated premorbid IQ as assessed via the WRAT 3. The sample of healthy controls had significantly more males than the sample of patients and the sample of unaffected siblings. Although all of the patients were clinically stable at the time of the study, the patient sample demonstrated significantly elevated symptoms of depression and mania compared to the siblings and healthy controls. Siblings and healthy controls did not differ in mania scores but siblings reported significantly elevated symptoms of depression compared to the healthy controls.
Table 1.
Sociodemographic and clinical characteristics of bipolar patients (BPD), unaffected siblings (UAS) and healthy controls (HC).
| Subject Characteristic | BPD (n = 55) | UAS (n = 53)a | HC (n = 113) | Statistic | p value | Group Differenceb |
|---|---|---|---|---|---|---|
| Sex: male/female | 19/36 | 19/34 | 60/53 | χ2 = 7.2 | .027 | BD=UAS<HC |
| Race: white/nonwhite | 31/24 | 30/23 | 74/39 | χ2 = 1.89 | .390 | BD=UAS=HC |
| Age | 39.4 (12.2) | 40.7 (11.7) | 38.0 (11.6) | F = 1.02 | .361 | BD=UAS=HC |
| Estimated IQ | 98.7 (10.5) | 99.3 (10.2) | 98.8 (12.4) | F = 0.05 | .952 | BD=UAS=HC |
| CARS-M | 4.1 (4.7) | 1.6 (2.4) | 0.8 (3.1) | F = 24.44 | <.001 | BD>UAS=HC |
| HDRS | 6.6 (5.0) | 3.1 (2.9) | 1.8 (4.0) | F = 33.69 | <.001 | BD>UAS>HC |
Note: All data are reported as mean (standard deviation) unless otherwise noted.
Data were missing for two unaffected sibling participants, who were subsequently dropped from the analysis.
Group differences are reported based on Bonferroni-corrected post-hoc Mann-Whitney U tests.
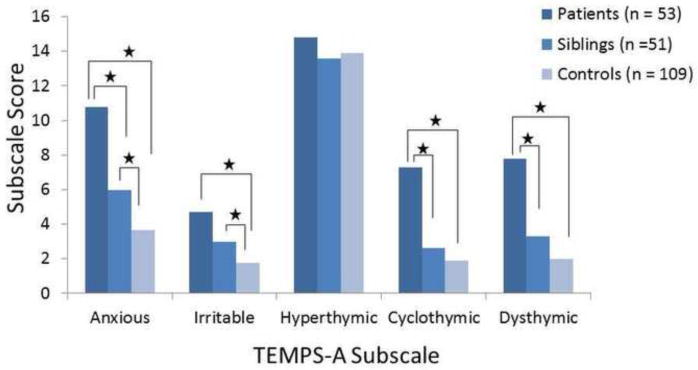
Results from the non-parametric analysis for the TEMPS-A and SPQ data are reported in Table 2. For the TEMPS-A data, patients with BPD scored significantly higher than healthy controls on all of the subscales except for the hyperthymia subscale (Figure 1). Based on the results from post-hoc testing using a conservative Bonferroni correction for mutliple comparisons, patients with BPD scored significantly higher than unaffected siblings on the anxious, cyclothymic, and dysthymic subscales of the TEMPS-A, whereas there was no difference between patients and siblings on the irritable or hyperthymic subscales. Unaffected siblings demonstrated significantly elevated scores compared to controls on the anxious and irritable subscales. On the anxious subscale, unaffected siblings had scores that were significantly intermediate between patients and controls.
Table 2.
TEMPS and SPQ scores in patients with bipolar disorder (BPD), unaffected siblings (UAS), and healthy controls (HC)
| TEMPS-A Subscale | BPD (n = 53)a | UAS (n = 51)b | HC (n = 109)c | Kruskal-Wallis Statistic | p value | Group Difference* |
|---|---|---|---|---|---|---|
| Anxious | 10.79 (5.8) | 5.98 (4.32) | 3.68 (3.3) | 63.00 | <.001 | BPD>UAS>HC |
| Irritable | 4.70 (3.4) | 3.00 (2.5) | 1.75 (1.8) | 32.40 | <.001 | BPD=UAS>HC |
| Hyperthymic | 14.80 (4.8) | 13.59 (5.3) | 13.88 (5.1) | 0.67 | .714 | BPD=UAS=HC |
| Cyclothymic | 7.28 (3.8) | 2.62 (2.5) | 1.88 (2.3) | 72.36 | <.001 | BPD>UAS=HC |
| Dysthymic | 7.77 (4.5) | 3.31 (3.6) | 2.00 (2.38) | 65.43 | <.001 | BPD>UAS=HC |
| SPQ Subscale | BPD (n = 55) | UAS (n = 51)d | HC (n = 107)e | Kruskal-Wallis Statistic | p value | Group Difference* |
|---|---|---|---|---|---|---|
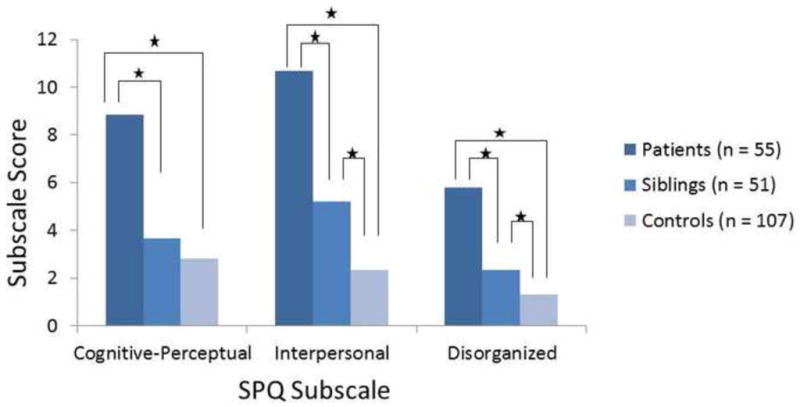
| Cognitive-Perceptual | 8.83 (7.6) | 3.65(3.4) | 2.81(4.1) | 37.20 | <.001 | BPD>UAS=HC |
| Interpersonal Deficits | 10.67(7.7) | 5.20(4.9) | 3.26(4.3) | 42.70 | <.001 | BPD>UAS>HC |
| Disorganized | 5.18(3.7) | 2.33(2.5) | 1.30(2.2) | 60.05 | <.001 | BD>UAS>HC |
Note: Data are presented as mean (standard deviation).
TEMPS-A data were missing for 2 patients with BPD.
TEMPS-A data were missing for 2 unaffected siblings.
TEMPS-A data were missing for 4 healthy controls.
SPQ data were missing for 2 unaffected siblings.
SPQ data were missing for 6 healthy controls.
Group differences are reported based on Bonferroni-corrected Mann-Whitney U tests.
Figure 1.
Similarly, patients scored significantly higher than controls on the three subscales (cognitive-perceptual, interpersonal deficits, disorganized) of the SPQ (Figure 2). After correcting for multiple comparisons, patients scored significantly higher than unaffected siblings on all three subscales. Unaffected siblings scored significantly higher than healthy controls on the interpersonal deficits and disorganized subscales, whereas there was no difference between the groups on the cognitive-perceptual subscale.
Figure 2.
As the groups differed on sex, and current symptoms of depression and mania, these were entered as covariates in a parametric (MANCOVA) analysis of the SPQ and TEMPS-A data. Previous work has demonstrated that scores on the TEMPS-A may be affected by age (Mendlowicz et al., 2005); thus, this variable was also entered as a covariate. The resulting MANCOVA indicated that the groups differed significantly on all subscales of the TEMPS-A except for hyperthymia and all subscales of the SPQ when controlling for age, sex, race, and symptoms of mania and depression.
The correlational analysis indicated that the subscales of the TEMPS-A and the SPQ were all significantly and positively correlated with each other within and across the groups with the exception of the hyperthymia subscale (see Table 3). After Bonferroni correction, the hyperthymia subscale was significantly positively correlated with the cyclothymic subscale of the TEMPS-A and the cognitive-perceptual subscale of the SPQ; no other significant correlations were found between this subscale and any others. There were significant positive correlations between depression as measured by the HDRS and all subscales of the SPQ and the TEMPS-A across the entire sample. Symptoms of mania, as measured by the CARS-M, were significantly positively correlated with all subscales of the TEMPS-A and the SPQ across the entire sample with the exception of the hyperthymia subscale of the TEMPS-A.
Table 3.
Correlations between the TEMPS-A and the SPQ across all three groups.
| Subscale | TEMPS-A Anxious | TEMPS-A Cyclothymic | TEMPS-A Dysthymic | TEMPS-A Irritable | TEMPS-A Hyperthymic | SPQ Cognitive -Perceptual | SPQ Inter person al Deficits | SPQ Disorganized |
|---|---|---|---|---|---|---|---|---|
| TEMPS-A Anxious | — | .624* | .677* | .528* | .131 | .604* | .616* | .576* |
|
|
||||||||
| TEMPS-A Cyclothymic | — | — | .719* | .558* | .263* | .630* | .586* | .677* |
|
|
||||||||
| TEMPS-A Dysthymic | — | — | — | .586* | .053 | .595* | .630* | .592* |
|
|
||||||||
| TEMPS-A Irritable | — | — | — | — | .136 | .471* | .517* | .524* |
|
|
||||||||
| TEMPS-A Hyperthymic | — | — | — | — | — | .225* | .037 | .158 |
|
|
||||||||
| SPQ Cognitive-Perceptual | — | — | — | — | — | — | .789* | .651* |
|
|
||||||||
| SPQ Interpersonal Deficits | — | — | — | — | — | — | — | .682* |
Correlations are significant at p <.001
The majority of the patients with BPD had experienced psychosis at some point in their illness (n = 42) whereas the remaining 13 patients had not. Thus, we had limited statistical power to detect any differences on the TEMPS-A or the SPQ as a function of psychosis in patients or siblings. We did not find any statistically significant differences on the SPQ or the TEMPS-A between patients with or without psychosis or between siblings of probands with or without psychosis. Siblings of probands with psychosis demonstrated nonsignificantly elevated scores on all three subscales of the SPQ compared to siblings of probands without psychosis (cognitive-perceptual [effect size = .39]; interpersonal deficits [effect size = .37]; disorganized [effect size = .30]), suggesting that statistical significance may have been reached with a larger sample size.
DISCUSSION
The present results broadly confirm previous findings (Akiskal et al., 1985; Evans et al., 2005; Mendlowicz et al., 2005; Vázquez et al., 2008) and support the concept of a dimensional phenotype, in which individuals at high genetic risk for BPD exhibit elevated levels of traits associated with the full clinical manifestation of the disorder. Our findings also expand upon previous work in that we demonstrate a strong association between affective temperament and psychosis proneness across patients, unaffected siblings, and control participants. Finally, although we did not have sufficient statistical power to fully investigate the differences in affective temperament and psychosis proneness between siblings of probands with and without psychosis, our data suggest that siblings of probands with psychosis scored higher on a measure of psychosis proneness than siblings of probands without a psychosis history.
Several previous reports have investigated affective temperament between patients, relatives, and controls and have documented inconsistent results. In particular, several groups have found increased levels of cyclothymic temperament in relatives compared to controls (Chiaroni et al., 2005; Mendlowicz et al., 2005; Vázquez et al., 2008) whereas others have not (Evans et al., 2005; Gandotra et al., 2011; Kesebir et al., 2005). Using a strict statistical correction for multiple comparisons, we did not find a difference between relatives and controls for the cyclothymic temperament, although we replicate previous findings of elevated cyclothymia in patients compared to controls (Mendlowicz et al., 2005). Consistent with several prior reports (Mendlowicz et al., 2005; Vázquez et al., 2008), we also found that relatives demonstrated significantly elevated scores on the anxious and irritable subscales of the TEMPS-A compared to controls. Prior work has demonstrated that relatives were elevated compared to controls on the dysthymic subscale (Vázquez et al., 2008). The current results suggest that relatives and controls do not differ on this subscale, as has also been previously reported (Evans et al., 2005; Mendlowicz et al., 2005), although patients were elevated compared to controls. As the anxious subscale was the only one that demonstrated the expected gradient (i.e., patients < siblings < controls), this temperament may represent the most suitable candidate for a temperamental endophenotype in BPD. However, it must be kept in mind when interpreting our results that we used a conservative correction for multiple comparisons. Without such a correction, unaffected siblings demonstrated scores that were significantly intermediate between patients and healthy controls on all subscales of the TEMPS-A except for the hyperthymia subscale.
The only affective temperament that did not differ between any of the groups was the hyperthymic temperament. This is consistent with a previous report that did not find any differences between patients, relatives, and controls on this subscale (Mendlowicz et al., 2005) and with another study that found that patients and controls do not differ on this subscale (Vázquez et al., 2008), nor do patients and relatives (Aguiar Ferreira et al., 2012). As noted above, however, there are several studies with contrasting results for the hyperthymia subscale, with some studies reporting that controls score higher than relatives (Evans et al., 2005) and other studies reporting that patients and relatives are elevated on hyperthymia compared to controls (Gandotra et al., 2011; Kesebir et al., 2005). Although these results at first glance may appear to be in conflict, the differences among the studies appear less significant when considering differences in the methodology used to obtain such results. The two studies (Gandotra et al., 2011; Kesebir et al., 2005) that report higher levels of hyperthymia in patients and relatives compared to controls found this to be the case when assessing this subscale categorically. In the study by Gandotra and colleagues (2011), participants were classified as being either high (score greater than or equal to 7 on this subscale) or low (score less than or equal to 6) on hyperthymia. When dichotomized in this way, patients and relatives did show elevated levels of this temperament compared to controls. Similarly, in the study by Kesebir and colleagues (2005), differences in hyperthymia were found only when the dominant affective temperament was determined using a z-score cut-off and rates of this temperament were compared across the groups. When the subscale scores were examined as a continuous variable, there were no differences between the groups. Regarding the apparent discrepancy between studies, such as the current report, that have reported null results for the hyperthymic subscale and those that have found elevations in controls, this may be related to the sample size of the study.
Just as the anxious affective temperament demonstrated a gradient among probands, siblings, and controls, we also found that levels of schizotypy were significantly intermediate among siblings compared to probands and controls. Previous work has demonstrated that patients with BPD have higher levels of schizotypy compared to unaffected relatives (Savitz et al., 2009). To our knowledge, this is the first study to measure schizoptypy directly in a sample of patients, relatives, and controls. Our finding that siblings were significantly intermediate on the interpersonal deficits and disorganized subscales of the SPQ suggests that schizotypy is an endophenotype for BPD. In a previous study examining schizotypy in patients and relatives (but not controls), patients with BPD with psychosis demonstrated higher levels of schizotypy compared to BPD patients without psychosis (Savitz et al., 2009). There is also evidence that relatives of patients with BPD with psychosis have schizotypy scores that are higher than relatives of BPD patients without psychosis (Schürhoff et al., 2005). Given the small number of patients without psychosis in our sample (n = 13), we were unable to detect any statistically significant differences between the two patient groups on any subscale of the SPQ. Numerically, siblings of probands with psychosis had higher mean scores on each subscale of the SPQ compared to siblings of probands without psychosis; however we were unable to detect any statistically significant differences between the two groups. Further examination in a larger sample is required to determine whether SPQ scores are higher in siblings of patients with versus without psychosis.
Correlational analyses suggest that schizotypy and affective temperament are strongly related within the entire sample, with the exception of the hyperthymia subscale, as has been shown previously (Morvan et al., 2011). These results suggest that both affective temperament and tendency toward schizotypy are related aspects of a larger vulnerability to psychopathology. We also found that current symptoms of depression and mania were strongly and positively associated with both affective temperament and schizotypy. The only non-significant correlation regarding mood state was that between symptoms of mania and the hyperthymic subscale of the TEMPS-A. The lack of correlation between the hyperthymia subscale and the majority of the subscales of the TEMPS-A and the SPQ, along with the null results for differences between the groups on the hyperthymic subscale and a lack of correlation with manic symptoms, suggest that this affective temperament may not be as strongly associated with the broader bipolar phenotype as are the other affective temperaments, or that it may be associated with different aspects of the broader bipolar spectrum that were not well-represented in the current sample of predominantly BPD I patients. Several other reports have found that increased hyperthymia may even be a protective factor for patients with BPD. One study found that patients with higher hyperthymia and lower dysthymia scores had lower rates of white matter hyperintensities in the brain and had less risk for suicide than patients with lower hyperthymia and higher dysthymia scores (Serafini et al., 2011). Several other studies have found that hyperthymia appears to be protective against hopelessness (Pompili et al., 2008) and suicidality (Pompili et al., 2012; Rihmer et al., 2009; Vázquez et al., 2010). Rybakowski and colleagues (2013) recently reported a significant positive correlation between lithium response and hyperthymia. Further work is needed to determine what other factors are associated with hyperthymia and how it may function as a protective factor in BPD.
This study has clear strengths, including a sample size as large or larger than that of previous studies that have examined unaffected relatives, as well as a robust design restricting unaffected first-degree relatives to siblings past the age of bipolar illness onset in related probands. Moreover, we used a stringent Bonferroni correction for multiple comparisons, minimizing the likelihood of false positive findings.
There are several limitations to the current study that should be addressed. We did not have a sufficient number of patients without psychosis to be able to examine temperament and psychosis proneness as a function of psychosis history in patients or siblings. Furthermore, in interpreting our results it must be kept in mind that the vast majority of the patients included in this study were diagnosed with BPD I (n = 47 out of 55 total patients) and thus the results cannot necessarily be generalized to the bipolar spectrum. Due to the conservative nature of our statistical correction, the possibility of Type II error (false negative findings) cannot be discounted. Without Bonferroni correction, unaffected siblings demonstrated scores on all subscales of the SPQ and the TEMPS-A, with the exception of the hyperthymia subscale, that were significantly intermediate between patients and controls. Thus, our conservative statistical approach may have resulted in false negative findings regarding the intermediacy of the unaffected siblings.
Nevertheless, the current study replicates and extends previous work demonstrating that unaffected relatives of patients with BPD demonstrate attenuated levels of affective temperament compared to patients and elevated levels compared to controls. In addition, we provide evidence that psychosis proneness is an endophenotype for BPD by demonstrating that siblings have levels of this trait that are intermediate between patients and controls. Further work is required to investigate the potential protective effects of the hyperthymic affective temperament, as well as to understand the relationship between psychosis status, affective temperament, and psychosis proneness in both patients and relatives.
Acknowledgments
This research is supported by grants from the National Institute of Mental Health (NIMH) including 1R03MH079995 and 1K23MH077807 (to KEB). Additional support (to MPR) was provided by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the Veterans Affairs James J Peters VAMC, and the Department of Veterans Affairs NY/NJ (VISN3) Mental Illness Research, Education, and Clinical Center (MIRECC).
Footnotes
Conflicts of Interests:
The authors affirm that they have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ferreira Aguiar, de A, Vasconcelos AG, Neves FS, Laks J, Correa H. Affective temperaments: Familiality and clinical use in mood disorders. J Affect Disord. 2012;148:53–6. doi: 10.1016/j.jad.2012.11.047. [DOI] [PubMed] [Google Scholar]
- Akiskal HS. Toward a definition of generalized anxiety disorder as an anxious temperament type. Acta Psychiatr Scand Suppl. 1998;393:66–73. doi: 10.1111/j.1600-0447.1998.tb05969.x. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, Downs J, Jordan P, Watson S, Daugherty D, Pruitt DB. Affective disorders in referred children and younger siblings of manic-depressives. Mode of onset and prospective course. Arch Gen Psychiatry. 1985;42:996–1003. doi: 10.1001/archpsyc.1985.01790330076009. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, Mallya G. Criteria for the “soft” bipolar spectrum: treatment implications. Psychopharmacol Bull. 1987;23:68–73. [PubMed] [Google Scholar]
- Akiskal HS, Mendlowicz MV, Jean-Louis G, Rapaport MH, Kelsoe JR, Gillin JC, Smith TL. TEMPS-A: validation of a short version of a self-rated instrument designed to measure variations in temperament. J Affect Disord. 2005;85:45–52. doi: 10.1016/j.jad.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Altman EG, Hedeker DR, Janicak PG, Peterson JL, Davis JM. The Clinician-Administered Rating Scale for Mania (CARS-M): development, reliability, and validity. Biol Psychiatry. 1994;36:124–134. doi: 10.1016/0006-3223(94)91193-2. [DOI] [PubMed] [Google Scholar]
- Bellivier F, Golmard JL, Rietschel M, Schulze TG, Malafosse A, Preisig M, McKeon P, Mynett-Johnson L, Henry C, Leboyer M. Age at onset in bipolar I affective disorder: further evidence for three subgroups. Am J Psychiatry. 2003;160:999–1001. doi: 10.1176/appi.ajp.160.5.999. [DOI] [PubMed] [Google Scholar]
- Cassano GB, Akiskal HS, Perugi G, Musetti L, Savino M. The importance of measures of affective temperaments in genetic studies of mood disorders. J Psychiatr Res. 1992;26:257–268. doi: 10.1016/0022-3956(92)90032-j. [DOI] [PubMed] [Google Scholar]
- Chiaroni P, Hantouche E-G, Gouvernet J, Azorin J-M, Akiskal HS. The cyclothymic temperament in healthy controls and familially at risk individuals for mood disorder: endophenotype for genetic studies? J Affect Disord. 2005;85:135–145. doi: 10.1016/j.jad.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Claridge G, McCreery C, Mason O, Bentall R, Boyle G, Slade P, Popplewell D. The factor structure of “schizotypal’ traits: a large replication study. Br J Clin Psychol Br Psychol Soc. 1996;35(Pt 1):103–115. doi: 10.1111/j.2044-8260.1996.tb01166.x. [DOI] [PubMed] [Google Scholar]
- Evans L, Akiskal HS, Keck PE, McElroy SL, Jr, Sadovnick AD, Remick RA, Kelsoe JR. Familiality of temperament in bipolar disorder: support for a genetic spectrum. J Affect Disord. 2005;85:153–168. doi: 10.1016/j.jad.2003.10.015. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Willians JBW. Structured Clinical Interview for DSM IV TR Axis I Disorders, Patient Edition (SCID-I/P) NewYork: Biometrics Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- Gandotra S, Ram D, Kour J, Praharaj SK. Association between affective temperaments and bipolar spectrum disorders: preliminary perspectives from a controlled family study. Psychopathology. 2011;44:216–224. doi: 10.1159/000322691. [DOI] [PubMed] [Google Scholar]
- Goodwin F, Jamison K. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. Oxford University Press; New York: 2007. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, McGuire M, Gruenberg AM, Walsh D. Schizotypal symptoms and signs in the Roscommon Family Study. Their factor structure and familial relationship with psychotic and affective disorders. Arch Gen Psychiatry. 1995;52:296–303. doi: 10.1001/archpsyc.1995.03950160046009. [DOI] [PubMed] [Google Scholar]
- Kesebir S, Vahip S, Akdeniz F, Yüncü Z, Alkan M, Akiskal H. Affective temperaments as measured by TEMPS-A in patients with bipolar I disorder and their first-degree relatives: a controlled study. J Affect Disord. 2005;85:127–133. doi: 10.1016/j.jad.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Mendlowicz MV, Jean-Louis G, Kelsoe JR, Akiskal HS. A comparison of recovered bipolar patients, healthy relatives of bipolar probands, and normal controls using the short TEMPS-A. J Affect Disord. 2005;85:147–151. doi: 10.1016/j.jad.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Morvan Y, Tibaoui F, Bourdel MC, Lôo H, Akiskal KK, Akiskal HS, Krebs MO. Confirmation of the factorial structure of temperamental autoquestionnaire TEMPS-A in non-clinical young adults and relation to current state of anxiety, depression, and to schizotypal traits. J Affect Disord. 2011;131:37–44. doi: 10.1016/j.jad.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Placidi GF, Signoretta S, Liguori A, Gervasi R, Maremmani I, Akiskal HS. The semi-structured affective temperament interview (TEMPS-I). Reliability and psychometric properties in 1010 14–26-year old students. J Affect Disord. 1998;47:1–10. doi: 10.1016/s0165-0327(97)00122-5. [DOI] [PubMed] [Google Scholar]
- Pompili M, Rihmer Z, Akiskal HS, Innamorati M, Iliceto P, Akiskal KK, Lester D, Narciso V, Ferracuti S, Tatarelli R, et al. Temperament and personality dimensions in suicidal and nonsuicidal psychiatric inpatients. Psychopathology. 2008;41:313–321. doi: 10.1159/000146069. [DOI] [PubMed] [Google Scholar]
- Pompili M, Innamorati M, Rihmer Z, Gonda X, Serafini G, Akiskal H, Amore M, Niolu C, Sher L, Tatarelli R, et al. Cyclothymic-depressive-anxious temperament pattern is related to suicide risk in 346 patients with major mood disorders. J Affect Disord. 2012;136:405–411. doi: 10.1016/j.jad.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Rihmer A, Rozsa S, Rihmer Z, Gonda X, Akiskal KK, Akiskal HS. Affective temperaments, as measured by TEMPS-A, among nonviolent suicide attempters. J Affect Disord. 2009;116:18–22. doi: 10.1016/j.jad.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Rossi A, Daneluzzo E. Schizotypal dimensions in normals and schizophrenic patients: a comparison with other clinical samples. Schizophr Res. 2002;54:67–75. doi: 10.1016/s0920-9964(01)00353-x. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Dembinska D, Kliwicki S, Akiskal KK, Akiskal HH. TEMPS-A and long-term lithium response: positive correlation with hyperthymic temperament. J Affect Disord. 2013;145:187–189. doi: 10.1016/j.jad.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Savitz JB, Ramesar RS. Personality: is it a viable endophenotype for genetic studies of bipolar affective disorder? Bipolar Disord. 2006;8:322–337. doi: 10.1111/j.1399-5618.2006.00309.x. [DOI] [PubMed] [Google Scholar]
- Savitz J, van der Merwe L, Ramesar R. Dysthymic and anxiety-related personality traits in bipolar spectrum illness. J Affect Disord. 2008a;109:305–311. doi: 10.1016/j.jad.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Savitz J, van der Merwe L, Ramesar R. Hypomanic, cyclothymic and hostile personality traits in bipolar spectrum illness: a family-based study. J Psychiatr Res. 2008b;42:920–929. doi: 10.1016/j.jpsychires.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Savitz J, van der Merwe L, Stein DJ, Solms M, Ramesar R. Neuropsychological status of bipolar I disorder: impact of psychosis. Br J Psychiatry J Ment Sci. 2009;194:243–251. doi: 10.1192/bjp.bp.108.052001. [DOI] [PubMed] [Google Scholar]
- Schürhoff F, Laguerre A, Szöke A, Méary A, Leboyer M. Schizotypal dimensions: continuity between schizophrenia and bipolar disorders. Schizophr Res. 2005;80:235–242. doi: 10.1016/j.schres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Serafini G, Pompili M, Innamorati M, Fusar-Poli P, Akiskal HS, Rihmer Z, Lester D, Romano A, de Oliveira IR, Strusi L, et al. Affective temperamental profiles are associated with white matter hyperintensity and suicidal risk in patients with mood disorders. J Affect Disord. 2011;129:47–55. doi: 10.1016/j.jad.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Vázquez GH, Kahn C, Schiavo CE, Goldchluk A, Herbst L, Piccione M, Saidman N, Ruggeri H, Silva A, Leal J, et al. Bipolar disorders and affective temperaments: a national family study testing the “endophenotype” and “subaffective” theses using the TEMPS-A Buenos Aires. J Affect Disord. 2008;108:25–32. doi: 10.1016/j.jad.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Vázquez GH, Gonda X, Zaratiegui R, Lorenzo LS, Akiskal K, Akiskal HS. Hyperthymic temperament may protect against suicidal ideation. J Affect Disord. 2010;127:38–42. doi: 10.1016/j.jad.2010.04.015. [DOI] [PubMed] [Google Scholar]