Abstract
Purpose
Compare performance of normalized reflectance index (NRI) and retinal nerve fiber layer thickness (RNFLT) parameters determined from OCT images for glaucoma and glaucoma suspect diagnosis.
Methods
Seventy-five eyes from seventy-one human subjects were studied: 33 controls, 24 glaucomatous, and 18 glaucoma-suspects. RNFLT and NRI maps were measured using two custom-built OCT systems and the commercial instrument RTVue. Using area under the receiver operating characteristic (ROC) curve, RNFLT and NRI measured in seven RNFL locations were analyzed to distinguish between control, glaucomatous, and glaucoma-suspect eyes.
Results
The mean NRI of the control group was significantly larger than the means of glaucomatous and glaucoma-suspect groups in most RNFL locations for all three OCT systems (p<0.05 for all comparisons). NRI performs significantly better than RNFLT at distinguishing between glaucoma-suspect and control eyes using RTVue OCT (p=0.008). The performances of NRI and RNFLT for classifying glaucoma-suspect vs. control eyes were statistically indistinguishable for PS-OCT-EIA (p=0.101) and PS-OCT-DEC (p=0.227). The performances of NRI and RNFLT for classifying glaucomatous vs. control eyes were statistically indistinguishable (PS-OCT-EIA: p=0.379; PS-OCT-DEC: p=0.338; RTVue OCT: p=0.877).
Conclusions
NRI is a promising measure for distinguishing between glaucoma-suspect and control eyes and may indicate disease in the pre-perimetric stage. Results of this pilot clinical study warrant a larger study to confirm the diagnostic power of NRI for diagnosing pre-perimetric glaucoma.
Keywords: glaucoma, optical coherence tomography, retinal nerve fiber layer
Introduction
Glaucoma is a progressive disease characterized by loss of retinal ganglion cells and their axons in the retinal nerve fiber layer (RNFL). Multiple clinical approaches are employed for glaucoma diagnosis, including morphological assessment of the optic nerve and visual field testing. Optic nerve imaging devices such as GDx VCC (Carl Zeiss Meditec, Inc, Dublin, CA), Heidelberg Retinal Tomography (HRT, Heidelberg Engineering, GmbH, Dossenheim, Germany), and Optical Coherence Tomography (OCT) (e.g., RTVue, Optovue, Inc., Fremont, CA) are widely used to assist in glaucoma diagnosis and monitoring. Early detection of glaucoma or disease progression is important because effective treatments are available to preserve visual function.
Optical Coherence Tomography (OCT) is a noninvasive imaging method that provides high-resolution quantitative morphological information about the RNFL and optic nerve. A recent study reports a sensitivity of 85% and specificity of 94% for distinguishing between glaucoma and control eyes using RNFLT measured by the Cirrus OCT instrument.1 Wu et al. reports that statistical RNFLT parameters for evaluating the diagnostic performance of the Spectralis OCT system (Heidelberg Engineering, Heidelberg, Germany) are good for diagnosing early perimetric glaucoma (AUC=0.895) and excellent for moderately advanced glaucoma (AUC=0.952).2 Polarization Sensitive Optical Coherence Tomography (PS-OCT) has emerged as a candidate technique for glaucoma diagnosis. PS-OCT provides both depth-resolved morphological images, RNFLT and RNFL birefringence (Δn).3 Studies using OCT to distinguish glaucoma-suspect vs. control eyes are inconclusive. Some studies using OCT did not find significant RNFLT differences between control and ocular hypertensive or glaucoma-suspect eyes.4, 5 Other studies using OCT reported promising results for using changes in RNFLT to detect early structural damage in glaucoma-suspect eyes.6–8 Determining the RNFL properties that best distinguish between control and glaucoma-suspect eyes requires further study.
In a longitudinal glaucoma study involving non-human primates using OCT, a RNFL reflectance parameter, reflectivity index (RI) was introduced for distinguishing between early onset glaucomatous vs. control eyes.9 Study results suggest that RNFL reflectance (RI) might be an earlier indicator of glaucoma onset than RNFLT, phase retardation (PR), or birefringence (Δn).9 A recent human study suggested that a RNFL reflectance parameter can be used for glaucoma assessment.10 We report results of a cross-sectional study on human eyes using a normalized RNFL reflectance index (NRI) which is RI * RNFLT. Performance of RNFLT and NRI, are compared for distinguishing glaucomatous vs. control eyes and glaucoma-suspect vs. control eyes.
METHODS
Subjects and study protocol
Two study groups are presented. The first group consisted of 34 eyes (13 control, 9 glaucomatous, and 12 glaucoma-suspect) from 33 human subjects enrolled at the Eye Institute of Austin (EIA). The first study group was imaged with a custom polarization-sensitive OCT system (PS-OCT-EIA) and a commercial OCT system (RTVue). The second group consisted of 41 eyes (20 control, 15 glaucomatous, and 6 glaucoma-suspect) from 38 human subjects enrolled at the Duke Eye Center (DEC). The second group was imaged with a second custom polarization-sensitive OCT system (PS-OCT-DEC). Both studies were designed to evaluate RNFL birefringence, RNFLT, RI, and NRI for glaucoma diagnostics. We report only the RNFLT and NRI since birefringence and RI were found to be less useful for detecting glaucoma (supplement Table e4–7). Both eyes of each study participant were imaged. For each subject with the same diagnosis for both eyes (e.g., glaucomatous), the eye providing the best quality images was selected for further analysis. For data recorded with PS-OCT instruments, three imaging measurements were recorded from each eye. The image with least number of un-processable clusters (e.g., A-scans affected by eye blinking or cases when the RNFL is outside the effective imaging depth) was selected and processed. For RTVue OCT, the eye with highest scan score index (SSI) was selected. For glaucoma patients with one glaucomatous eye and one glaucoma-suspect eye, both eyes were included in the data analysis since the study does not directly compare glaucomatous vs. glaucoma-suspect eyes. Mean-age and standard deviation together with gender distribution, mean and standard deviation of visual field mean deviation (VF MD) and visual field pattern standard deviation (VF PSD) are indicated in Table 1.
Table 1.
Mean-age and standard deviation together with gender distribution, mean and standard deviation of visual field mean deviation (VF MD) and visual field pattern standard deviation (VF PSD) of control (ct), glaucomatous (gl), and glaucoma-suspect (gs) eyes imaged by PS-OCT-EIA, PS-OCT-DEC and RTVue OCT.
| PS-OCT-EIA | ct (n=13) | gl (n=9) | gs (n=12) | |
| Age (years) | 54.46 ± 7.60 | 65.56 ± 6.39 | 66.17 ± 8.30 | |
| Gender | ||||
| Male | 3 | 4 | 6 | |
| Female | 10 | 5 | 6 | |
| VF MD | -0.33 ± 1.49 | -3.02 ± 2.13 | -0.57 ± 1.65 | |
| VF PSD | 1.98 ± 1.07 | 4.83 ± 3.17 | 2.24 ± 1.93 | |
| PS-OCT-DEC | ct (n=20) | gl (n=15) | gs (n=6) | |
| Age (years) | 58.25 ± 7.47 | 67.27 ± 6.40 | 66.00 ± 8.15 | |
| Gender | ||||
| Male | 9 | 4 | 2 | |
| Female | 11 | 11 | 4 | |
| VF MD | -0.54 ± 1.86 | -5.00 ± 4.82 | 0.58 ± 1.99 | |
| VF PSD | 2.17 ± 1.45 | 6.14 ± 4.46 | 1.80 ± 0.29 | |
| RTVue OCT | ct (n=13) | gl (n=9) | gs (n=12) | |
| Age (years) | 54.46 ± 7.60 | 65.56 ± 6.39 | 66.17 ± 8.30 | |
| Gender | ||||
| Male | 3 | 4 | 6 | |
| Female | 10 | 5 | 6 | |
| VF MD | 0.10 ± 1.20 | -3.00 ± 2.15 | -0.35 ± 1.58 | |
| VF PSD | 1.98 ± 1.10 | 4.42 ± 3.39 | 1.73 ± 0.53 | |
Eligibility to participate in the study was based on medical and ocular history and a comprehensive eye examination including standard disc photography and results of a Humphrey-Zeiss 24-2 (Carl Zeiss Meditec, Inc, Dublin, CA) visual field test. Inclusion and exclusion criteria and definitions are given in Table 2 and Table 3. Using the definitions in Table 3, each eye was classified as normal, glaucoma, or glaucoma suspect by a glaucoma expert at either EIA or DEC. The two studies are considered separately because the expert classification and the instrumentation were different at the two study sites.
Table 2.
Inclusion and exclusion criteria for the study.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Table 3.
Definitions of control, glaucoma, and glaucoma-suspect.
| Control | Glaucoma | Glaucoma-suspect* |
|---|---|---|
| Has an intraocular pressure (IOP) less than 21 mmHg with no history of elevated IOP, normal visual fields [mean deviation and pattern standard deviation (PSD) within 95% confidence limits and Glaucoma Hemifield Test (GHT) within normal limits], and no optic disc abnormalities judged by a glaucoma specialist (H.G.R. at EIA and S.J.M. at DEC). |
Has history of elevated IOP, two consecutive abnormal visual fields (PSD outside the 95% confidence limits, abnormal GHT, or any typical visual field defect), and an abnormal optic disc. |
Ocular hypertension: Has an IOP higher than 21 mmHg but less than 30 mmHg measured in at least three separate office visits and have normal optic nerve head appearance. Preperimetric glaucoma: Has an asymmetric cup-to-disc ratio and show early glaucomatous optic disc abnormality, including thinning of the neuroretinal rim and notching. |
All subjects belonging to the glaucoma-suspect group have normal visual field test results as defined in the control group.
This study was approved by the Institutional Review Boards at The University of Texas at Austin and at Duke University Medical Center (NCT #01222065).
Instrumentation
Two custom built polarization-sensitive OCT instruments (PS-OCT-EIA and PS-OCT-DEC) and one commercial OCT instrument (RTVue OCT) were employed for retinal imaging (see supplemental eTable 1). The basic design of the two custom OCT systems operating at 1060nm was described previously3. The RTVue uses a superluminescent diode light source with a center wavelength of 840 nm.
RNFLT and NRI calculation
For PS-OCT-EIA and PS-OCT-DEC, RNFLT(r,θ) in µm was calculated by a custom LabVIEW software program (National Instruments, Austin, Texas) to automatically detect RNFL and RPE region boundaries in each B-scan.3, 11,12 RNFLT(r,θ) of RTVue OCT is provided by RTVue software version 4.0.5.39.
The normalized RNFL reflectance index (NRI) is defined as the ratio of the integrated OCT RNFL intensities (IRNFL) to the average OCT intensity of a thin layer centered on the retinal pigment epithelium (RPE). NRI is the intensity of back-reflected light summed over the RNFL normalized by the intensity of back-reflected light measured over the RPE. The RI reported in the primate study is NRI/RNFLT. Advantages of NRI over RI are: 1) the measure is unitless whereas the RI has units of inverse length; 2) NRI is somewhat less prone to error since the pixel intensities in the RNFL are simply summed whereas in computing RI, errors in RNFLT can be introduced; 3) because NRI is a measure of the composite RNFL reflectivity, this measure is sensitive to reductions in either thickness or reflectivity.
For OCT data, we define NRI(r,θ) for one cluster at radius r and azimuth angle θ as:
| (1) |
where,
| (2) |
corresponding to the summed OCT signal intensity IRNFLi in the RNFL in one cluster, where Na is number of pixels in cluster ‘c’ located at r and θ.
Average OCT signal intensity within a thin layer about the retinal pigment epithelium is calculated as:
| (3) |
where Na is the number of pixels in the band containing the RPE in one B-scan, IRPEa is the OCT signal in this band, Nb is the number of B-scans in each image collection, and NRPE is the number of pixels (7 pixels or 33 µm) in the band containing the RPE in one B-scan.
Since the RTVue OCT does not record clustered data, we define NRI(r,θ) for one A-scan at radius r and azimuth angle θ as:
| (4) |
Where Ni is number of pixels in the RNFL in one A-scan, IRNFLi is the image intensity value in the RNFL and
| (5) |
is the average OCT image intensity in the RPE averaged over all B-scans in one image collection, where Na is the number of pixels in the band containing the RPE in one B-scan, IRPEa is the image intensity in this band, Nb is the number of B-scans in each image collection, and NRPE is the number of pixels (7 pixels or 70 µm) in the band containing the RPE. For one imaging session, we calculate NRI for A-scans in one retinal scan and then construct an NRI map for that scan.
For both EIA and DEC OCT systems, seven RNFL locations were analyzed: all-rings, inner 5 rings, outer 5 rings, temporal (T), superior (S), nasal (N), and inferior (I) quadrants (Figure 1). For the RTVue OCT system, seven RNFL locations were analyzed: all-rings, inner 7 rings, outer 6 rings, temporal (T), superior (S), nasal (N), and inferior (I) quadrants.
Figure 1.
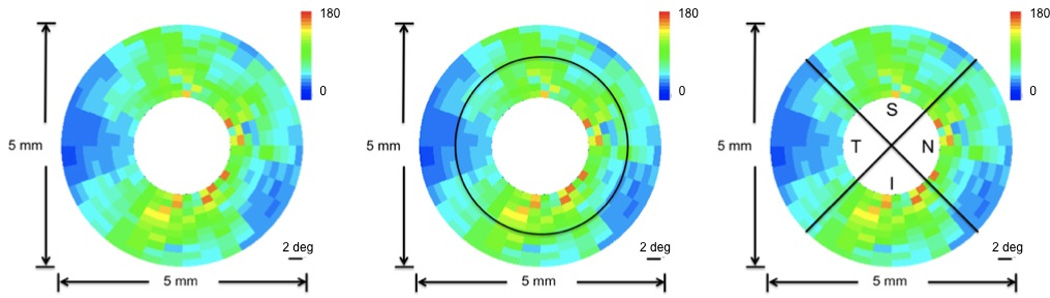
Definitions of analyzed RNFL locations of EIA and DEC OCT datasets illustrated on a clustered RNFLT map of a human eye (OD). Average computed across all-rings (left panel). Averages computed over 5 inner rings (inner) and 5 outer rings (outer) (middle panel). Averages computed over the temporal (T), superior (S), nasal (N) and inferior (I) quadrants (right panel).
The equations used to calculate RNFLT and NRI in the seven RNFL locations are given in Table 4.
Table 4.
The calculation of average values of RNFLT(r,θ) and NRI(r,θ) in seven RNFL locations.
| RNFL location |
Equation | Instrument-specific RNFL properties | |
|---|---|---|---|
| All rings |
|
P(rθ) – RNFL properties including RNFLT(rθ) or NRI(rθ). rin – the radius of the innermost scanning ring. rout – the radius of the outermost scanning ring. Na – total number of A-scans in one measurement. NINNER – number of A-scans in inner rings. NOUTER – number of A-scans in outer rings. NS – number of A-scans in superior quadrant. NI – number of A-scans in inferior quadrant. NN – number of A-scans in nasal quadrant. NT – number of A-scans in temporal quadrant. PS-OCT-EIA: rin = 1 mm rout = 2.5 mm Na = 36000 (100 A-scan/cluster × 36 clusters/ring × 10 rings) PS-OCT-DEC: rin = 0.75 mm rout = 2.5 mm Na = 36000 (100 A-scan/cluster × 36 clusters/ring × 10 rings) RTVue OCT: rin = 0.65 mm rout = 2.45 mm Na = 8681 (425 A-scan/ring × 4 rings + 587 A-scan/ring × 3 rings + 775 A-scan/ring × 3 rings + 965 A-scan/ring × 3 rings) |
|
| Inner rings |
|
||
| Outer rings |
|
||
| Superior |
|
||
| Inferior |
|
||
| Nasal | OS: |
||
|
OD: |
|||
| Temporal | OS: |
||
|
OD: |
Statistical analysis
The area under the Receiver Operating Characteristic (ROC) curve (AUC) was used to compare the performance of RNFLT and NRI for distinguishing between glaucoma and control subjects as well as between glaucoma-suspect and control subjects. Differences between areas under ROC curves were compared using a non-parametric method based on bootstrap sampling (n=2000 resamples). We used the pROC package13 in the R statistical programming language (v2.15.10; http://www.R-project.org/, R Development Core Team, 2012, R Foundation for Statistical Computing, Vienna, Austria) and R studio (v0.94, RStudio, Inc.) for the ROC analysis. PASS 11 software (NCSS, Kaysville, Utah 84037) was used for statistical power and sample size calculations. Two sample t-test with equal variance was used for comparisons of the means of NRI and RNFLT of the glaucomatous, glaucoma-suspect, and control groups.
Results
We calculated the average NRI and RNFLT in seven RNFL locations. Pairwise comparisons among average NRI and RNFLT in different RNFL locations for distinguishing between glaucomatous and control eyes as well as between glaucoma-suspect and control eyes were made in terms of the area under the ROC curve. The average and standard deviation of NRI and RNFLT measured by PS-OCT-EIA, PS-OCT-DEC, and RTVue OCT in 7 RNFL locations of glaucomatous, glaucoma-suspect and control groups are shown in Table 5 and Table 6. For all three OCT instruments, NRIs of the control group are significantly larger than those of the glaucomatous group in all RNFL locations (p value shown in Table 6). NRIs of the control group are significantly larger than those of the glaucoma-suspect group in most RNFL locations (p value in Table 6).
Table 5.
The average and standard deviation of RNFLT measured by PS-OCT-EIA, PS-OCT-DEC and RTVue OCT in 7 RNFL locations of glaucomatous (gl), glaucoma-suspect (gs) and control (ct) groups.
| PS-OCT-EIA | RNFLT (µm) | ct (n=13) | gl (n=9) | gs (n=12) |
p value of gl vs. ct |
p value of gs vs. ct |
| ALL rings | 84.058 ± 13.539 | 65.652 ± 2.962 | 70.387 ± 11.168 | 0.00036* | 0.00583* | |
| INNER rings | 93.343 ± 14.182 | 73.150 ± 4.469 | 77.586 ± 11.740 | 0.00028* | 0.00311* | |
| OUTER rings | 72.9 27 ±12.748 | 57.672 ± 3.174 | 63.226 ± 11.466 | 0.00115* | 0.02907* | |
| Superior | 99.292 ± 17.518 | 77.462 ± 8.426 | 75.939 ± 11.742 | 0.00126* | 0.00038* | |
| Inferior | 94.074 ± 16.764 | 70.147 ± 14.651 | 77.062 ± 13.814 | 0.00124* | 0.00563* | |
| Nasal | 75.399 ± 20.390 | 64.803 ± 10.015 | 65.919 ± 5.705 | 0.08324 | 0.06702 | |
| Temporal | 65.693 ± 13.826 | 49.648 ± 10.009 | 62.562 ± 32.709 | 0.00375* | 0.37731 | |
| PS-OCT-DEC | RNFLT (µm) | ct (n=20) | gl (n=15) | gs (n=6) | ||
| ALL rings | 67.511 ± 4.479 | 58.730 ± 5.410 | 62.484 ± 3.664 | 0.00000* | 0.00984* | |
| INNER rings | 70.736 ± 7.045 | 61.604 ± 6.965 | 65.314 ± 3.534 | 0.00029* | 0.04226* | |
| OUTER rings | 64.454 ± 4.780 | 55.651 ± 4.866 | 59.939 ± 5.556 | 0.00000* | 0.03093* | |
| Superior | 76.470 ± 12.235 | 63.502 ± 10.656 | 67.817 ± 7.511 | 0.00124* | 0.05821 | |
| Inferior | 73.212 ± 9.293 | 61.957 ± 7.774 | 60.951 ± 8.425 | 0.00030* | 0.00404* | |
| Nasal | 62.939 ± 12.728 | 57.342 ± 6.319 | 62.791 ± 8.115 | 0.06406 | 0.48946 | |
| Temporal | 57.215 ± 9.665 | 52.179 ± 6.498 | 57.775 ± 9.485 | 0.04547* | 0.54918 | |
| RTVue OCT | RNFLT (µm) | ct (n=13) | gl (n=9) | gs (n=12) | ||
| ALL rings | 125.293 ± 12.514 | 98.673 ± 12.624 | 114.804 ± 17.194 | 0.00004* | 0.04635* | |
| INNER rings | 153.603 ± 19.016 | 117.046 ± 16.828 | 140.348 ± 24.304 | 0.00008* | 0.07039* | |
| OUTER rings | 92.264 ± 6.793 | 77.234 ± 8.474 | 85.003 ± 9.349 | 0.00008* | 0.01772* | |
| Superior | 157.290 ± 18.123 | 124.313 ± 18.901 | 139.969 ± 22.671 | 0.00026* | 0.02258* | |
| Inferior | 160.741 ± 13.343 | 122.047 ± 22.750 | 144.199 ± 20.848 | 0.00003* | 0.01292* | |
| Nasal | 94.652 ± 18.365 | 69.255 ± 9.165 | 87.178 ± 22.398 | 0.00054* | 0.18470 | |
| Temporal | 88.487 ± 12.132 | 79.074 ± 16.933 | 87.864 ± 14.667 | 0.07164 | 0.45429 |
Table 6.
The average and standard deviation of NRI measured by PS-OCT-EIA, PS-OCT-DEC and RTVue OCT in 7 RNFL locations of glaucomatous (gl), glaucoma-suspect (gs) and control (ct) groups.
| PS-OCT-EIA | NRI | ct (n=13) | gl (n=9) | gs (n=12) |
p value of gl vs. ct |
p value of gs vs. ct |
| ALL rings | 2226.369 ± 219.516 | 1633.200 ± 224.668 | 1720.683 ± 178.260 | 0.00000* | 0.00000* | |
| INNER rings | 2489.908 ± 257.980 | 1812.722 ± 312.250 | 1896.192 ± 224.131 | 0.00001* | 0.00000* | |
| OUTER rings | 1909.754 ± 174.941 | 1455.778 ± 242.103 | 1543.258 ± 155.460 | 0.00003* | 0.00001* | |
| Superior | 2549.269 ± 440.515 | 1867.433 ± 192.533 | 1845.283 ± 270.317 | 0.00016* | 0.00004* | |
| Inferior | 2540.662 ± 316.212 | 1786.278 ± 592.825 | 1903.775 ± 322.501 | 0.00046* | 0.00002* | |
| Nasal | 2049.346 ± 399.788 | 1525.800 ± 197.058 | 1626.658 ± 158.909 | 0.00086* | 0.00118* | |
| Temporal | 1720.054 ± 295.773 | 1297.898 ± 310.042 | 1486.797 ± 606.125 | 0.00211* | 0.11402 | |
| PS-OCT-DEC | NRI | ct (n=20) | gl (n=15) | gs (n=6) | ||
| ALL rings | 1562.440± 190.372 | 1100.141± 191.161 | 1221.933± 170.761 | 0.00000* | 0.00032* | |
| INNER rings | 1635.695± 259.396 | 1148.511± 228.728 | 1316.150± 201.610 | 0.00000* | 0.00541* | |
| OUTER rings | 1497.005± 198.606 | 1043.759± 169.603 | 1148.588± 223.010 | 0.00000* | 0.00060* | |
| Superior | 1801.783± 429.505 | 1217.025± 333.845 | 1368.400± 215.503 | 0.00006* | 0.01339* | |
| Inferior | 1710.158± 330.698 | 1114.308± 270.276 | 1198.192± 327.767 | 0.00000* | 0.00139* | |
| Nasal | 1407.556± 383.236 | 1057.143± 180.055 | 1213.545± 312.360 | 0.00125* | 0.13529 | |
| Temporal | 1299.774± 301.778 | 979.251± 184.080 | 1090.285± 263.628 | 0.00047* | 0.06960 | |
| RTVue OCT | NRI | ct (n=13) | gl (n=9) | gs (n=12) | ||
| ALL rings | 13.001± 2.066 | 8.110± 2.166 | 9.957± 1.772 | 0.00002* | 0.00033* | |
| INNER rings | 15.230± 2.999 | 9.106± 2.402 | 11.411± 2.522 | 0.00003* | 0.00114* | |
| OUTER rings | 10.401± 1.343 | 6.948± 2.148 | 8.260± 1.143 | 0.00008* | 0.00014* | |
| Superior | 16.066± 2.889 | 9.777± 3.083 | 11.736± 2.965 | 0.00004* | 0.00059* | |
| Inferior | 17.149± 2.519 | 10.495± 3.313 | 12.585± 1.864 | 0.00002* | 0.00002* | |
| Nasal | 9.614± 2.385 | 5.890± 1.773 | 7.304± 2.069 | 0.00037* | 0.00843* | |
| Temporal | 9.174± 1.798 | 6.278± 1.841 | 8.202± 2.587 | 0.00074* | 0.14166 |
Glaucomatous vs. control eyes
We identified the RNFL location that provided the largest AUC for each RNFL property for all three OCT instruments (values superscripted with “*” in Table 7,8 and 9; ROC curves shown in Figure 2). For the PS-OCT-EIA dataset, the all-rings average of NRI (NRIALL) and the inner-rings average of RNFLT (RNFLTINNER) gave the largest AUCs. For PS-OCT-EIA data, a significant difference between AUC of RNFLTINNER and NRIALL was not observed (p=0.379) for the task of distinguishing between glaucomatous and control eyes.
Table 7.
AUC and its standard error of NRI and RNFLT averaged over seven RNFL locations for PS-OCT-EIA dataset for distinguishing glaucomatous vs. control eyes.
| RNFL location |
Glaucomatous vs. Control | |
|---|---|---|
| NRI | RNFLT | |
| AUC | AUC | |
| ALL rings | 0.983 ± 0.021* | 0.932 ± 0.056 |
| INNER rings | 0.923 ± 0.078 | 0.940 ± 0.050* |
| OUTER rings | 0.966 ± 0.032 | 0.897 ± 0.071 |
| Superior | 0.923 ± 0.060 | 0.872 ± 0.076 |
| Inferior | 0.872 ± 0.086 | 0.889 ± 0.068 |
| Nasal | 0.872 ± 0.076 | 0.735 ± 0.112 |
| Temporal | 0.838 ± 0.096 | 0.838 ± 0.089 |
Table 8.
AUC and its standard errors of NRI and RNFLT averaged over seven RNFL locations for the PS-OCT-DEC dataset for distinguishing glaucomatous vs. control eyes.
| RNFL location |
Glaucomatous vs. Control | |
|---|---|---|
| NRI | RNFLT | |
| AUC | AUC | |
| ALL rings | 0.957 ± 0.032 | 0.880 ± 0.065 |
| INNER rings | 0.947 ± 0.037 | 0.790 ± 0.083 |
| OUTER rings | 0.963 ± 0.026* | 0.910 ± 0.058* |
| Superior | 0.860 ± 0.063 | 0.783 ± 0.081 |
| Inferior | 0.920 ± 0.044 | 0.837 ± 0.071 |
| Nasal | 0.813 ± 0.075 | 0.635 ± 0.097 |
| Temporal | 0.823 ± 0.073 | 0.657 ± 0.095 |
Table 9.
AUC and standard errors for distinguishing glaucomatous vs. control eyes of NRI and RNFLT averaged over seven RNFL locations from the RTVue OCT dataset.
| RNFL location |
Glaucomatous vs. Control | |
|---|---|---|
| NRI | RNFLT | |
| AUC | AUC | |
| ALL rings | 0.957 ± 0.037* | 0.949 ± 0.043* |
| INNER rings | 0.957 ± 0.037 | 0.940 ± 0.046 |
| OUTER rings | 0.949 ± 0.043 | 0.932 ± 0.054 |
| Superior | 0.949 ± 0.043 | 0.897 ± 0.065 |
| Inferior | 0.949 ± 0.043 | 0.906 ± 0.074 |
| Nasal | 0.923 ± 0.059 | 0.915 ± 0.061 |
| Temporal | 0.872 ± 0.075 | 0.726 ± 0.124 |
Figure 2.
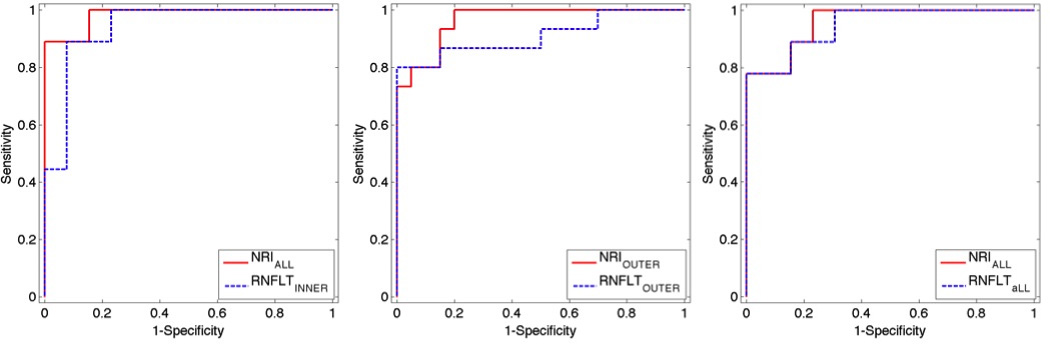
ROCs of NRIALL and RNFLTINNER for distinguishing glaucomatous vs. control eyes for PS-OCT-EIA dataset (left). ROCs of NRIOUTER and RNFLTOUTER for distinguishing glaucomatous vs. control eyes for PS-OCT-DEC dataset (middle). ROC curves of NRIALL and RNFLTALL for distinguishing glaucomatous vs. control eyes for RTVue OCT dataset (right).
The results for data collected by the PS-OCT-DEC system are similar to that collected by the PS-OCT-EIA system (Table 8). The AUCs of RNFLTOUTER and NRIOUTER were not statistically significantly different (p=0.338) at distinguishing between glaucomatous and control eyes.
The RTVue OCT dataset was analyzed to investigate if the parameters for glaucoma diagnosis might vary for different OCT systems. For distinguishing control and glaucomatous eyes, the all-rings average of NRI (NRIALL) and RNFLTALL provided the largest AUCs among all RNFL locations of NRI and RNFLT (superscripted with “*” in Table 9). A statistically significant difference between the AUC of NRIALL and that of RNFLTALL for distinguishing glaucomatous and control eyes (p=0.877) was not observed. For the RTVue dataset, NRIALL and RNFLTALL had similar performance for the task of distinguishing glaucomatous vs. control eyes. Thus, the results derived from the RTVue OCT dataset were consistent with those obtained from the other OCT datasets.
Glaucoma-suspect vs. control eyes
We also selected the RNFL location that provided the largest AUC value for each RNFL property (values superscripted with “*” in Table 10,11 and 12, ROC curves in Figure 3). For distinguishing glaucoma-suspect vs. control eyes, NRIINNER and RNFLTS exhibited the largest AUCs for the PS-OCT-EIA dataset. The comparisons between full AUCs of NRIINNER and RNFLTS did not show any statistically significant difference (p=0.101).
Table 10.
AUC and standard errors for distinguishing glaucoma-suspect vs. control eyes of NRI and RNFLT from the PS-OCT-EIA dataset averaged over seven RNFL locations.
| RNFL location |
Control vs. Glaucoma-suspect | |
|---|---|---|
| NRI | RNFLT | |
| AUC | AUC | |
| ALL rings | 0.968 ± 0.034 | 0.821 ± 0.094 |
| INNER rings | 0.981 ± 0.022* | 0.808 ± 0.089 |
| OUTER rings | 0.955 ± 0.041 | 0.788 ± 0.101 |
| Superior | 0.897 ± 0.064 | 0.833 ± 0.092* |
| Inferior | 0.936 ± 0.047 | 0.801 ± 0.089 |
| Nasal | 0.782 ± 0.101 | 0.756 ± 0.107 |
| Temporal | 0.769 ± 0.104 | 0.660 ± 0.117 |
Table 11.
AUC and standard errors for distinguishing glaucoma-suspect vs. control eyes of NRI and RNFLT from the PS-OCT-DEC dataset averaged over seven RNFL locations.
| RNFL location |
Control vs. Glaucoma-suspect |
|
|---|---|---|
| NRI | RNFLT | |
| AUC | AUC | |
| All rings | 0.933 ± 0.052* | 0.850 ± 0.077 |
| Inner rings | 0.833 ± 0.117 | 0.800 ± 0.110 |
| Outer rings | 0.908 ± 0.063 | 0.742 ± 0.130 |
| Superior | 0.808 ± 0.085 | 0.750 ± 0.134 |
| Inferior | 0.867 ± 0.088 | 0.875 ± 0.077* |
| Nasal | 0.583 ± 0.145 | 0.483 ± 0.133 |
| Temporal | 0.667 ± 0.124 | 0.450 ± 0.142 |
Table 12.
AUC and standard errors for distinguishing glaucoma-suspect vs. control eyes based on NRI and RNFLT averaged over seven RNFL locations from the RTVue OCT dataset.
| RNFL location |
Glaucoma-suspect vs. Control | |
|---|---|---|
| NRI | RNFLT | |
| AUC | AUC | |
| ALL rings | 0.885 ± 0.066 | 0.679 ± 0.118 |
| INNER rings | 0.833 ± 0.081 | 0.673 ± 0.116 |
| OUTER rings | 0.878 ± 0.073 | 0.744 ± 0.106 |
| Superior | 0.846 ± 0.085 | 0.724 ± 0.106 |
| Inferior | 0.929 ± 0.052* | 0.744 ± 0.110* |
| Nasal | 0.763 ± 0.104 | 0.667 ± 0.118 |
| Temporal | 0.622 ± 0.125 | 0.487 ± 0.124 |
Figure 3.
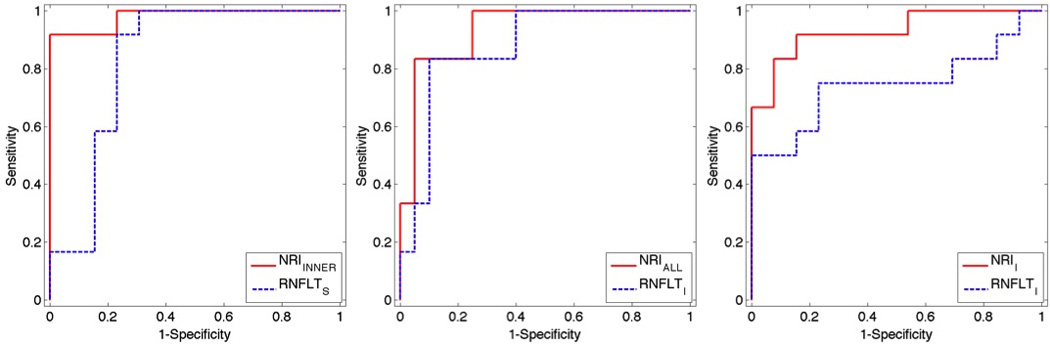
ROCs of NRIINNER and RNFLTS for distinguishing glaucoma-suspect vs. control eyes from PS-OCT-EIA data (left). ROCs of NRIALL and RNFLTI for distinguishing glaucoma-suspect vs. control eyes from PS-OCT-DEC data (middle). ROC curves of NRII and RNFLTI for distinguishing glaucoma-suspect vs. control eyes for the RTVue OCT dataset (right).
For the PS-OCT-DEC dataset, NRIALL and RNFLTI had the largest AUCs for distinguishing glaucoma-suspect vs. control eyes The AUCs of NRIALL and RNFLTI did not show any statistically significant difference (p=0.227).
For RTVue OCT dataset, the inferior RNFL location gave the largest AUC for both NRI (NRII) and RNFLT (RNFLTI) for distinguishing glaucoma-suspect from control eyes. The AUC of NRII was significantly larger than that of RNFLTI (p=0.008). Thus, NRII performs significantly better than RNFLTI at distinguishing glaucoma-suspect vs. control eyes.
DISCUSSION
In this human clinical study, a new OCT measured RNFL parameter, NRI, is introduced for glaucoma diagnosis. The diagnostic potential of NRI and RNFLT measured in seven RNFL locations (all-rings, inner-rings, outer-rings, and TSNI quadrants) is assessed to distinguish glaucomatous vs. control eyes as well as glaucoma-suspect vs. control eyes using data recorded by two custom-built PS-OCT systems and a commercial OCT system.
Since NRI can be computed similarly for all three OCT systems used in this study, measurement of NRI does not require introduction of new instrumentation or hardware modifications of existing systems. Computation of NRI requires only a software addition. Regardless of which OCT system was used to record retinal data, AUCs of NRI were always larger than those of RNFLT for distinguishing glaucoma-suspect vs. control eyes. The larger AUCs for NRI compared with RNFLT may be because both RNFL reflectance (RI) and RNFLT decrease with glaucoma. NRI is a unitless hybrid parameter that may also be less sensitive to RNFL boundary detection errors than either reflectance or RNFLT alone. RI as calculated in the primate study9 does not improve glaucoma detection in this clinical study (see supplemental eTable 4–7). Results of this pilot clinical study suggest the need for a larger clinical study to validate the diagnostic power of NRI for identifying glaucoma at the pre-perimetric stage.
The average age of control subjects is significantly less than that of both the glaucoma and glaucoma-suspect groups as tested by two-sample t-test for two independent samples with equal variance in datasets from the Eye Institute of Austin (EIA) and Duke Eye Center (DEC) (Table 1). Therefore, RNFLT and NRI measurements might be biased by age difference in different groups since RNFLT decreases as age increases14–17. The relationship between NRI and age is unknown. However, the Pearson's correlation coefficient was evaluated between NRI measured by both custom OCT and RTVue OCT and age in the control, glaucoma, and glaucoma-suspect groups, respectively, and no statistically significant correlations were observed (p>0.5 for all groups as shown in supplemental eTable 2). From eTable 3, we observe no statistically significant correlations between RNFLT, measured by either of the PS-OCT instruments or RTVue OCT, and patient age in control, glaucoma, and glaucoma-suspect groups in our study. However, the finding that neither NRI nor RNFLT is statistically significant correlated with age may be due to the small sample size in our study. For example, for the control group in DEC, with the current sample size of 20 patients (which is the largest in our study) we achieve only an 11% power to detect a difference between a correlation of 0 and the observed correlation of −0.167 with a significance level of 0.05.
NRI as defined here is different than an RNFL reflectance parameter introduced in a previous study involving non-human primates.9 Because NRI includes both RNFLT and reflectance, NRI can be considered as a combination feature of RNFLT and reflectance. Candidate cellular mechanisms that motivate why RNFL reflectance can be used as an early indicator of glaucoma were discussed previously.9 The observed decrease in RNFL reflectance might be due to reduced collected backscatter due to intensified mitochondrial fission in early glaucoma resulting in increased large-angle scattering.9
In conclusion, a new parameter, NRI is introduced that may outperform RNFLT for distinguishing between glaucoma-suspect and control eyes. Results of this pilot clinical study suggest that NRI derived from OCT retinal images is a promising measure to detect pre-perimetric glaucoma.
Supplementary Material
Acknowledgements
Supported by National Eye Institute at the National Institutes of Health (Grant R01EY016462) and Research to Prevent Blindness (S.J.M.). Involved in design and conduct of the study (S.L., B.W., B.Y., T.E.M., M.K.M., S.J.M., H.G.R.); collection, management, analysis, and interpretation of the data (S.L., B.W., B.Y., T.E.M., M.K.M., S.J.M., H.G.R.); and preparation, review, or approval of the manuscript (S.L., B.W., B.Y., T.E.M., M.K.M., S.J.M., H.G.R.). The authors would like to thank Andrew W. Ross, M.D. for coordinating the study at EIA and Sara Crowell, M.S., CCRP for coordinating the study at DEC. The authors also thank Amit S. Paranjape, Ph.D. for his contribution to data collection at EIA and Andrew Klotz for his contribution to image processing of DEC data.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chang RT, Knight OJ, Feuer WJ, Budenz DL. Sensitivity and specificity of time-domain versus spectral-domain optical coherence tomography in diagnosing early to moderate glaucoma. Ophthalmology. 2009 Dec;116(12):2294–2299. doi: 10.1016/j.ophtha.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Wu H, de Boer JF, Chen TC. Diagnostic capability of spectral-domain optical coherence tomography for glaucoma. Am J Ophthalmol. 2012 May;153(5):815–826. e812. doi: 10.1016/j.ajo.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elmaanaoui B, Wang B, Dwelle JC, et al. Birefringence measurement of the retinal nerve fiber layer by swept source polarization sensitive optical coherence tomography. Opt Express. 2011 May 23;19(11):10252–10268. doi: 10.1364/OE.19.010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoh ST, Greenfield DS, Mistlberger A, Liebmann JM, Ishikawa H, Ritch R. Optical coherence tomography and scanning laser polarimetry in normal, ocular hypertensive, and glaucomatous eyes. Am J Ophthalmol. 2000 Feb;129(2):129–135. doi: 10.1016/s0002-9394(99)00294-9. [DOI] [PubMed] [Google Scholar]
- 5.Sugimoto M, Ito K, Goto R, Uji Y. Symmetry analysis for detecting early glaucomatous changes in ocular hypertension using optical coherence tomography. Jpn J Ophthalmol. 2004 May-Jun;48(3):281–286. doi: 10.1007/s10384-003-0058-3. [DOI] [PubMed] [Google Scholar]
- 6.Anton A, Moreno-Montanes J, Blazquez F, Alvarez A, Martin B, Molina B. Usefulness of optical coherence tomography parameters of the optic disc and the retinal nerve fiber layer to differentiate glaucomatous, ocular hypertensive, and normal eyes. J Glaucoma. 2007 Jan;16(1):1–8. doi: 10.1097/01.ijg.0000212215.12180.19. [DOI] [PubMed] [Google Scholar]
- 7.Caprioli J, Nouri-Mahdavi K, Law SK, Badala F. Optic disc imaging in perimetrically normal eyes of glaucoma patients with unilateral field loss. Trans Am Ophthalmol Soc. 2006;104:202–211. [PMC free article] [PubMed] [Google Scholar]
- 8.Choi MG, Han M, Kim YI, Lee JH. Comparison of glaucomatous parameters in normal, ocular hypertensive and glaucomatous eyes using optical coherence tomography 3000. Korean J Ophthalmol. 2005 Mar;19(1):40–46. doi: 10.3341/kjo.2005.19.1.40. [DOI] [PubMed] [Google Scholar]
- 9.Dwelle J, Liu S, Wang B, et al. Thickness, phase retardation, birefringence, and reflectance of the retinal nerve fiber layer in normal and glaucomatous non-human primates. Invest Ophthalmol Vis Sci. 2012 Aug;53(8):4380–4395. doi: 10.1167/iovs.11-9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermeer KA, van der Schoot J, Lemij HG, de Boer JF. RPE-normalized RNFL attenuation coefficient maps derived from volumetric OCT imaging for glaucoma assessment. Invest Ophthalmol Vis Sci. 2012 Aug 14; doi: 10.1167/iovs.12-9933. [DOI] [PubMed] [Google Scholar]
- 11.Wang B, Paranjape A, Yin B, et al. Optimized Retinal Nerve Fiber Layer Segmentation Based on Optical Reflectivity and Birefringence for Polarization-Sensitive Optical Coherence Tomography. Paper presented at: Proc. SPIE. 2011 [Google Scholar]
- 12.Kass M, Witkin A, Terzopoulos D. Snakes: Active contour models. International Journal of Computer Vision. 1988;1(4):321–331. [Google Scholar]
- 13.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alamouti B, Funk J. Retinal thickness decreases with age: an OCT study. Br J Ophthalmol. 2003 Jul;87(7):899–901. doi: 10.1136/bjo.87.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowd C, Zangwill LM, Blumenthal EZ, et al. Imaging of the optic disc and retinal nerve fiber layer: the effects of age, optic disc area, refractive error, and gender. J Opt Soc Am A Opt Image Sci Vis. 2002 Jan;19(1):197–207. doi: 10.1364/josaa.19.000197. [DOI] [PubMed] [Google Scholar]
- 16.Varma R, Skaf M, Barron E. Retinal nerve fiber layer thickness in normal human eyes. Ophthalmology. 1996 Dec;103(12):2114–2119. doi: 10.1016/s0161-6420(96)30381-3. [DOI] [PubMed] [Google Scholar]
- 17.Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology. 2012 Apr;119(4):731–737. doi: 10.1016/j.ophtha.2011.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


