Abstract
Background
The District of Columbia Department of Health (DCDOH) funds facilities to provide HIV medical case management (MCM), inclusive of linkage, engagement in care, and treatment adherence support. The objective of this analysis was to identify differences in clinical outcomes among HIV-infected persons receiving care at MCM-funded facilities compared to non-funded facilities.
Methods
Newly diagnosed and prevalent HIV-infected persons were identified from the DCDOH surveillance system. Clinical outcomes of interest were linkage, retention in care, and viral suppression. Bivariate analyses and random effects logistic regression were used to examine differences in demographics and clinical outcomes of persons receiving care at MCM-funded and non-funded facilities.
Results
Among 5,631 prevalent cases, 56.7% received care at MCM-funded facilities of which 76.2% were retained in care, and 70.6% achieved viral suppression. Those receiving care in MCM-funded facilities were significantly more likely to be retained in care (aOR 4.13; 95%CI: 1.93-8.85) and as likely (aOR 1.06; 95%CI: 0.68-1.62) to be virally suppressed than persons receiving care in non-funded facilities. Among 789 newly diagnosed persons, those diagnosed in MCM-funded facilities were not significantly more likely to be linked to care within 3 months (aOR 0.50; 95%CI: 0.21-1.18) than those diagnosed in non-funded facilities.
Discussion
This study provides evidence that medical case management may be beneficial to HIV-infected persons in DC, as it improves retention in care. Further identification of the specific services providing the most benefit to clients is needed, as well as a better understanding of the complex relationship between retention and viral suppression.
Keywords: HIV, medical case management, linkage to care, viral suppression, engagement in care
Introduction
HIV viral suppression slows HIV progression to AIDS, reduces mortality,1,2,3 and can prevent transmission to uninfected partners.4 Linkage to care, generally defined as entry into HIV medical care after an HIV diagnosis, and engagement in HIV care, generally defined as regular receipt of HIV primary care services after diagnosis, among HIV-infected individuals are crucial steps to achieving and maintaining viral suppression. Routinely seeing an HIV care provider offers frequent opportunities to monitor HIV viral load and provides physicians the chance to focus on secondary HIV prevention efforts.5 Data from the Centers for Disease Control and Prevention (CDC) however suggest 66% of persons living with HIV infection in the United States were linked to care, 37% were retained in care, and only 25% were virally suppressed.6
The District of Columbia Department of Health (DCDOH) HIV/AIDS, Hepatitis, STD, and Tuberculosis Administration (HAHSTA) routinely uses HIV surveillance data to measure linkage to HIV care, engagement in HIV care, and viral suppression among HIV cases diagnosed and living in Washington, DC. Although linkage to care within 3 months of HIV diagnosis has increased since 2005 and was above 75% for newly diagnosed HIV cases in 2010, engagement in care and viral suppression rates remain lower.7 In 2010, 40% of persons diagnosed and living with HIV were retained in care, 37% received sporadic or non-regular care, and among those receiving some care, 56% had achieved viral suppression.7 Given the burden of HIV in Washington, DC with 2.7% of the population living with HIV in 20107; it is important to identify programs or services that improve these outcomes.
Structural interventions, such as medical case management (MCM), have been shown to facilitate entry and engagement in care among persons living with HIV. The Health Resources Services Administration HIV/AIDS Bureau (HRSA HAB) defines medical case management as “as a range of client-centered services that link clients with health care, psychosocial, and other services.”8 Case management may be delivered through in-person meetings with clients, telephone contacts or through other forms of communication.8 HRSA HAB distinguishes non-medical case management from medical case management as provision of assistance to clients in obtaining medical, social, community, legal, financial, or other necessary services without assistance in the coordination of medical treatments.8
Antiretroviral Treatment Access Study (ARTAS) I and II demonstrated that receipt of strengths-based case management services increased the likelihood of linking into HIV medical care over the traditional passive referral system.9,10 The International Association of Physicians in AIDS Care recently released evidence-based guidelines for improving entry into and retention in care and recommended case management be used for individuals with new HIV diagnoses, for the homeless who experience multiple barriers to adherence, and for adolescents and young adults living with HIV.11 Services which can often be arranged by a case manager, such as mental health and substance abuse ancillary services, have also been shown to increase retention in care and increase the mean number of HIV medical visits received.12 Similarly, patient navigation services, which may be a component of medical case management, have been found to significantly reduce unmet needs and structural barriers to accessing care, such as payment for medical care and getting an appointment at a convenient time.13
DCDOH HAHSTA currently funds health care provider organizations in Washington, DC to provide HIV medical case management services through the Ryan White Care Act. For an organization to receive funding, it must respond to a request for application, and successfully compete for funding after having its application externally reviewed and scored. In fiscal year 2010 (October 1, 2009 through September 30, 2010) 22 distinct organizations in the District were funded by the DCDOH HAHSTA to provide MCM services to HIV-infected clients through Ryan White Part A and Part B funding. These organizations included three hospitals, nine community-based clinics, and ten community-based organizations.
The DC DOH HAHSTA has a defined Operational Model for MCM.14 Medical case management core services and interventions are outlined in a guidelines document and include: an initial intake and assessment of service needs using a defined acuity scale; development of a comprehensive individualized service plan; linkages and coordination of services required to implement the plan; client monitoring to assess the efficacy of the plan; and periodic reassessment and modification of the plan as necessary based on a client's medical and psychosocial outcomes; and treatment adherence support at every stage of the process.14 Local tools have been developed and are available to assist sites with implementation of each of the above mentioned interventions. There are four levels of acuity management: intensive for high need clients, moderate, basic, and self-management. The level of acuity determines the interventions and the frequency of receipt of the interventions.14 In 2011, 22% of clients received intensive case management, 13% moderate, 30% basic and 35% self-management.
At MCM-funded sites, services are delivered by medical case managers who, as per DC regulations, must be licensed social workers or registered nurses with the exception of individuals who do not hold these degrees but were previously providing these services and have been grandfathered into this category. Training of medical case managers is conducted through monthly treatment adherence roundtables, quarterly in collaboration with the Case Management Operating Committee, and through one-on-one trainings are organized on an as needed basis. Since the introduction of the MCM guidelines in 2010, more than 350 medical case managers in DC have been trained on how to provide these services.
DC DOH HAHSTA has adopted the HRSA HAB performance measures and quality indicators from MCM funded sites are collected monthly and quarterly with an emphasis on measuring retention in care, viral suppression, and CD4 counts. From March through May of 2010, 42% and 47% of MCM programs were monitoring viral loads and CD4 counts, respectively and by 2011, during this same time period, those proportions had increased to 91% and 87%, respectively. Data reported to the DCDOH HAHSTA from the individual clinics have also shown that 55% of persons with at least one primary care visit during 2011 were engaged in care and 67% of these clients were virally suppressed.15 To date, these outcomes have not yet been compared to clinics that are not funded to provide these services in Washington, DC.
During fiscal year 2010, there were 47 medical facilities providing HIV care in Washington, DC that did not receive MCM funding. These facilities included 20 private providers, 13 community-based clinics, and 11 hospitals. Some of these facilities did not offer any type of linkage or navigation services while others participated in other DCDOH HAHSTA linkage to care programs and may have been funded by the CDC for case management and other evidenced-based interventions. To our knowledge, none of these programs were as comprehensive as the Ryan White funded Medical Case Management program.
DCDOH HAHSTA is participating in the Enhanced Comprehensive HIV Prevention Planning (ECHPP) project, a three year demonstration project aimed at improving program planning and implementation to: 1) reduce new HIV infections; 2) link people with HIV to care and treatment and improve health outcomes; 3) reduce HIV-related health disparities; and 4) achieve a more coordinated response to the HIV epidemic.16 Program evaluation is a key component of the ECHPP project, and the DCDOH HAHSTA and local researchers are working together to examine the efficacy of a variety of linkage to care and navigation programs currently in use in Washington, DC in response to the ECHPP strategy to “implement interventions or strategies promoting retention in or re-engagement in care for HIV-positive persons”. One of the programs identified for evaluation was the Ryan White funded Medical Case Management program. The objective of this study was to assess whether there were differences in clinical outcomes of HIV-infected persons diagnosed and receiving care at MCM funded facilities compared to those of non-MCM funded facilities in Washington, DC in the year prior to full implementation of ECHPP.
Methods
In order to assess the associations between MCM services and HIV clinical outcomes, this study compared linkage to care, engagement in care, and viral suppression rates among MCM and non-MCM funded sites. Data for 14 of the MCM-funded organizations in fiscal year 2010 were available and analyzed representing three community-based organizations, two hospitals, and nine community-based clinics. Data were abstracted from the HIV testing and counseling data (PEMS), routine HIV surveillance data (eHARS) and routinely collected laboratory data, inclusive of CD4+ T-Cell and HIV RNA viral load laboratory values. Cases newly diagnosed between October 2009 and September 2010 and prevalent cases diagnosed as of April 2009 and alive as of December 31, 2010 were included in this analysis.
Data Sources
PEMS Data
The DCDOH Program Evaluation and Monitoring System (PEMS) data were used to identify persons who tested for HIV and had a reactive test during the funding period, October 1, 2009 to September 30, 2010 (Fiscal Year 2010). The facilities that performed the reactive HIV tests were classified into MCM funded and non-MCM funded facilities. This was determined by whether a facility received funding from DCDOH HAHSTA for MCM services. Inclusion of PEMS data provided an opportunity to assess HIV testing outcomes among community-based organizations, as well as community-based clinics and hospitals that were and were not funded for MCM services.
PEMS data also include information for referrals to HIV primary care when an HIV test is reactive. A referral is defined as referring a client with a reactive HIV test to access HIV confirmatory testing and care services but does not necessarily include following up to determine if those services were actually received. In order to confirm that the referral resulted in linkage to HIV primary care, persons referred for care based on PEMS data were matched to HIV/AIDS case surveillance data from the DCDOH HAHSTA enhanced HIV/AIDS Reporting System (eHARS). For confidentiality reasons, PEMS data do not record the full first and last name of persons who receive an HIV test; however, a unique code for each person based upon the first and last letters of their first name, the first and last letters of their last name, and their date of birth is maintained. Using the elements of this unique code, persons were matched between PEMS and eHARS with SAS v. 9.2. Possible matches were manually reviewed for accuracy.
HIV/AIDS Surveillance Data
HIV/AIDS case surveillance data from DCDOH HAHSTA eHARS were used to identify Washington, DC residents 13 years of age and older at the time of HIV diagnosis. Persons diagnosed in the District of Columbia Jail were excluded. Demographic information including date of birth, sex at birth, race/ethnicity, mode of HIV transmission, insurance type, and address at time of diagnosis are routinely collected for HIV cases and recorded in eHARS. These data and corresponding HIV-related laboratory data were also extracted for these cases.
Cases were considered to be ‘newly diagnosed’ if they were diagnosed with HIV during the funding period, October 1, 2009 through September 30, 2010 (Fiscal Year 2010). Cases were considered to be ‘prevalent’ if they were diagnosed with HIV by April 1, 2009, alive as of December 31, 2010, and had at least one HIV lab reported during the funding period from a MCM funded or non-MCM funded facility. This 6-month lag time was chosen for prevalent cases to reduce the likelihood that these persons were either establishing themselves in HIV care or had more severe disease progression, both of which could result in more frequent visits to a medical facility and affect our ability to correctly capture the clinical outcomes of interest. Prevalent cases with HIV labs reported from both MCM funded and non-MCM funded facilities during the funding period were also excluded from this analysis.
Clinical Outcomes
The clinical outcomes of interest included linkage to care, engagement in care, and viral suppression. These outcomes were determined from laboratory data routinely reported to eHARS. Laboratories must report all CD4 cell count, CD4 percentage, and viral load results from patients diagnosed with HIV to the DCDOH HAHSTA as required by District of Columbia Municipal Code.
Linkage to care was defined separately for PEMS and eHARS cases. For persons identified in PEMS, linkage to care was defined as the first CD4 or viral load result reported to eHARS on or after the date of the reactive HIV test. For newly diagnosed cases identified in eHARS, linkage to care was defined as the first CD4 or viral load result on or after the date of confirmed HIV diagnosis. Linkage to care was assessed within 3 months and within 6 months of the reactive HIV test or diagnosis. The 3 and 6 month intervals were not mutually exclusive.
Engagement in care was defined as being either retained in care or in sporadic care. Retention in care was defined as having two CD4 and/or VL labs reported to DCDOH HAHSTA at least 3 months apart during the funding period from the same facility. This definition was based upon the HRSA HAB performance measure for retention in HIV care.17 Those persons determined to be in sporadic care had at least one lab reported but not within the HRSA- specified time interval for retention in care. Viral suppression was defined as <200 copies/mL for the last HIV viral load test performed during the funding period (Fiscal Year 2010).
Statistical Analysis
Pearson's chi-square test was performed to determine if the proportion of persons referred to HIV primary care, and the proportion of confirmed linkages within 3 months and 6 months of a reactive HIV test, differed significantly between MCM funded facilities and non-MCM funded facilities.
Pearson's chi-square tests were also performed to identify differences in demographics among newly diagnosed HIV cases at MCM funded and non-MCM funded facilities. The student's T-test was performed to determine if mean age at diagnosis differed and the Kruskal-Wallis test was performed to determine if median CD4 cell count at diagnosis differed among these populations as well. The first CD4 cell count result reported within 6 months of diagnosis was considered to be the CD4 count at diagnosis.
Pearson's chi-square tests were performed to identify differences in the demographics among prevalent HIV cases receiving care at MCM funded and non-MCM funded facilities. The student's T-test was used to determine if there was a difference in current age, i.e. age as of December 2009, among those receiving care at MCM funded and non-MCM funded facilities.
The demographics of interest for all bivariate analyses included age, sex, race/ethnicity, mode of HIV transmission, insurance type, and ward of residence at the time of HIV diagnosis. Washington, DC is divided into eight geopolitical regions called ‘wards,’ each with approximately 75,000 people. Ward information was determined by geocoding the address at time of HIV diagnosis collected from eHARS case data.
Random effects logistic regression models were used to control individual clinic factors, such as performance and types of services available, particularly among non-MCM funded facilities. Unadjusted and adjusted random effects logistic regression models were used to examine the association between linkage to care and diagnosis within MCM funded and non-MCM funded facilities among newly diagnosed HIV cases. Two models were created; the first model examined the outcome of linkage to care within 3 months and the second examined the outcome of linkage to care within 6 months. Unadjusted and adjusted random effects logistic regression models were also used to examine the associations between engagement in care and viral suppression and receiving care within MCM funded and non-MCM funded facilities among prevalent HIV cases. All adjusted models included the demographic variables listed above. In order to account for HIV disease progression at the time of diagnosis, an additional variable, concurrent HIV and AIDS diagnosis, was included in the linkage to care models among newly diagnosed cases. Concurrent HIV and AIDS diagnosis was defined by having an AIDS diagnosis date within 3 months of the initial HIV diagnosis date in eHARS.
All analyses were conducted using SAS v. 9.2, random effects models were performed using the proc glimmix procedure, and a p-value of 0.05 was set as the threshold for statistical significance. This study was approved by both the George Washington University and the District of Columbia Department of Health Institutional Review Boards.
Results
Engagement in Care and Viral Suppression among Prevalent Cases
Of persons living with HIV during the study period, 6,463 cases had at least one HIV related lab reported; 832 of these cases were excluded as they had labs reported from both MCM funded and non-MCM funded facilities. Therefore 5,631 HIV cases were considered to be receiving care during fiscal year 2010 and 3,192 (56.7%) were receiving care at MCM funded facilities (Table 1). Persons receiving care at MCM funded facilities were more likely to be female, black, Hispanic, have an HIV mode of transmission attributed to injection drug use or heterosexual contact, publically insured, and living in Wards 7 and 8 (all p<0.0001).
Table 1.
Demographics of Persons Living with HIV and Receiving Care in Medical Case Management and Non-Medical Case Management Facilities between October 1, 2009 and September 30, 2010
| Medical Case Management n=3,192 | Non-Medical Case Management n=2,439 | p-value | |
|---|---|---|---|
| Continuous Characteristics | |||
| Mean Current Age | 45.7 | 47.5 | <0.0001* |
| Categorical Characteristics | N (%) | N (%) | |
| Sex | |||
| Male | 2,088 (65.4) | 1,838 (75.4) | <0.0001 |
| Female | 1,104 (34.6) | 601 (24.6) | |
| Race/Ethnicity | |||
| White | 188 (5.9) | 762 (31.2) | <0.0001 |
| Black | 2,710 (85.0) | 1,526 (62.6) | |
| Hispanic | 239 (7.5) | 94 (3.9) | |
| Other | 55 (1.7) | 57 (2.3) | |
| Mode of Transmission | |||
| MSM | 1,051 (32.9) | 1,296 (53.1) | <0.0001 |
| IDU | 601 (18.8) | 181 (7.4) | |
| MSM/IDU | 135 (4.2) | 53(2.2) | |
| Heterosexual | 1,084 (34.0) | 593 (24.3) | |
| RNI** | 319 (10.0) | 311 (12.8) | |
| Other | 2 (0.06) | 5 (0.2) | |
| Insurance at Diagnosis | |||
| Private | 92 (2.9) | 386 (15.8) | <0.0001 |
| Public | 539 (16.9) | 152 (6.2) | |
| Other | 64 (2.0) | 11 (0.5) | |
| None | 50 (1.6) | 8 (0.3) | |
| Unknown | 2,447 (76.7) | 1,882 (77.2) | |
| Ward of Residence at Diagnosis | |||
| Ward 1 | 455 (14.3) | 371 (15.2) | <0.0001 |
| Ward 2 | 248 (7.8) | 455 (18.7) | |
| Ward 3 | 36 (1.1) | 76 (3.1) | |
| Ward 4 | 304 (9.5) | 251 (10.3) | |
| Ward 5 | 510 (16.0) | 317 (13.0) | |
| Ward 6 | 421 (13.2) | 359 (14.7) | |
| Ward 7 | 496 (15.5) | 259 (10.6) | |
| Ward 8 | 566 (17.7) | 239 (9.8) | |
| Homeless/Missing residence at diagnosis | 156 (4.9) | 112 (4.6) |
T-test p-value
RNI: risk not identified
Persons living with HIV and receiving care in MCM funded facilities were more likely to be retained in care (76.2% vs. 59.9%) (Table 2) than persons receiving care at non-MCM facilities. The adjusted random effects logistic regression also indicated that persons receiving care in MCM funded facilities were significantly more likely (aOR 4.13, 95% CI 1.93, 8.85) to be retained in HIV care when compared to persons receiving care at non-MCM funded facilities, after adjusting for demographic characteristics and the individual clinic characteristics.
Table 2.
Engagement in Care and Viral Suppression among Persons Living with HIV and Receiving Care in Medical Case Management and Non-Medical Case Management Facilities between October 1, 2009 and September 30, 2010
| Medical Case Management n=3,192 | Non-Medical Case Management n=2,439 | Odds Ratio (95% CI)* | Adjusted Odds Ratio (95% CI)* | |
|---|---|---|---|---|
| N(%) | N(%) | |||
| Engagement in Care | ||||
| Retained in care | 2,431 (76.2) | 1,462 (59.9) | 3.50 (1.54, 7.95) | 4.13 (1.93, 8.85) |
| Sporadic care | 761 (23.8) | 977 (40.1) | ref | ref |
| Viral Suppression** | ||||
| Suppressed | 2,197 (70.6) | 1,722 (75.7) | 0.87 (0.48,1.58) | 1.06 (0.68, 1.62) |
| Not suppressed | 915 (29.4) | 554 (24.3) | ref | ref |
Odds ratios are based on random effects models and adjusted for facility of care, sex, current age, race/ethnicity, mode of transmission, insurance, and ward.
Excludes 243 cases with missing viral load data.
The bivariate analyses indicated that higher proportions of cases receiving care in non-MCM funded facilities were virally suppressed (75.7% vs. 70.6%) than in MCM funded sites. Adjusted random effects logistic regression, however, demonstrated however that persons receiving care in MCM funded facilities were no more likely to be virally suppressed than persons receiving care at non-MCM funded facilities (aOR 1.06, 95% CI 0.68, 1.62) during fiscal year 2010 after adjusting for demographic characteristics and the individual clinic characteristics.
Referral and Linkage to Care among Newly Diagnosed PEMS Cases
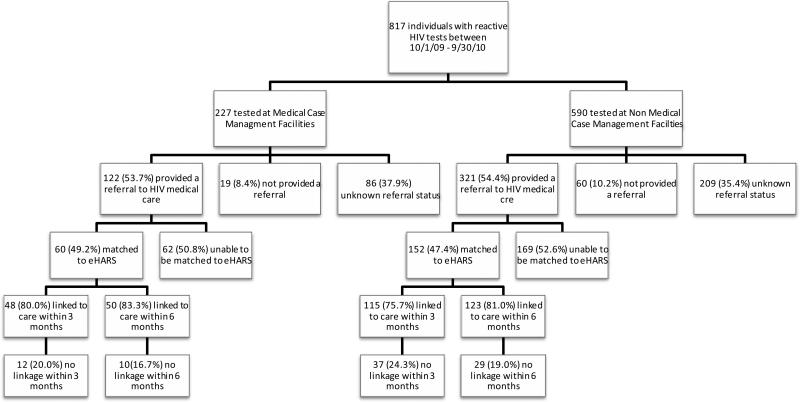
Based upon PEMS testing data, 817 people had a reactive HIV test result during the funding period; 227 (27.8%) of these individuals were tested at a MCM funded facility (Figure 1). Overall, 443 (54.2%) individuals were referred to HIV medical care. The proportion of individuals referred to HIV medical care did not differ significantly between MCM and non-MCM funded facilities, with 53.7% and 54.4% of individuals provided referrals, respectively (p=0.66).
Figure 1.
Referrals and Linkage to Care among Persons with a Reactive HIV Test in Medical Case Management and Non-Medical Case Management Facilities between October 1, 2009 and September 30, 2010
In order to confirm linkage of these persons to HIV medical care, the PEMS cases were matched to eHARS. After matching, 212 (47.7%) of the 443 individuals referred to HIV medical care based on PEMS data were matched to eHARS case information; 60 (28.3%) of these individuals were tested in a MCM funded facility. Of those tested in a MCM funded facility, 80.0% were linked to care within 3 months and 83.3% were linked to care within 6 months. These proportions did not differ significantly from non-MCM funded facilities, where 75.7% were linked to care within 3 months and 81.0% were linked to care within 6 months (p=0.50 and p=0.68, respectively).
Linkage to Care among Newly Diagnosed Surveillance Cases
Linkage to care was also measured at a population-based level using HIV surveillance data from eHARS. During the funding period, there were 789 adult and adolescent HIV cases diagnosed and 406 (51.5%) of these cases were diagnosed at 10 medical facilities funded for MCM (Table 3). Those diagnosed at MCM funded facilities were significantly younger, with a mean age at diagnosis of 37.5 years vs. 40.7 years at non-MCM funded facilities (p=0.0004). A significantly greater proportion of the cases diagnosed in MCM funded facilities were black or Hispanic (p<0.0001) and publically insured (p<0.0001).
Table 3.
Demographics among HIV Cases Diagnosed in Medical Case Management and Non-Medical Case Management Facilities between October 1, 2009 and September 30, 2010
| Medical Case Management n=406 | Non-Medical Case Management n=383 | p-value | |
|---|---|---|---|
| Continuous Characteristics | |||
| Mean Age at Diagnosis | 37.3 | 40.7 | 0.0004* |
| Median CD4 Count at Diagnosis(cells/μl) | 391 | 384 | 0.1750** |
| Categorical Characteristics | N (%) | N (%) | |
| Sex | |||
| Male | 282 (69.5) | 268 (70.0) | 0.8748 |
| Female | 124 (30.5) | 115 (30.0) | |
| Race/Ethnicity | |||
| White | 28 (6.9) | 66 (17.2) | <0.0001 |
| Black | 333 (82.0) | 290 (75.7) | |
| Hispanic | 34 (8.4) | 16 (4.2) | |
| Other | 11 (2.7) | 11 (2.9) | |
| Mode of Transmission | |||
| MSM | 153 (37.7) | 131 (34.2) | 0.0051 |
| IDU | 30 (7.4) | 16 (4.2) | |
| MSM/IDU | 8 (2.0) | 6 (1.6) | |
| Heterosexual | 131 (32.3) | 108 (28.2) | |
| RNI*** | 84 (20.7) | 122 (31.9) | |
| Insurance at Diagnosis | |||
| Private | 38 (9.4) | 169 (44.1) | <0.0001 |
| Public | 141 (34.7) | 102 (26.6) | |
| Other | 95 (23.4) | 28 (7.3) | |
| None | 25 (6.2) | 14 (3.7) | |
| Unknown | 107 (26.4) | 70 (18.3) | |
| Concurrent HIV/AIDS Diagnosis | |||
| Yes | 76 (18.7) | 96 (25.1) | 0.0309 |
| No | 330 (81.3) | 287 (74.9) | |
| Ward of Residence at Diagnosis | |||
| Ward 1 | 41 (10.1) | 29 (7.6) | 0.0005 |
| Ward 2 | 25 (6.2) | 27 (7.1) | |
| Ward 3 | 8 (2.0) | 22 (5.7) | |
| Ward 4 | 43 (10.6) | 28 (7.3) | |
| Ward 5 | 56 (13.8) | 50 (13.1) | |
| Ward 6 | 20 (4.9) | 35 (9.1) | |
| Ward 7 | 31 (7.6) | 49 (12.8) | |
| Ward 8 | 79 (19.5) | 51 (13.3) | |
| Homeless/Missing residence at diagnosis | 103 (25.4) | 92 (24.0) |
T-test p-value
Kruskal Wallis p-value
RNI: risk not identified
The proportion of cases diagnosed in MCM funded facilities linked to care within 3 months was significantly lower compared to those at non-MCM funded facilities (72.4% vs. 80.4% in non-MCM funded facilities, p=0.0082) (Table 4). Although the proportion of cases linked to care within 6 months was slightly higher among non-MCM funded facilities, it was not significantly different (80.0% vs. 85.1%, p=0.061). The odds ratios and 95% confidence intervals from the random effects models indicate that there was no statistically significant association between diagnoses within a MCM funded facility and linkage to care within 3 months or within 6 months (linkage within 3 months: aOR 0.47, 95% CI 0.19, 1.15; linkage within 6 months: aOR 0.57, 95% CI 0.23, 1.43) when compared with diagnoses in non-MCM funded facilities.
Table 4.
Linkage to Care among HIV Cases Diagnosed in Medical Case Management and Non-Medical Case Management Facilities between October 1, 2009 and September 30, 2010
| Medical Case Management n=406 | Non-Medical Case Management n=383 | Odds Ratio (95% CI)* | Adjusted Odds Ratio (95% CI)* | |
|---|---|---|---|---|
| Linkage to Care in 3 months | ||||
| < 3 months | 294 (72.4) | 308 (80.4) | 0.50 (0.21, 1.18) | 0.47 (0.19, 1.15) |
| ≥ 3 months | 112 (27.6) | 75 (19.6) | ref | ref |
| Linkage to Care in 6 months | ||||
| < 6 months | 325 (80.0) | 326 (85.1) | 0.55 (0.22, 1.38) | 0.57 (0.23, 1.43) |
| ≥ 6 months | 81 (20.0) | 57 (14.9) | ref | ref |
Odds ratios are based on random effects models and are adjusted for facility of care, sex, age, race/ethnicity, mode of transmission, insurance, concurrent HIV and AIDS diagnosis, and ward of residence.
Discussion
This study used HIV testing and population-based HIV surveillance data to examine differences in linkage to care, engagement in care, and viral suppression rates among HIV-infected persons diagnosed and receiving care at MCM funded facilities and non-MCM facilities in Washington, DC prior to full ECHPP implementation. Although there were no differences in linkage to care within 3 or 6 months among persons who had a reactive HIV test and who were diagnosed with HIV, we found persons living with HIV and receiving HIV medical care within MCM funded facilities were significantly more likely to be engaged in care and equally likely to be virally suppressed when compared to persons receiving care at non-MCM funded facilities.
Linkage to care services are one of the core services provided by Ryan White Medical Case Management programs, yet linkage to care after a reactive HIV test or HIV diagnosis was not significantly greater at MCM funded facilities. In addition, these facilities were no more likely to provide a referral to HIV primary care than non-MCM funded facilities and approximately one-half of persons, regardless of facility type, received this referral. There may be several reasons for the lack of improved referral and linkage to care rates associated with MCM funded facilities. First, several of the facilities funded to provide MCM and included in this analysis were community-based organizations and did not have any licensed medical providers on staff. These organizations must refer and link newly identified HIV positive individuals to medical clinics not associated with their organization. Even though many of the community-based organizations routinely work with medical clinics to ensure these linkages, setting up an appointment with a new doctor and traveling to a new clinic or organization may be daunting to patients and testing in non-medical settings has been associated with delayed linkage to care.18,19
In order to further assess the potential effect of CBOs on linkage rates, we recalculated linkage excluding the community-based organizations and found a marginal but insignificant increase in linkage rates among both MCM and non MCM-funded sites. This implies that reasons other than clinic factors may be possible for the delay in linkage. These factors might include patient factors such as distrust of medical providers and denial of HIV status.20 Furthermore, city-wide data in Washington, DC show that linkage to care within 3 months of diagnosis is currently above 75% among all newly diagnosed HIV infected persons in Washington, DC and 90% of all diagnosed cases are linked eventually.7 Therefore, though there was a slight delay in linkage among these cases, the vast majority of persons will eventually link to HIV care.
Persons living with HIV and receiving care at MCM funded facilities were more than four times as likely to be retained in care compared to persons at non-MCM funded facilities. This association was true even after controlling for demographic information and the individual clinic in which persons were receiving HIV care. This finding is similar to research that demonstrates improved retention in care after receipt of services similar to those utilized by the Ryan White Medical Case Management program.21 For example, Andersen et al. found that use of a nurse case manager and provision of transportation to and from medical appointments increased the proportion of women who did not miss any HIV medical appointments from 10% pre-intervention to over 50% at follow-up. 22 A similar study that provided patients with access to a non-clinical patient navigator who accompanied them to appointments, coordinated specialty care, and provided referrals significantly increased the proportion of participants with two or more HIV medical appointments in a 6 month time period.13 Finally, ARTAS I demonstrated that participants who met with a case manager that worked with them to identify and address barriers to health care were significantly more likely to visit an HIV clinician at least twice in a 12 month period compared to those who did not meet with a case manager.9 While the type of support provided in these other studies was variable, they all include elements of medical case management that may influence one's ability to remain consistently engaged in care.
Retention in HIV care has been shown to increase viral suppression rates in a variety of settings23,24,25,26 as has the provision of treatment adherence support.27 Given the positive effect of medical case management on retention in care, we had not anticipated persons living with HIV and receiving care at MCM funded facilities would be equally likely to be virally suppressed than persons receiving care at non-MCM funded facilities. This lack of association between MCM services and viral suppression may be explained in part by the fact that these data are reflective of baseline information measuring the influence of MCM on clinical outcomes. These MCM sites were funded prior to full implementation of the National HIV/AIDS Strategy (NHAS) and ECHPP at which time there was not as intense a focus on earlier initiation of antiretroviral therapy and achievement of viral suppression. However, despite the lack of difference in viral suppression rates, it is important to note that among this particular cohort of persons, rates of viral suppression were higher than those observed in the city overall (56%) and nationally (25%).5,7
There are several limitations to this study worth noting. First, missing data on insurance, risk and incomplete linkages were notable. Second, HIV surveillance lab data was used as a proxy for medical care visits to determine linkage to care and engagement in care and we may have missed medical care visits in which an HIV lab was not performed. However, a recent study found using lab visits as a proxy for HIV medical care visits was a reliable measure.28 Therefore, at the very least, this study provides a conservative estimate of the number or proportion of persons who were linked to care and engaged in care. Additionally, there may have been differential reporting of laboratory results from MCM funded sites compared to non-funded sites, however; there is no reason reporting from these sites would be different as all major laboratories routinely report to the DCDOH HAHSTA. Next, HIV surveillance data does not provide information on the number of HIV-infected persons receiving MCM services or the frequency in which they receive them at funded facilities, however; DCDOH HAHSTA data show that among people accessing at least one core medical service, 56% received medical case management in 2011. DCDOH HAHSTA also encourages all MCM funded facilities to perform an intake assessment on everyone receiving care within their facility and tailors the level of services to each person's needs, therefore it is likely persons were exposed to at least one of the core MCM services provided.8 Finally, it is possible exposure to medical case management among newly diagnosed HIV cases was misclassified by using surveillance data resulting in an underestimate of the association diagnosis in a MCM funded facility has on linkage to care. Among the 789 persons who were diagnosed within MCM funded and non-MCM funded facilities during the funding period, 13% of these cases were linked to care at a different facility than the one which performed their diagnostic test and of those cases, 70% of them linked to care in a different type of facility. Either they were diagnosed within a MCM funded facility and linked to care within a non-MCM funded facility or vice versa. We conducted a sensitivity analysis however, and linkage to care within 3 and 6 months did not differ significantly when the 105 cases that linked to a different facility, or when the 73 cases that linked to a different type of facility, were excluded from the analysis. It is likely that the limitations of the study identified here would attenuate the association between medical case management and engagement in care and viral suppression, rather than strengthen these associations.
In conclusion, although we cannot demonstrate causality, these findings are consistent with other studies occurring nationally and suggest that medical case management may be beneficial to HIV-infected persons living in Washington, DC, as case management was associated with higher levels of retention in care. For community-based organizations, developing programs to ensure that referrals to care result in actual linkage and engagement in care are also warranted and will improve patient outcomes. Such programs are currently being developed in Washington, DC and include an emphasis on establishing comprehensive patient-centered HIV medical homes and the development of partnerships between community-based organizations and HIV clinics to provide services ranging from HIV testing to retention in care. Currently, under the Affordable Care Act and Medicaid expansion in DC, medical case management services are not covered or reimbursed thereby reinforcing the importance of Ryan White funding to continue to provide these services to clients to improve outcomes such as retention in care. While more extensive research is needed to identify the specific services that provide the most benefit to clients, it will also be important to continue to examine the relationship between retention in care and viral suppression in the years after ECHPP and NHAS were fully implemented in Washington, DC. With increased focus on all aspects of the continuum of HIV care, data from later years may show more benefit for viral suppression from MCM, thereby decreasing HIV morbidity and mortality, and reducing new HIV infections.
Acknowledgments
The authors would like to acknowledge the contributions of Gunther Freehill who passed away during the writing of this manuscript. Gunther was the Chief of the CARE Bureau at the DC Department of Health HIV/AIDS, Hepatitis, STD,TB Administration and was a lifelong advocate for HIV-infected persons and instrumental in the implementation of the Ryan White CARE Act. His dedication and passion will be missed by many. The authors would also like to thank the DC developmental Center for AIDS Research (P30AI087714), the ECHPP study team, and surveillance staff at the DC Department of Health HIV/AIDS, Hepatitis, STD, TB Administration. They would also like to thank Drs Alan Greenberg, Irene Kuo, Manya Magnus, Sam Simmens, and Marlene Smurzynski of George Washington University, and Dr. Julia Dombrowksi of the University of Washington, for their review and comments regarding earlier versions of this manuscript.
source of funding: This analysis was funded through supplemental funding for the Enhanced Comprehensive HIV Prevention Planning (ECHPP) Initiative through the District of Columbia Developmental Center for AIDS Research, an NIH-funded Program (P30AI087714) and in support of the Public Health/Academic Partnership between the District of Columbia Department of Health, HIV/AIDS, Hepatitis, STD, TB Administration and The George Washington University School of Public Health and Health Services, Department of Epidemiology and Biostatistics (Contract Number POHC-2006-C-0030).
Footnotes
Conflicts of interest The authors have no conflicts of interest to declare. All authors from the George Washington University, as well as the District of Columbia Department of Health, reviewed and approved the final draft of the paper. Additionally, under the Partnership contract, the District of Columbia Department of Health had the right to review and approve the final version of the manuscript.
Portions of this paper were presented at the 2012 National Summit on HIV and Viral Hepatitis Diagnosis, Prevention, and Access to Care held in Washington, DC in November 2012. “Linkage, Engagement and Viral Suppression Rates among HIV-Infected Persons Receiving Care at Medical Case Management Programs in Washington, DC”, (Abstract #124).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stone V. Strategies for optimizing adherence to highly active antiretroviral therapy: lessons from research and clinical practice. Clin Infect Dis. 2001;33:65–72. doi: 10.1086/322698. [DOI] [PubMed] [Google Scholar]
- 2.Paterson D, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:1–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 3.Ickovics J, Meade C. Adherence to HAART among patients with HIV: breakthroughs and barriers. AIDS Care. 2002;14:9–18. doi: 10.1080/09540120220123685. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aberg JA, Kaplan JE, Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:651–681. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention HIV in the United States: The Stages of Care. 2012 Jul; [Google Scholar]
- 7.District of Columbia Department of Health [June 15, 2013];District of Columbia HIV/AIDS, Hepatitis, STD, and TB annual report 2011. 2012 Available at http://doh.dc.gov/sites/default/files/dc/sites/doh/publication/attachments/HAHSTA_ANNUAL_REPOR_2011.pdf.
- 8.Health Resources and Services Administration [July 23, 2013];HRSA CARE Action. Redefining Case Management. 2008 Nov; Available at http://hab.hrsa.gov/newspublications/careactionnewsletter/november2008.pdf.
- 9.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to Care. AIDS. 2005;19:423–431. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 10.Craw JA, Gardner LI, Marks G, et al. Brief strengths-based case management promotes entry into HIV medical care. J Acquir Immune Defic Syndr. 2008;47(5):597–606. doi: 10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- 11.Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care Panel. Ann Intern Med. Jun. 2012;156(11):817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashman JJ, Conviser R, Pounds B. Associations between HIV-positive individuals’ receipt of ancillary services and medical care receipt and retention. AIDS Care. 2002;14(1):S109–S118. doi: 10.1080/09540120220149993a. [DOI] [PubMed] [Google Scholar]
- 13.Bradford JB, Coleman S, Cunningham W. HIV System Navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS. 2007;21(Suppl 1):S49–S58. doi: 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]
- 14.District of Columbia Department of Health [June 15, 2013];HIV medical case management guidelines. 2010 Available at http://doh.dc.gov/sites/default/files/dc/sites/doh/publication/attachments/mcm_march_19.pdf.
- 15.District of Columbia Department of Health DOH care notes: A newsletter from HAHSTA's Care, Housing, and Support Bureau. 2012 Apr;1(1) [Google Scholar]
- 16.Centers for Disease Control and Prevention [June 17, 2013];Enhanced comprehensive HIV prevention planning and implementation for metropolitan statistical areas most affected by HIV/AIDS. Available at http://www.cdc.gov/hiv/prevention/demonstration/echpp/index.html.
- 17.Health Resources and Services Administration [June 15, 2013];HAB HIV Core Clinical Performance Measures for Adult/Adolescent Clients: Group 1. 2008 Jul; Available at http://hab.hrsa.gov/deliverhivaidscare/files/habgrp1pms08.pdf.
- 18.Torian LV, Wiewel EW, Liu KL, et al. Risk factors for delayed initiation of medical care after diagnosis of human immunodeficiency virus. Arch Intern Med. 2008;168(11):1181–1187. doi: 10.1001/archinte.168.11.1181. [DOI] [PubMed] [Google Scholar]
- 19.Jenness SM, Myers JE, Neaigus A, et al. Delayed entry into HIV medical care after HIV diagnosis: risk factors and research methods. AIDS Care. 2012;24(10):1240–1248. doi: 10.1080/09540121.2012.656569. [DOI] [PubMed] [Google Scholar]
- 20.Jenness SM, Hanna DB, Murrill CS. Barriers to HIV medical care among adults newly diagnosed with HIV in New York City.. Presented at: National HIV Prevention Conference; Atlanta, GA. 2007. [Google Scholar]
- 21.Higa DH, Marks G, Crepaz N, et al. Interventions to improve retention in HIV primary care: a systematic review of U.S. studies. Curr HIV/AIDS Rep. 2012;9:313–325. doi: 10.1007/s11904-012-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen M, Hockman E, Smereck G, et al. Retaining women in HIV medical care. J Assoc Nurses AIDS Care. 2007;18(3):33–41. doi: 10.1016/j.jana.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Mugavero MJ, Amico KR, Westfall AO, et al. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr. 2012;59(1):86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Axelrad JE, Mimiaga MJ, Grasso C, et al. Trends in the spectrum of engagement in HIV care and subsequent clinical outcomes among men who have sex with men (MSM) at a Boston community health center. AIDS Patient Care STDS. 2013;27(5):287–296. doi: 10.1089/apc.2012.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castel AD, Willis S, Griffin A, et al. Linkage and engagement in care as predictors of achieving and maintaining viral suppression.. Presented at: HIV Treatment and Prevention Adherence Conference; Miami, FL. 2012. [Google Scholar]
- 26.Willis S, Castel AD, Griffin A, et al. Factors associated with achieving viral suppression among newly diagnosed HIV/AIDS cases in Washington, DC.. Presented at: XIX International AIDS Conference; Washington, DC. 2012. [Google Scholar]
- 27.Fatti G, Meintjes G, Shea J, et al. Improved survival and antiretroviral treatment outcomes in adults receiving community-based adherence support: 5-year results from a multicentre cohort study in South Africa. J Acquir Immune Defic Syndr. 2012;61(4):50–58. doi: 10.1097/QAI.0b013e31826a6aee. [DOI] [PubMed] [Google Scholar]
- 28.Dean B, Debes R, Bozzette S, et al. HIV laboratory tests used as a proxy for medical visits for defining engagement in care.. Presented at: 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]