Abstract
Nearly a half-century ago, the thrice-weekly hemodialysis schedule was empirically established as a means to provide an adequate dialysis dose while also treating the greatest number of end-stage renal disease patients using limited resources. Landmark trials of hemodialysis adequacy have historically been anchored to thrice-weekly regimens, but a recent randomized controlled trial demonstrated that frequent hemodialysis (six times per week) confers cardiovascular and survival benefits. Based on these collective data and experience, clinical practice guidelines advise against a less than thrice-weekly treatment schedule in patients without residual renal function, yet provide limited guidance on the optimal treatment frequency when substantial native kidney function is present. Thus, during the transition from Stage 5 chronic kidney disease to end-stage renal disease, the current paradigm is to initiate hemodialysis on a “full dose” thrice-weekly regimen even among patients with substantial residual renal function. However, emerging data suggests that frequent hemodialysis accelerates residual renal function decline, and infrequent regimens may provide better preservation of native kidney function. Given the high mortality rates during the first 90 days of hemodialysis and the survival benefits of preserved native kidney function, initiation with twice-weekly treatment schedules (“infrequent hemodialysis”) with an incremental increase in frequency over time may provide an opportunity to optimize patient survival. This review outlines the clinical benefits of post-hemodialysis residual renal function, studies of twice-weekly treatment regimens, and the potential risks and benefits of infrequent hemodialysis.
Over 380,000 people in the US receive hemodialysis (HD) for end-stage renal disease (ESRD), among whom approximately 105,000 are newly initiated on therapy.1 ESRD patients have a 7-fold higher risk of death compared to the general population,1 and the first 6 months following dialysis initiation represents a critical transition period during which there is a heightened mortality risk.2
Early recommendations from the 1997 Kidney Disease Outcomes Quality Initiative (KDOQI) Hemodialysis Adequacy Group supported dialysis initiation at a glomerular filtration rate (GFR) of approximately 10ml/min/1.73m2.3 Updated 2006 KDOQI guidelines have subsequently advised that dialysis initiation may be warranted at higher levels of GFR (<15ml/min/1.73m2) in the context of symptoms or declining health related to loss of kidney function.3 In fact, 29% and 16% of patients initiate dialysis with an estimated GFR (eGFR) of 10 to <15ml/min/1.73m2 and >15ml/min/1.73m2, respectively.1 Residual renal function (RRF) at HD commencement may significantly contribute to solute clearance and fluid balance,4–6 and recent data suggests that preservation of urine output (UOP) is associated with a survival benefit.7 Although aggressive measures are taken to preserve RRF in the pre-dialysis and peritoneal dialysis (PD) settings, there is comparatively less focus on the protection of RRF after HD initiation. In comparison to PD, HD has been shown to result in a more rapid decline of RRF, which may be due to intradialytic hypotension, renal ischemia, and exposure to nephrotoxic inflammatory mediators during treatment.8,9 Yet some studies have shown that RRF is better preserved among HD patients than previously believed, with up to 70% and 14–20% of HD patients retaining RRF after 1 and 3 to 5 years of therapy, respectively.10 Indeed, KDOQI guidelines have ranked preservation of RRF in HD as a grade A recommendation and have emphasized clinical outcomes investigation on RRF as a critical research priority.11
Despite these recommendations, the current US paradigm is to initiate HD patients on “full-dose,” thrice-weekly therapy irrespective of RRF and to continue the same frequency of treatment even after RRF has declined. Within US and European prevalent HD cohorts, limited data suggest that ~4% of patients or less are on twice-weekly therapy.12,13 In contrast, data from non-Western countries suggest that prescription of twice-weekly HD is highly prevalent (9% of prevalent and 25% of incident patients in Japan,14 43% of prevalent patients in Iran,15 and 75% of prevalent patients in Sudan16). Although decisions to administer once- or twice-weekly treatment may stem from dialytic resource and financial constraints, emerging data suggest that, in comparison to thrice-weekly therapy, “infrequent dialysis” does not confer greater mortality risk13,17 and may in fact be associated with greater conservation of RRF.18 These findings provide incentive to reevaluate twice-weekly therapy as a new paradigm for HD initiation. Although some experts have suggested that twice-weekly therapy may be acceptable in the context of substantial RRF,19 studies of HD adequacy have historically focused on thrice-weekly regimes in cohorts without RRF.20,21 Given the impact of RRF on survival, twice-weekly therapy may provide an opportunity to reduce the markedly high mortality rates observed during the transition period from Stage 5 chronic kidney disease (CKD) to ESRD.
History of Thrice-Weekly Therapy
Historical narratives indicate that the frequency and duration of HD prescribing patterns were empirically determined when this therapy first came into use following Scribner’s invention of a permanent access device at the University of Washington in 1960.22,23 The first chronic HD patients in Seattle dialyzed once every 5 to 7 days until symptoms of uremia recrudesced. The development of malignant hypertension due to hypervolemia and uremia-associated peripheral neuropathy necessitated an intensification of treatment to twice-weekly therapy. However, the 12 to 20 hour twice-weekly schedule proved to be burdensome on patients and families, and a 6 to 8 hour thrice-weekly overnight schedule was subsequently adopted in Seattle based on early experiences with overnight HD first developed by Shaldon in London.24 When the Medicare ESRD Program came into being in 1973, thrice-weekly dialysis was the usual practice and provided a concession between delivery of adequate therapy and treatment of the most patients using limited resources.23
Hemodialysis Adequacy: Landmark Studies and Clinical Practice Guidelines
Landmark studies that have sought to define the optimal dialysis dose have largely focused on thrice-weekly regimens. In the first endeavor to establish an individualized, quantitative approach to dialysis prescription, the US National Cooperative Dialysis Study (NCDS) randomized patients using a 2 × 2 factorial design to two time-averaged blood urea nitrogen targets (50 vs 100 mg/dl) and two dialysis session lengths (2.5–3.5 vs 4.5–5.0 hours).18 Although the study demonstrated that a higher dialysis dose was associated with clinical benefit, the frequency of HD was fixed as a thrice-weekly schedule at the time of the protocol design to avoid the complexity of dealing with a variable that might vary across centers. Following the establishment of kt/v urea as an important predictor of clinical outcomes, the HEMO trial demonstrated that higher vs lower dialysis doses (single pool kt/v [spKt/v] 1.25 vs 1.65) conferred similar outcomes, but in this study dialytic prescriptions were also anchored to thrice-weekly schedules.11 Notably, the NCDS and HEMO cohorts were both restricted to patients with minimal to absent RRF (creatinine clearance ≤3ml/min and urea clearance≤1.5ml/min per 35L body water, respectively), thus reducing generalizability to those with preserved RRF.
Following the HEMO study, efforts to define optimal HD adequacy have shifted from augmenting the per-session dialytic dose to examining the impact of treatment duration and frequency on outcomes. The Frequent Hemodialysis Network (FHN) Daily trial randomized patients to frequent HD (6 times/week) and conventional HD (3 times/week) and demonstrated that those in the former group had significant benefits with respect to the composite primary outcome of death and 12-month change in left ventricular mass (with the majority of the treatment effect on the latter outcome given the low death rates), control of hypertension, and hyperphosphatemia.25 Although a higher number of access-related complications were observed in the frequent HD arm, there were no differences in terms of other secondary outcomes such as cognitive performance, depression, nutritional markers (eg, serum albumin) or use of erythropoietin-stimulating agents (ESAs). Although this study demonstrated a number of benefits on primary and secondary outcomes, it was not designed to evaluate the impact of treatment frequency among patients transitioning from Stage 5 CKD to ESRD with substantial RRF. Patients with urea clearance cutoffs <3ml/min/35L body water were excluded, and the vast majority of patients were anuric (60.0% and 72.0% in the conventional and frequent HD groups, respectively).
The most recent KDOQI guidelines recommend that the minimally adequate and target dialysis doses for patients without substantial RRF (residual urea clearance of <2ml/min/1.73m2) is an spKt/v of 1.2 to 1.4 per session, respectively, and advise against prescribing less than thrice-weekly therapy.11 In patients with a residual urea clearance of ≥2ml/min/1.73m2, allowances are made for a dose reduction to 60% of the minimum target of those without RRF. However no randomized controlled trials to date have examined the prognostic implications of reduction of dialysis dose or frequency in patients with substantial RRF, nor do current practice guidelines define the optimal frequency of therapy in this context.
Importance of Residual Renal Function
In stage 5 CKD and ESRD, UOP may variably be present at low levels of GFR due to 1) a urea osmotic diuresis, 2) volume expansion associated with sodium retention, and 3) tubular damage impairing sodium and water reabsorption.26–28 Hence a rapid reduction in UOP may be observed upon commencement of dialysis with the subsequent reversal of volume expansion and high urea load per nephron. Earlier studies have shown that HD patients experience an accelerated loss of RRF compared to those on PD (−3.6ml/min/1.73m2 vs −4.2ml/min/1.73m2 during the first 1 year of dialysis, respectively) which may be related to greater intradialytic hypotension resulting in renal ischemia and acute tubular necrosis, and activation of nephrotoxic inflammatory mediators (e.g., exposure to dialysis tubing and impurities, previously used bioincompatible membranes).8,9 However, it should be noted that with the advent of biocompatible membranes, replacement of vasodilatory acetate buffer with bicarbonate, and secular trends towards earlier initiation of dialysis therapy, contemporary HD cohorts may experience greater preservation of RRF than previously observed.29–31 A small study of 11 patients on twice-weekly HD demonstrated that urine production, inulin clearance, and fractional sodium clearance steadily increased over the 3-day interdialytic interval.32 Although this may be a result of increased osmotic load and extracellular volume accumulation over the long interdialytic period, examination of the impact of infrequent dialysis on long term RRF preservation is warranted.
RRF preservation following dialysis initiation has important implications for clinical outcomes in the ESRD population. At very low levels of GFR (~4–5ml/min/1.73m2) in ESRD patients, RRF has a significant impact on solute clearance given its continuous nature33; it may also provide greater clearance of middle and large molecular weight solutes in comparison to dialytic therapies.6,34,35 RRF also improves fluid balance,6,10,36 reducing the risk of large interdialytic weight gains and subsequent high ultrafiltration requirements, left ventricular hypertrophy, intradialytic hypotension, myocardial stunning, and cardiovascular mortality. In the PD population, RRF’s association with improved survival is well established. In a reanalysis of the CANUSA study, each 5L/week per 1.73m2 increment in RRF and each 250ml increase in UOP was associated with a 12% and 36% reduction in mortality, respectively.37 Similarly, in the ADEMEX trial, each 10L/week per 1.73m2 increment in RRF was associated with an 11% reduction in mortality.38 In both studies, peritoneal clearance was not associated with survival.
Recent observational data also suggests that RRF is associated with improved survival in HD patients. In a study of 650 incident HD patients, Vilar et al demonstrated that the presence of RRF was associated with improved survival at 6, 12, and 24 months follow-up in multivariable adjusted analyses; notably, patients with preserved RRF had lower kt/v values suggesting that native renal clearance was superior to dialytic clearance with respect to mortality benefit.10 In an evaluation of 740 incident HD patients from the NECOSAD cohort, each 1L/week increase in clearance was associated with a 56% reduction in risk of death, and RRF was a stronger predictor of survival than dialytic clearance.39 Most recently, in a study of 734 incident HD patients from the CHOICE cohort, preservation of UOP after 1 year was associated with lower all-cause mortality and a trend towards reduced cardiovascular mortality, and baseline UOP was associated with improved quality of life, improved cognition, dietary liberalization, and reduced inflammation.7 In both the PD and HD populations, RRF may be associated with improved nutritional parameters,10,40–42 decreased ESA requirements,10 improved phosphorus43,44 and potassium control,45 and reduced left ventricular hypertrophy,46 which may serve as underlying mechanistic links between RRF and improved mortality.
Infrequent Dialysis: Historical Precedents
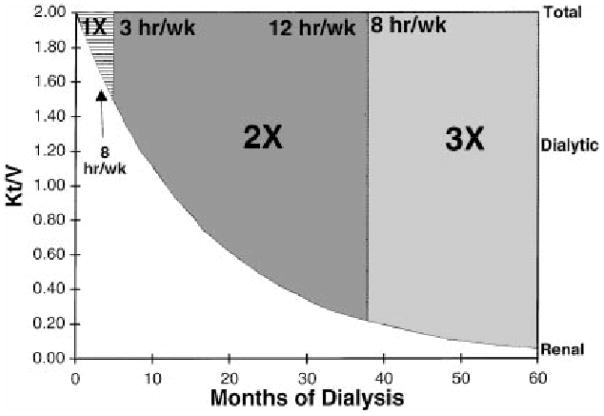
Emerging data suggests that RRF may be used as a guide to establish and adjust HD frequency and fluid status monitoring. This concept of “incremental dialysis” was initially described among PD patients, in whom RRF is critical to solute clearance, fluid balance, and survival.47,48 The ideal scenario for utilization of incremental dialysis is when significant RRF is present and can contribute to total renal replacement therapy, which may occur in the context of 1) HD or PD initiation, 2) resumption of dialysis with renal allograft failure, and 3) conversion from failing PD to HD.49 Using this approach, the dose of delivered dialysis is increased as RRF declines in such a manner that the sum of weekly residual renal plus artificial dialytic clearance is maintained above a certain minimum. Keshaviah et al have previously defined a urea kinetic model to show how the HD dose can be titrated to compensate for declining renal function while maintaining a constant total combined dose of renal and dialytic clearance.50 Using data on RRF decline from a previously described HD cohort, the authors demonstrated a model in which a hypothetical patient initiates once-weekly therapy and is gradually increased to twice-weekly and thrice-weekly dialysis as RRF declines after 5 and 36 months, respectively (Figure 1).
Figure 1.
Urea kinetic model in which a hypothetical patient initiates once-weekly therapy and is gradually increased to twice-weekly and thrice-weekly dialysis as residual renal function declines after 5 and 36 months, respectively. The renal and dialytic contributions to the dose of dialysis are shown as a function of the months on dialysis, and the total dose of delivered therapy is fixed at a set kt/v. Taken from Keshaviah PR, Emerson PF, Nolph KD. Timely initiation of dialysis: a urea kinetic approach. American journal of kidney diseases: the official journal of the National Kidney Foundation. Feb 1999;33(2):344–348.
Dietary protein restriction has also been employed as an adjunctive measure to reduce dialysis frequency in incremental treatment regimes. The Northern Cooperative Study Trial enrolled 69 patients with a weekly RRF kt/v urea of approximately 0.5 in an integrated diet dialysis program consisting of once-weekly HD and a low protein diet (0.4g/day) supplemented with essential amino acids in order to maintain a predialytic BUN of <90mg/dl.51 After 1 year, there was >50% dropout, and patients developed signs of worsened anthropometric status (eg, decreased serum creatinine) and uremia progression (eg, decreased distal nerve conduction velocity) leading authors to advise against broad application of this management strategy.
Twice-Weekly Therapy and Outcomes
Mortality
To date, there have been a limited number of observational studies that have evaluated the association between twice-weekly HD therapy and outcomes (Table 1). The sole US study examining the prognostic implications of twice-weekly therapy was a retrospective evaluation of 15,543 HD patients from the 1993 US Dialysis Morbidity and Mortality Study cohort.13 In this study, patients on twice-weekly therapy tended to be of shorter vintage, older age, female gender, and Caucasian, and at baseline had more favorable nutritional parameters (higher serum albumin) but worse anthropometric markers (lower serum creatinine and body mass index). Multivariable adjusted survival analyses restricted to incident patients demonstrated that twice-weekly therapy was associated with a mortality reduction that was attenuated with further adjustment for RRF. Examination of prevalent patients demonstrated that twice-weekly therapy was associated with reduced mortality risk compared to patients on thrice-weekly therapy; however these analyses did not account for differences in RRF, and the survival advantage associated with twice-weekly therapy was likely related to more favorable patient characteristics in the twice-weekly group.
Table 1.
Summary of observational studies examining the association between infrequent hemodialysis and outcomes.
| Study | Cohort description | n | Results | |
|---|---|---|---|---|
| Mortality | ||||
| Hanson (1999)13 | Incident and prevalent HD | 15,067 | Incident patients: Twice-weekly treatment similar mortality risk compared to thrice-weekly treatment. Prevalent patients: Twice- weekly therapy decreased mortality risk but unadjusted for RRF. |
|
| Lin (2012)17 | Incident and prevalent HD | 1288 (multivariable adjusted) | Overall cohort: Twice-weekly treatment similar mortality risk compared to thrice-weekly patients. Incident patients: Survival rates between twice and thrice-weekly patients similar in both subgroups. Prevalent (vintage>5 years) patients: Survival rates between twice and thrice-weekly patients similar in both subgroups. |
|
| Stankuviene52 (2010) | Incident HD | 2428 | Once and twice-weekly treatment with increased mortality risk compared to thrice-weekly treatment. | |
| Elamin (2012)16 | Prevalent HD | 2012 | Greater proportion of twice- weekly patients with 1-year mortality than thrice-weekly patients (but differences not statistically significant). | |
| Residual Renal Function | ||||
| Lin (1999)18 | Prevalent HD | 74 | Twice-weekly treatment with greater RRF preservation than thrice-weekly treatment. | |
| Chen (2012)53 | Incident HD | 51 | Twice-weekly treatment with thrice-weekly treatment. greater RRF preservation than thrice-weekly treatment. | |
| Daugirdas (2013)54 | Prevalent HD | 63 (Nocturnal trial) 83 (Daily trial) |
Nocturnal trial: Frequent HD with greater RRF decline than conventional HD. Daily trial: Frequent HD with similar RRF decline as conventional HD. |
|
| Nutritional Parameters | ||||
| Supasyndh (2009)57 | Prevalent HD | 142 | Twice-weekly treatment similar nutritional laboratory parameters and daily protein intake but greater daily energy intake than thrice-weekly treatment. | |
| Lin (1999)18 | Prevalent HD | 74 | Twice-weekly treatment with similar nutritional laboratory parameters as thrice-weekly treatment. | |
Abbreviations: HD, hemodialysis; RRF, residual renal function.
* Unpublished data.
† Comparison between frequent HD (6 times/week) and conventional HD (3 times/week).
Notably, prevalent patients on twice-weekly therapy had shorter dialysis session lengths and marginally higher delivered kt/v per session, resulting in lower weekly kt/v compared to their thrice-weekly counterparts. These findings among prevalent patients are noteworthy as they suggest that infrequent dialysis despite lower weekly delivered kt/v is not per se harmful, and that twice-weekly schedules may not only serve as a temporary transition strategy but also as a long-term dialytic approach.
Similarly, in a smaller but more contemporary cohort of 1288 incident and prevalent HD patients from the Shanghai Renal Registry, multivariable adjusted survival analyses demonstrated an equivalent mortality risk between twice-weekly and thrice-weekly patients, although interpretation is limited by absence of RRF data and residual confounding.17 Case-mix differences within this cohort (ie, high prevalence of glomerulonephritis and low prevalence of diabetes and hypertension as the primary cause of kidney disease) may also limit generalizability to US populations.
In contrast, other studies from developing countries have shown that twice-weekly therapy is associated with increased mortality. Data from 2063 incident HD patients in Lithuania demonstrated that patients on once- and twice-weekly therapy (~36% and 5% of cohort, respectively) had a nearly two-fold higher risk in mortality compared to those on thrice-weekly regimens, although there was limited adjustment for covariates that did not include RRF.52
In another study of 1011 prevalent HD patients in Sudan, a greater proportion of patients on thrice-weekly therapy survived over a 1-year follow-up period compared to those on twice-weekly treatment although differences did not reach statistical significance (89% vs 85%, respectively; p=0.06); however these findings are limited by lack of time-to-event analysis, residual confounding, and incomplete data on dialysis adequacy or RRF.16 In contrast to the Dialysis Morbidity and Mortality Study, resource constraints (ie, limited HD accessibility) among non-US cohorts likely had a stronger influence on decisions to prescribe twice-weekly therapy regimes than physician- or patient-related factors, and may have also impacted outcomes.
Residual Renal Function
Given the hastened decline in RRF that may be observed with HD initiation, there has been increasing interest in how the frequency of dialytic therapy impacts RRF preservation. In a study of 74 prevalent HD patients in Taiwan who maintained the same HD frequency without cross-over, the rate of RRF decline was compared among patients on twice vs thrice-weekly therapy with similar baseline creatinine clearance and UOP levels.18 After a mean follow-up of 18 months, those on twice-weekly therapy had higher creatinine clearance and UOP levels and a slower rate of RRF decline compared to their thrice-weekly counterparts. Additionally, patients on twice-weekly treatment had greater clearance of large molecular weight solutes (as measured by serum beta-2 microglobulin levels), less intradialytic hypotension, and fewer hospitalizations for infections; however, no differences in nutritional or inflammatory parameters, total spKt/v or AV fistula dysfunction were observed between groups. However, analyses did not account for differences in demographics or case-mix covariates between the two groups with multivariable adjustment. Unpublished data from Shanghai have also shown that in a cohort of 165 HD patients, 45.5% received less than thrice-weekly HD; in a subgroup of 51 patients with GFR follow-up within one year of HD initiation, increased HD frequency was associated with greater decline in RRF in multivariable adjusted analyses.53
A corollary study from the FHN Daily and companion Nocturnal trials comparing frequent HD vs conventional HD suggests that the impact of dialytic frequency on RRF decline exists on a spectrum.54 Among non-anuric patients in the Nocturnal trial arm, patients receiving frequent HD had a greater decline in RRF as measured by urine volume, urea clearance, and creatinine clearance compared to those receiving conventional HD at 4 and 12 months follow-up. Notably, patients in the frequent HD group had a significantly lower nadir in intradialytic systolic blood pressure compared to the conventional group, suggesting a hemodynamic etiology as the mechanistic link. However, in the Daily trial aim, there was no significant difference in RRF at 4 and 12 month follow-up between the frequent HD and conventional HD patients with non-zero RRF. It should be noted however that the Daily trial excluded patients with higher levels of RRF in comparison to the Nocturnal trial (urea clearance cutoffs <3ml/min/35L body water and <10ml/min/35L body water, respectively), which may have resulted in underpowered analyses with which to ascertain differences between the frequent and conventional groups in the Daily trial.
Other Potential Benefits
There may be a number of additional benefits that infrequent treatment confers to incident HD patients. For example, it has been suggested that frequent therapy may contribute to malnutrition through dialytic losses of vital nutrients (eg, carnitine, vitamin C).55 However, inadequate uremic toxin clearance associated with infrequent therapy could theoretically exacerbate malnutrition, and studies have shown that switching patients from conventional to daily HD increases appetite and protein intake.56 In a cross-sectional study of 142 prevalent HD patients all with a weekly spKt/v>3.6 from the National Kidney Foundation dialysis unit of Thailand, analyses unadjusted for difference in patients’ case-mix covariates or RRF demonstrated no significant differences in anthropometric measurements, laboratory nutrition markers (ie, serum albumin, total cholesterol, nPNA), or survey-ascertained dietary protein intake between those receiving twice- and thrice-weekly therapy; notably, twice-weekly therapy patients reported greater dietary energy intake than thrice-weekly patients.57 As noted above, Taiwanese patients receiving twice-weekly therapy had similar nutritional parameters (serum albumin, normalized protein catabolic rate) as their thrice-weekly counterparts.18
Extrapolation of findings from studies evaluating greater than thrice-weekly HD regimens suggest that more frequent HD regimens may be associated with greater vascular access loss25 (possibly due to inflammation associated with the dialytic procedure and more frequent access cannulation). Additionally, frequent HD may predispose to increased ESA resistance and from dialytic blood loss and iron deficiency, and repeated exposure to dialysis tubing and membranes could potentially increase inflammation, oxidative stress, and subsequent cardiovascular risks.55 Initiation of HD with twice-weekly schedules and gradual transition to thrice-weekly therapy as RRF declines may also reduce lifestyle and employment status interruptions, minimize psychological, and enhance patients’ quality of life and acceptance of treatment.48,55
It is possible that some patients who undergo an infrequent treatment strategy may be reluctant to increase HD frequency as RRF declines, resulting in non-compliance and inadequate dialysis; thus it is imperative that providers and patients have an understanding that the dialysis frequency and dose will inevitably increase over time.49 Lastly, given the global epidemic of ESRD, appropriate allocation of infrequent HD to patients with substantial RRF who can thus achieve adequate total (renal plus dialytic) clearance may provide the opportunity to allocate limited dialytic resources to a broader population. Further study of the direct impact of infrequent HD regimens on ESRD patients’ mortality, RRF, vascular access preservation, cardiovascular outcomes, ESA resistance and usage, quality of life, and cost-benefits are needed.
Potential Harms of Infrequent Dialysis
Despite achievement of adequate or supratherapeutic spKt/v targets with adjunctive native solute clearance, there may be certain scenarios in which intermittent infrequent HD is ill-advised. For example, patients with excess interdialytic weight gains may not be suitable for less than thrice-weekly HD schedules, irrespective of presence of RRF.17 High interdialytic weight gains and chronic extracellular fluid volume overload predispose to hypertension, left ventricular hypertrophy, and congestive heart failure which negatively impact dialysis patients’ survival.58 Additionally, higher interdialytic weight gains in the context of infrequent HD may necessitate high ultrafiltration rates that result in intradialytic hypotension, myocardial stunning, cardiac ischemia,59 and inability to achieve dry weight due to cessation of ultrafiltration and infusion of saline that perpetuating the volume overload cycle.60 Hence, “permissive hypervolemia” with the intention in preserving native kidney function to survival benefit may have detrimental effects on cardiovascular health that offset the benefits of RRF.6
Protracted interdialytic intervals with infrequent HD may also pose harm. Data from 32,065 US patients from the ESRD Clinical Performance Measures Project has shown higher rates of death and cardiovascular-related hospital admissions on the day after the long 2-day interdialytic interval presumably due to accumulation and/or rapid dialytic reduction of electrolytes, fluid, and various uremic toxins.61 Although RRF data was not available, >75% of patients had a vintage of ≥1 year, and it is likely that the majority of patients were anuric and lacked adjunctive capacity to clear uremic toxins and maintain fluid and electrolyte balance. Data from 22,163 patients from the Dialysis Outcomes and Practice Patterns Study showed similar findings in non-US populations in Europe and Japan.62 Nonetheless protracting the long interdialytic interval from 2 days with thrice-weekly dialysis to 3–4 days with infrequent HD may be deleterious in patients who are prone to hyperkalemia and other electrolyte derangements.
Lastly, it is possible that some subgroups may be unsuitable for twice-weekly therapy due to higher dialysis dose requirements. In a subgroup analysis of the HEMO study, women experienced a survival benefit with higher dialysis dose whereas men did not.20 Additionally, patients with a high burden of comorbidities or hypercatabolic state may require more frequent HD regimens to maximize solute clearance. Thus prescribing HD frequency using an individualized approach in lieu of a “one size fits all” strategy, and examining the differential effects of infrequent therapy across subgroup populations is warranted.
Conclusion
For the past 50 years, usual practice has been to initiate HD with a thrice-weekly treatment schedule irrespective of patients’ RRF. The presence of native kidney function at even low levels provides fluid and electrolyte balance, and it is associated with more favorable quality of life indicators and survival benefit. However, native kidney function is often neglected following HD initiation, and there are a paucity of studies examining ways to preserve this intrinsic source of solute clearance and ultrafiltration. In contrast, PD initiation has typically employed a more individualized approach that incorporates both native kidney and dialysis clearance into the total weekly clearance target until RRF is lost at which time the dialytic prescription is intensified. Ideally, assessment of HD adequacy should encompass per-session dialysis dose and length, frequency of treatment, and the contribution of RRF. It is our opinion that initiating HD with a twice-weekly regimen with an incremental increase over time may provide an opportunity to preserve RRF, optimize quality of life, and decrease mortality. Emerging observational data that suggests infrequent HD schedules may be beneficial, but interpretation is limited by residual confounding, lack of data on native kidney function, and inclusion of anuric prevalent HD patients. At this time, randomized controlled trials are needed to determine the adequacy, safety, and cost-benefits of infrequent HD regimens.
Acknowledgments
Support: KKZ is supported by research grants from the NIH/NIDDK (R01 DK078106, K24 DK091419), a philanthropist grant from Mr. Harold Simmons, and a research grant from DaVita Clinical Research. CMR was supported by an NIH/NIDDK grant (F32 DK093201). CPK is supported by an NIH/NIDDK grant (R01 DK096920).
References
- 1.U.S. Renal Data System. USRDS 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2012. [Google Scholar]
- 2.Lukowsky LR, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K. Patterns and predictors of early mortality in incident hemodialysis patients: new insights. Am J Nephrol. 2012;35(6):548–558. doi: 10.1159/000338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NKF-DOQI clinical practice guidelines for peritoneal dialysis adequacy. National Kidney Foundation. Am J Kidney Dis. 1997;30(3 Suppl 2):S67–136. doi: 10.1016/s0272-6386(97)70028-3. [DOI] [PubMed] [Google Scholar]
- 4.Canaud B. Residual renal function: the delicate balance between benefits and risks. Nephrol Dial Transplant. 2008;23(6):1801–1805. doi: 10.1093/ndt/gfn089. [DOI] [PubMed] [Google Scholar]
- 5.Chandna SM, Farrington K. Residual renal function: considerations on its importance and preservation in dialysis patients. Semin Dial. 2004;17(3):196–201. doi: 10.1111/j.0894-0959.2004.17306.x. [DOI] [PubMed] [Google Scholar]
- 6.Vilar E, Farrington K. Emerging importance of residual renal function in end-stage renal failure. Semin Dial. 2011;24(5):487–494. doi: 10.1111/j.1525-139X.2011.00968.x. [DOI] [PubMed] [Google Scholar]
- 7.Shafi T, Jaar BG, Plantinga LC, Fink NE, Sadler JH, Parekh RS, Powe NR, Coresh J. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56(2):348–358. doi: 10.1053/j.ajkd.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62(3):1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 9.Lysaght MJ, Vonesh EF, Gotch F, Ibels L, Keen M, Lindholm B, Nolph KD, Pollock CA, Prowant B, Farrell PC. The influence of dialysis treatment modality on the decline of remaining renal function. ASAIO Trans. 1991;37(4):598–604. [PubMed] [Google Scholar]
- 10.Vilar E, Wellsted D, Chandna SM, Greenwood RN, Farrington K. Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant. 2009;24(8):2502–2510. doi: 10.1093/ndt/gfp071. [DOI] [PubMed] [Google Scholar]
- 11.Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48 (Suppl 1):S2–90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 12.Couchoud C, Kooman J, Finne P, Leivestad T, Stojceva-Taneva O, Ponikvar JB, Collart F, Kramar R, de Francisco A, Jager KJ. From registry data collection to international comparisons: examples of haemodialysis duration and frequency. Nephrol Dial Transplant. 2009;24(1):217–224. doi: 10.1093/ndt/gfn442. [DOI] [PubMed] [Google Scholar]
- 13.Hanson JA, Hulbert-Shearon TE, Ojo AO, Port FK, Wolfe RA, Agodoa LY, Daugirdas JT. Prescription of twice-weekly hemodialysis in the USA. Am J Nephrol. 1999;19(6):625–633. doi: 10.1159/000013533. [DOI] [PubMed] [Google Scholar]
- 14.Japanese Society for Dialysis Therapy. An overview of regular dialysis treatment in Japan as of December 31, 1997. Tokyo: Bunkyo-ku; 1997. p. 266. [Google Scholar]
- 15.Haghighi AN, Broumand B, D’Amico M, Locatelli F, Ritz E. The epidemiology of end-stage renal disease in Iran in an international perspective. Nephrol Dial Transplant. 2002;17(1):28–32. doi: 10.1093/ndt/17.1.28. [DOI] [PubMed] [Google Scholar]
- 16.Elamin S, Abu-Aisha H. Reaching target hemoglobin level and having a functioning arteriovenous fistula significantly improve one year survival in twice weekly hemodialysis. Arab J Nephrol Transplant. 2012;5(2):81–86. [PubMed] [Google Scholar]
- 17.Lin X, Yan Y, Ni Z, Gu L, Zhu M, Dai H, Zhang W, Qian J. Clinical outcome of twice-weekly hemodialysis patients in shanghai. Blood Purif. 2012;33(1–3):66–72. doi: 10.1159/000334634. [DOI] [PubMed] [Google Scholar]
- 18.Lin YF, Huang JW, Wu MS, Chu TS, Lin SL, Chen YM, Tsai TJ, Wu KD. Comparison of residual renal function in patients undergoing twice-weekly versus three-times-weekly haemodialysis. Nephrology (Carlton) 2009;14(1):59–64. doi: 10.1111/j.1440-1797.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 19.Daugirdas JT, Ing TS. Handbook of Dialysis. Chapter 6. Boston: Little, Brown; 1994. Chronic hemodialysis prescription: A urea kinetic approach; pp. 99–103. [Google Scholar]
- 20.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 21.Lowrie EG, Laird NM, Parker TF, Sargent JA. Effect of the hemodialysis prescription of patient morbidity: report from the National Cooperative Dialysis Study. N Engl J Med. 1981;305(20):1176–1181. doi: 10.1056/NEJM198111123052003. [DOI] [PubMed] [Google Scholar]
- 22.Blagg CR. The early history of dialysis for chronic renal failure in the United States: a view from Seattle. Am J Kidney Dis. 2007;49(3):482–496. doi: 10.1053/j.ajkd.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Scribner BH, Cole JJ, Ahmad S, Blagg CR. Why thrice weekly dialysis? Hemodial Int. 2004;8(2):188–192. doi: 10.1111/j.1492-7535.2004.01094.x. [DOI] [PubMed] [Google Scholar]
- 24.Shaldon S. Experience to date with home hemodialysis. In: Scribner BH, editor. Proceedings of the Working Conference on Chronic Dialysis. Seattle, WA: University of Washington; 1964. pp. 66–69. [Google Scholar]
- 25.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363(24):2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danovitch GM, Bourgoignie J, Bricker NS. Reversibility of the “salt-losing” tendency of chronic renal failure. N Engl J Med. 1977;296(1):14–19. doi: 10.1056/NEJM197701062960104. [DOI] [PubMed] [Google Scholar]
- 27.Feinfeld DA, Danovitch GM. Factors affecting urine volume in chronic renal failure. Am J Kidney Dis. 1987;10(3):231–235. doi: 10.1016/s0272-6386(87)80179-8. [DOI] [PubMed] [Google Scholar]
- 28.Yeh BP, Tomko DJ, Stacy WK, Bear ES, Haden HT, Falls WF., Jr Factors influencing sodium and water excretion in uremic man. Kidney Int. 1975;7(2):103–110. doi: 10.1038/ki.1975.15. [DOI] [PubMed] [Google Scholar]
- 29.McKane W, Chandna SM, Tattersall JE, Greenwood RN, Farrington K. Identical decline of residual renal function in high-flux biocompatible hemodialysis and CAPD. Kidney Int. 2002;61(1):256–265. doi: 10.1046/j.1523-1755.2002.00098.x. [DOI] [PubMed] [Google Scholar]
- 30.Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, Hulbert-Shearon T, Jones CA, Bloembergen WE. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11(3):556–564. doi: 10.1681/ASN.V113556. [DOI] [PubMed] [Google Scholar]
- 31.Van Stone JC. The effect of dialyzer membrane and etiology of kidney disease on the preservation of residual renal function in chronic hemodialysis patients. ASAIO J. 1995;41(3):M713–716. doi: 10.1097/00002480-199507000-00105. [DOI] [PubMed] [Google Scholar]
- 32.van Olden RW, van Acker BA, Koomen GC, Krediet RT, Arisz L. Time course of inulin and creatinine clearance in the interval between two haemodialysis treatments. Nephrol Dial Transplant. 1995;10(12):2274–2280. doi: 10.1093/ndt/10.12.2274. [DOI] [PubMed] [Google Scholar]
- 33.Rottembourg J. Residual renal function and recovery of renal function in patients treated by CAPD. Kidney Int Suppl. 1993;40:S106–110. [PubMed] [Google Scholar]
- 34.Babb AL, Ahmad S, Bergstrom J, Scribner BH. The middle molecule hypothesis in perspective. Am J Kidney Dis. 1981;1(1):46–50. doi: 10.1016/s0272-6386(81)80011-x. [DOI] [PubMed] [Google Scholar]
- 35.Bargman JM, Golper TA. The importance of residual renal function for patients on dialysis. Nephrol Dial Transplant. 2005;20(4):671–673. doi: 10.1093/ndt/gfh723. [DOI] [PubMed] [Google Scholar]
- 36.Konings CJ, Kooman JP, Schonck M, Struijk DG, Gladziwa U, Hoorntje SJ, van der Wall Bake AW, van der Sande FM, Leunissen KM. Fluid status in CAPD patients is related to peritoneal transport and residual renal function: evidence from a longitudinal study. Nephrol Dial Transplant. 2003;18(4):797–803. doi: 10.1093/ndt/gfg147. [DOI] [PubMed] [Google Scholar]
- 37.Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12(10):2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 38.Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13(5):1307–1320. doi: 10.1681/ASN.V1351307. [DOI] [PubMed] [Google Scholar]
- 39.Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15(4):1061–1070. doi: 10.1097/01.asn.0000117976.29592.93. [DOI] [PubMed] [Google Scholar]
- 40.Suda T, Hiroshige K, Ohta T, Watanabe Y, Iwamoto M, Kanegae K, Ohtani A, Nakashima Y. The contribution of residual renal function to overall nutritional status in chronic haemodialysis patients. Nephrol Dial Transplant. 2000;15(3):396–401. doi: 10.1093/ndt/15.3.396. [DOI] [PubMed] [Google Scholar]
- 41.Szeto CC, Lai KN, Wong TY, Law MC, Leung CB, Yu AW, Li PK. Independent effects of residual renal function and dialysis adequacy on nutritional status and patient outcome in continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1999;34(6):1056–1064. doi: 10.1016/S0272-6386(99)70011-9. [DOI] [PubMed] [Google Scholar]
- 42.Wang AY, Sea MM, Ip R, Law MC, Chow KM, Lui SF, Li PK, Woo J. Independent effects of residual renal function and dialysis adequacy on actual dietary protein, calorie, and other nutrient intake in patients on continuous ambulatory peritoneal dialysis. J Am Soc Nephrol. 2001;12(11):2450–2457. doi: 10.1681/ASN.V12112450. [DOI] [PubMed] [Google Scholar]
- 43.Wang AY, Woo J, Sea MM, Law MC, Lui SF, Li PK. Hyperphosphatemia in Chinese peritoneal dialysis patients with and without residual kidney function: what are the implications? Am J Kidney Dis. 2004;43(4):712–720. [PubMed] [Google Scholar]
- 44.Wang M, You L, Li H, Lin Y, Zhang Z, Hao C, Chen J. Association of Circulating Fibroblast Growth Factor-23 with Renal Phosphate Excretion among Hemodialysis Patients with Residual Renal Function. Clin J Am Soc Nephrol. 2012 doi: 10.2215/CJN.00230112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morduchowicz G, Winkler J, Zabludowki JR, Boner G. Effects of residual renal function in hemodialysis patients. Int Urol Nephrol. 1994;26:125–131. doi: 10.1007/BF02768252. [DOI] [PubMed] [Google Scholar]
- 46.Wang AY, Wang M, Woo J, Law MC, Chow KM, Li PK, Lui SF, Sanderson JE. A novel association between residual renal function and left ventricular hypertrophy in peritoneal dialysis patients. Kidney Int. 2002;62:639–647. doi: 10.1046/j.1523-1755.2002.00471.x. [DOI] [PubMed] [Google Scholar]
- 47.Agrawal A, Saran R, Nolph KD. Continuum and integration of pre-dialysis care and dialysis modalities. Perit Dial Int. 1999;19 (Suppl 2):S276–280. [PubMed] [Google Scholar]
- 48.Mehrotra R, Nolph KD, Gotch F. Early initiation of chronic dialysis: role of incremental dialysis. Perit Dial Int. 1997;17(5):426–430. [PubMed] [Google Scholar]
- 49.Golper TA. Incremental dialysis. J Am Soc Nephrol. 1998;9(12 Suppl):S107–111. [PubMed] [Google Scholar]
- 50.Keshaviah PR, Emerson PF, Nolph KD. Timely initiation of dialysis: a urea kinetic approach. Am J Kidney Dis. 1999;33(2):344–348. doi: 10.1016/s0272-6386(99)70310-0. [DOI] [PubMed] [Google Scholar]
- 51.Locatelli F, Andrulli S, Pontoriero G, Di Filippo S, Bigi MC. Supplemented low-protein diet and once-weekly hemodialysis. Am J Kidney Dis. 1994;24(2):192–204. doi: 10.1016/s0272-6386(12)80181-8. [DOI] [PubMed] [Google Scholar]
- 52.Stankuviene A, Ziginskiene E, Kuzminskis V, Bumblyte IA. Impact of hemodialysis dose and frequency on survival of patients on chronic hemodialysis in Lithuania during 1998–2005. Medicina (Kaunas) 2010;46(8):516–521. [PubMed] [Google Scholar]
- 53.Chen J. Association between twice-weekly hemodialysis therapy and outcomes. Unpublished data. [Google Scholar]
- 54.Daugirdas JT, Greene T, Rocco MV, Kaysen GA, Depner TA, Levin NW, Chertow GM, Ornt DB, Raimann JG, Larive B, Kliger AS. Effect of frequent hemodialysis on residual kidney function. Kidney Int. 2013;83(5):949–958. doi: 10.1038/ki.2012.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molnar MZ, Ojo AO, Bunnapradist S, Kovesdy CP, Kalantar-Zadeh K. Timing of dialysis initiation in transplant-naive and failed transplant patients. Nat Rev Nephrol. 2012;8(5):284–292. doi: 10.1038/nrneph.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galland R, Traeger J, Arkouche W, Delawari E, Fouque D. Short daily hemodialysis and nutritional status. Am J Kidney Dis. 2001;37(1 Suppl 2):S95–98. doi: 10.1053/ajkd.2001.20758. [DOI] [PubMed] [Google Scholar]
- 57.Supasyndh O, Satirapoj B, Seenamngoen S, Yongsiri S, Choovichian P, Vanichakarn S. Nutritional status of twice and thrice-weekly hemodialysis patients with weekly Kt/V > 3.6. J Med Assoc Thai. 2009;92(5):624–631. [PubMed] [Google Scholar]
- 58.Fagugli RM, Pasini P, Quintaliani G, Pasticci F, Ciao G, Cicconi B, Ricciardi D, Santirosi PV, Buoncristiani E, Timio F, Valente F, Buoncristiani U. Association between extracellular water, left ventricular mass and hypertension in haemodialysis patients. Nephrol Dial Transplant. 2003;18(11):2332–2338. doi: 10.1093/ndt/gfg371. [DOI] [PubMed] [Google Scholar]
- 59.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4(5):914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chazot C, Jean G. The advantages and challenges of increasing the duration and frequency of maintenance dialysis sessions. Nat Clin Pract Nephrol. 2009;5(1):34–44. doi: 10.1038/ncpneph0979. [DOI] [PubMed] [Google Scholar]
- 61.Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med. 2011;365(12):1099–1107. doi: 10.1056/NEJMoa1103313. [DOI] [PubMed] [Google Scholar]
- 62.Zhang H, Schaubel DE, Kalbfleisch JD, Bragg-Gresham JL, Robinson BM, Pisoni RL, Canaud B, Jadoul M, Akiba T, Saito A, Port FK, Saran R. Dialysis outcomes and analysis of practice patterns suggests the dialysis schedule affects day-of-week mortality. Kidney Int. 2012;81(11):1108–1115. doi: 10.1038/ki.2011.481. [DOI] [PMC free article] [PubMed] [Google Scholar]