Abstract
Scope
A key event in the development of plaque in the arteries is the migration and proliferation of smooth muscle cells (SMCs) from the media to the intima of the blood vessel. This study was conducted to evaluate the effects of bisdemethoxycurcumin, a naturally occurring structural analog of curcumin, on PDGF-stimulated migration and proliferation of SMCs.
Methods and results
Demethoxycurcumin were synthesized by condensing vanillin and 4-hydroxybenzaldehyde. SMCs isolated from adult rat aorta were stimulated with PDGF in the presence or absence of curcumin or bisdemethoxycurcumin following which cell migration and proliferation were assessed by monolayer wound healing assay and [3H]-thymidine incorporation respectively. PDGF-induced phosphorylation of PDGF-receptor-β and its downstream effector Akt were assessed by Western blotting. Intracellular reactive oxygen species (ROS) was assessed using the fluorescent dye DCFDA. Bisdemethoxycurcumin elicited a concentration-dependent inhibition of PDGF-stimulated phosphorylation of PDGFR-β, Akt and Erk as well as the PDGF-stimulated SMC migration and proliferation. Bisdemethoxycurcumin was more potent than curcumin in inhibiting migration and proliferation and suppressing PDGF-signaling in SMCs. Both compounds were equipotent in inhibiting PDGF-stimulated intracellular ROS-generation.
Conclusion
Bisdemethoxycurcumin may be of potential use in the prevention or treatment of vascular disease.
Keywords: Curcumin, bisdemethoxycurcumin, migration, proliferation, platelet-derived growth factor, vascular smooth muscle cell
1. Introduction
Migration of vascular smooth muscle cells (VSMC) from the media to the intima of the blood vessels play a key role in intimal thickening in vascular diseases such as atherosclerosis and restenosis following coronary angioplasty [1]. VSMC migration is triggered by a variety of stimuli, including platelet-derived growth factor (PDGF) that are released by the injured blood vessel and blood/vascular cells associated with the injured site [2]. In contrast to the ‘quiescent’ VSMCs in the media, the migrated VSMC is proliferative in nature and exhibits a ‘synthetic’ phenotype capable of secreting extracellular matrix which contributes to intimal hyperplasia. Consequently, strategies to inhibit PDGF-stimulated VSMC migration and proliferation represent a potential strategy to overcome intimal thickening in atherosclerosis and restenosis.
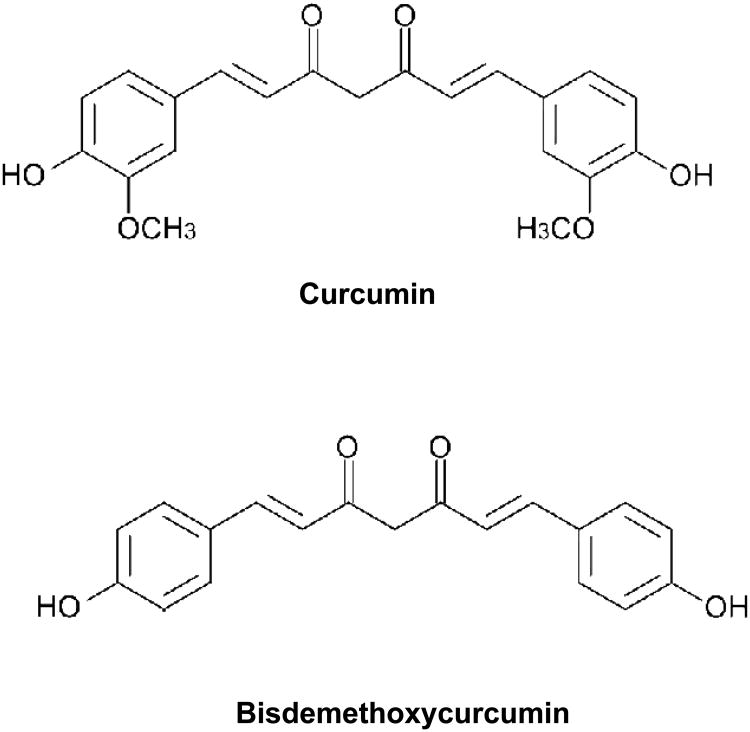
Curcumin (diferuloyl methane) (Figure 1; diferuloyl methane; 1,7-bis(4-hydroxy-3-methoxy-phenyl)-1,6-heptadiene-3-5-dione), the major yellow pigment extracted from spice turmeric (Curcuma longa) that has been used in cooking and in traditional Indian medicine for centuries [3]. It has been credited with antiinflammatory, immunomodulatory and anticancer properties [4]. Several molecular mechanisms have been proposed as an explanation for the pluripotent activities attributed to curcumin [5]. Our previous studies have shown that curcumin is a potent inhibitor of PDGF-stimulated migration, proliferation, and collagen synthesis in the VSMCs [6]. At the molecular level curcumin blocked PDGF-induced reorganization of the actin-cytoskeleton and attenuated PDGF- mediated signal transduction in the VSMCs [6]. More importantly curcumin inhibited balloon-catheter induced neointima formation in the rat artery, indicating its protective function in vascular injury [6]. However, the low solubility of curcumin in biological fluids, its poor bioavailability and rapid metabolism remains some of the challenges in developing curcumin as a drug to treat cardiovascular diseases [7, 8].
Figure 1.
Chemical structures of curcumin and bisdemethoxycurcumin.
In a view understand the structural requirements of curcumin for its biological activity, our lab has evaluated similar molecules such as dehydrozingerone (which chemically is half the molecule of curcumin without the diketone system) and isoeugenol (a molecule devoid of the keto group as compared to dehydrozingerone) [9]. Recent studies from other lab have shown that bisdemethoxycurcumin (Figure 1), a natural analog of curcumin which is devoid of the two methoxy groups on the phenolic nucleus of curcumin has superior biological activity in a variety of disease models including Alzheimer's disease, inflammation, and tumor suppression [9-12]. In the present study, we have evaluated the effect of bisdemethoxycurcumin on PDGF-stimulated migration and proliferation and signaling events in VSMC and compared it with curcumin. Our studies show that bisdemethoxycurcumin is a more potent than curcumin in inhibiting PDGF-mediated vascular events.
2. Materials and methods
2.1. Chemicals
Curcumin was synthesized by condensing vanillin with acetyl acetone as a boron complex as previously reported [13]. Essentially the same procedure was used to synthesize demethoxycurcumin except that vanillin was replaced by 4-hydroxy-benzaldehyde. The purity and the chemical structure were confirmed by melting point, elemental analysis, and spectral studies. PDGF-BB was obtained from Sigma Chemical Co (St Louis, MO). Antibodies against phospho-PDGF receptor-β (Tyr751), PDGF receptor β (28E1), phospho-Akt (Ser473), phospho-Erk, total-Erk and pan-Akt were obtained from Cell Signaling Technology (Boston, MA). Fetal bovine serum, penicillin-streptomycin, Dulbecco's Minimum Essential Media without phenol red, insulin-transferrin-selenous acid, and 5-(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA) were from Gibco/Invitrogen (Carlsbad, CA).
2.2. Smooth muscle cell isolation and culture
Thoracic aorta SMCs were obtained from male Sprague-Dawley rats weighing between 100 and 150 g using collagenase and elastase as described by us previously [14]. This protocol was approved by the Institutional Animal Care and Use Committee of the University of Wyoming. Each isolate consisted of cells pooled from 2 to 4 individual aortas. Early passages (< 5) of smooth muscle cells were used for all experiments. All cultures were maintained in a humidified atmosphere of 5% CO2-95% air. Isolated cells were seeded on tissue culture plates in DMEM/F-12 media supplemented with penicillin (50 U/ml), streptomycin (50 mg/ml), insulin (5 mg/ml), transferrin (5 mg/ml), and selenous acid (5 ng/ml) at a density of 3 × 104 cells/cm2 and were grown to confluence in 10% fetal bovine serum. Cultures were treated with serum-free medium for 24 hours before experiments were initiated.
2.3. Cell migration assay
Cell migration was assessed using a monolayer-wounding protocol in which cells migrated from a confluent area into an area that was mechanically denuded of cells [6]. For monolayer-wounding cell migration assay, confluent cells were treated with serum-free medium containing hydroxyurea (5 mM) for 24 hours before the start of the experiments. Hydroxyurea was used to prevent proliferation of cells [15]. Hydroxyurea by itself did not have any effect on cell migration (data not shown). Area of migration was photographed with a video camera system using Scion Image software at the intersection of the previously marked line and wound edge at 0 hours (WW0) and at 24 hours (WW24) after treatment with PDGF (10 ng/mL) in the presence or absence of test compound. Cell migration was calculated as wound width covered at time t(WW0 – WW24) and expressed as percentage.
2.4. Cell proliferation assay
Cell proliferation was assessed by [3H]-thymidine incorporation in mitogenically quiescent VSMCs [6]. Cells were incubated for 18 hours with or without PDGF (10 ng/mL) and various concentrations of test compounds and then pulse-labeled with 1mCi/mL of [3H] thymidine for 6 hours. Cells were washed 3 times with phosphate-buffered saline, precipitated with 10% (wt/vol) ice-cold trichloroacetic acid, and rinsed with absolute ethanol and air dried. For analysis, the monolayer was dissolved in 250 mL of 0.5 M sodium hydroxide per well at room temperature overnight. Duplicate samples of 100 μL were counted in scintillation fluid in a liquid scintillation counter (Beckmann LC 6000IC). Cell viability was assessed by MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Briefly, VSMCs growing on a 96-well plate were treated with either curcumin or bisdemethoxycurcumin at 10 and 25μM concentrations. Thirty minutes following the treatment, 100 μL of MTT solution (5 mg/mL, Sigma, St. Louis, MO) was added to the SMCs and incubate for 1 hour. MTT solution was removed and DMSO was used to dissolve formazan and the absorbance was measured at 570 nm.
2.5. SDS-PAGE and immunoblotting
Western blotting for protein was performed as described previously [16]. Briefly, cells were lysed in RIPA buffer containing 1 mM sodium vanadate, 1 mM phenyl-methyl-sulfonyl fluoride, 5 mg/ml aprotinin, and 5 mg/ml leupeptin. Protein concentration was determined by the bicinchoninic acid method (Pierce Biotechnology Inc., Rockford, IL). Lysates corresponding to equal amounts of proteins were boiled in Laemmli sample buffer, and the supernatants were loaded onto gels for SDS-PAGE. Proteins were transferred onto nitrocellulose membranes and probed with the following primary antibodies: anti-phospho PDGF receptor and anti-phospho Akt (Thr308) (at dilutions of 1:1000). Appropriate horseradish peroxidase–coupled secondary antibodies (HRPO-conjugated) were used at 1:5000. Immunoreactive bands were detected by using the Super Signal West Dura Extended Duration Substrate (Pierce, Milwaukee, WI). The intensity of bands was measured with a scanning densitometer using a Molecular Imager Gel Doc XR+ System (Bio Rad, Hercules, CA).
2.6. Analysis of ROS production
Production of intracellular ROS was evaluated by changes in fluorescence intensity resulting from oxidation of the intracellular fluorophore, DCF-DA [17]. VSMCs were subcultured and grown in 200μL culture medium/well in 96-well plates until 95% confluence. Following a 16-h quiescence incubation period in serum free medium cells were washed with Krebs–Ringer– HEPES (KRH) buffer and then incubated for 30 min DCF-DA (25 μM) with or without curcumin or bisdemethoxycurcumin. After three additional washes with KRH buffer, the cells were treated with PDGF (10 ng/ml) in a total volume of 200 μl of KRH. After 10 min, the fluorescence of each well was measured and recorded at excitation 485 nm, emission 530 nm with microplate fluorescence reader (Spectra GEMINIXS, Molecular Device, Sunnyvale, CA).
2.7. Cellular-uptake
Cultured VSMCs were grown to 70% confluence and were incubated with curcumin and demethoxycurcumin for 30 min following which, the media was removed and cells were washed three times with PBS. The cells were treated with ethanol, sonicated, centrifuged and the absorbance of the supernatant was measured at measured at 434 nm using a spectrophotometer. Standard curves for curcumin and bisdemethoxycurcumin obtained in an alcoholic solution was used for calculating the concentrations.
2.8. Statistical analysis
Data are expressed as mean ± SEM and statistically evaluated using one-way ANOVA followed by Bonferroni post hoc test using Graph Pad Prism (Graph Pad Software, Inc., San Diego, CA, USA). A ‘p’ value of less than 0.05 was considered to be statistically significant.
3. Results
3.1. Bisdemethoxycurcumin inhibits PDGF-stimulated VSMC migration
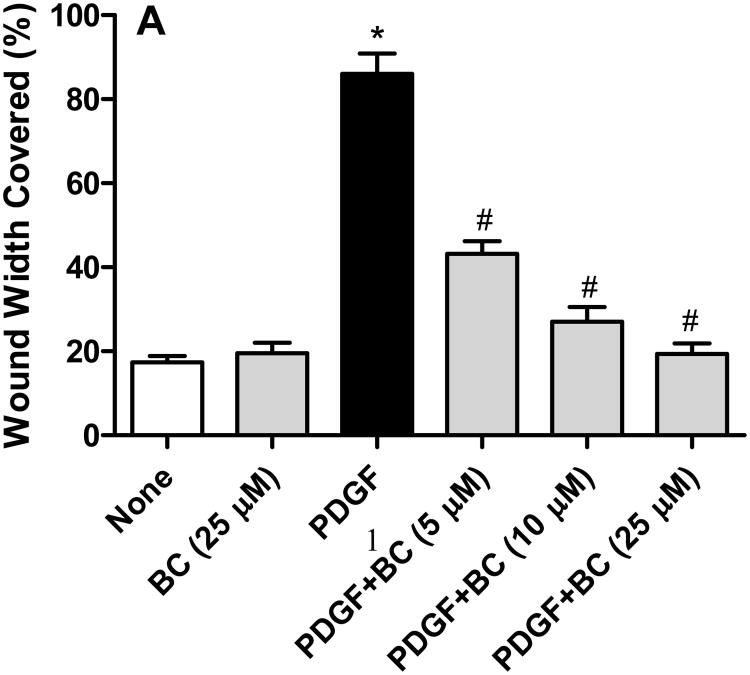
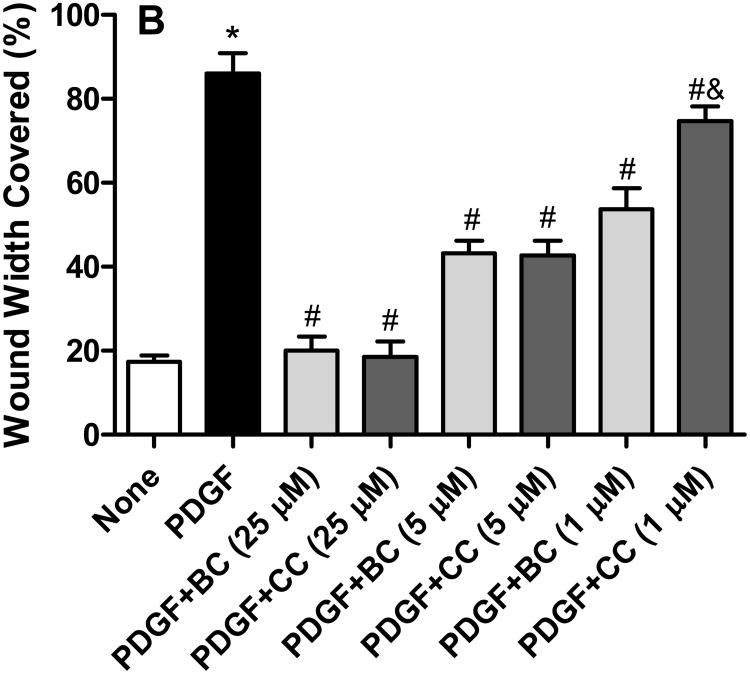
PDGF (10 ng/mL) induced a robust motogenic effect (>4 folds over control) on cultured VSMCs so as to cover about 90% of the wound width in 24 h. This cell migration induced by PDGF was inhibited by bisdemethoxycurcumin in a concentration-dependent manner, with a near complete inhibition observed at 10 μM bisdemethoxycurcumin (Figure 2A). At concentrations of 5 μM and above both curcumin and bisdemethoxycurcumin were equipotent at inhibiting PDGF-stimulated VSMC (Figure 2B). However, when the concentration was lowered to 1 μM bisdemethoxycurcumin exhibited significantly higher inhibitory activity compared to curcumin. Neither curcumin nor bisdemethoxycurcumin by at the concentrations studied had any effect on cell viability (data not shown).
Figure 2.
A. Bisdemethoxycurcumin (BC) attenuates PDGF (10 ng/mL)-stimulated VSMC motility VSMC motility the monolayer wound-healing assay. Data are mean ± SEM (n=6) and represents the average wound width covered in each treatment group *p<0.001 compared to untreated, #p<0.01 compared to PDGF. B. Inhibition of PDGF (10 ng/mL)-stimulated cell migration by BC and curcumin (CC). Data are mean ± SEM and represents the average wound width covered in each treatment group *p<0.001 compared to untreated, #p<0.01 compared to PDGF, &p<0.05 compared to PDGF +CC.
3.2. Bisdemethoxycurcumin inhibits PDGF-stimulated VSMC proliferation
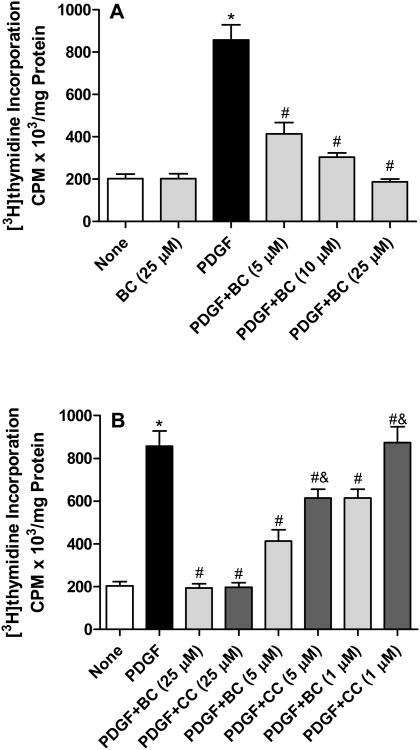
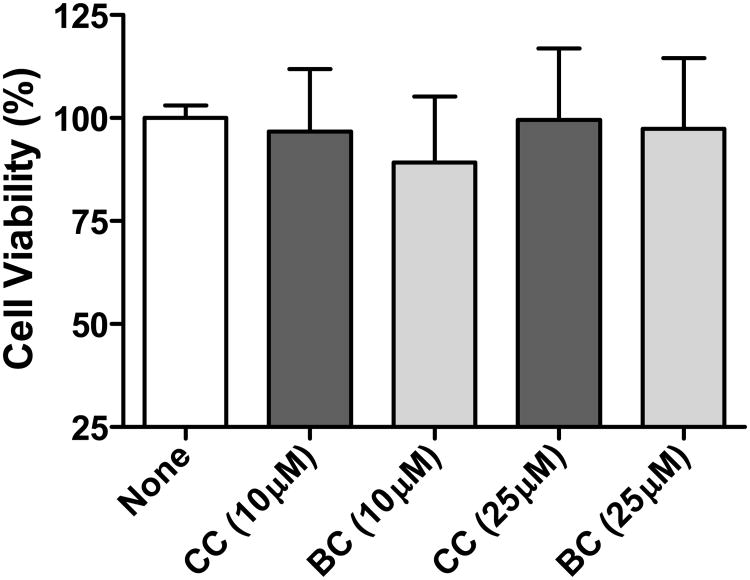
We next determined the ability of bisdemethoxycurcumin to inhibit PDGF-stimulated VSMC proliferation. As illustrated in Figure 3A, treatment with PDGF (10 ng/mL) resulted in ∼5 fold increase in the incorporation of [3H]-thymidine. Bisdemethoxycurcumin (1-25 μM) inhibited PDGF-stimulated cell proliferation in a concentration-dependent manner, with near complete inhibition at 25 μM). When compared to curcumin, bisdemethoxycurcumin was superior at inhibiting PDGF-stimulated proliferation at both 1 μM and 5 μM concentrations (Figure 3B). Comparison of activities revealed that 28% inhibition in PDGF-induced cell proliferation was observed with 5 μM concentration of curcumin whereas demethoxycurcumin elicited the same extent of inhibition at 1 μM concentration. However, at a concentration of 25 μM both the compounds abrogated the effect of PDGF on cell proliferation. When quiescent cells were treated with the test compounds in the absence of PDGF, no significant differences (from control) were observed in the extent of 3[H]-thymidine incorporation. Furthermore, at the concentrations tested neither demethoxycurcumin nor curcumin caused overt cell death (Figure 4) as assessed by the MTT assay.
Figure 3.
A. Bisdemethoxycurcumin (BC) inhibits PDGF-stimulated VSMC proliferation. Cells were treated with PDGF (10ng/mL) for 24 hours in the presence or absence of BC, and incorporation of [3H]-thymidine was monitored and normalized to protein. Results are the mean ± SE from 3 independent experiments. *p<0.001 compared to untreated, #p<0.01 compared to PDGF. B. Inhibition of PDGF (10 ng/mL)-stimulated cell proliferation by BC and curcumin (CC). Data are mean ± SEM *p<0.001 compared to untreated, #p<0.01 compared to PDGF, &p<0.05 compared to PDGF + corresponding concentrations of CC.
Figure 4.
Effect of curcumin and bisdemethoxycurcumin viability of VSMC. Curcumin and bisdemethoxycurcumin were tested at concentrations of 10 and 25 μM for its effect on cell viability using the MTT assay. Mean ± SEM, n = 4.
3.3. Bisdemethoxycurcumin attenuates PDGF-signal transduction in cultured VSMCs
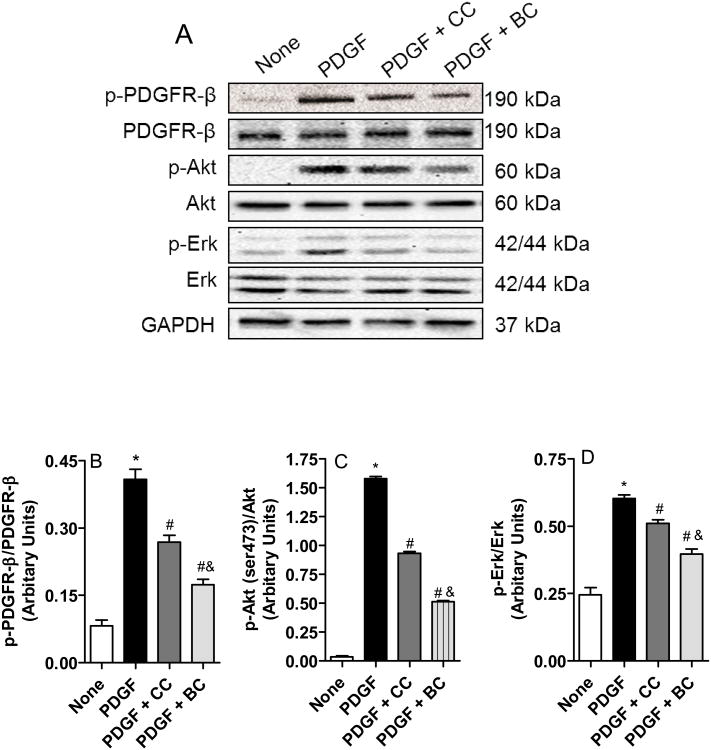
Binding of PDGF to its cognate receptor elicits autophosphorylation and activation of the intracellular tyrosine kinase domain receptor [18]. The activated PDGF-receptor phosphorylates multiple residues on downstream effectors, a key being Akt. Figure 5A shows that both curcumin and bisdemethoxycurcumin inhibits PDGF-stimulated phosphorylation of PDBF-receptor-β, phosphorylation of Akt at the Serine 473 residue and the phosphorylation of Erk. Consistent with the migration and proliferation data, bisdemethoxycurcumin was superior to curcumin in inhibiting these signaling events mediated by PDGF (Figure 5B-D).
Figure 5.
Effect of bisdemethoxycurcumin (BC) and curcumin (CC) on PDGF (10 ng/mL)– stimulated tyrosine phosphorylation of PDGFR β, serine (473) phosphorylation Akt and phospho Erk in VSMCs. Cells were treated with or without (control) CC of BC (10 μM) for 30 minutes and stimulated with PDGF (10 ng/mL) for 10 minutes. Cells were lysed, and lysates were immuno-blotted with antibodies directed against phospho-PDGF receptor β, phospho-Akt and phospho-Erk. A. Representative Western blot image showing phospho-PDGF receptor β, phospho-Akt and phospho-Erk together with the total proteins. B-D. Relative densities of phosphorylated PDGF receptor β, phosphorylated Akt and phospho-Erk normalized to total proteins. Data are mean + SEM (n=3) of the *p<0.001 compared to untreated, #p<0.01 compared to PDGF, &p<0.05 compared to PDGF compared to PDGF + CC.
3.4. Bisdemethoxycurcumin inhibits PDGF-induced reactive oxygen species production
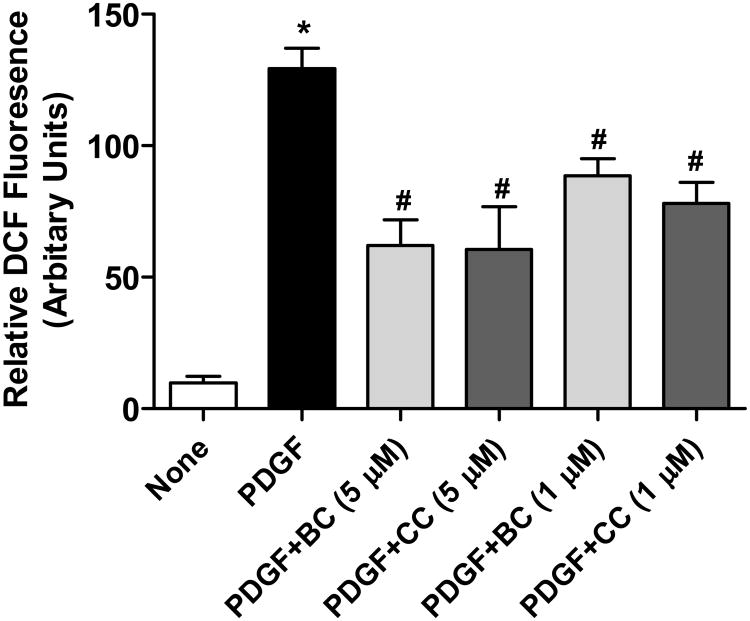
Small amount of ROS generated when PDGF binds to its receptor has been shown to reversibly oxidize a critical cysteine residue in the active site of phosphates which aids in the transduction of PDGF-signaling [19, 20]. Consequently, antioxidants have been shown to relieve this phosphatase inhibition and reduce PDGF signaling [21]. Because curcumin has antioxidant properties [22], we investigated the effects of bisdemethoxycurcumin on PDGF-stimulated ROS generation in cultured VSMC. Figure 6 shows that transient incubation of VSMCs with PDGF resulted in ROS generation which was inhibited by both curcumin and bisdemethoxycurcumin to similar extent, suggesting that the differential effects of these two compounds on PDGF-signaling may not be mediated through its antioxidant properties.
Figure 6.
Scavenging of PDGF (10 ng/mL)-generated intracellular ROS by bisdemethoxycurcumin (BC) and curcumin (CC) assessed as DCF fluorescence. Data are mean ± SEM (n=3) of the relative fluorescence intensities. *p<0.001 compared to untreated, #p<0.01 compared to PDGF.
3.5. Bisdemethoxycurcumin exhibited better cellular-uptake compared to curcumin
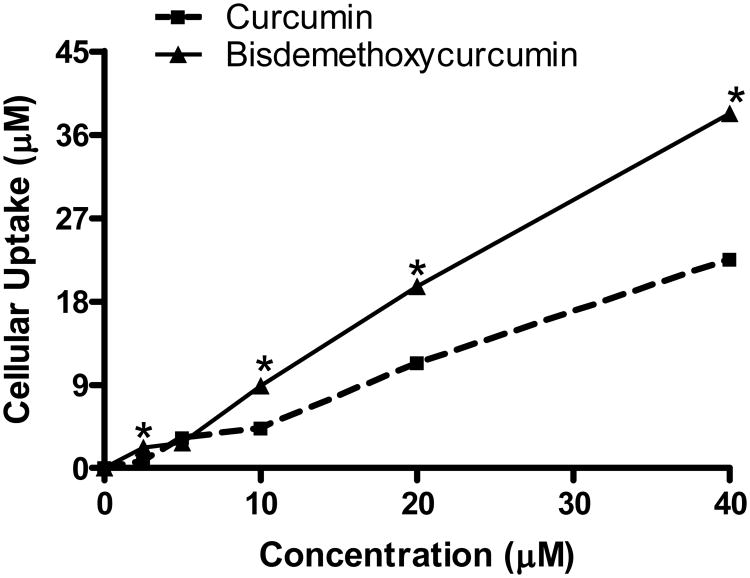
Cellular-uptakes of curcumin and bisdemethoxycurcumin are illustrated in figure 7. As evidenced by the figure bisdemethoxycurcumin exhibited better cellular uptake at concentrations above 5 μM. At the maximum concentration tested (40 μM), 76% of bisdemethoxycurcumin was taken-up by the cells whereas only 58.7% of curcumin uptake was observed the same concentration. However BC and CC exhibited almost identical in uptake at the concentration range in which efficacy was evaluated in this study (1-10 μM) suggesting that improved cellular uptake of bisdemethoxycurcumin may not account for the enhancement of BC activity in the proliferation and wound healing studies.
Figure 7.
Cellular-uptake of curcumin and bisdemethoxycurcumin. VSMCs were treated with various concentrations of curcumin and the cellular uptake of the compounds was assessed by spectrophotometry at 400 nm. The concentrations were calculated for a standard curve generated by using various concentrations of these compounds in ethanol.
4. Discussion
The major finding from this study is that bisdemethoxycurcumin is a potent inhibitor of PDGF-induced migration and proliferation of VSMCs. Furthermore, bisdemethoxycurcumin inhibited PDGF-stimulated PDGF-receptor phosphorylation and the phosphorylation of the downstream effector Akt. Compared to curcumin, bisdemethoxycurcumin exhibited greater potency in inhibiting PDGF-stimulated migration and proliferation of VSMCs and the PDGF-signaling cascade. Both curcumin and bisdemethoxycurcumin exhibited equi-potency in scavenging intracellular ROS generated by PDGF indicating that the enhanced potency of bisdemethoxycurcumin may be independent of its antioxidant properties. Cellular uptake studies show that bisdemethoxycurcumin is better absorbed into the cells compared to curcumin at higher concentrations. However, at concentrations wherein they exhibit their function effects the cellular uptake of these compounds is comparable. This suggests that the inherent biological activity of bismethoxycurcumin on PDFG-mediated migration and proliferation and migration may be superior compared to curcumin. In addition, our results suggest that the methoxy groups on the phenolic ring on curcumin may not be necessary for its inhibition of PDGF-mediated migration and proliferation of VSMCs. Given the documented short-comings in the in-vivo bioavailability of curcumin, its extreme hydrophobicity and instability, it may be worthwhile to consider developing bismethoxycurcumin as a viable alternative template for designing molecules that can interfere with the abnormal smooth muscle functions associated with vascular diseases.
Structure activity studies on the naturally occurring curcumin derivatives have resulted in varying results. Yodkeeree and coworkers compared curcumin with its dimethoxy derivatives for their anti-metastatic activity and found that bisdemethoxycurcumin was superior to curcumin in inhibiting tumor the invasiveness of human fibrosarcoma cells [11]. Consistent with these observations Matsunaga and coworkers have shown that bisdemethoxycurcumin exhibited higher potency and selectivity compared to curcumin in inhibiting aldo-keto reductase, a potential therapeutic target for a variety of cancers [23]. Fiala and coworkers using active fractions from curcuminoids and found that bisdemethoxycurcumin is the most potent in enhancing defective phagocytosis of amyloid-beta [24]. In contrast however, curcumin was found to be more active than bisdemethoxycurcumin in tumor necrosis factor-induced activation of nuclear factor kappa B, although the suppression of proliferation by curcuminoids in a variety of tumor cells was comparable [25]. Interestingly, Zhongfa and coworkers have recently shown that the oral bioavailability of the naturally occurring dimethoxy derivatives of curcumin is higher than that of curcumin [26]. Even if the biological activities of the two molecules are comparable, the beneficial pharmacokinetic profile of demethoxycurcumin may be an added advantage over curcumin.
Enhanced PDGF signaling has been implicated in the pathophysiology of a variety of diseases involving the remodeling of the vascular wall [18, 27, 28]. In the arteries, PDGF receptors are over-expressed by several folds following endothelial injury associated with angioplasty or early stages of atherosclerosis [29, 30]. Conversely, inhibiting the PDGF pathway either by using antisense oligonucleotides or blocking antibody has been used to successfully suppress intimal thickening [30, 31]. Our previous studies have demonstrated that curcumin is a potent inhibitor of PDGF-induced VSMC migration, proliferation and signal transduction [6]. Curcumin inhibited neointima formation following balloon injury in rats [6]. However, the poor solubility and bioavailability together with its quick metabolism limits its potential as a therapeutic agent [7, 8]. The present study shows that bisdemethoxycurcumin has better cellular penetration, retains the antioxidant properties of curcumin and is more potent at inhibiting PDGF-mediated abnormal events in the VSCMs. Recent studies in other model systems have also indicated that bisdemethoxycurcumin is more potent than curcumin [9, 10, 24]. Further in-vivo studies are necessary to ascertain the benefits of bisdemethoxycurcumin over curcumin.
In addition to vascular remodeling, upregulated PDGF signaling has been implicated in a variety of diseases such as cancer, fibrosis and pulmonary hypertension [32]. Small molecule inhibitors of PDGF-signaling such as imatinib mesylate (imatinib, ST1571, or Gleevec) is clinically used to treat patients with myelogenous leukemia and gastrointestinal stromal tumor [33]. However, many of the molecules developed for selective PDGF-receptor inhibition are limited by their serious adverse effects [34]. In this context developing a molecule such as bisdemethoxycurcumin which is a naturally occurring spice product with a wide margin of safety is worthy of further studies for its therapeutic value in stopping or preventing diseases related to over-activation of PDGF signaling.
In summary, this study demonstrates that a natural analog of curcumin, bisdemethoxycurcumin is a more potent inhibitor of PDGF-stimulated VSMC migration and proliferation compared to curcumin. These effects of bisdemethoxycurcumin may be mediated via its inhibitory effects on PDGF-receptor signaling in VSMCs. Given the pharmacokinetic limitations of curcumin, and the better cellular uptake of bisdemethoxycurcumin, it may be worthwhile to further explore the beneficial effects of bisdemethoxycurcumin in vascular diseases.
Acknowledgments
This work was supported by a grant from the NIHI NBRE (P20RR016474).
Abbreviations
- MTT
(3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PDGF
platelet-derived growth factor
- ROS
reactive oxygen species
- VSMC
vascular smooth muscle cells
- DCFDA
5-(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
References
- 1.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190:300–309. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 2.Willis AI, Pierre-Paul D, Sumpio BE, Gahtan V. Vascular smooth muscle cell migration: current research and clinical implications. Vasc Endovascular Surg. 2004;38:11–23. doi: 10.1177/153857440403800102. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr. 2010;103:1545–1557. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 5.Lin JK. Molecular targets of curcumin. Adv Exp Med Biol. 2007;595:227–243. doi: 10.1007/978-0-387-46401-5_10. [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Thomas DP, Zhang X, Culver BW, et al. Curcumin inhibits platelet-derived growth factor-stimulated vascular smooth muscle cell function and injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2006;26:85–90. doi: 10.1161/01.ATV.0000191635.00744.b6. [DOI] [PubMed] [Google Scholar]
- 7.Basnet P, Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurien BT, Scofield RH. Increasing aqueous solubility of curcumin for improving bioavailability. Trends Pharmacol Sci. 2009;30:334–335. doi: 10.1016/j.tips.2009.04.005. author reply 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YL, Yang HP, Gong L, Tang CL, Wang HJ. Hypomethylation effects of curcumin, demethoxycurcumin and bisdemethoxycurcumin on WIF-1 promoter in non-small cell lung cancer cell lines. Mol Med Report. 2011;4:675–679. doi: 10.3892/mmr.2011.473. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed T, Enam SA, Gilani AH. Curcuminoids enhance memory in an amyloid-infused rat model of Alzheimer's disease. Neuroscience. 2011;169:1296–1306. doi: 10.1016/j.neuroscience.2010.05.078. [DOI] [PubMed] [Google Scholar]
- 11.Yodkeeree S, Chaiwangyen W, Garbisa S, Limtrakul P. Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit cancer cell invasion through the down-regulation of MMPs and uPA. The Journal of nutritional biochemistry. 2009;20:87–95. doi: 10.1016/j.jnutbio.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Pugazhenthi S, Akhov L, Selvaraj G, Wang M, Alam J. Regulation of heme oxygenase-1 expression by demethoxy curcuminoids through Nrf2 by a PI3-kinase/Akt-mediated pathway in mouse beta-cells. Am J Physiol Endocrinol Metab. 2007;293:E645–655. doi: 10.1152/ajpendo.00111.2007. [DOI] [PubMed] [Google Scholar]
- 13.Sreejayan, Rao MN. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol. 1994;46:1013–1016. doi: 10.1111/j.2042-7158.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- 14.Sreejayan N, Lin Y, Hassid A. NO attenuates insulin signaling and motility in aortic smooth muscle cells via protein tyrosine phosphatase 1B-mediated mechanism. Arterioscler Thromb Vasc Biol. 2002;22:1086–1092. doi: 10.1161/01.atv.0000020550.65963.e9. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar R, Meinberg EG, Stanley JC, Gordon D, Webb RC. Nitric oxide reversibly inhibits the migration of cultured vascular smooth muscle cells. Circ Res. 1996;78:225–230. doi: 10.1161/01.res.78.2.225. [DOI] [PubMed] [Google Scholar]
- 16.Dong F, Yang X, Sreejayan N, Ren J. Chromium (D-phenylalanine)3 improves obesity-induced cardiac contractile defect in ob/ob mice. Obesity (Silver Spring) 2007;15:2699–2711. doi: 10.1038/oby.2007.322. [DOI] [PubMed] [Google Scholar]
- 17.Stratton MS, Yang X, Sreejayan N, Ren J. Impact of insulin-like growth factor-I on migration, proliferation and Akt-ERK signaling in early and late-passages of vascular smooth muscle cells. Cardiovasc Toxicol. 2007;7:273–281. doi: 10.1007/s12012-007-9006-7. [DOI] [PubMed] [Google Scholar]
- 18.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 19.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 20.Rhee SG, Kang SW, Jeong W, Chang TS, et al. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Kappert K, Sparwel J, Sandin A, Seiler A, et al. Antioxidants relieve phosphatase inhibition and reduce PDGF signaling in cultured VSMCs and in restenosis. Arterioscler Thromb Vasc Biol. 2006;26:2644–2651. doi: 10.1161/01.ATV.0000246777.30819.85. [DOI] [PubMed] [Google Scholar]
- 22.Sreejayan N, Rao MN. Free radical scavenging activity of curcuminoids. Arzneimittelforschung. 1996;46:169–171. [PubMed] [Google Scholar]
- 23.Matsunaga T, Endo S, Soda M, Zhao HT, et al. Potent and selective inhibition of the tumor marker AKR1B10 by bisdemethoxycurcumin: probing the active site of the enzyme with molecular modeling and site-directed mutagenesis. Biochemical and biophysical research communications. 2009;389:128–132. doi: 10.1016/j.bbrc.2009.08.107. [DOI] [PubMed] [Google Scholar]
- 24.Fiala M, Liu PT, Espinosa-Jeffrey A, Rosenthal MJ, et al. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer's disease patients are improved by bisdemethoxycurcumin. Proc Natl Acad Sci U S A. 2007;104:12849–12854. doi: 10.1073/pnas.0701267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandur SK, Pandey MK, Sung B, Ahn KS, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–1773. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 26.Zhongfa L, Chiu M, Wang J, Chen W, et al. Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. Cancer chemotherapy and pharmacology. 2012;69:679–689. doi: 10.1007/s00280-011-1749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 28.Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Majesky MW, Reidy MA, Bowen-Pope DF, Hart CE, et al. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990;111:2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferns GA, Raines EW, Sprugel KH, Motani AS, et al. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991;253:1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- 31.Sirois MG, Simons M, Edelman ER. Antisense oligonucleotide inhibition of PDGFR-beta receptor subunit expression directs suppression of intimal thickening. Circulation. 1997;95:669–676. doi: 10.1161/01.cir.95.3.669. [DOI] [PubMed] [Google Scholar]
- 32.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchdunger E, O'Reilly T, Wood J. Pharmacology of imatinib (STI571) Eur J Cancer. 2002;38(5):S28–36. doi: 10.1016/s0959-8049(02)80600-1. [DOI] [PubMed] [Google Scholar]
- 34.Yardley DA, Burris HA, 3rd, Markus T, Spigel DR, et al. Phase II trial of docetaxal plus imatinib mesylate in the treatment of patients with metastatic breast cancer. Clin Breast Cancer. 2009;9:237–242. doi: 10.3816/CBC.2009.n.040. [DOI] [PubMed] [Google Scholar]