Abstract
Background
In response to rising pharmaceutical costs, many state Medicaid programs have implemented policies requiring prior authorization for high-cost medications, even for established users. However, little is known about the impact of these policies on the use of antihypertensive medicines in the United States.
Objective
The aim of this longitudinal, population-based study was to assess comprehensive prior-authorization programs for antihypertensives on drug use and costs in a vulnerable Medicaid population in Michigan and Indiana.
Methods
A prior-authorization policy for anti-hypertensives was implemented in Michigan in March 2002 and in Indiana in September 2002; Indiana also implemented an antihypertensive stepwise-therapy requirement in July 2003. Our study cohort included individuals aged ≥18 years in Michigan and Indiana who were continuously enrolled in both Medicaid and Medicare from July 2000 through September 2003. Claims data were obtained from the Centers for Medicare and Medicaid Services. We included all antihypertensive medications, including diuretics, angiotensin-converting enzyme inhibitors, calcium channel blockers, β-blockers, α-blockers, and angiotensin II receptor blockers. We used interrupted time-series analysis to study policy-related changes in the total number and cost of anti-hypertensive prescriptions.
Results
Overall, 38,684 enrollees in Michigan and 29,463 in Indiana met our inclusion criteria. Slightly more than half of our cohort in both states was female (53.29% in Michigan and 56.32% in Indiana). In Michigan, 20.23% of patients were aged ≥65 years; 77.44% were white, 20.11% were black, and the remainder were Hispanic, Native American, Asian, or of other or unknown race. In Indiana, 20.07% were aged ≥65 years; 84.93% were white, 13.64% were black, and the remainder were Hispanic, Native American, Asian, or of other or unknown race. The implementation of both policies was associated with large and immediate reductions in the use of nonpreferred medications: 83.33% reduction in the use of such drugs in Michigan (−84.30 prescriptions per 1000 enrollees per month; P < 0.001) and 35.76% in Indiana (−64.45 prescriptions per 1000 enrollees per month; P < 0.001). As expected, use of preferred medications also increased substantially in both states (P < 0.001). Overall, antihypertensive therapy immediately dropped 0.16% in Michigan (P = 0.04) and 1.82% in Indiana (P = 0.02). Implementation of the policies was also associated with reductions in pharmacy reimbursement of $616,572.43 in Michigan and $868,265.97 in Indiana in the first postpolicy year.
Conclusions
Prior authorization was associated with lower use of nonpreferred antihypertensive drugs that was largely offset by increases in the use of preferred drugs. The possible clinical consequences of policy-induced drug switching for individual patients remain unknown because the present study did not include access to medical record data. Further research is needed to establish whether large-scale switches in medicines following the inception of prior-authorization policies have any long-term health effects.
Keywords: prior authorization, Medicaid, anti-hypertensive, drug utilization, costs
INTRODUCTION
Rising drug costs are a major concern in nearly all US health insurance systems, including Medicaid programs.1 Between 1994 and 2004, Medicaid drug expenditures grew ~15.4% annually, leading many states to implement cost-control measures.2 One popular approach has been prior-authorization policies that require prescribers to receive permission before dispensing specific medications.3,4 In particular, many Medicaid programs and Medicare Part D plans have instituted prior-authorization requirements for drugs that treat hypertension. For example, at least 32 state Medicaid programs have prior-authorization programs in place for angiotensin II receptor blockers (ARBs).5 These policies often require stepwise therapy in which patients are required to try one medication class before being permitted to receive another. Antihypertensives are a natural target for such policies because there are many different therapies within each class, often with comparable therapeutic effects and a wide range of costs.6
Drugs for cardiovascular conditions are the most widely prescribed agents in Medicaid, representing 17.9% of all prescriptions in 2004.7 Hypertension is a highly prevalent condition that forms a major component of these costs. An estimated 65 million US adults had hypertension in 1999 and 2000, and rates of control among community-dwelling people aged >60 years range from 23% to 38%.8,9 This high prevalence has created a considerable burden for the health care system, with costs totaling US $63.5 billion in 2006.10 Approximately one third of this expenditure was for medications, and evidence suggests that closer adherence to evidence-based clinical guidelines could significantly reduce costs, making them an obvious target for cost-control measures.6
Research on other medication classes suggests prior-authorization requirements may reduce both medication use and costs. For example, the establishment of a prior-authorization requirement for nongeneric NSAIDs in the Tennessee Medicaid system was associated with a 19% drop in the number of days of therapy prescribed and a 53% reduction in costs.11 Similarly, prior authorization for cyclooxygenase-2 inhibitors was associated with an 18% reduction in per-prescription costs across several Medicaid programs.12 Finally, a prior-authorization requirement for proton pump inhibitors implemented in the Georgia Medicaid system was associated with a 7% reduction in the number of prescriptions and a 50% reduction in expenditures.13 However, the effect of prior-authorization policies on drug utilization and expenditure is not universal across drug classes. Studies of prior-authorization requirements for antipsychotic medications in 3 states did not find any significant reduction in pharmacy costs.14,15 Moreover, a study of prior authorization of controlled-release oxycodone found only small cost savings.16 Thus, the effects of prior-authorization policies on drug use and costs appear to be class specific. There is also some evidence that the impact of a prior-authorization policy can vary, based on when and how it is implemented.17
Previous research has not thoroughly explored the longitudinal impact of prior-authorization policies on antihypertensive use and expenditures. One serious concern about prior authorization is that limited formularies might lead to suboptimal matching of patients with appropriate medication, leading to poorer disease management.18 For asymptomatic conditions such as hypertension, administrative restrictions may be disruptive to treatment, even with a range of therapeutic alternatives.19 One previously published study of prior authorization for ARBs found that stepwise therapy policies were associated with small decreases in utilization, whereas less restrictive policies were not.2 However, this study only examined one class of antihypertensive medications. As policies are often implemented across many classes simultaneously and users may switch between classes, it is important to assess their overall impact. The objective of this longitudinal, population-based study was to assess the effects of comprehensive prior-authorization programs for antihypertensives on drug use and costs in a vulnerable Medicaid population in Michigan and Indiana.
PATIENTS AND METHODS
Study Setting
We examined the impact of prior-authorization requirements targeting antihypertensives that were implemented in 2 different Medicaid programs: Michigan and Indiana. The prior-authorization policy for anti-hypertensives in Michigan was implemented in March 2002 and affected a number of agents across a range of drug classes. However, an analysis of the Michigan policy found that the prior-authorization requirements were particularly restrictive for cardiovascular medicines in comparison with other drug classes.19 There were also concerns that the Michigan prior-authorization policy was poorly implemented, leading to confusion among both patients and providers.19
Indiana Medicaid first required prior authorization for some antihypertensive agents 6 months later, in September 2002. Initially, the policy affected only certain angiotensin-converting enzyme (ACE) inhibitors, but it was quickly expanded to cover numerous antihypertensive classes, including ARBs, β-blockers (BBs), and calcium channel blockers (CCBs). Indiana also coupled its prior-authorization requirement for ARBs with a stepwise-therapy requirement started in July 2003. Under this program, patients were required to try an ACE inhibitor before being granted prior authorization to obtain an ARB.5 Such stepwise-therapy requirements are a common component of Medicaid preferred drug lists in the United States.14
Data Sources
Our study cohort included all individuals aged ≥18 years in Michigan and Indiana, who were dually enrolled in Medicaid and Medicare programs continuously from July 2000 through September 2003. This time frame provided ≥20 months of observation leading up to the implementation of either policy and ≥1 year of follow-up after implementation. As in previous work, we excluded dually enrolled patients covered by either Medicaid or Medicare managed-care programs because the data capture used in this study design might not have represented the complete claims history of such patients.20 For the same reason, we also excluded individuals enrolled solely in Medicaid in both states, because managed-care programs covered the majority of these individuals. We used administrative claims data from Medicaid and Medicare fee-for-services enrollees in 2 states purchased from the Centers for Medicare and Medicaid Services. This data use was reviewed and found to meet federal regulatory criteria exempting it from review by the institutional review boards at both Harvard Pilgrim Health Care Institute and Kaiser Permanente Northern California.
In our analysis of drug utilization, we included all antihypertensive medications, including diuretics, ACE inhibitors, CCBs, BBs, α-blockers, and ARBs. Based on the individual state policies, we classified each drug into the following categories: (1) nonpreferred drugs that were subject to prior authorization; (2) preferred drugs for which prior authorization was not required; and (3) unlisted antihypertensives that were not explicitly designated as either nonpreferred or preferred drugs in the state’s Medicaid system, and were therefore not subject to prior authorization.
Measures
To estimate the impact of both state policies on overall antihypertensive utilization in a comparable manner, we analyzed the number of prescriptions per 1000 enrollees per month in both states. Second, we analyzed the same rate of preferred, nonpreferred, and unlisted drugs separately to assess changes in their use resulting from the policies. Finally, we calculated the total reimbursement for antihypertensive medications per 1000 enrollees per month to assess pharmacy reimbursement. We converted all costs to 2003 US dollars using the Consumer Price Index.21
Statistical Analysis
We used interrupted time-series analysis to assess the impact of prior authorization in both states.22 This method simultaneously controls for the baseline (prepolicy) level and trend of each outcome and estimates postpolicy changes in both the level and the trend. Because the 2 states’ policies were implemented 6 months apart, we analyzed them as 2 distinct interventions in separate models, but assessed all outcomes as rates to make them directly comparable. All models were fit using maximum likelihood generalized least squares models, and we controlled for autocorrelation by including any significant autoregressive parameters up to 12 months. All analyses were conducted using SAS 9.1 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Sample Characteristics
Descriptive characteristics of our cohort in both states are detailed in Table I. There were 38,684 enrollees in Michigan and 29,463 in Indiana who met our study inclusion criteria. Slightly more than half of our cohort in both states was female (53.29% [20,614/38,684] in Michigan and 56.32% [16,595/29,463] in Indiana), consistent with the previously published observation that Medicaid traditionally covers more adult females than males.23 In Michigan, 20.23% of subjects (7827/ 38,684) were aged ≥65 years; 77.44% (29,957 subjects) were white, 20.11% (7781) were black, and the remainder were Hispanic (0.90% [348]), Native American (0.56% [218]), Asian (0.25% [96]), or of other (0.54% [207]) or unknown (0.20% [77]) race. In Indiana, 20.07% (5913/29,463) were aged ≥65 years; 84.93% were white (25,022 subjects), 13.64% (4018) were black, and the remainder were Hispanic (0.55% [163]), Native American (0.14% [41]), Asian (0.21% [61]), or of other (0.33% [97]) or unknown (0.21% [61]) race.
Table I.
Descriptive characteristics of subjects in Michigan and Indiana aged ≥18 years who were dually enrolled in both Medicare and Medicaid programs continuously from July 2000 through September 2003, and were included in a retrospective data analysis to assess the impact of implementing prior-authorization policies for antihypertensive agents in these states’ Medicaid programs in March 2002 and September 2002, respectively. Values are shown as number (%) of individuals.
| Characteristic | Michigan (n = 38,684) | Indiana (n = 29,463) |
|---|---|---|
| Sex | ||
| Female | 20,614 (53.29) | 16,595 (56.32) |
| Male | 18,070 (46.71) | 12,868 (43.68) |
| Age group, y | ||
| 18–44 | 13,936 (36.03) | 10,229 (34.72) |
| 45–64 | 16,921 (43.74) | 13,321 (45.21) |
| ≥65 | 7827 (20.23) | 5913 (20.07) |
| Race | ||
| White | 29,957 (77.44) | 25,022 (84.93) |
| Black | 7781 (20.11) | 4018 (13.64) |
| Hispanic | 348 (0.90) | 163 (0.55) |
| Native American | 218 (0.56) | 41 (0.14) |
| Asian | 96 (0.25) | 61 (0.21) |
| Other | 207 (0.54) | 97 (0.33) |
| Unknown | 77 (0.20) | 61 (0.21) |
| Antihypertensive use in January 2002* | ||
| Overall | 12,484 (32.27) | 9378 (31.83) |
| Diuretic | 5391 (13.94) | 4464 (15.15) |
| ACE inhibitor | 4637 (11.99) | 3155 (10.71) |
| CCB | 4142 (10.71) | 3013 (10.23) |
| β-Blocker | 3323 (8.59) | 2398 (8.14) |
| ARB | 1047 (2.71) | 1037 (3.52) |
| α-Blocker | 586 (1.51) | 441 (1.50) |
| Other antihypertensive | 925 (2.39) | 627 (2.13) |
ACE = angiotensin-converting enzyme; CCB = calcium channel blocker; ARB = angiotensin II receptor blocker.
Patients may have received >1 antihypertensive agent.
Prepolicy Medication Use
Table I also shows the overall level of treatment with antihypertensive medicines in our study cohort. Two months before the implementation of the policy in Michigan (January 2002), the overall proportion of patients receiving ≥1 antihypertensive was 32.27% (12,484/38,684) in Michigan and 31.83% (9378/29,463) in Indiana. The proportion of patients receiving ≥1 prescription within the major antihypertensive classes was also similar between states (Table I). However, there were several differences in utilization of the specific drugs that were subsequently subject to prior authorization under the different state policies. Table II shows the top 20 antihypertensive drugs by pharmacy reimbursement from July 2000 through February 2002 (ie, the prepolicy period in Michigan). During this time period, drugs subsequently subject to prior authorization represented 19.30% of all antihypertensive prescriptions and 31.36% of antihypertensive pharmacy reimbursements. These numbers varied by class. For example, 89.12% of ARB prescriptions in the prepolicy period were for agents that were later subject to prior authorization, whereas diuretics did not appear to be affected by the policy. The policy in Indiana was comparatively more restrictive; 30.88% of all anti-hypertensive prescriptions received in the prepolicy period were subject to subsequent prior authorization, representing 46.13% of total prepolicy pharmacy reimbursements.
Table II.
Reimbursement for antihypertensive medications among subjects in Michigan and Indiana aged ≥18 years who were dually enrolled in both Medicare and Medicaid programs continuously from July 2000 through September 2003, and were included in a retrospective data analysis to assess the impact of implementing prior-authorization (PA) policies for antihypertensive agents in these states’ Medicaid programs in March 2002 and September 2002, respectively. Table shows top 20 antihypertensives before implementation of the PA requirement.
| Drug | Baseline Use From July 2000 Through February 2002
|
Subsequent PA Status
|
||
|---|---|---|---|---|
| Total Reimbursement* | Prescriptions, No. | Michigan | Indiana | |
| Calcium channel blockers | ||||
| Amlodipine | 2,528,277 | 48,591 | – | PA required |
| Diltiazem† | 764,043 | 17,536 | – | PA required |
| Nifedipine | 711,765 | 11,385 | – | – |
| Diltiazem‡ | 555,727 | 10,489 | – | – |
| Amlodipine/benazepril | 482,320 | 7733 | PA required | PA required |
| Nifedipine | 414,624 | 6498 | PA required | PA required |
| Verapamil | 332,999 | 18,906 | – | – |
| ACE inhibitors | ||||
| Lisinopril§ | 1,368,245 | 39,122 | – | PA required |
| Quinapril | 755,776 | 20,444 | PA required | PA required |
| Enalapril | 711,275 | 21,593 | – | – |
| Lisinopril|| | 577,257 | 17,205 | PA required | PA required |
| Ramipril | 317,986 | 7834 | PA required | PA required |
| Angiotensin II receptor blockers | ||||
| Losartan | 569,171 | 11,299 | PA required | – |
| Valsartan | 306,714 | 6944 | PA required | – |
| β-Blockers | ||||
| Metoprolol | 501,066 | 18,921 | PA required | – |
| Propranolol | 332,747 | 17,334 | – | – |
| Atenolol | 314,631 | 44,878 | – | – |
| α-Blockers | ||||
| Carvedilol | 426,276 | 4692 | – | – |
| Diuretics | ||||
| Furosemide | 596,391 | 110,240 | – | – |
| Torsemide | 371,659 | 9362 | – | – |
ACE = angiotensin-converting enzyme.
Adjusted to year-2003 US $ using the Consumer Price Index.21
Diltiazem brands requiring prior authorization in Indiana were Dilacor XR® (Watson Pharmaceuticals, Inc., Corona, California); Cardizem® (Abbott Laboratories, North Chicago, Illinois); and Tiazac® (Forest Pharmaceuticals, Inc., St. Louis, Missouri).
The diltiazem brand not requiring prior authorization in Indiana was Cartia XT ® (Watson Pharmaceuticals, Inc.).
The lisinopril brand not requiring prior authorization in Michigan was Zestril® (AstraZeneca Pharmaceuticals LP, Wilmington, Delaware).
The lisinopril brand requiring prior authorization in Michigan was Prinvil® (Merck & Co., Inc., Whitehouse Station, New Jersey).
For some drug classes, the differences in the scope of the prior-authorization policies were substantial. For instance, Indiana’s preferred drug list included only generic ACE inhibitors, whereas Michigan’s list included some branded drugs. As a result, 85.31% of the pre-policy prescriptions for ACE inhibitors in Indiana were subject to prior authorization after the policy, versus only 36.34% in Michigan.
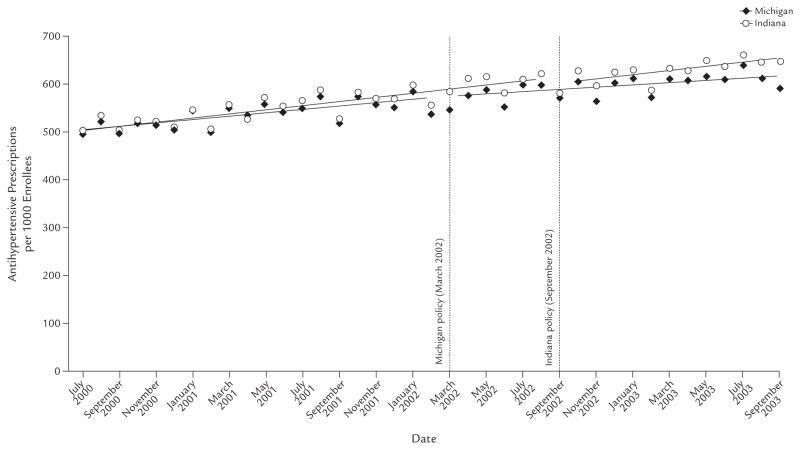
Overall Antihypertensive Use
The implementation of the prior-authorization policies appeared to have only a small effect on the overall extent of antihypertensive use in either state. Figure 1 shows the time series of the rate of antihypertensive prescribing in both states, before and after their respective policies were enacted. Use of antihypertensives rose over the study period in both states. Our time-series analyses indicated that there was no statistically significant immediate change in utilization (−4.07 prescriptions per 1000 enrollees per month [95% CI, −13.34 to 5.20]; P = NS) in Michigan, but there was a significant decline in trend of prescriptions per 1000 enrollees per month (−0.94 [95% CI, −1.85 to −0.04]; P = 0.04), representing a drop of 0.16% in the first month. In contrast, our model suggested an immediate decrease in utilization of 15.67 prescriptions per 1000 enrollees per month in Indiana (95% CI, −29.15 to −2.19; P = 0.02); this represented a lasting 1.82% reduction in the number of antihypertensive prescriptions in Indiana. However, there was no significant change in the trend (0.60; 95% CI, −1.16 to 2.35).
Figure 1.
Number of antihypertensive prescriptions per 1000 subjects in Michigan and Indiana among those aged ≥18 years who were dually enrolled in both Medicare and Medicaid programs continuously from July 2000 through September 2003, and were included in a retrospective data analysis to assess the impact of implementing prior-authorization policies for antihypertensive agents in these states’ Medicaid programs in March 2002 and September 2002, respectively.
Use of Preferred and Nonpreferred Agents
In contrast to the relatively small effect on the overall level of antihypertensive use, the policies had a marked effect on drug choice between preferred and nonpreferred agents.
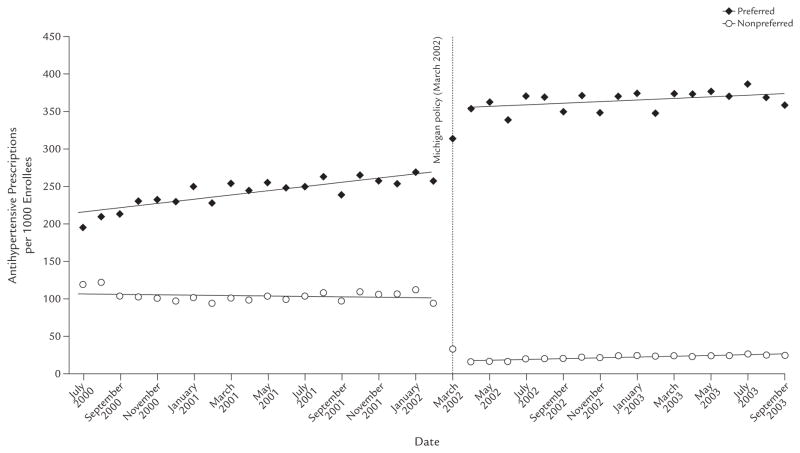
As shown in Figure 2, controlling for baseline trends, use of nonpreferred drugs in Michigan dropped 84.30 prescription per 1000 enrollees per month (95% CI, −91.92 to −76.77; P < 0.001), an immediate reduction of 83.33%. There was a slight increase in trend of 0.81 prescription per 1000 enrollees per month (95% CI, 0.14 to 1.48; P = 0.02). The drop in the utilization of nonpreferred drugs was offset by an almost identical increase in the prescription rate of preferred drugs of 82.65 per 1000 enrollees per month (95% CI, 67.38 to 97.92; P < 0.001), with a slight decrease in trend of 1.77 prescriptions per 1000 enrollees per month (95% CI, −3.12 to −0.42; P = 0.01). Finally, there were no significant changes in either the level or trend in the rate of drugs that were not listed on the formulary (data not shown).
Figure 2.
Number of antihypertensive prescriptions per 1000 subjects, by prior-authorization status, in Michigan among those aged ≥18 years who were dually enrolled in both Medicare and Medicaid programs continuously from July 2000 through September 2003, and were included in a retrospective data analysis to assess the impact of implementing prior-authorization policies for antihypertensive agents in the state’s Medicaid programs in March 2002.
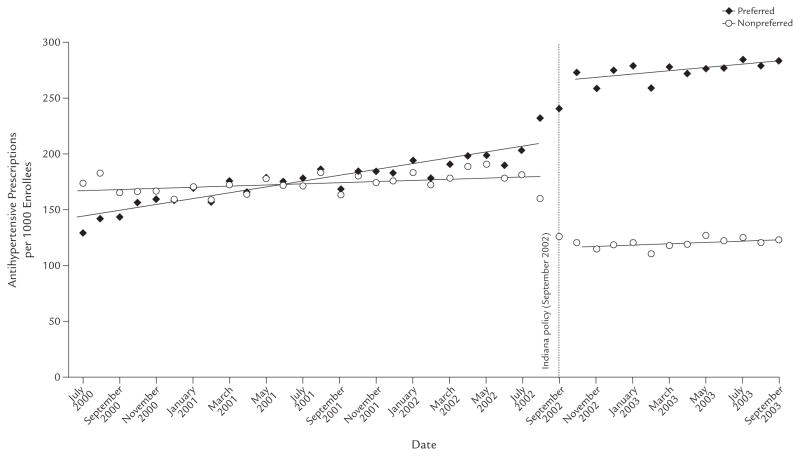
A similarly large shift between nonpreferred and preferred drugs occurred in Indiana, as shown in Figure 3. Following the implementation of prior authorization, there was an immediate drop in utilization of nonpreferred agents of 64.45 prescriptions per 1000 enrollees per month (95% CI, −75.31 to −53.60; P < 0.001), a reduction of 35.76%. We found no significant change in trend (estimate 0.15 prescription per 1000 enrollees per month; 95% CI, −1.14 to 1.44). This drop in the use of non-preferred agents was partially offset by an immediate level increase in the use of preferred medications of 54.91 prescriptions per 1000 enrollees per month (95% CI, 42.56 to 67.27; P < 0.001). There was a small but nonsignificant decrease in trend (−1.30 prescriptions per 1000 enrollees per month; 95% CI, −2.77 to 0.18). There was no change in the utilization level or trend of drugs not listed in the formulary (for both; data not shown). These estimates did not total the overall uncontrolled change estimates because of the inclusion of autoregressive parameters.
Figure 3.
Number of antihypertensive prescriptions per 1000 subjects, by prior-authorization status, in Indiana among those aged ≥18 years who were dually enrolled in both Medicare and Medicaid programs continuously from July 2000 through September 2003, and were included in a retrospective data analysis to assess the impact of implementing prior-authorization policies for antihypertensive agents in the state’s Medicaid programs in September 2002.
Pharmacy Reimbursements
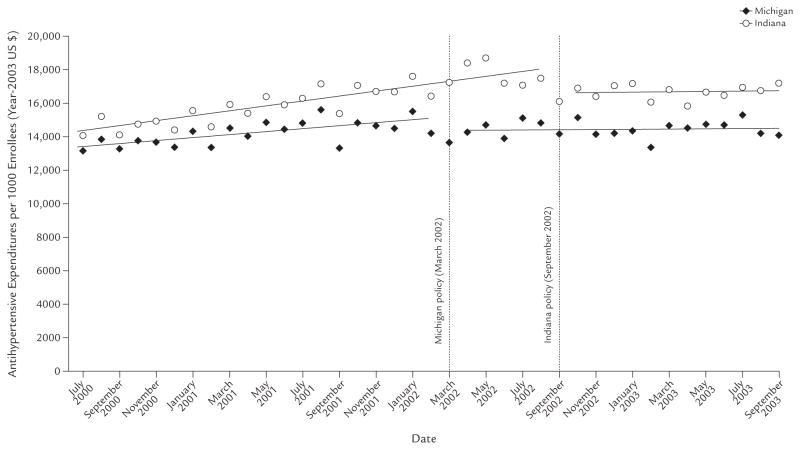
This switch from nonpreferred to preferred drugs was associated with reductions in pharmacy reimbursements. Figure 4 shows the overall pharmacy reimbursement per 1000 enrollees per month in both states. After prior authorization was introduced, and controlling for baseline trends, there was an immediate reduction in cost in both states and a leveling of reimbursements over time. In Michigan, the immediate reduction in cost was −$808.63 per 1000 enrollees per month (95% CI, −$1261.27 to −$356.00; P < 0.001) and a further reduction in trend of −$83.16 per month afterward (95% CI, −$127.02 to −$39.30; P < 0.001). Similarly, in Indiana, there was an immediate reduction of −$1559.00 per 1000 enrollees per month (95% CI, −$2414.03 to −$703.97; P < 0.001) and a further reduction in trend of −$137.97 per month thereafter (95% CI, −$239.74 to −$36.20; P = 0.01). Overall, in the year after the policies were introduced, these reductions equated to pharmacy reimbursement savings of $616,572.43 in Michigan and $868,265.97 in Indiana, or $16,189.80 and $29,469.71 per 1000 enrollees, respectively.
Figure 4.
Total reimbursement of antihypertensive medications per 1000 subjects in Michigan and Indiana among those aged ≥18 years who were dually enrolled in both Medicare and Medicaid programs continuously from July 2000 through September 2003, and were included in a retrospective data analysis to assess the impact of implementing prior-authorization policies for antihypertensive agents in these states’ Medicaid programs in March 2002 and September 2002, respectively.
DISCUSSION
Prior-authorization policies in Medicaid have been controversial because they could potentially inhibit the use of evidence-based therapies.18 Our analysis of 2 distinct policies at the population level found evidence of only minor changes in overall antihypertensive use, despite major shifts between preferred and nonpreferred medications. However, we could not assess the appropriateness of these medication choices. These findings are consistent with research on reference pricing policies for ACE inhibitors, for which policy-prompted medication switches were not associated with decreases in drug use, but rather with substantial reductions in drug costs.24,25 The comparatively more restrictive policy in Indiana was associated with a small decrease in overall antihypertensive use, similar to previous findings on more restrictive prior-authorization policies for ARBs.5 However, the more restrictive policy in Indiana was associated with an almost 2-fold greater decrease in pharmacy reimbursement per enrollee.
This study has several limitations. First, we examined only the impact of the policies on population-level indicators of drug use and costs. Whether or not the policy-induced drug switching we observed had clinical consequences for individual patients remains unknown. However, a previously published study of reference pricing for ACE inhibitors found no evidence that switching led to negative clinical outcomes.24 Similarly, we did not have access to detailed medical record data, so were unable to assess the impact of the policy on such clinical outcomes as blood pressure or incidence of side effects. Furthermore, our follow-up periods were 18 months and 12 months for Michigan and Indiana, respectively, so we could not observe changes in outcomes beyond these relatively short periods. If the policy led to the use of less optimal medications for particular patients, it might influence long-term outcomes, such as myocardial infarction or stroke. Data limitations also limited our ability to study individuals who were not dually eligible for Medicare and Medicaid, so it is unclear whether our results would extend to the remainder of the Medicaid population.
Second, there are additional costs and benefits of prior authorization that we could not observe. For instance, patients and their physicians likely faced time costs because of the forms and telephone calls necessitated by these policies.18 A 2005 survey of physicians in 9 states estimated they faced a mean annual cost of $1569 per year to comply with Medicaid prior-authorization policies for antihypertensives and statins.26 Also, we did not include any estimates of the administrative cost of prior authorization for state Medicaid agencies. Our study did not include any data about supplemental rebates that Michigan and Indiana may have negotiated with manufacturers in exchange for placement of their products as preferred drugs on state formularies. Such rebates, which are confidential, would likely make the net cost savings to each state higher than we estimated.
We hypothesize that the difference in policy effects between this class of medications and other classes results from unique differences between medicines and the conditions that they treat. For drugs primarily used for symptomatic conditions, such as NSAIDs and cyclooxygenase-2 inhibitors, previous interrupted time-series studies of Medicaid enrollees have reported reductions in use after the introduction of prior-authorization policies.11,13 In contrast, prior-authorization policies for atypical antipsychotics, anticonvulsants, and anti-depressants have been associated with smaller changes in overall utilization in interrupted time-series studies of Medicaid patients.14,15,20,27,28 However, these policies have been associated with small but significant unintended consequences for the seriously mentally ill, such as decreased adherence, discontinuation of essential medications, and reductions in use of necessary drugs during new episodes of illness.14,20,27,28 Thus, it would appear that prior authorization may not sharply influence overall level of drug use when the drug is for a chronic, rather than symptomatic, condition, and when there are readily available, clinically comparable medicines that do not require prior authorization. The differences in policy impact between drug classes may also be due to the different patient populations in question; individuals with serious mental illness may be more vulnerable to changes in medication accessibility.
CONCLUSIONS
Prior authorization was associated with lower use of nonpreferred antihypertensive drugs that was largely offset by increases in the use of preferred drugs. The possible clinical consequences of policy-induced drug switching for individual patients remain unknown because the present study did not include access to medical record data. Further research is needed to establish whether large-scale switches in medicines following the inception of prior-authorization policies have any long-term health effects.
Acknowledgments
This study used data obtained through grant 5R01MH069776-03 from the National Institute for Mental Health and was conducted at the Department of Population Medicine at Harvard Medical School and the Harvard Pilgrim Health Care Institute. This analysis was completed while Dr. Law was supported by the Thomas O. Pyle Fellowship and the Fellowship in Pharmaceutical Policy at Harvard Medical School and the Harvard Pilgrim Health Care Institute. Dr. Lu was also supported by the Fellowship Program in Pharmaceutical Policy Research at Harvard Medical School and the Harvard Pilgrim Health Care Institute. Drs. Soumerai, Zhang, Ross-Degnan, and Adams are investigators in the Health Maintenance Organization Research Network Centers for Education and Research on Therapeutics, supported by the Agency for Healthcare Research and Quality (Grant No. U18HS010391) and the Harvard Pilgrim Health Care Institute. Dr. Adams also received core support from Community Benefit, Kaiser Permanente Northern California. The study sponsors had no control over the study design; collection, management, analysis, and interpretation of data; and preparation, review, or approval of the manuscript.
Drs. Soumerai, Zhang, Ross-Degnan, and Adams, and Mr. LeCates and Ms. Graves previously received research funding for a separate study from a public/ private partnership program supported by the US Agency for Healthcare Research and Quality, Eli Lilly and Company, and the Harvard Pilgrim Health Care Foundation. Dr. Zhang has served as a statistical consultant for Policy Analysis Inc.
The authors have indicated that they have no other conflicts of interest regarding the context of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mello MM, Studdert DM, Brennan TA. The pharmaceutical industry versus Medicaid—limits on state initiatives to control prescription-drug costs. N Engl J Med. 2004;350:608–613. doi: 10.1056/NEJMlim035683. [DOI] [PubMed] [Google Scholar]
- 2.Catlin A, Cowan C, Heffler S, Washington B for the National Health Expenditure Accounts Team. National health spending in 2005: The slowdown continues [published correction appears in Health Aff (Millwood). 2007; 26:595] Health Aff (Millwood) 2007;26:142–153. doi: 10.1377/hlthaff.26.1.142. [DOI] [PubMed] [Google Scholar]
- 3.Owens M. State Medicaid Program Issues: Preferred Drug Lists. Reston, Va: National Pharmaceutical Council; 2003. [Google Scholar]
- 4.Soumerai SB. Benefits and risks of increasing restrictions on access to costly drugs in Medicaid. Health Aff (Millwood) 2004;23:135–146. doi: 10.1377/hlthaff.23.1.135. [DOI] [PubMed] [Google Scholar]
- 5.Fischer MA, Choudhry NK, Winkelmayer WC. Impact of Medicaid prior authorization on angiotensin-receptor blockers: Can policy promote rational prescribing? Health Aff (Millwood) 2007;26:800–807. doi: 10.1377/hlthaff.26.3.800. [DOI] [PubMed] [Google Scholar]
- 6.Fischer MA, Avorn J. Economic implications of evidence-based prescribing for hypertension: Can better care cost less? JAMA. 2004;291:1850–1856. doi: 10.1001/jama.291.15.1850. [DOI] [PubMed] [Google Scholar]
- 7.Lied TR, Gonzalez J, Taparanskas W, Shukla T. Trends and current drug utilization patterns of Medicaid beneficiaries. Health Care Financ Rev. 2006;27:123–132. [PMC free article] [PubMed] [Google Scholar]
- 8.Fields LE, Burt VL, Cutler JA, et al. The burden of adult hypertension in the United States 1999 to 2000: A rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: Current outcomes and control in the community. JAMA. 2005;294:466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- 10.Thom T, Haase N, Rosamond W, et al. for the American Heart Association Statistics Committee and Stroke Statistics Committee. Heart disease and stroke statistics—2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee [published corrections appear in Circulation. 2006;113: e696 and Circulation. 2006;114:e630] Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 11.Smalley WE, Griffin MR, Fought RL, et al. Effect of a prior-authorization requirement on the use of nonsteroidal antiinflammatory drugs by Medicaid patients. N Engl J Med. 1995;332:1612–1617. doi: 10.1056/NEJM199506153322406. [DOI] [PubMed] [Google Scholar]
- 12.Fischer MA, Schneeweiss S, Avorn J, Solomon DH. Medicaid prior-authorization programs and the use of cyclooxygenase-2 inhibitors. N Engl J Med. 2004;351:2187–2194. doi: 10.1056/NEJMsa042770. [DOI] [PubMed] [Google Scholar]
- 13.Delate T, Mager DE, Sheth J, Motheral BR. Clinical and financial outcomes associated with a proton pump inhibitor prior-authorization program in a Medicaid population. Am J Manag Care. 2005;11:29–36. [PubMed] [Google Scholar]
- 14.Soumerai SB, Zhang F, Ross-Degnan D, et al. Use of atypical antipsychotic drugs for schizophrenia in Maine Medicaid following a policy change. Health Aff (Millwood) 2008;27:w185–w195. doi: 10.1377/hlthaff.27.3.w185. [DOI] [PubMed] [Google Scholar]
- 15.Law MR, Ross-Degnan D, Soumerai SB. Effect of prior authorization of second-generation antipsychotic agents on pharmacy utilization and reimbursements. Psychiatr Serv. 2008;59:540–546. doi: 10.1176/ps.2008.59.5.540. [DOI] [PubMed] [Google Scholar]
- 16.Morden NE, Zerzan JT, Rue TC, et al. Medicaid prior authorization and controlled-release oxycodone. Med Care. 2008;46:573–580. doi: 10.1097/MLR.0b013e31816493fb. [DOI] [PubMed] [Google Scholar]
- 17.Roughead EE, Zhang F, Ross-Degnan D, et al. Differential effect of early or late implementation of prior authorization policies on the use of Cox II inhibitors. Med Care. 2006;44:378–382. doi: 10.1097/01.mlr.0000204056.31664.36. [DOI] [PubMed] [Google Scholar]
- 18.Hamel MB, Epstein AM. Prior-authorization programs for controlling drug spending. N Engl J Med. 2004;351:2156–2158. doi: 10.1056/NEJMp048294. [DOI] [PubMed] [Google Scholar]
- 19.Bernasek C, Farkas J, Felman H, et al. Case Study: Michigan’s Medicaid Prescription Drug Benefit. Washington, DC: Kaiser Family Foundation; 2003. [Google Scholar]
- 20.Adams AS, Zhang F, LeCates RF, et al. Prior authorization for antidepressants in Medicaid: Effects among disabled dual enrollees. Arch Intern Med. 2009;169:750–756. doi: 10.1001/archinternmed.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Dept of Labor. Bureau of Labor Statistics. [Accessed April 24, 2008];Consumer Price Index. http://www.bls.gov/cpi/
- 22.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 23.The Henry J. Kaiser Family Foundation. Medicaid’s Role for Women. Menlo Park, Calif: The Henry J. Kaiser Family Foundation; 2007. [Accessed July 10, 2009]. http://www.kff.org/womenshealth/upload/7213_03.pdf. [Google Scholar]
- 24.Schneeweiss S, Walker AM, Glynn RJ, et al. Outcomes of reference pricing for angiotensin-converting-enzyme inhibitors. N Engl J Med. 2002;346:822–829. doi: 10.1056/NEJMsa003087. [DOI] [PubMed] [Google Scholar]
- 25.Schneeweiss S, Soumerai SB, Glynn RJ, et al. Impact of reference-based pricing for angiotensin-converting enzyme inhibitors on drug utilization. CMAJ. 2002;166:737–745. [PMC free article] [PubMed] [Google Scholar]
- 26.Ketcham JD, Epstein AJ. Which physicians are affected most by Medicaid preferred drug lists for statins and antihypertensives? Pharmaco Economics. 2006;24(Suppl 3):27–40. doi: 10.2165/00019053-200624003-00003. [DOI] [PubMed] [Google Scholar]
- 27.Lu CY, Soumerai SB, Ross-Degnan D, et al. Unintended impacts of a Medicaid prior authorization policy on access to medications for bipolar illness. Med Care. 2010;48:4–9. doi: 10.1097/MLR.0b013e3181bd4c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Adams AS, Ross-Degnan D, et al. Effects of prior authorization on medication discontinuation among Medicaid beneficiaries with bipolar disorder. Psychiatr Serv. 2009;60:520–527. doi: 10.1176/ps.2009.60.4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]