Abstract
Long-term potentiation of NMDA-receptor-mediated synaptic transmission (NMDAR-LTP) is a little-understood form of plasticity. In the present study, we investigated whether NMDAR-LTP in the dentate gyrus involves recruitment of extrasynaptic NMDARs, because NMDARs are expressed both synaptically and extrasynaptically with evidence for subtype differences at different locations. We show that before induction of NMDAR-LTP, pharmacological inhibition of glutamate transporters resulted in glutamate spillover from the synapse and activation of extrasynaptic NMDARs. After the induction of NMDAR-LTP, such activation of extrasynaptic NMDARs was absent. Activation of extrasynaptic NMDARs after glutamate uptake inhibition also occurred when synaptic NMDARs were inhibited with MK801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate], and this extrasynaptically mediated NMDAR-EPSC was strongly reduced by prior induction of NMDAR-LTP. The extrasynaptic NMDARs were shown to be NR2D-containing, because the activation of extrasynaptic NMDARs by glutamate spillover was prevented by the NR2D-selective antagonists PPDA [(2R*,3S*)-1-(phenanthrenyl-2-carbonyl)piperazine-2,3-dicarboxylic acid] and UBP141. Further studies using selective antagonists for NR2A- and NR2B-containing NMDARs demonstrated that synaptic NMDARs are predominantly NR2A-containing and NR2B-containing receptors, whereas the extrasynaptic NMDARs are complex multimeric receptors with NR2A, NR2B, or NR2D subunits. Our results show that LTP of NMDAR-EPSCs involves movement of NMDARs from an extrasynaptic to a synaptic location and suggest a novel physiological role for extrasynaptic NMDARs.
Keywords: NMDA receptor, synaptic plasticity, LTP, dentate gyrus, glutamate transporters, patch clamp
Introduction
Activity-dependent modulation of NMDA receptor (NMDAR) trafficking is a dynamic and powerful mechanism underlying regulation of synaptic transmission and neuronal excitability. Because activation of NMDARs evokes EPSCs with very slow kinetics and a large Ca2+ influx, modulation of NMDA-receptor-mediated transmission will alter repetitive synaptic activity and Ca2+-dependent intracellular processes. Numerous studies have shown that long-term potentiation (LTP) and long-term depression (LTD) of NMDAR transmission can be induced by increased presynaptic activity. In particular, high-frequency stimulation (HFS) rapidly induces LTP of NMDAR-EPSPs/EPSCs in the hippocampal dentate gyrus (O'Connor et al., 1994, 1995; Harney et al., 2006), CA1 (Bashir et al., 1991; Berretta et al., 1991; Asztely et al., 1992; Xie et al., 1992; Clark and Collingridge, 1995; Kullmann et al., 1996; Jia et al., 1998; Grosshans et al., 2002; Kotecha et al., 2003; Lozovaya et al., 2004), and CA3 (Kwon and Castillo, 2008; Rebola et al., 2008) (for review, see Lau and Zukin, 2007). LTD of NMDAR-EPSCs can also be induced by low-frequency stimulation (Morishita et al., 2005) or by HFS under conditions of relatively high intracellular Ca2+ buffering (Harney et al., 2006).
The induction of NMDAR-LTP involves several stages and requires activation of three types of postsynaptic receptor: first, NMDARs (O'Connor et al., 1995; Kwon and Castillo, 2008; Rebola et al., 2008); second, group I mGluRs, and specifically mGluR5 (O'Connor et al., 1994; Harney et al., 2006; Kwon and Castillo, 2008; Rebola et al., 2008), with costimulation of mGluR5 and NMDARs also being required for potentiation of exogenously evoked NMDAR currents (Kotecha et al., 2003); third, adenosine A2A receptor activation (Rebola et al., 2008). NMDAR-LTP also requires activation of a G-protein (Rebola et al., 2008) and a rise in intracellular Ca2+ (Harney et al., 2006; Kwon and Castillo, 2008; Rebola et al., 2008) and release of Ca2+ from internal IP3-sensitive stores (Kwon and Castillo, 2008), followed by activation of the intracellular messengers PKC (O'Connor et al., 1994; Kwon and Castillo, 2008) and src (Rebola et al., 2008). Enhancement of exogenously evoked NMDAR currents also requires src (Kotecha et al., 2003).
All NMDARs require two NR1 subunits, assembled with NR2 subunits either as dimers containing NR1/NR2A or NR1/NR2B, or as heterotrimers containing NR1/NR2A/NR2B or NR1/NR2A/NR2D (Cull-Candy and Leszkiewicz, 2004). NR2B receptors are strongly expressed early in development, and NR2A expression increases during development (Monyer et al., 1994). NR2A and NR2B subunits are expressed at synapses (Tovar and Westbrook, 1999; Janssen et al., 2005; Thomas et al., 2006) (for review, see Cull-Candy and Leszkiewicz, 2004), whereas expression of NR2D subunits is mainly restricted to extrasynaptic locations (Momiyama et al., 1996; Misra et al., 2000b; Momiyama, 2000; Brickley et al., 2003).
Here we report that the induction of NMDAR-LTP in the medial perforant path of the dentate gyrus involves the movement of NMDARs from an extrasynaptic to a synaptic site. We show that before induction of LTP, the extrasynaptic, but not the synaptic, NMDARs are NR2D-containing complexes. After the induction of NMDAR-LTP, synaptic NMDAR-mediated transmission involves activation of NR2D-containing subunits.
Materials and Methods
Slice preparation.
Transverse hippocampal slices (350 μm) were prepared from the brains of male Wistar rats (3–4 weeks old, 40–80 g; Harney et al., 2006). All animal procedures were performed in accordance with local and national regulations. Slices were cut in ice-cold artificial CSF (ACSF) solution containing (in mm) 75 sucrose, 87 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 7 MgCl2, 10 d-glucose, 1 ascorbic acid, and 3 pyruvic acid. During incubation and experiments, slices were perfused with ACSF containing (in mm) 125 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, and 25 d-glucose. ACSF solutions were bubbled with 95% O2 and 5% CO2. All salts used were obtained from Sigma-Aldrich.
Patch-clamp electrophysiology.
Whole-cell patch-clamp recordings were made from granule cells of the dentate gyrus. Patch pipettes were filled with intracellular solution containing (in mm) 140 K-gluconate, 10 HEPES, 0.2 EGTA, 20 phosphocreatine, 2 Mg2ATP, 0.3 NaGTP (pH 7.3, 290–300 mOsm). Slices were maintained at 32−34°C during recordings. Granule cells were voltage clamped at −70 mV, and EPSCs were evoked by stimulation with a bipolar tungsten wire electrode placed in the middle one-third of the dentate gyrus molecular layer to activate the medial perforant pathway. Recordings were made with an Axopatch 1D amplifier (Molecular Devices), and signals were filtered at 5 kHz using a four-pole Bessel filter and digitized at 10 kHz using a Digidata 1320A analog–digital interface (Molecular Devices). Series resistance was typically 6–18 MΩ before compensation of ∼80%. NMDAR-EPSCs were recorded in picrotoxin (100 μm), 6-cyano-7-quinoxaline-2, 3-dione (CNQX; 10 μm), (2S)-3-{[(15)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl}(phenylmethyl)phosphinic acid (CGP55845; 2 μm), and glycine (20 μm). HFS consisted of eight trains of eight stimuli at 200 Hz, at an intertrain interval of 2 s, repeated three times, and was applied under current-clamp conditions. LTP amplitude was expressed as the percentage of control amplitude at 35–40 min after HFS. Picrotoxin, CNQX, ifenprodil, and (+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate (MK801) were from Sigma-Aldrich, CGP55845 was from Tocris Bioscience, and (2R*,3S*)-1-(phenanthrenyl-2-carbonyl)piperazine-2,3-dicarboxylic acid (PPDA) was obtained from Ascent Scientific.
Data analysis.
Data were acquired and analyzed using pClamp 9.0, Clampfit (Molecular Devices) and Strathclyde Electrophysiology software (J. Dempster, University of Strathclyde, Glasgow, UK). The decay of averaged NMDAR-EPSCs were fit with one or two exponential functions, and for EPSCs best described by two exponentials, weighted decay time constants were calculated as τW = τ1 [A1/(A1 + A2)] + τ2 [A2(A1 + A2)], where τ is the fitted time constant and A is amplitude. Data are mean ± SEM, and statistical significance was evaluated using paired t tests or ANOVA with post hoc Tukey's test for multiple comparisons (p < 0.05). Error bars in all figures represent mean ± SEM.
Results
Induction of LTP of NMDAR-mediated transmission
LTP of NMDAR-mediated transmission (NMDAR-LTP) has been reported previously in numerous studies in the hippocampal dentate gyrus (O'Connor et al., 1994, 1995;Harney et al., 2006) and CA1 (Bashir et al., 1991; Berretta et al., 1991; Asztely et al., 1992; Xie et al., 1993; Clark and Collingridge, 1995; Kullmann et al., 1996; Jia et al., 1998; Grosshans et al., 2002; Kotecha et al., 2003; Lozovaya et al., 2004) (for review, see Lau and Zukin, 2007). Relatively little is understood about the underlying mechanisms of NMDAR-LTP; however, there is recent evidence for lateral glutamate receptor movement between the extrasynaptic and synaptic membrane (for review, see Newpher and Ehlers, 2008). We investigated whether NMDAR-LTP was generated by movement of extrasynaptic NMDARs to a synaptic location. Isolated NMDAR-EPSCs were recorded from the medial perforant path of the dentate gyrus at a test frequency of 0.033 Hz, at 34°C. Recordings were performed at −70 mV rather than at positive potentials to preserve normal physiological postsynaptic neuronal glutamate uptake. LTP of isolated NMDAR-EPSCs was induced by HFS, resulting in an increase in EPSC amplitude measuring 160 ± 14% of control (n = 5) (Fig. 1A,B) and total charge transfer increasing to 140 ± 6% of control. NMDAR-EPSC 10–90% rise time was reduced after induction of LTP (4.4 ± 0.6 ms and 3.6 ± 0.4 ms before and after LTP, p = 0.03, n = 5), perhaps indicating increased activation of NMDARs located centrally in the postsynaptic membrane after LTP induction (Steigerwald et al., 2000) or insertion of receptors with faster activation kinetics (Erreger et al., 2005; Dravid et al., 2008). The decay time constant was unchanged (τD = 36 ± 3 ms and 35 ± 3 ms before and after LTP, respectively, p = 0.7).
Figure 1.
Activation of extrasynaptic NMDARs by glutamate spillover is reduced after NMDAR-LTP. A, Averaged EPSC traces showing control NMDAR-LTP with increased NMDAR-EPSC amplitude at 40 min after HFS, compared with control. B, Graph of LTP of NMDAR-EPSCs induced by HFS; n = 5. C, Traces showing enhanced NMDAR-EPSC amplitude after perfusion with TBOA (30 μm). Inset, EPSCs scaled to peak and scaled EPSCs on expanded timescale to illustrate the prolonged rise time in TBOA. D, Plots of NMDAR-EPSC amplitude during perfusion with TBOA for 30 min (filled symbols, n = 5) and 15 min (open symbols; n = 4). The enhancement of NMDAR-EPSCs by TBOA was completely reversible with washout. E, Traces showing averaged NMDAR-EPSCs in control, after induction of LTP and in TBOA after induction of LTP. F, Graph showing occlusion of the TBOA enhancement of NMDAR-EPSCs after HFS-induced LTP; n = 5.
Previous studies have shown that presynaptic LTP is associated with changes in paired pulse ratio (PPR) and a reduced coefficient of variation (CV). In the present study, NMDAR-LTP was not associated with any changes in PPR (1.0 ± 0.05 in control and 0.98 ± 0.03 after LTP, p = 0.25, n = 17) or CV (0.12 ± 0.01 in control and 0.11 ± 0.01 after LTP, p = 0.7, n = 17). These results, in addition to our previous studies showing the block of NMDAR-LTP with high postsynaptic intracellular calcium buffering (Harney et al., 2006), suggest that NMDAR-LTP is expressed postsynaptically.
Activation of extrasynaptic NMDARs by glutamate spillover is reduced after NMDAR-LTP
We first investigated whether extrasynaptic NMDARs were present by using the glutamate uptake inhibitor DL-threo-β-benzyloxyaspartic acid (TBOA) to induce spillover of synaptically released glutamate. Bath perfusion of TBOA (30 μm) evoked a rapidly reversible enhancement of NMDAR-EPSC amplitude (162 ± 10%, p < 0.001, n = 17) (Fig. 1C,D), prolonged EPSC rise time (192 ± 18% of control; 4.7 ± 0.3 ms and 8.9 ± 0.9 ms in control and TBOA, respectively; p < 0.001, n = 17) and resulted in a small but significant prolongation of EPSC decay kinetics (117 ± 7% of control; tD = 32 ± 1 ms and 37 ± 2 ms in control and TBOA respectively; p = 0.01, n = 17). Total charge transfer was increased by 199 ± 17% of control in the presence of TBOA. The prolonged rise time in the presence of TBOA indicates an increased distance between the glutamate release site and the activation of a population of NMDARs, and is consistent with glutamate spillover from the synapse and activation of an extrasynaptic population of NMDARs.
We compared the activation of extrasynaptic NMDARs before and after LTP, by applying TBOA at 10 min after the induction of LTP. The TBOA enhancement of NMDAR-EPSCs was occluded by LTP induction. Thus, perfusion of TBOA after the induction of LTP failed to enhance the NMDAR EPSCs (37 ± 8 pA after LTP, 160% of control and 37 ± 5 pA, 169% of control in TBOA; p = 0.9, n = 5) (Fig. 1E,F). The lack of effect of TBOA on NMDAR-EPSCs after LTP induction demonstrates that extrasynaptic NMDARs are strongly reduced after the induction of NMDAR-LTP.
Block of synaptic NMDARs using MK801 reveals that the extrasynaptic NMDAR-mediated current is reduced after induction of NMDAR-LTP
We also studied the effect of TBOA after inhibition of synaptic NMDARs with the use-dependent NMDAR antagonist MK801 (Huettner and Bean, 1988; Tovar and Westbrook, 1999). Such conditions allowed the effect of LTP to be observed on isolated extrasynaptically evoked NMDAR-EPSCs. Synaptic NMDAR-EPSCs were blocked with the irreversible use-dependent NMDAR antagonist MK801 (40 μm). Perfusion of MK801 caused a progressive block of NMDAR-EPSCs during stimulation at a frequency of 0.1 Hz, which attained ∼85% inhibition of charge transfer after 180 stimuli (Fig. 2A,B). Although a higher frequency stimulation and/or depolarization may have induced a more complete block, we stimulated at a low frequency and at −70 mV to avoid induction of NMDAR-LTP. After block of synaptic NMDARs by MK801 (and washout for 10 min, without stimulation) an increase in NMDAR-EPSCs induced by TBOA will result from the activation of extrasynaptic NMDARs by synaptically released glutamate. We compared NMDAR-EPSCs recorded after MK801 treatment under 3 different conditions: first, MK801 followed by 10 min washout; second, MK801, 10 min washout, and application of TBOA; and third, LTP induction followed by MK801, 10 min washout, and application of TBOA. Charge transfer measurements were compared, and illustrated in Figure 2, as they reflect changes in both NMDAR-EPSC amplitude and kinetics after perfusion with MK801. EPSCs recorded after MK801 block and washout showed a small recovery from MK801 block to 23 ± 5% of the control EPSC charge transfer (n = 6) (Fig. 2B,G). The residual EPSC after MK801 application and washout was blocked by d-AP5 (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). As predicted, MK801 washout followed by application of TBOA induced a much larger NMDAR-EPSC, with a charge transfer of 54 ± 11% of the control EPSC (p = 0.014, ANOVA with post hoc Tukey's test, n = 7) (Fig. 2C,D,G). In contrast, LTP induction before MK801 treatment inhibited the enhancement of NMDAR-EPSCs by TBOA after washout of MK801 (charge transfer of 31 ± 8% of control, not significantly different from recovery after MK801, without TBOA, p = 0.4, ANOVA, n = 7) (Fig. 2E–G). These results further demonstrate that there is a reduction in the current mediated by extrasynaptic NMDARs after LTP induction. Moreover, they eliminate the possibility that the TBOA enhancement of the NMDAR-EPSCs was attributable to activation of synaptic NMDARs and that inhibition of the TBOA enhancement resulted from saturation of synaptic NMDARs during LTP.
Figure 2.
Inhibition of synaptic receptors with MK801 reveals a reduction in extrasynaptic NMDAR-mediated current after LTP. A, Averaged NMDAR-EPSCs in control and after perfusion of MK801 (40 μm) and washout, showing little recovery of the synaptic current. Insets show scaled EPSCs. B, Graph illustrating that perfusion of MK801 strongly reduces NMDAR-EPSC total charge transfer; n = 6. Washout of MK801 (with no stimulation during washout) resulted in only a small recovery of the NMDAR-EPSCs. C, Traces showing NMDAR-EPSCs in control, after washout of MK801 and in the presence of TBOA, after MK801 washout. D, Graph showing averaged data for similar experiments to B except that TBOA was perfused after washout of MK801, resulting in a much larger recovery of the NMDAR-EPSCs caused by activation of extrasynaptic NMDARs; n = 7. E, Traces showing NMDAR-EPSCs in control, after LTP induction, after MK801 perfusion and washout, and in TBOA (after MK801 treatment). F, Graph illustrating that LTP induction followed by MK801 treatment, to block synaptic receptors, resulted in a much smaller recovery of NMDAR-EPSCs with TBOA application; n = 7. G, Bar graph illustrating the mean NMDAR-EPSC charge transfer after block of synaptic NMDARs with MK801, in the presence of TBOA after MK801 treatment and in the presence of MK801 after LTP induction plus MK801 treatment. *p < 0.05, ANOVA plus post hoc Tukey's test.
The NR2D antagonists PPDA and UBP141 inhibit activation of extrasynaptic NMDARs
We postulated that the extrasynaptic NMDARs may be complex heterotrimers containing the NR2D subunit because NR2D subunits are widely expressed in the brain (Ishii et al., 1993) and NR2D-containing receptors appear to be confined to the extrasynaptic membrane in the neuronal cell types in which they have been studied (Momiyama et al., 1996; Misra et al., 2000a,b; Momiyama, 2000; Brickley et al., 2003). Triheteromeric NR1/2B/2D receptors have been identified in the soma of dentate gyrus granule cells in single-channel recordings (Pina-Crespo and Gibb, 2002). Here we studied the role of NR2D-containing receptors in synaptic transmission using the competitive antagonists PPDA (Feng et al., 2004) and UBP141 (Morley et al., 2005). We initially compared the action of PPDA on control synaptic NMDAR-EPSCs and on the extrasynaptic NMDAR-EPSCs evoked in the presence of TBOA. PPDA inhibited control synaptic NMDAR-EPSCs only at very high concentrations of 5–10 μm (40% inhibition at 5 μm, n = 5 and 65% inhibition at 10 μm, n = 6). PPDA is nonselective at such high concentrations, inhibiting NR2A and NR2B as well as NR2D (Hrabetova et al., 2000; Feng et al., 2004). The relative insensitivity of synaptic NMDARs to PPDA demonstrates an absence of NR2D-containing NMDARs at the synapse, with the inhibition of synaptic NMDAR-EPSCs at high concentrations of PPDA being attributable to a nonselective action of PPDA on NR1/NR2A- or NR1/NR2B-containing receptors. The TBOA enhancement of NMDAR-EPSCs, mediated by extrasynaptic NMDARs activated by glutamate spillover, was much more sensitive to PPDA than control synaptic NMDAR-EPSCs. The threshold concentration for PPDA inhibition of the extrasynaptic component of NMDAR-EPSCs in TBOA was ∼0.08 μm, with an IC50 value of 0.2 μm (supplemental Fig. 2, available at www.jneurosci.org as supplemental material), 30-fold lower than the IC50 for PPDA at synaptic NMDARs. Comparisons of the potency of an antagonist on EPSCs generated synaptically and extrasynaptically are qualitative, because the glutamate concentration is not steady state and may differ between synaptic and extrasynaptic location. However, the observed differential PPDA sensitivity can be attributed to the expression of distinct NMDAR subtypes. The IC50 value for PPDA at extrasynaptic NMDARs found in the present study is very similar to the Ki of ∼0.1 μm for the action of PPDA at NR1A/NR2D recombinant receptors shown in previous studies (Hrabetova et al., 2000; Feng et al., 2004), and strongly supports the identification of extrasynaptic NMDARs as NR2D-containing receptors.
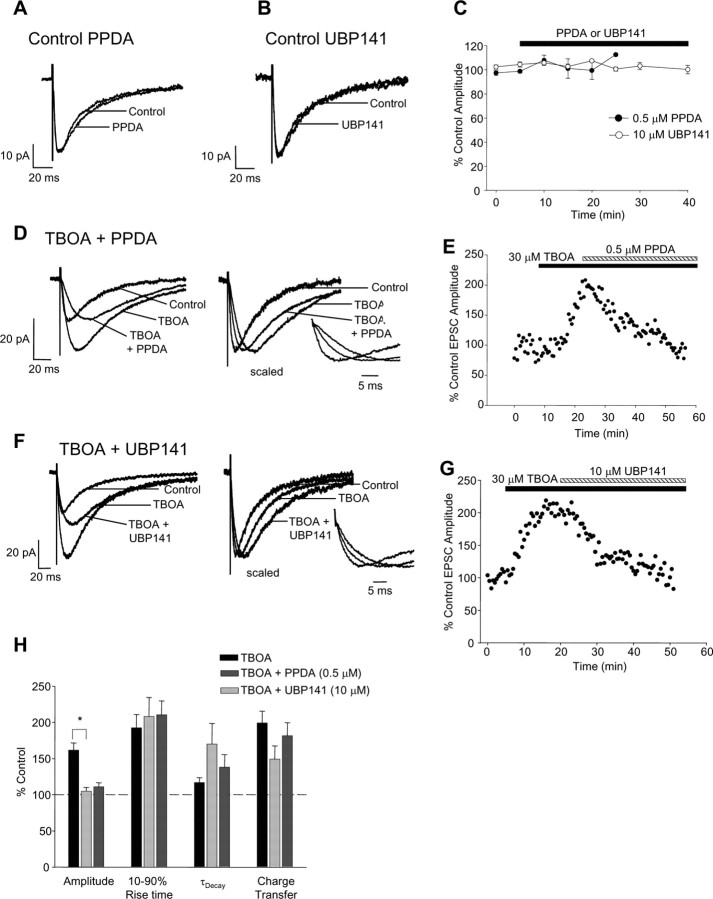
Detailed studies of the involvement of NR2D-containing receptors (see below) were performed using PPDA at 0.5 μm, a concentration that had no effect on the amplitude, rise time, or decay time of control synaptic NMDAR-EPSCs (amplitude 102 ± 5% of control, 10–90% rise time 4.3 ± 3 ms in control and 4.5 ± 0.3 in PPDA, p = 0.5; decay time constant, τD = 34 ± 2 ms in control and 40 ± 4 ms in PPDA, p = 0.16, n = 9) (Fig. 3A,C,H) but completely inhibited the TBOA enhancement of NMDAR-EPSC amplitude (168 ± 25% of control in TBOA, 105 ± 4% of control in TBOA + PPDA, p = 0.017 for control vs TBOA and p = 0.026 for TBOA vs TBOA + PPDA, n = 5) (Fig. 3D,E,H). Total charge transfer was also reduced by 0.5 μm PPDA, from 228 ± 42% of control in TBOA (p = 0.01, control vs TBOA) to 151 ± 14% of control in PPDA (p = 0.4, control vs TBOA + PPDA, n = 5) (Fig. 3H). As in the initial studies above, a small prolongation of decay kinetics was observed in TBOA (116 ± 7% of control, τD = 32 ± 1 ms and 37 ± 2 ms in control and TBOA, respectively; p = 0.01; n = 17). In TBOA and PPDA, the decay time constant was also prolonged (165 ± 23% of control, τD = 43 ± 3 ms in TBOA and 56 ± 9 ms in TBOA and PPDA, p = 0.034, n = 5) (Fig. 3H).
Figure 3.
NR2D-containing extrasynaptic NMDARs are activated when glutamate uptake is inhibited. A, Traces showing no effect of 0.5 μm PPDA on control NMDAR-EPSCs. B, Traces showing no effect of 10 μm UBP141 on control NMDAR-EPSCs. C, Graph of NMDAR-EPSC amplitude during incubation with 0.5 μm PPDA (filled symbols; n = 9) and 10 μm UBP141 (open symbols; n = 4). D, Traces showing PPDA inhibition of the TBOA enhancement of NMDAR-EPSCs, inset illustrates EPSCs on an expanded timescale. E, Representative experiment showing the PPDA inhibition of the TBOA enhancement of NMDAR-EPSCs. F, Traces showing the UBP141 inhibition of the TBOA enhancement of NMDAR-EPSCs, inset illustrates EPSCs on an expanded timescale. G, Representative experiment showing the UBP141 inhibition of the TBOA enhancement of NMDAR-EPSCs. H, Bar graph illustrating mean percentage changes in NMDAR-EPSC properties with TBOA alone (n = 17), TBOA + PPDA (n = 5) and TBOA + UBP141 (n = 4). *p < 0.05, ANOVA plus post hoc Tukey's test.
Similar results were obtained with UPB141, a NR2D-selective antagonist with approximately five-fold to seven-fold selectivity for NR2D over NR2A/NR2B-containing receptors, with a Ki of ∼0.1 μm for the inhibitory action of PPDA at NR1A/NR2D recombinant receptors (Morley et al., 2005). UBP141 (10 μm) had no effect on control NMDAR-EPSCs (EPSC amplitude 99 ± 3% of control, τD 97 ± 6% of control, rise time 90 ± 6% of control) (Fig. 3B,C,H) but inhibited the TBOA enhancement of NMDAR-EPSC amplitude (179 ± 11% of control in TBOA and 111 ± 6% of control in TBOA + UBP141, n = 4) (Fig. 3F–H). In agreement with our results using PPDA, the prolongation of NMDAR-EPSC kinetics in TBOA was unaffected by UBP141 (τD 138 ± 18% of control and rise time 170 ± 28% of control, p = 0.005, n = 4) (Fig. 3H).
NR2D-containing NMDARs are essential for the expression of NMDAR-LTP
We postulated that the large reduction in extrasynaptic receptors after induction of NMDAR-LTP (Fig. 2) was caused by the movement of extrasynaptic NR2D-containing NMDARs into the synapse. Such a movement of NR2D-containing NMDARs would be expected to make the expression of LTP sensitive to a NR2D receptor antagonist. Perfusion of PPDA (0.5 μm) at 10 min after the induction of NMDAR-LTP resulted in rapid block of the maintenance of LTP, with NMDAR-EPSC amplitude being reduced to the control pre-LTP level within 10 min of PPDA application (from 156 ± 9% of control 5–10 min after HFS to 103 ± 8% of control amplitude in PPDA, n = 7) (Fig. 4A,B,E). The rise time was reduced after LTP induction, similar to the change observed in control LTP (rise time of 4.8 ± 0.4 ms and 3.7 ± 0.3 ms in control and in PPDA after LTP induction, respectively, p = 0.04 for control vs LTP, p = 0.09 for control vs PPDA, ANOVA plus post hoc Tukey's test, n = 7), and there was no change in decay time constant (τD = 30 ± 1 ms and 28 ± 2 ms in control and PPDA, respectively, p = 0.2, ANOVA, n = 7) (Fig. 4E). These experiments show that the expression of NMDAR-LTP is mediated by activation of NR2D-containing NMDARs. Before LTP induction, synaptic NMDAR-EPSCs are mediated only by NR2A-/NR2B-containing receptors, but this is altered after LTP induction when NR2D-containing NMDARs contribute to synaptically evoked EPSCs.
Figure 4.
PPDA inhibits the maintenance of LTP of NMDAR-EPSCs but does not prevent the induction of NMDAR-LTP. A, Traces showing NMDAR-EPSCs in control conditions, 10 min after induction of LTP and in the presence of PPDA after LTP induction. B, Graph showing that perfusion of PPDA inhibits the maintenance of LTP of NMDAR-EPSCs, n = 7. C, Incubation in and subsequent washout of PPDA did not inhibit LTP induction. Averaged traces show no change in NMDAR-EPSC amplitude after HFS in the presence of 0.5 μm PPDA, compared with control and increased amplitude with subsequent washout of PPDA 10 min after HFS. D, Averaged data from five experiments showing no plasticity after HFS in the presence of PPDA and subsequent LTP after washout of PPDA. E, Bar graph illustrating the mean percentage changes in NMDAR-EPSC properties after LTP (n = 5), with PPDA perfusion after LTP induction (n = 5), after HFS in the presence of PPDA and after washout of PPDA; n = 5. *p < 0.05, **p < 0.01, ANOVA + post hoc Tukey's test. F, HFS does not activate NR2D-containing NMDARs. Graph of normalized summated NMDAR-EPSC amplitude during trains of 8 stimuli delivered at 200 Hz in control (closed circles) and in the presence of PPDA (open circles); n = 7. Inset, Voltage-clamp records of summated NMDAR-EPSCs evoked by 200 Hz trains in control and in the presence of PPDA. Bar graphs show no significant differences in mean summated EPSC amplitude or weighted decay time constant (τw) fitted to the decay of the summated EPSC.
Activation of NR2D-containing NMDARs is not required for the induction of NMDAR-LTP
Having established that the expression of NMDAR-LTP involves putative NR2D-containing NMDARs, we investigated whether the induction of NMDAR-LTP requires activation of this population of NMDARs. To test whether activation of NR2D-containing NMDARs is required for the induction of NMDAR-LTP, PPDA was perfused before HFS and then washed out 5 min after HFS. If the induction as well as the expression of LTP was blocked by PPDA, then washout of PPDA would not result in recovery of LTP. However, if PPDA was blocking only the expression of LTP and was not required for the induction of LTP, then LTP would be established after PPDA washout. PPDA inhibited the rapid induction of LTP in all experiments, and in five of seven experiments, washout of PPDA resulted in the establishment of LTP (Fig. 4C–E). NMDAR-EPSC amplitude measured 109 ± 8% of control 5 min after HFS and 190 ± 20 of control 25–30 min after HFS and washout of PPDA (p = 0.001 for control vs PPDA washout, ANOVA with post hoc Tukey's test, n = 5) (Fig. 4C–E). NMDAR-EPSC rise time did not change after HFS and was slightly, but not significantly, reduced during LTP expression after washout of PPDA (110 ± 15% of control post-HFS and 86 ± 13% of control after PPDA washout, p = 0.8 for control vs post-HFS and p = 0.7 for control vs PPDA washout, n = 5) (Fig. 4E). There were no changes in decay time constant (Fig. 4E).
HFS does not activate NR2D-containing NMDARs
We tested whether NR2D-containing NMDARs were activated during high frequency trains of stimuli at 200 Hz, identical to those used to induce NMDAR-LTP. Under voltage-clamp conditions, NMDAR-EPSCs summated during 200 Hz trains (Fig. 4F) but there was no significant difference in summated EPSC amplitude between control and in 0.5 μm PPDA (ratio of EPSClast/EPSCfirst, 8 ± 1 in control and 6.8 ± 1 in PPDA, p = 0.2, unpaired t test, n = 7) (Fig. 4F). The decay time course of the summated EPSC was not significantly different between control and in PPDA, with weighted decay time constants of 90 ± 20 ms in control and 80 ± 18 ms in PPDA (p = 0.7, n = 7) (Fig. 4F). In current clamp (which was normally used for delivery of HFS to induce plasticity), PPDA did not significantly reduce the summated EPSP depolarization evoked by 200 Hz trains (33 ± 4 mV in control and 29 ± 6 mV in PPDA, p = 0.5, n = 6 control and 7 PPDA) (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). In addition, there were no significant differences in burst half-width or the weighted decay time constant fitted to the decay of the summated EPSP (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). These experiments suggest that glutamate uptake efficiently limits spillover during HFS.
Effects of the NR2B antagonist ifenprodil and the NR2A antagonist (3-((+)-2-carboxypiperazin-4-yl)-propyl-1-phosphonate)
To further investigate the receptor subtypes that mediate synaptic and extrasynaptic NMDAR EPSCs, we used the NR2B-selective antagonist ifenprodil and the NR2A-selective antagonist (3-((+)-2-carboxypiperazin-4-yl)-propyl-1-phosphonate) (R-CPP), in addition to PPDA. We examined whether NR2B-containing NMDARs contributed to the extrasynaptic NMDAR-mediated current using ifenprodil, a noncompetitive NMDAR inhibitor which has a >100-fold selectivity for NR2B-containing receptors (Williams, 1993; Hess et al., 1998; Hatton and Paoletti, 2005). Ifenprodil (10 μm) inhibited the synaptic NMDAR-EPSC by 28 ± 6% of control (p = 0.01, n = 5) (Fig. 5A), with no change in decay kinetics (τD = 45 ± 5 ms and 42 ± 4 ms in control and ifenprodil, respectively, p = 0.16, n = 5), similar to that described previously in dentate gyrus (Dalby and Mody, 2003; Ye et al., 2005) and CA1 (Kirson and Yaari, 1996), and demonstrating the presence of NR2B-containing synaptic receptors. There was no change in paired pulse ratio (0.9 ± 0.06 and 0.99 ± 0.05 in control and ifenprodil, respectively, p = 0.07, n = 5) indicating that the action of ifenprodil is postsynaptic rather than presynaptic. The effect of ifenprodil on extrasynaptic NMDARs was tested in slices that were preincubated in ifenprodil and then perfused with TBOA. Ifenprodil did not inhibit the TBOA enhancement of NMDAR-EPSC amplitude (166 ± 36% of control amplitude, n = 5, similar to the TBOA effect in the absence of ifenprodil, 162 ± 10% of control) (Fig. 5A,B,F). However, the NMDAR-EPSC decay was significantly prolonged in the presence of TBOA and ifenprodil (156 ± 11% of control, τD = 36 ± 5 ms in ifenprodil and 55 ± 6 ms in ifenprodil + TBOA, p = 0.002, n = 5) (Fig. 5F) and rise time was also increased (4.8 ± 0.8 ms in ifenprodil and 9.5 ± 2 ms in ifenprodil + TBOA, p = 0.004, n = 5) (Fig. 5F). Thus the NR2B antagonist ifenprodil does not inhibit the TBOA enhancement of amplitude but prolongs the kinetics of the enhanced NMDAR-EPSC in TBOA.
Figure 5.
Ifenprodil and R-CPP inhibit control NMDAR-EPSCs but do not prevent the TBOA-induced enhancement of NMDAR-EPSCs. A, Traces on the left show NMDAR-EPSCs in control and in ifenprodil (10 μm). Traces on the right show NMDAR-EPSCs recorded from a cell incubated in ifenprodil and enhanced EPSCs in ifenprodil and TBOA. B, Representative experiment showing the TBOA enhancement of NMDAR-EPSCs during incubation in ifenprodil. C, Traces showing NMDAR-EPSCs in control, in R-CPP and ifenprodil and in R-CPP, ifenprodil, and TBOA. D, Representative experiment showing the strong block of synaptic NMDAR-EPSCs by R-CPP and ifenprodil and the enhancement of the residual EPSC by TBOA. E, Traces showing NMDAR-EPSCs in control, ifenprodil, R-CPP, PPDA and TBOA, with no TBOA-enhancement of NMDAR-EPSCs. F, Bar graph illustrating mean percentage changes in NMDAR-EPSC properties with TBOA (n = 17), ifenprodil + TBOA (n = 5), R-CPP, ifenprodil + TBOA (n = 11) and R-CPP, ifenprodil, PPDA + TBOA (n = 4).
The contribution of NR2A-containing receptors to synaptic NMDAR-EPSCs was tested using R-CPP, which has ∼7-fold and 40-fold greater selectivity for the NR2A subunit compared with NR2B- and NR2D-containing NMDARs, respectively (Feng et al., 2005). At a concentration of 2 μm, R-CPP inhibited control synaptic NMDAR-EPSCs by 39 ± 6% of control (p = 0.05, n = 5), with no change in decay kinetics (τD = 35 ± 4 ms and 42 ± 7 ms in control and R-CPP, respectively, p = 0.1, n = 5). A combination of ifenprodil and R-CPP depressed control NMDAR-EPSCs by 62 ± 3% of control (n = 10). The concentration of ifenprodil used (10 μm), is expected to be a saturating concentration and when higher concentrations of R-CPP (5–10 μm) were tested, we failed to further block the synaptic NMDAR-EPSC, however the residual EPSC was abolished by d-AP5 (supplemental Fig. 4, available at www.jneurosci.org as supplemental material). The maximum efficacy for ifenprodil inhibition is ∼80–90% (Williams, 1993; Neyton and Paoletti, 2006) so this may explain the ifenprodil-resistant synaptic EPSC component. Alternatively, the residual EPSC may be mediated by triheteromeric NMDARs, which form up to 30% of the synaptic NMDAR population (Al-Hallaq et al., 2007) and which have a reduced sensitivity to ifenprodil (Hatton and Paoletti, 2005).
To further examine the pharmacology of extrasynaptic NMDARs, we applied TBOA in the presence of ifenprodil and R-CPP. With the synaptic NMDAR-EPSC component substantially blocked by ifenprodil and R-CPP, we observed a large increase in NMDAR-EPSC amplitude with perfusion of TBOA (167 ± 17% of the EPSC in ifenprodil + R-CPP, p < 0.001, n = 11) (Fig. 5C,D,F). NMDAR-EPSC decay kinetics and rise time were also prolonged in TBOA, similar to the effects seen in controls (τD measuring 147 ± 13% of the ifenprodil + R-CPP control, p < 0.001 and 10–90% rise time of 183 ± 38% of the ifenprodil + R-CPP control, p = 0.004, n = 11) (Fig. 5F). As illustrated in Figure 3, PPDA and UBP141 prevented the increase in NMDAR-EPSC amplitude induced by TBOA but did not alter the prolongation of rise time and decay kinetics. Coapplication of PPDA, R-CPP, and ifenprodil completely inhibited the effects of TBOA on NMDAR EPSC amplitude (91 ± 4% of the EPSC in R-CPP, ifenprodil and PPDA, p = 0.01) (Fig. 5F), decay time constant (83 ± 9% of the R-CPP + ifenprodil + PPDA EPSC, p = 0.7). and rise time (88 ± 4% of the R-CPP + ifenprodil + PPDA EPSC, p = 0.5, n = 4) (Fig. 5F). The I-V curve for NMDAR-EPSCs recorded in R-CPP, ifenprodil and TBOA was similar to that of control EPSCs (ratio of the NMDA-EPSC at −50 mV to that at +50 mV was 0.32 for controls and 0.36 in R-CPP, ifenprodil + TBOA, n = 3) (supplemental Fig. 5, available at www.jneurosci.org as supplemental material), indicating that the Mg2+ sensitivity of the extrasynaptic receptors was identical to synaptic NMDARs.
In summary, although the TBOA enhancement of the NMDAR-EPSC was inhibited by PPDA, the effects of glutamate uptake inhibition on NMDAR-EPSC amplitude, rise time and decay kinetics were completely prevented only by coapplication of R-CPP, ifenprodil and PPDA. These results suggest that the extrasynaptic NMDARs may be a mixed population of heterotrimers which are very sensitive to PPDA and have a reduced sensitivity to R-CPP and ifenprodil compared with synaptic NMDARs.
Discussion
In the present study, extrasynaptic NMDARs were activated by glutamate spillover when glutamate transporters were pharmacologically inhibited with TBOA. The rise time of NMDAR-EPSCs was increased in the presence of TBOA, as expected, because of the increased diffusion time caused by the longer distance between the glutamate release site and the extrasynaptically located receptors, in agreement with that found previously in CA1 (Arnth-Jensen et al., 2002). The presence of extrasynaptic NMDARs has been well documented (Tovar and Westbrook, 1999; Harris and Pettit, 2007), and recent studies have shown the existence of a high density of extrasynaptic NMDARs comprising 36% of the total dendritic pool (Harris and Pettit, 2007). The extrasynaptic NMDARs were shown to be relatively stable in the present study because after strong inhibition of synaptic NMDARs with the use-dependent blocker MK801, little recovery occurred of the synaptic NMDAR-EPSC occurred under control conditions. A similar stable population of extrasynaptic receptors was also shown in CA1 using MK801 in acute slices (Harris and Pettit, 2007), although the extrasynaptic NMDARs are highly mobile in cultured neurons (Tovar and Westbrook, 1999).
The present experiments show that the expression of NMDAR-LTP is mediated by activation of NR2D-containing NMDARs. Before LTP induction, synaptic NMDAR-EPSCs are mediated only by NR2A- and NR2B-containing receptors, but this was altered after LTP induction when the synaptic NMDAR-EPSCs became sensitive to low concentrations of PPDA, thus demonstrating the presence of NR2D-containing NMDARs. Extrasynaptic NR2D-containing NMDARs were detected, before LTP induction, when activated by glutamate spillover in TBOA. However, after LTP induction, extrasynaptic NMDARs were not activated by uptake inhibition. We postulate that LTP induction involves lateral movement of NMDARs from an extrasynaptic site to the synapse. Before LTP, the relevant NR2D-containing receptors may be situated in a perisynaptic location very close to the synaptic area, and therefore only quite small lateral movement of NMDARs would be required to induce LTP. Recent studies using single-molecule fluorescent microscopy tracking of receptors have shown that NMDARs can rapidly cycle into and out of synapses by lateral diffusion in the plane of the membrane. NMDARs have low mobility under basal conditions, but the diffusion rate of both synaptic and extrasynaptic NMDARs is greatly increased by activation of PKC (Groc et al., 2004). Interestingly, NMDAR-LTP also requires activation of PKC (O'Connor et al., 1994; Kwon and Castillo, 2008). NMDARs can also move into and out of synapses by trafficking from internal sites involving soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-dependent exocytosis (for review, see Lau and Zukin, 2007). Kwon and Castillo (2008) recently showed that NMDAR-LTP at mossy fiber synapses involved SNARE-dependent exocytosis because NMDAR-LTP was blocked by inhibitors of SNARE complex proteins. Thus, it is also possible that the movement of NMDARs from the extrasynaptic to synaptic membrane could involve a combination of lateral diffusion and intracellular cycling, because previous studies have demonstrated that NMDAR trafficking can have complex mechanisms involving exo/endocytosis in conjunction with transport intracellularly or within the membrane (Washbourne et al., 2004).
Activation of extrasynaptic NR2D-containing NMDARs was not required for the induction of NMDAR-LTP, because LTP induction was not blocked by PPDA. Therefore, it is likely that LTP induction is mediated by activation of synaptic NR2A- and NR2B-containing NMDARs (Dalby and Mody, 2003; Thomas et al., 2006; Harney et al., unpublished observations) in conjunction with group I mGluRs (O'Connor et al., 1994). We suggest that Ca2+ influx via synaptic NR2A/2B-containing NMDARs (Harney et al., 2006) together with PKC stimulation after activation of mGluR5 results in a lateral movement of extrasynaptic NR2D-containing NMDARs, which are likely to be located very close to the synaptic area, probably at a perisynaptic site. We found no evidence for NR2D-containing receptors contributing to baseline NMDAR-mediated synaptic transmission before induction of LTP, suggesting that the inclusion of NR2D into the synapse upon LTP induction may be a transient phenomenon, with the synaptic NR2D-containing receptors being replaced over a period of time by NR2A- and/or NR2B-containing receptors. These results present a novel physiological role for extrasynaptic NMDARs acting as a reserve pool of receptors that are recruited to the synapse during LTP.
The extrasynaptic NMDARs were identified pharmacologically using the known NR2A-, NR2B-, and NR2D-selective antagonists R-CPP, ifenprodil, and PPDA, respectively. Our studies indicate that extrasynaptic NMDARs are most likely to be a population of multimeric receptors containing NR2D and NR2B subunits, and possibly NR2A. The extrasynaptic NMDARs were identified as NR2D-containing receptors because they were inhibited by low concentrations of the competitive NR2D antagonists PPDA and UBP141 and at concentrations that did not inhibit synaptic NMDAR-EPSCs. PPDA had an IC50 of 0.2 μm at the NR2D-containing NMDARs, similar to the Ki of ∼0.1 μm for the action of PPDA at NR1A/NR2D recombinant receptors (Hrabetova et al., 2000; Feng et al., 2004). In previous experiments on recombinant receptors, PPDA was found to have a three-fold to six-fold higher affinity for NR1A/NR2D receptors compared with NR1/NR2A or NR1/NR2B (Hrabetova et al., 2000; Feng et al., 2004). PPDA inhibited synaptic NMDARs in the present study only at a much higher concentration (5–10 μm), which is nonselective and blocks NR2A- and NR2B-containing NMDARs. Although low concentrations of PPDA are selective for both NR2C- and NR2D-containing receptors compared with NR2A- and NR2B-containing subtypes, we believe the PPDA-sensitive currents we report in dentate gyrus are most likely to be mediated by NR2D-containing receptors. In situ mRNA hybridization (Ishii et al., 1993; Monyer et al., 1994; Wenzel et al., 1996) and protein immunohistochemistry (Okabe et al., 1998; Thompson et al., 2002) show the presence of NR2D, in addition to NR2A, NR2B, mRNA, and protein, in the hippocampus. Native NMDARs containing NR2D are highly expressed in the early postnatal brain including the hippocampus, with levels reaching a peak at ∼7–14 d postnatal and then decreasing two-fold in adulthood (Dunah et al., 1996; Hrabetova et al., 2000; Feng et al., 2004). In contrast, NR2C is confined almost entirely to the cerebellum, thalamus, and olfactory bulb (Ishii et al., 1993; Wenzel et al., 1997). However, the extrasynaptic NMDARs investigated in the present study are unlikely to be dimeric NR1/NR2D because such receptors give rise to currents with very slow deactivation kinetics (τD ∼ 3–4 s) (Monyer et al., 1994; Vicini et al., 1998; Wyllie et al., 1998; Misra et al., 2000a,b) and the evoked extrasynaptic NMDAR-EPSCs in the present study have only a slightly prolonged EPSC decay time course compared with synaptic EPSCs. This suggests the presence of multimeric extrasynaptic NMDARs (see below). Despite the presence of functional NR1/NR2D-containing NMDARs in many central neurons, there is a notable absence of NMDAR-mediated synaptic responses with the very prolonged decay times associated with activation of dimeric NR2D receptors. NR2D-containing receptors appear to be confined to the extrasynaptic membrane in most neurons, for example, in several types of cerebellar cells, where they have been studied intensively (Momiyama et al., 1996; Misra et al., 2000b), and in dorsal horn spinal cord neurons (Momiyama et al., 1996; Momiyama, 2000). In the present studies, even HFS at 200 Hz did not activate the PPDA-sensitive NR2D-containing NMDARs, showing that glutamate uptake efficiently limits spillover during HFS (Diamond and Jahr, 2000). Although a previous study purported to show that HFS in CA1 resulted in activation of extrasynaptic NR2D-containing NMDAR (Lozovaya et al., 2004), the NR2D-containing receptors were detected using 10 μm PPDA, a concentration that we find also blocks synaptic NMDARs.
The NR2D-selective antagonists PPDA and UBP141 were not sufficient to completely block the TBOA enhancement of EPSCs, which was only prevented by R-CPP and ifenprodil in addition to PPDA. Our experiments using MK801 revealed that block of synaptic NMDARs did not prevent the enhancement of NMDAR-EPSCs by TBOA, indicating that the receptors activated in TBOA are not synaptic. Therefore, the requirement for NR2A-, NR2B-, and NR2D-selective antagonists to inhibit the extrasynaptic NMDARs suggests that a mixed population of receptor subtypes is located extrasynaptically. Triheteromeric NR1/2B/2D receptors have been identified in the soma of dentate gyrus granule cells using single-channel recordings (Pina-Crespo and Gibb, 2002). Moreover, functional evidence for the expression of NR1/NR2B/NR2D in addition to NR1/NR2D has been demonstrated at extrasynaptic sites in cerebellar Golgi cells (Brickley et al., 2003) and by immunohistochemical data indicating that NR2D-containing receptors in the adult midbrain are commonly present as triheteromeric NR1/NR2B/NR2D receptors (Dunah et al., 1998). It is possible that a combination of triheteromeric NR1/NR2B/NR2D and either triheteromeric NR1/NR2A/NR2B or dimeric NR1/NR2A and NR1/NR2B receptors are expressed extrasynaptically. The PPDA sensitivity of multimeric NR2D-containing NMDARs has not been investigated, and little is known about the kinetics of currents mediated by such receptors. It is interesting to note that in a study of triheteromeric NR1/NR2A/NR2B NMDARs, the efficacy of selective antagonists was reduced and superadditive inhibition was observed when antagonists for both receptor subtypes were coapplied (Hatton and Paoletti, 2005).
Footnotes
This work was supported by Science Foundation Ireland. The work to develop UBP141 was supported by National Institutes of Health Grant MH060252.
References
- Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci. 2007;27:8334–8343. doi: 10.1523/JNEUROSCI.2155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnth-Jensen N, Jabaudon D, Scanziani M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake. Nat Neurosci. 2002;5:325–331. doi: 10.1038/nn825. [DOI] [PubMed] [Google Scholar]
- Asztely F, Wigström H, Gustafsson B. The relative contribution of NMDA receptor channels in the expression of long-term potentiation in the hippocampal CA1 region. Eur J Neurosci. 1992;4:681–690. doi: 10.1111/j.1460-9568.1992.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Bashir ZI, Alford S, Davies SN, Randall AD, Collingridge GL. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991;349:156–158. doi: 10.1038/349156a0. [DOI] [PubMed] [Google Scholar]
- Berretta N, Berton F, Bianchi R, Brunelli M, Capogna M, Francesconi W. Long-term potentiation of NMDA receptor-mediated EPSPs in guinea-pig hippocampal slices. Eur J Neurosci. 1991;3:850–854. doi: 10.1111/j.1460-9568.1991.tb00096.x. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Misra C, Mok MH, Mishina M, Cull-Candy SG. NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J Neurosci. 2003;23:4958–4966. doi: 10.1523/JNEUROSCI.23-12-04958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KA, Collingridge GL. Synaptic potentiation of dual-component excitatory postsynaptic currents in the rat hippocampus. J Physiol. 1995;482:39–52. doi: 10.1113/jphysiol.1995.sp020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004 doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Dalby NO, Mody I. Activation of NMDA receptors in rat dentate gyrus granule cells by spontaneous and evoked transmitter release. J Neurophysiol. 2003;90:786–797. doi: 10.1152/jn.00118.2003. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Synaptically released glutamate does not overwhelm transporters on hippocampal astrocytes during high-frequency stimulation. J Neurophysiol. 2000;83:2835–2843. doi: 10.1152/jn.2000.83.5.2835. [DOI] [PubMed] [Google Scholar]
- Dravid SM, Prakash A, Traynelis SF. Activation of recombinant NR1/NR2C NMDA receptors. J Physiol. 2008;586:4425–4439. doi: 10.1113/jphysiol.2008.158634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Yasuda RP, Wang YH, Luo J, Davila-García M, Gbadegesin M, Vicini S, Wolfe BB. Regional and ontogenic expression of the NMDA receptor subunit NR2D protein in rat brain using a subunit-specific antibody. J Neurochem. 1996;67:2335–2345. doi: 10.1046/j.1471-4159.1996.67062335.x. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Luo J, Wang YH, Yasuda RP, Wolfe BB. Subunit composition of N-methyl-D-aspartate receptors in the central nervous system that contain the NR2D subunit. Mol Pharmacol. 1998;53:429–437. doi: 10.1124/mol.53.3.429. [DOI] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJA, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT. Structure-activity analysis of a novel NR2C//NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol. 2004;141:508–516. doi: 10.1038/sj.bjp.0705644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Morley RM, Jane DE, Monaghan DT. The effect of competitive antagonist chain length on NMDA receptor subunit selectivity. Neuropharmacology. 2005;48:354–359. doi: 10.1016/j.neuropharm.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cognet L, Brickley K, Stephenson FA, Lounis B, Choquet D. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat Neurosci. 2004;7:695–696. doi: 10.1038/nn1270. [DOI] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci. 2002;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- Harney SC, Rowan M, Anwyl R. Long-term depression of NMDA receptor-mediated synaptic transmission is dependent on activation of metabotropic glutamate receptors and is altered to long-term potentiation by low intracellular calcium buffering. J Neurosci. 2006;26:1128–1132. doi: 10.1523/JNEUROSCI.2753-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AZ, Pettit DL. Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. J Physiol. 2007;584:509–519. doi: 10.1113/jphysiol.2007.137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton CJ, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron. 2005;46:261–274. doi: 10.1016/j.neuron.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Hess SD, Daggett LP, Deal C, Lu CC, Johnson EC, Veliçelebi G. Functional characterization of human N-methyl-d-aspartate subtype 1A/2D receptors. J Neurochem. 1998;70:1269–1279. doi: 10.1046/j.1471-4159.1998.70031269.x. [DOI] [PubMed] [Google Scholar]
- Hrabetova S, Serrano P, Blace N, Tse HW, Skifter DA, Jane DE, Monaghan DT, Sacktor TC. Distinct NMDA receptor subpopulations contribute to long-term potentiation and long-term depression induction. J Neurosci. 2000;20(RC81):1–6. doi: 10.1523/JNEUROSCI.20-12-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner JE, Bean BP. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, et al. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- Janssen WG, Vissavajjhala P, Andrews G, Moran T, Hof PR, Morrison JH. Cellular and synaptic distribution of NR2A and NR2B in macaque monkey and rat hippocampus as visualized with subunit-specific monoclonal antibodies. Exp Neurol. 2005;191(Suppl 1):S28–S44. doi: 10.1016/j.expneurol.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn Mem. 1998;5:331–343. [PMC free article] [PubMed] [Google Scholar]
- Kotecha SA, Jackson MF, Al-Mahrouki A, Roder JC, Orser BA, MacDonald JF. Co-stimulation of mGluR5 and N-methyl-D-aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. J Biol Chem. 2003;278:27742–27749. doi: 10.1074/jbc.M301946200. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Erdemli G, Asztély F. LTP of AMPA and NMDA receptor-mediated signals: evidence for presynaptic expression and extrasynaptic glutamate spill-over. Neuron. 1996;17:461–474. doi: 10.1016/s0896-6273(00)80178-6. [DOI] [PubMed] [Google Scholar]
- Kwon HB, Castillo PE. Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron. 2008;57:108–120. doi: 10.1016/j.neuron.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Lozovaya NA, Grebenyuk SE, Tsintsadze TSh, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. J Physiol. 2004;558:451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra C, Brickley SG, Wyllie DJ, Cull-Candy SG. Slow deactivation kinetics of NMDA receptors containing NR1 and NR2D subunits in rat cerebellar Purkinje cells. J Physiol. 2000a;525:299–305. doi: 10.1111/j.1469-7793.2000.t01-1-00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra C, Brickley SG, Farrant M, Cull-Candy SG. Identification of subunits contributing to synaptic and extrasynaptic NMDA receptors in Golgi cells of the rat cerebellum. J Physiol. 2000b;524:147–162. doi: 10.1111/j.1469-7793.2000.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama A. Distinct synaptic and extrasynaptic NMDA receptors identified in dorsal horn neurones of the adult rat spinal cord. J Physiol. 2000;523:621–628. doi: 10.1111/j.1469-7793.2000.t01-1-00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama A, Feldmeyer D, Cull-Candy SG. Identification of a native low-conductance NMDA channel with reduced sensitivity to Mg2+ in rat central neurones. J Physiol. 1996;494:479–492. doi: 10.1113/jphysiol.1996.sp021507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morishita W, Marie H, Malenka RC. Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nat Neurosci. 2005;8:1043–1050. doi: 10.1038/nn1506. [DOI] [PubMed] [Google Scholar]
- Morley RM, Tse HW, Feng B, Miller JC, Monaghan DT, Jane DE. Synthesis and pharmacology of N1-substituted piperazine-2,3-dicarboxylic acid derivatives acting as NMDA receptor antagonists. J Med Chem. 2005;48:2627–2637. doi: 10.1021/jm0492498. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JJ, Rowan MJ, Anwyl R. Long-lasting enhancement of NMDA receptor-mediated synaptic transmission by metabotropic glutamate receptor activation. Nature. 1994;367:557–559. doi: 10.1038/367557a0. [DOI] [PubMed] [Google Scholar]
- O'Connor JJ, Rowan MJ, Anwyl R. Tetanically induced LTP involves a similar increase in the AMPA and NMDA receptor components of the excitatory postsynaptic current: investigations of the involvement of mGlu receptors. J Neurosci. 1995;15:2013–2020. doi: 10.1523/JNEUROSCI.15-03-02013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Collin C, Auerbach JM, Meiri N, Bengzon J, Kennedy MB, Segal M, McKay RDG. Hippocampal Synaptic Plasticity in Mice Overexpressing an Embryonic Subunit of the NMDA Receptor. J Neurosci. 1998;18:4177–4188. doi: 10.1523/JNEUROSCI.18-11-04177.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina-Crespo JC, Gibb AJ. Subtypes of NMDA receptors in new-born rat hippocampal granule cells. J Physiol. 2002;541:41–64. doi: 10.1113/jphysiol.2001.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Köhr G. C-terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci. 2000;20:4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Drewery DL, Atkins HD, Stephenson FA, Chazot PL. Immunohistochemical localization of N-methyl–aspartate receptor subunits in the adult murine hippocampal formation: evidence for a unique role of the NR2D subunit. Mol Brain Res. 2002;102:55–61. doi: 10.1016/s0169-328x(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Liu XB, Jones EG, McAllister AK. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J Neurosci. 2004;24:8253–8264. doi: 10.1523/JNEUROSCI.2555-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, Villa M, Mohler H, Benke D. Developmental and regional expression of NMDA receptor subtypes containing the NR2D subunit in rat brain. J Neurochem. 1996;66:1240–1248. doi: 10.1046/j.1471-4159.1996.66031240.x. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Fritschy JM, Mohler H, Benke D. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem. 1997;68:469–478. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Wyllie DJ, Béhé P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J Physiol. 1998;510:1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Berger TW, Barrionuevo G. Isolated NMDA receptor-mediated synaptic responses express both LTP and LTD. J Neurophysiol. 1992;67:1009–1013. doi: 10.1152/jn.1992.67.4.1009. [DOI] [PubMed] [Google Scholar]