Abstract
Background
Little is currently known about how maternal depression symptoms and unhealthy nutrition during pregnancy may developmentally interrelate to negatively affect child cognitive function.
Aims
To test whether prenatal maternal depression symptoms predict poor prenatal nutrition, and whether this in turn prospectively associates with reduced postnatal child cognitive function.
Method
In 6979 mother-offspring pairs participating in the Avon Longitudinal Study of Parents and Children (ALSPAC) in the UK, maternal depression symptoms were assessed five times between 18 weeks gestation and 33 months old. Maternal reports of the nutritional environment were assessed at 32 weeks gestation and 47 months old, and child cognitive function was assessed at age 8 years.
Results
During gestation, higher depressive symptoms were related to lower levels of healthy nutrition and higher levels of unhealthy nutrition, each of which in turn was prospectively associated with reduced cognitive function. These results were robust to postnatal depression symptoms and nutrition, as well as a range of potential prenatal and postnatal confounds (i.e. poverty, teenage mother, low maternal education, parity, birth complications, substance use, criminal lifestyle, partner cruelty towards mother).
Conclusions
Prenatal interventions aimed at the well-being of children of parents with depression should consider targeting the nutritional environment.
Maternal depression can negatively affect the development of children.1 For the purpose of prevention, research has sought to identify the different mechanisms through which maternal depression can negatively affect a child.2 Postnatal mechanisms that link maternal depression to impaired child development include maladaptive parenting and negative maternal cognitions,3,4 as well as increased interpersonal stress of the mother.5 Equally important, however, is the degree to which depression can alter the intra-uterine environment, and hence affect fetal development.6 For example, depression is reported to be associated with higher levels of circulating cortisol,7 which can affect the development of the biological stress system of a child.8,9 Less well researched, however, is the potential for depression to associate with an unhealthy nutritional prenatal environment. A recent review10 has highlighted the need for research, examining the potential synergistic impact of depression and nutrition: stress and mental health problems can contribute to unhealthy eating patterns during pregnancy,11,12 which can affect the neurocognitive development of a child.10 Indeed, as indexed by the DSM-IV-TR,13 symptoms of depression include a significant weight loss or gain. This suggests a change in dietary habits to which children of mothers with depression presumably would be exposed, particularly during pregnancy and the early postnatal years, when infants and young children are particularly dependent on the environment provided by a caregiver.14 In the current study, we examined the extent to which prenatal depression symptoms were associated with an unhealthy nutritional intake during pregnancy, which, in turn, would prospectively associate with reduced child cognitive function at 8 years.
Method
Sample
The Avon Longitudinal Study of Children and Parents (ALSPAC) was established to understand how genetic and environmental characteristics influence health and development in parents and children. All pregnant women resident in a defined area in the south west of England, with an expected date of delivery between 1 April 1991 and 31 December 1992, were eligible, and 13 761 women (contributing 13 867 pregnancies) were recruited. These women have been followed over the past 19-22 years.15 When compared with 1991 national census data, the ALSPAC sample was found to be similar to the UK population as a whole.16 Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee and the local research ethics committees.
Measures
Sample characteristics and birth information
Descriptive statistics for the overall sample are presented in Table 1. Maternal ethnicity was recorded at 18 weeks gestation.
Table 1.
Sample characteristics and birth information
| n/Na | ||
|---|---|---|
| Ethnicity of mother, White: % | 94.4 | 6590/6979 |
| Multiparous, % | 54.9 | 3150/6741 |
| At least one birth complication, % | 14.9 | 1042/5229 |
| Infant birth weight, g: mean (s.d.) | 3426.86 (544.35) | 6896/6979 |
| Female children, % | 50.1 | 3499/6979 |
n = number of mothers in category; N = number of mothers with responses from the selected sample of 6979.
Infant birth weight and birth complications (e.g. abruption, preterm rupture, cervical suture) were recorded at birth. Birth complications were dichotomised to compare mothers with any complications (1) v. those without (0). Parity was obtained at 18 weeks gestation using a series of questions about previous pregnancies. Multiparous mothers were coded 1 and primiparous mothers were coded 0.
Maternal depression symptoms
Symptoms were repeatedly assessed (at 32 weeks prenatally, and 8 weeks, 8 months, 21 months and 33 months postnatally) with the Edinburgh Postnatal Depression Scale (EPDS), a widely used 10-item self-report questionnaire that has been shown to be valid in and outside the postnatal period.17,18 In the overall model, latent depression scores were created for the prenatal and postnatal periods, with higher values indicating greater symptoms of depression.
Maternal diet
Data on maternal diet were collected via the Food Frequency Questionnaire (FFQ),19 completed by women at 32 weeks gestation and when the child was 47 months old. The FFQ contains a set of questions about the frequency of consumption of a wide variety of food and drink. The women indicated how often they were currently consuming these foods and drinks; possible responses were: never or rarely; once in 2 weeks; once to three times per week; four to seven times per week; and more than once daily.
We delineated both ‘healthy’ and ‘unhealthy’ food groups, and then sought to examine the internal reliability of these groupings through a confirmatory factor analysis, deleting items with low correlations as needed. In instances where there were two indicators of a latent nutrition factor, we constrained the loadings to be equal. Prenatal healthy diet showed acceptable fit to the data (χ2[19] = 435.53; comparative fit index (CFI) = 0.946; Tucker-Lewis index (TLI) = 0.920; root mean square error of approximation (RMSEA) = 0.058, 90% CI 0.053-0.062), and consisted of second-order latent factor defined by three first-order latent factors: fish (i.e. white fish, oily fish), non-meat protein (i.e. pulses, nuts) and vegetables (i.e. cabbage, green vegetables, carrots, other root vegetables). Postnatal healthy diet also showed acceptable fit to the data (χ2[32] = 285.681; CFI = 0.973; TLI = 0.962; RMSEA = 0.036, 90% CI 0.032-0.040) and was defined similarly: fish (i.e. white fish, other fish, tuna), non-meat protein (i.e. pulses, nuts, soya meat) and vegetables (i.e. cabbage, green vegetables, carrots, other root vegetables).
Prenatal unhealthy diet showed acceptable fit to the data (χ2[13] = 306.880; CFI = 0.936; TLI = 0.897; RMSEA = 0.059, 90% CI 0.053-0.064), and consisted of second-order latent factor defined by two first-order latent factors: processed food (i.e. fried food, meat pies or pasties, chips, crisps) and junk food (i.e. chocolate bars, cakes or buns, biscuits). Postnatal unhealthy food also showed acceptable fit to the data (χ2[13] = 203.622; CFI = 0.965; TLI = 0.944; RMSEA = 0.049, 90% CI 0.043-0.055), and was similarly defined by processed food (i.e. sausages or burgers, meat pies, fried potatoes) and junk food (i.e. chocolate coated biscuits, other biscuits, chocolate). Although these dietary factors were identified by the use of a confirmatory analytic method, the latent factors identified here are highly similar to those identified in ALSPAC, on the FFQ, using exploratory data reduction methods.19 In order to reduce complexities of the overall models, the latent scores underlying the healthy and unhealthy factors were saved and used as observed variables.
Child cognitive function
Child cognitive function was assessed at age 8, and consisted of the performance and verbal IQ assessments of the Wechsler Intelligence Scale for Children (WISC-III).20 Scores were age-normed in accordance with standard procedures. We assessed these two scales in a latent factor of ‘cognition’ in the overall model. The distribution of the performance IQ (mean = 99.71 (s.d. = 17.09), Skew = 0.016) and verbal IQ (mean = 107.22 (s.d. = 16.75), Skew = –0.021) were normal and within the range of age-appropriate norms.
Control variables
Control variables were summated into an index and regressed on all study variables, including the cognitive outcome. The controls, at birth, consisted of parity and birth complications (described earlier). Prenatal controls included mothers’ involvement with police (pregnancy: assessed 18 weeks), substance use (any indication of hard drugs, alcoholism, consumption of alcohol at frequency equal to or greater than two drinks a day (assessed during pregnancy between 18 and 32 weeks)), and mother experiencing cruelty from partner (e.g. any indication of emotional and/or physical abuse from partner (pregnancy: assessed 18 weeks)). We also controlled for repeated measures of contextual risk factors, via mother reports, that we have previously shown to be prospectively related to child emotional and behavioural dysregulation.5,14 These assessments spanned pregnancy, age 0-2 years, and 2-4 years. At each age, there were seven total risks (scored 1 with indication, 0 without indication):
inadequate basic living conditions - not having a working bath/shower, no hot water, no indoor toilet and/or no working kitchen (pregnancy: assessed at 8 weeks; age 0-2: assessed at 2, 8 and 21 months; age 2-4: assessed at 33 and 47 months);
inadequate housing - any indication of crowding (pregnancy: at 8 weeks; age 0-2: 21 months; age 2-4: 33 months) and/or homelessness (pregnancy: at 18 weeks; age 0-2: 2, 8 and 21 months; age 2-4: 33 months);
housing defects - any indication of mould, roof leaks, and rats, mice or cockroaches (pregnancy: at 18 weeks; age 0-2: 8 and 21 months; age 2-4: 33 months);
poverty, coded via the Registrar General’s Social Class Scale21 (pregnancy: at 32 weeks; age 0-2: 8 and 21 months; age 2-4: 33 months);
being a single caregiver - not cohabiting, not in a relationship (pregnancy: at 32 weeks; age 0-2: 6 and 21 months; age 2-4: 33 and 47 months);
early parenthood - 19 years or younger (at 18 weeks in pregnancy);
low educational attainment - did not finish mandatory schooling (pregnancy: at 32 weeks; age 0-2: 21 months; age 2-3: 33 months).
Selected sample of ALSPAC mothers and children
Of the original 13 761 mother and child pairs, a total of 6979 children completed the cognitive assessments at age 8. These were the mothers and children included in the present study.
Analysis
The analysis proceeded in two main steps. In the first step, we examined a path analysis examining the interrelations between maternal depression symptoms, unhealthy diet and child cognitive function. We also examined the degree to which maternal depression might indirectly relate to reduced child cognitive function indirectly via an unhealthy prenatal diet. In the second step, we examined a path analysis examining the interrelations between maternal depression symptoms, healthy diet and child cognitive function, as well as examining the degree to which maternal depression might indirectly relate to reduced child cognitive function via a low prenatal healthy diet.
Indirect pathways were programmed in model constraint statements in Mplus. The indirect effects tested here assessed the extent to which maternal depression symptoms might relate to reduced cognitive function via the nutritional environment (i.e. higher unhealthy, lower healthy). Therefore, the indirect effects were defined by the product term of the two pathways of interest (i.e. maternal depression to nutrition×nutrition to child cognitive function). Because standard errors underlying indirect effects (i.e. product terms) are known to be skewed, we bootstrapped all indirect effects 10 000 times with bias-corrected 95% confidence intervals. The indirect pathways reported below are based on the bootstrapped variability around the product of non-standardised path coefficient estimates.
Model fit was established using RMSEA (acceptable fit ⩽0.08), as well as the CFI and TLI (acceptable fit ⩾0.90).22,23 Maximum likelihood estimation with robust standard errors was used to estimate the model parameters, and missing data were handled through full information maximum likelihood. All analyses were conducted using Mplus version 7.0 for Windows.24
Results
Step 1: Maternal depression symptoms, unhealthy diet and child cognitive function
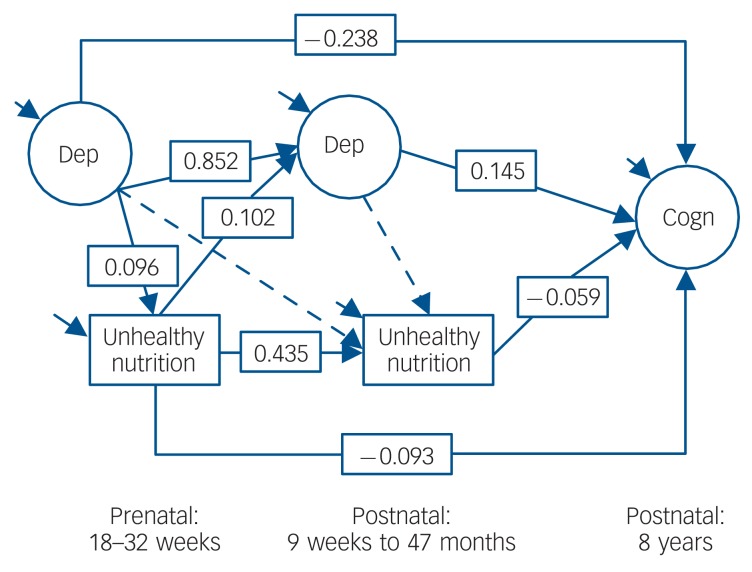
Figure 1 contains the unhealthy diet model, which showed acceptable fit to the data (χ2[27] = 311.786; CFI = 0.980; TLI = 0.967; RMSEA = 0.039, 90% CI 0.035-0.043). Above and beyond postnatal depression, postnatal unhealthy diet and the controls, maternal prenatal depression symptoms were related to more unhealthy diet, which, in turn, prospectively associated with reduced cognitive function. The bias-corrected confidence intervals (via 10 000 bootstraps) for the indirect pathway of maternal depression symptoms relating to reduced cognitive function via unhealthy nutrition did not cross zero (b = –0.010; 95% CI –0.015 to –0.006), which suggests that symptoms of depression in pregnancy can affect child development via a more unhealthy nutritional environment. Moreover, within this model, prenatal maternal depression symptoms (above and beyond postnatal depression symptoms) were prospectively associated with reduced cognitive function, whereas postnatal maternal depression symptoms (above and beyond prenatal depression symptoms) were associated with higher cognitive function. In addition, prenatal and postnatal unhealthy diets were associated with reduced child cognitive function.
Fig. 1.
Latent path model for maternal depression, unhealthy nutrition and child cognitive function. Using Cohen’s population effect size statistics,33 an effect of 0.10 is a small effect, an effect of 0.24 is a medium effect, and an effect of 0.37 is a large effect. Dep, depression; Cogn, cognitive function. Solid arrow, P<0.05; dashed arrow, P>0.05.
Step 2: Maternal depression symptoms, healthy diet and child cognitive function
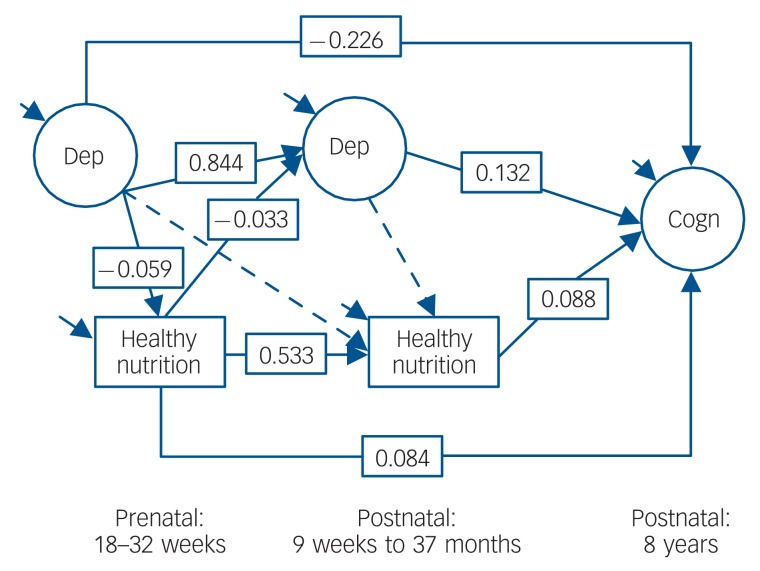
Figure 2 contains the healthy diet model, which showed acceptable fit to the data (χ2[28] = 678.952; CFI = 0.956; TLI = 0.929; RMSEA = 0.058, 90% CI 0.054-0.062). Above and beyond postnatal depression symptoms, postnatal healthy diet and the controls, maternal prenatal depression symptoms were related to lower prenatal healthy diet. Maternal prenatal diet, however, was prospectively associated with higher child cognitive function (and lower postnatal depression symptoms). The bias-corrected confidence intervals (via 10 000 bootstraps) of maternal depression relating to reduced child cognitive function via less healthy diet did not cross zero (b = –0.005; 95% CI –0.009 to –0.003), which suggests that maternal depression symptoms in pregnancy can affect child development via a less healthy nutritional environment.
Fig. 2.
Latent path model for maternal depression, healthy nutrition and child cognitive function. Using Cohen’s population effect size statistics, an effect of 0.10 is a small effect, an effect of 0.24 is a medium effect, and an effect of 0.37 is a large effect. Dep, depression; Cogn, cognitive function. Solid arrow, P<0.05; dashed arrow, P>0.05.
Discussion
Using a large longitudinal cohort, we provide evidence that prenatal maternal depression symptoms can relate to both more unhealthy and less healthy prenatal diets, which, in turn, can prospectively associate with reduced child cognitive function. These results increase the current knowledge about mechanisms through which maternal depression can affect child development.2
In the present study, prenatal maternal depression symptoms were not only directly associated with lowered cognitive postnatal function of the child, but they also did so indirectly via the nutritional environment. For some time it has been recognised that children are affected not only by the symptoms of maternal depression (e.g. as measured by the EPDS: anxious or worried, so unhappy that they have been crying),25 but also indirectly in that depression can affect the immediate rearing environment of a child.2 To date, a majority of published studies have focused on how depression associates with maladaptive parenting and maternal cognitions,4 whereas relatively fewer studies have focused on how maternal depression might lead to poor nutrition.10
During pregnancy the diet of the mother will directly influence the nutritional environment of a fetus, which presumably will affect the development of the fetal nervous system, including the brain.10 We separated the prenatal diet, as reported by the mothers, into unhealthy and healthy factors. Healthy eating can be specified as a diet of nutrient-rich foods, with limited intake of salt, solid fats and added sugar.26 Healthy foods were thus defined as those high in protein (e.g. fish, pulses), dietary fibre (e.g. pulses) and in important nutrients such as folate, magnesium, potassium, and vitamins A, C and K (e.g. vegetables).26 Unhealthy foods were defined as being high in saturated fat (e.g. fast food), trans fat (e.g. junk food), salt (e.g. processed foods) and added sugar,26 which have been associated with obesity, poor health and sedentary lifestyles.27
Given that there are many nutrients of critical importance in the regulation of fetal brain development, and that the timing and dosage of these nutrients is of great consequence,10 the fact that this sample showed continuity in healthy/unhealthy diets across individual pregnancies is important. It is worth highlighting research showing the relation between maternal mental health/stress and poor prenatal nutrition,10 with stress contributing directly to unhealthy eating patterns. This research, combined with the findings of the current study, underscores the potential for interventions at the level of nutrition. In addition, poor nutrition during pregnancy necessarily affects different aspects of brain development dependent on the timing of the insults.28
Although the present study did not examine biological mechanisms that might explain how prenatal maternal depression symptoms and nutrition can affect child cognitive development, Monk and colleagues10 highlighted that maternal consumption of certain nutrients (e.g. protein, B vitamins, folate) not only show effects on brain development, but can also have a role in stress response, as their metabolism can be altered by exposure to stress. Hence, it appears possible that maternal depression (as a proxy of stress) could affect brain development via the biological ‘cross-talk’ between stress and nutrition.10 Given the non-biological nature of the data used in the current study, however, the above statement is speculative, but might be considered an important area of investigation for future research.
One counterintuitive finding was that postnatal maternal depression symptoms, which were distinguished from prenatal depression symptoms, and hence did not represent continuity in depression, were associated with increased child cognitive abilities. Although speculative, a tentative explanation for this time-specific effect is that a degree of postnatal symptoms (e.g. via the EPDS, anxiety and worry) might be conducive to sensitive and responsive parenting early in development. A biological analogue can be found in research by DiPietro,29 who found that a degree of stress specific to pregnancy (not with postnatal continuity) was associated with accelerated fetal neurological maturation. We suggest that non-chronic parental stress (or distress) in the first year of a child’s life may be a normative part of child rearing, and hence could promote adaptive parenting, which could lead to increased child cognitive functioning.
Considerations for intervention
Findings from the present study highlight pregnancy as being a promising window of opportunity for preventing certain effects that are at least partially attributable to the nutritional environment. This is encouraging, given that nutrition could be considered a malleable risk factor. Research (and the current findings) suggests that women of childbearing age are particularly susceptible to the adverse effects of poor nutrition on mood problems.30 Therefore, diet-based interventions could be highly effective in reducing the association between reduced postnatal cognitive function and prenatal maternal depression.
Limitations
The present results should be interpreted in the context of five main limitations. First, this research is correlational in nature; hence no causative relationship has been identified. In addition, effect sizes for the prospective association of maternal depression and unhealthy diet were not large, and should therefore not be interpreted as deterministic of a child’s cognitive function. Second, most measures were based on maternal reports, raising the possibility of shared method variance. Future studies should incorporate multiple informants and biological indicators of the nutritional intake of the mother. Third, we relied on self-reports of mothers with depression, which calls into question the accuracy of the reports. That said, studies have found that mothers with depression can be as accurate as other informants about their children’s behaviour,31 and a meta-analyses suggested that the size of the effect of maternal depression on child outcomes, as measured by maternal reports on scale formats v. clinical diagnoses, do not significantly differ.1 Fourth, although the mothers and children of ALSPAC represent a broad spectrum of socioeconomic backgrounds, the sample includes relatively low numbers of individuals from ethnic minority groups. The present results will need replication with more ethnically diverse samples. Fifth, although this study controlled for many potential confounding prenatal and postnatal factors, it did not assess the actual biological mechanisms (e.g. DNA methylation) that might explain the prenatal association with postnatal child cognitive function.10,32
Findings from our analyses may provide fresh insights about how depressed mood can lead to altered dietary intake during pregnancy and open new avenues for increasing the effectiveness of prenatal interventions.
Acknowledgments
The UK Medical Research Council (grant ref: 74883), the Wellcome Trust (grant ref: 0754567) and the University of Bristol provide core support for ALSPAC. We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. E.D.B had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Declaration of interest
None.
Funding
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R01HD068437 to E.D.B. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Goodman S, Rouse M, Connell A, Broth M, Hall C, Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev 2011; 14: 1–27 [DOI] [PubMed] [Google Scholar]
- 2. Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed parents: a developmental approach to the understanding of mechanisms. Psychol Rev 1999; 106: 458–90 [DOI] [PubMed] [Google Scholar]
- 3. Pawlby S, Hay D, Sharp D, Waters CS, Pariante CM. Antenatal depression and offspring psychopathology: the influence of childhood maltreatment. Br J Psychiatry 2011; 199: 106–12 [DOI] [PubMed] [Google Scholar]
- 4. Murray L. The impact of postnatal depression on infant development. J Child Psychol Psychiatry 1992; 33: 543–561 [DOI] [PubMed] [Google Scholar]
- 5. Barker ED. The duration and timing of maternal depression as a moderator of the relationship between dependent interpersonal stress, contextual risk and early child dysregulation. Psychol Med 2012; November 6: 1–10 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goodman SH, Rouse MH, Long Q, Ji S, Brand SR. Deconstructing antenatal depression: what is it that matters for neonatal behavioral functioning? Infant Ment Health J 2011; 32: 339–61 [DOI] [PubMed] [Google Scholar]
- 7. Field T, Diego M, Hernandez-Reif M, Figueiredo B, Deeds O, Ascencio A, et al. Comorbid depression and anxiety effects on pregnancy and neonatal outcome. Infant Behav Dev 2010; 33: 23–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 2008; 3: 97–106 [DOI] [PubMed] [Google Scholar]
- 9. O’Donnell KJ, Glover V, Jenkins J, Browne D, Ben-Shlomo Y, Golding J, et al. Prenatal maternal mood is associated with altered diurnal cortisol in adolescence. Psychoneuroendocrinology 2013; February 12 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Monk C, Georgieff MK, Osterholm EA. Research review: maternal prenatal distress and poor nutrition - mutually influencing risk factors affecting infant neurocognitive development. J Child Psychol Psychiatry 2013; 54: 115–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hurley KM, Caulfield LE, Sacco LM, Costigan KA, Dipietro JA. Psychosocial influences in dietary patterns during pregnancy. J Am Diet Assoc 2005; 105: 963–6 [DOI] [PubMed] [Google Scholar]
- 12. Teegarden SL, Bale TL. Effects of stress on dietary preference and intake are dependent on access and stress sensitivity. Physiol Behav 2008; 93: 713–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (4th edn, text revision) (DSM-IV-TR). APA, 2000. [Google Scholar]
- 14. Barker ED, Copeland W, Maughan B, Jaffee SR, Uher R. Relative impact of maternal depression and associated risk factors on offspring psychopathology. Br J Psychiatry 2012; 200: 124–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, et al. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 2013; 42: 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort Profile: the ‘children of the 90s’ - the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013; 42: 111–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: the development of the Edinburgh 10-item Postnatal Depression Scale. Br J Psychiatry 1987; 150: 782–6 [DOI] [PubMed] [Google Scholar]
- 18. Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br J Psychiatry 1990; 157: 288–90 [DOI] [PubMed] [Google Scholar]
- 19. Micali N, Northstone K, Emmett P, Naumann U, Treasure JL. Nutritional intake and dietary patterns in pregnancy: a longitudinal study of women with lifetime eating disorders. Br J Nutrition 2012; 108: 2093–9 [DOI] [PubMed] [Google Scholar]
- 20. Wechsler D. The Wechsler Intelligence Scale for Children - Third Edition (UK edition). Psychological Corporation, 1991. [Google Scholar]
- 21. Office of Population Censuses and Surveys Standard Occupational Classification Volume 3. HMSO, 1991. [Google Scholar]
- 22. Browne MW, Cudeck R. Alternative ways of assessing model fit. In Testing Structural Equation Models (eds Bollen KA, Lang JS.): 136–62 Sage, 1993. [Google Scholar]
- 23. Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychol Bull 1980; 88: 588–606 [Google Scholar]
- 24. Muthén LK, Muthén BO. MPlus: Statistical Analyses with Latent Variables. User’s Guide. Muthén & Muthén, 2012. [Google Scholar]
- 25. Cox AD, Puckering C, Pound A, Mills M. The impact of maternal depression in young children. J Child Psychol Psychiatry 1987; 28: 917–28 [DOI] [PubMed] [Google Scholar]
- 26. US Department of Agriculture, US Department of Health and Human Services Dietary Guidelines for Americans 2010 (7th edn). US Government Printing Office, 2010. [Google Scholar]
- 27. James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obes Res 2001; 9 (suppl 11): S228–33 [DOI] [PubMed] [Google Scholar]
- 28. Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr 2007; 85 (suppl): S614–20 [DOI] [PubMed] [Google Scholar]
- 29. DiPietro JA, Kivlighan KT, Costigan KA, Rubin SE, Shiffler DE, Henderson JL, et al. Prenatal antecedents of newborn neurological maturation. Child Dev 2010; 81: 115–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bodnar LM, Wisner KL. Nutrition and depression: implications for improving mental health among childbearing-aged women. Biol Psychiatry 2005; 58: 679–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Richters JE. Depressed mothers as informants about their children: a review of evidence for distorion. Psychol Bull 1992; 112: 485–99 [DOI] [PubMed] [Google Scholar]
- 32. Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 2010; 151: 4756–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd edn). Lawrence Erlbaum, 1988. [Google Scholar]