Abstract
Background:
To date, only a few risk factors for pancreatic cancer have been established. We examined prospectively relations between several medical conditions and pancreatic cancer incidence.
Methods:
In 1986, 120 852 participants completed a baseline questionnaire on cancer risk factors, including several self-reported physician diagnosed medical conditions. At baseline, a random subcohort of 5000 participants was selected using a case-cohort approach for analysis. After 16.3 years of follow-up, 448 pancreatic cancer cases (63% microscopically confirmed) were available for analysis.
Results:
Diabetes mellitus type II and hepatitis were positively associated with pancreatic cancer risk (multivariable-adjusted hazard ratio: 1.79; 95% confidence interval: 1.12–2.87 and hazard ratio: 1.37; 95% confidence interval: 1.04–1.81, respectively). Furthermore, a positive trend in risk with increasing years of diagnosis of diabetes (P=0.004) and of hepatitis (P=0.02) was observed. However, an inverse association was observed between hypertension and pancreatic cancer risk, this was found among microscopically confirmed cases only (hazard ratio: 0.66; 95% confidence interval: 0.49–0.90), while years since diagnosis of hypertension significantly decreased cancer risk (P for trend=0.02).
Conclusion:
In this prospective study, a positive association was observed between self-reported physician diagnosed diabetes mellitus type II and hepatitis and pancreatic cancer risk, whereas an inverse association was observed with hypertension.
Keywords: pancreatic cancer, cohort studies, diabetes mellitus type II, hypertension, hepatitis
Cancer of the pancreas is a less common form of cancer, with approximately 170 000 new cases occurring annually worldwide, around 2.1% of all cancer cases (Ghadirian et al, 2003). Due to its poor prognosis, pancreatic cancer is one of the most fatal cancers worldwide (Jemal et al, 2009). To date, only a few consistent risk factors for pancreatic cancer have been identified, including smoking and obesity (Lee et al, 1996; Anderson et al, 2009). This study aims to provide further insight into possible associations between several medical conditions and pancreatic cancer, which remain under debate in the literature, specifically the duration of diabetes mellitus type II (DMII) and hypertension. To our knowledge, the effect of the hepatitis B virus (HBV) and hepatitis C virus (HCV) has not been studied yet in a European population.
Previous studies have investigated the association between several medical conditions and pancreatic cancer risk. Most studies of DM II observed positive associations (Lee et al, 1996; Silverman, 2001; Ghadirian et al, 2003; Huxley et al, 2005; Wang et al, 2006; Hassan et al, 2007; Luo et al, 2007; Jamal et al, 2009; Ogunleye et al, 2009; Stevens et al, 2009; Chodick et al, 2010; Ben et al, 2011; Lai et al, 2013). The effect of duration of DM II on the onset of pancreatic cancer is, however, still unclear with some studies observing a positive association with longer duration (Silverman, 2001; Ghadirian et al, 2003) and others showing an inverse association (La Vecchia et al, 1994; Huxley et al, 2005; Wang et al, 2006; Ben et al, 2011). For hypertension, Lindgren et al, reported a positive association with pancreatic cancer risk in women (Lindgren et al, 2005), whereas Batty et al, reported an inverse effect of diastolic blood pressure on pancreatic cancer risk (Batty et al, 2003); other studies reported no association (Grove et al, 1991; Batty et al, 2009; Inoue et al, 2009). Furthermore, some studies have reported elevated pancreatic cancer risks for infection with HBV or HCV (Berrington de Gonzalez et al, 2008; Hassan et al, 2008; Iloeje et al, 2010) and a recent meta-analysis supports that HBV infection is associated with pancreatic cancer (Luo et al, 2013). A study performed in an Asian population demonstrated that elevated levels of liver enzymes alanine transaminase (ALT) and aspartate aminotransferase (AST) are both risk factor for pancreatic cancer (Berrington de Gonzalez et al, 2008). In the same study hepatitis B was associated with elevated levels of ALT and AST. Moreover, obesity, risk factors for pancreatic cancer, is associated with liver injury and elevated levels of ALT and AST levels too (Berrington de Gonzalez et al, 2006). Finally, a number of other studies have observed positive associations between pancreatic cancer risk and other medical conditions such as chronic pancreatitis (Ghadirian et al, 2003; Anderson et al, 2009; Apte et al, 2009; Greer and Whitcomb, 2009), cholecystectomy (Silverman, 2001; Ghadirian et al, 2003; Lin et al, 2012), and gastric ulcer (Bao et al, 2010).
The biological mechanism by which DM II may lead to pancreatic cancer relates to hyperinsulinemia. DM II is characterized by glucose intolerance caused by insulin resistance and/or relative insulin deficiency, which will finally result in hyperinsulinemia (Gapstur et al, 2000). Furthermore, in vitro research has revealed the presence of a dose-response relationship between elevated levels of insulin and the growth of pancreatic cancer cells (Gapstur et al, 2000). However, DM II can also be a result of pancreatic cancer; in vitro studies have suggested that blockage of insulin receptors, impaired insulin action and glucose transport were involved in pancreatic cancer induced insulin resistance (Ben et al, 2011). It has been established that HBV and HCV viruses travel through the bloodstream and can be deposited in non-liver tissue like the kidney or skin (Hassan et al, 2008). The fact that the liver and the pancreas share common blood vessels and ducts and the proximity of the liver and pancreas, make it plausible that pancreatic cancer risk is elevated for people with a HBV or HCV infection (Hassan et al, 2008). Hepatitis B surface antigen (HBsAg) is an indicator of a current HBV infection; Different studies have shown that HBsAg positive subjects have an elevated risk of pancreatic cancer (Ben et al, 2012; Wang et al, 2012b). The possibility of a causal relation between cholecystectomy and pancreatic cancer is supported by an experimental study showing that cholecystectomy induces elevated levels of cholecystokinin, which is a known promoter of pancreatic cancer (Howatson and Carter, 1985). A relation between gallstones and chronic pancreatitis is suggested to be the underlying cause for pancreatic cancer (Hardt et al, 2001). Peptic ulcers might be a source of nitrosamines and therefore be involved in carcinogenesis (Bao et al, 2010). In addition, the role of cytokines, generated by inflammation, might play a role in carcinogenesis (Bao et al, 2010). Finally, in a recent meta-analysis performed by Trikudanathan et al. a positive relation between Helicobacter pylori infection, a known cause of peptic ulcers (Mhaskar et al, 2013), and pancreatic cancer was found (Trikudanathan et al, 2011).
In this large prospective cohort study, we investigated the associations between the medical conditions DM II, hypertension, cholecystectomy, gallstones, peptic ulcer, hepatitis and pancreatic cancer risk.
Materials and Methods
Study population and cancer follow-up
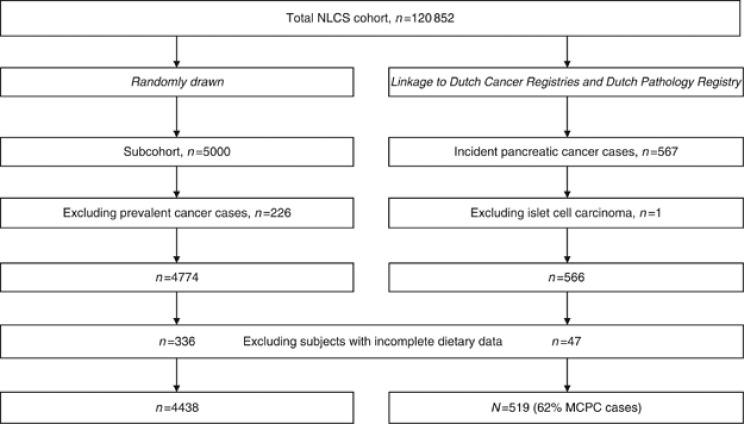
The design of the Netherlands Cohort Study (NLCS) has been reported in detail elsewhere (van den Brandt et al, 1990a); for a schematic presentation see Figure 1. This large-scale prospective cohort study was initiated in 1986 and included 58 279 men and 62 573 women aged 55–69 years from 204 Dutch municipalities with computerized population registries. A self-administered questionnaire on potential risk factors for cancer was completed at baseline. For reasons of efficiency, the case-cohort approach was chosen (weighting of study subjects based on Prentice's method) (Prentice, 1986). A random subcohort (n=5000) was selected immediately after identification of cohort members and followed biennially for migration and vital status to estimate the accumulated person-years of the whole cohort. The subcohort was selected as a simple random sample out of the total cohort at baseline (n=120 852), with a sampling fraction of 5000/120 852 (i.e., 0.04137). The entire cohort is being monitored for cancer occurrence through annual record linkage with the Netherlands Cancer Registry and the nationwide pathology registry PALGA (van den Brandt et al, 1990b; Casparie et al, 2007). For the current analysis, 16.3 years of follow-up have been used. Only one subcohort member was lost to follow-up, and completeness of nationwide cancer follow-up was estimated to be greater than 96% (Goldbohm et al, 1994b). All subcohort members with prevalent cancer (other than skin cancer) at baseline were excluded (n=226). Of the 567 incident cases of pancreatic cancer (ICD-O-3 code C25), persons with endocrine subtypes (islet-cell carcinoma; ICD-O-3 code C25.4; n=1) were excluded. Furthermore, we excluded participants with incomplete or inconsistent dietary data (47 incident cases and 336 subcohort members). Details are given elsewhere (Goldbohm et al, 1994a). The institutional review boards of the TNO Nutrition and Food Research Institute (Zeist, The Netherlands) and Maastricht University (Maastricht, The Netherlands) have approved the Netherlands Cohort Study protocol.
Figure 1.
Schematic presentation of the Netherlands Cohort Study Design. Abbreviations: MCPC=microscopically confirmed pancreatic cancer; NLCS=Netherlands Cohort Study.
Questionnaire
At baseline, all cohort members completed a mailed, self-administered questionnaire on dietary habits and other risk factors for cancer, including several medical conditions. Participants were asked to report whether a physician had ever diagnosed high blood pressure, diabetes (no subtypes), gallstones, cholecystectomy, hepatitis and/or jaundice or peptic ulcer and at what age. Furthermore, they were asked whether they ever had peptic ulcer surgery and at what age. The applicable age was recorded in 5-year age groups (from ‘younger than 30 years', ‘30–34 years' to ‘65–69 years').
Statistical analysis
We used Cox proportional hazards model to calculate age-adjusted and multivariable-adjusted hazard ratios (HR) and their corresponding 95% confidence intervals (95% CI). In our analyses we used the person-years of the subcohort to estimate the number of person-years of the full cohort and used these as the underlying time metric (van den Brandt et al, 1990a; Barlow et al, 1999). We estimated standard errors using the Huber-White sandwich estimator to account for the additional variance introduced by using the case-cohort approach (Lin and Wei, 1989). The proportional hazard assumption was tested using scaled Schoenfeld residuals and found to be justified (Schoenfeld, 1982).
We opted for two disease endpoints: microscopically confirmed pancreatic cancer (MCPC) cases (n=194 for men; n=156 for women) and all cases of pancreatic cancer (including both MCPC and non-microscopically confirmed pancreatic cancer (NMCPC) cases; n=298 for men; n=268 for women). The diagnosis of the NMCPC case group was made by the treating clinician and was based on clinical symptoms, physical examinations, and imaging results and recorded as discharge diagnosis. These cases were abstracted and recorded by a trained tumour registrar (van der Sanden et al, 1995).
To distinguish between subtypes of diabetes mellitus, the age of 30 years was used as a cut-off point; diabetes mellitus type I: diagnosed before age 30 years; DM II: diagnosed at age of ⩾30 years) as used in previous studies (Stevens et al, 2007). Due to low numbers, we did not analyse the possible association between diabetes mellitus type I and pancreatic cancer. Because diabetes can be both a cause and a sequel of pancreatic cancer, we excluded all cases who were diagnosed with DM II within five years prior to their diagnosis of pancreatic cancer (n=4), as per (Silverman, 2001). All medical conditions used in the analyses were dichotomized, with participants not exposed to the concerning medical condition used as a reference group. Additionally, years since diagnosis of DM II, hypertension and hepatitis/jaundice, was evaluated. Years since diagnosis was calculated by subtracting the midpoint age of the age groups of diagnosis from the age at baseline. Years since diagnosis of DM II was categorized, based on previous research (Hassan et al, 2007), into two broad categories: <10 years and 10 years or more. Years since diagnosis of hepatitis/jaundice and hypertension were categorized based on the median values among subcohort members: 29 years and 8.5 years, respectively.
We used two methods to determine which variables finally entered the multivariable-adjusted models: (1) is the potential confounder predefined, i.e. been used in other models in literature describing the association between the exposure of interest and pancreatic cancer?; or (2) is the potential confounder associated with pancreatic cancer risk and with the exposure of interest and does the potential confounder change the risk estimate by at least 10%? In the final models, we made use of two different sets of confounders. For the exposure variable DM II we used a model that included: age at baseline (years), sex, current cigarette smoking (yes/no), cigarettes smoked (number/day), years of cigarette smoking (years), alcohol consumption (g/day), body mass index (BMI; kg m−2), socio-economic status (based on education: lower vocational, second & medium vocational and university and higher vocational), and family history of pancreatic cancer (yes/no) (model 1). In our analyses concerning the medical conditions hypertension, cholecystectomy, gallstones, peptic ulcer and hepatitis/jaundice, we included all confounders from model 1 plus the variable DM II (yes/no). This model will be referred to as model 2. To permit comparison, we restricted age-adjusted analyses to participants included in the multivariable-adjusted analyses, which left 3962 subcohort members (1944 men and 2018 women) and 448 incident cases of pancreatic cancer (63% microscopically confirmed). Trends were evaluated by fitting the median value for each level of the categorical exposure variable among the subcohort members as a continuous term. For all medical conditions, except DM II, we evaluated whether early symptoms of pancreatic cancer before diagnosis could have influenced the results by excluding early cases (diagnosed within 2 years after baseline) in additional analyses. Furthermore, we investigated the interaction between DM II (yes/no) and smoking status (never/ex/current smoking). Interaction on a multiplicative scale between sex and any of the medical conditions examined in the current study were tested for pancreatic cancer and were not found to be statistically significant (P for interaction>0.05). Therefore, results for analyses on medical conditions are presented for both sexes combined. All analyses were performed using STATA statistical software package version 9. We considered a two-sided P-value of <0.05 as statistically significant.
Results
As Table 1 shows, the differences in most baseline characteristics between the pancreatic cancer cases and the subcohort, were small. A notable difference is that 6.0% of all pancreatic cancer cases were diagnosed with DM II compared to 3.3% in the subcohort. Also, there were more participants with a family history of pancreatic cancer among cases than among subcohort members, especially in women.
Table 1. Description of the exposure variables and confounders, the Netherlands Cohort Study on diet and cancer, 1986-2003.
| Men | Women | |||||
|---|---|---|---|---|---|---|
|
Characteristic |
Total pancreatic cancer cases |
Microscopically confirmed pancreatic cancer cases |
Subcohort |
Total pancreatic cancer cases |
Microscopically confirmed pancreatic cancer cases |
Subcohort |
| Number |
239 |
160 |
1.944 |
209 |
123 |
2.018 |
| Age, mean (s.d.) (years) |
62.0 (3.9) |
61.6 (3.9) |
61.2 (4.2) |
62.2 (4.3) |
61.3 (4.3) |
61.4 (4.3) |
| History of DM IIa,b (%) |
15 (6.4%) |
9 (5.7%) |
61 (3.1%) |
8 (3.8%) |
6 (4.9%) |
66 (3.3%) |
|
Years since diagnosis of DM IIa,b (%) | ||||||
| <10 years | 9 (60.0%) | 5 (55.6%) | 42 (68.9%) | 4 (50.0%) | 3 (50.0%) | 45 (68.2%) |
| 10–19 years | 4 (26.7%) | 2 (22.2%) | 13 (21.3%) | 3 (37.5%) | 2 (33.3%) | 18 (27.3%) |
| ⩾20 years | 2 (13.3%) | 2 (22.2%) | 6 (9.8%) | 1 (12.5%) | 1 (16.7%) | 3 (4.5%) |
| History of hypertension (%) |
51 (21.3%) |
27 (16.9%) |
469 (24.1%) |
64 (30.6%) |
29 (23.6%) |
584 (28.9%) |
|
Years since diagnosis of hypertension (%) | ||||||
| <8.5 years | 28 (54.9%) | 14 (51.9%) | 246 (52.5%) | 25 (39.7%) | 15 (51.7%) | 257 (44.9%) |
| ⩾8.5 years | 23 (45.1%) | 13 (48.2%) | 223 (47.6%) | 38 (60.3%) | 14 (48.3%) | 316 (55.2%) |
| History of hepatitis (%) |
33 (13.8%) |
22 (13.8%) |
214 (11.0%) |
37 (17.7%) |
22 (17.9%) |
287 (14.2%) |
|
Years since diagnosis of hepatitis (%) | ||||||
| <29 years | 13 (39.4%) | 10 (45.5%) | 98 (46.0%) | 11 (30.6%) | 6 (27.3%) | 129 (45.6%) |
| ⩾29 years |
20 (60.6%) |
12 (54.6%) |
115 (54.0%) |
25 (69.4%) |
16 (72.7%) |
154 (54.4%) |
| History of gallstones (%) |
11 (4.6%) |
5 (3.1%) |
101 (5.2%) |
34 (16.3%) |
17 (13.8%) |
282 (14.0%) |
| History of cholecystectomy (%) |
8 (3.4%) |
4 (2.5%) |
87 (4.5%) |
32 (15.3%) |
16 (13.0%) |
266 (13.2%) |
| History of peptic ulcer (%) |
30 (12.6%) |
21 (13.1%) |
229 (11.8%) |
9 (4.3%) |
2 (1.6%) |
94 (4.7%) |
| Current cigarette smoker (%) |
101 (42.3%) |
67 (41.9%) |
660 (34.0%) |
52 (24.9%) |
32 (26.0%) |
417 (20.7%) |
| Number of cigarettes smoked per day, mean (s.d.)c | 16.9 (11.1) | 16.6 (9.8) | 17.2 (10.6) | 11.3 (8.2) | 10.7 (7.4) | 11.6 (8.4) |
| Years of smoking, mean (s.d.; years)c |
33.6 (11.9) |
35.6 (12.0) |
33.5 (11.7) |
28.5 (12.2) |
28.3 (11.9) |
27.8 (12.4) |
| Ex cigarette smoker (%) |
119 (49.8%) |
82 (51.3%) |
1018 (52.4%) |
46 (22.0%) |
28 (22.8%) |
407 (20.2%) |
| Number of cigarettes smoked per day, mean (s.d.)d | 15.6 (11.6) | 15.5 (10.4) | 14.8 (11.5) | 5.3 (8.0) | 5.2 (7.4) | 4.7 (7.9) |
| Years of smoking, mean (s.d.; years)d |
32.8 (14.9) |
33.1 (14.7) |
28.9 (15.8) |
13.4 (16.5) |
13.8 (16.4) |
11.4 (15.8) |
| BMI, mean (s.d.; kg m−2) |
25.3 (3.0) |
22.5 (2.7) |
24.9 (2.6) |
25.5 (3.5) |
25.9 (3.8) |
25.1 (3.5) |
| Alcohol, mean (s.d.; g/day) |
18.0 (18.0) |
17.3 (18.1) |
14.9 (16.9) |
6.5 (10.3) |
6.9 (10.0) |
5.9 (9.6) |
|
Level of education (%) | ||||||
| Low | 108 (45.2%) | 72 (45.0%) | 854 (43.9%) | 174 (56.0%) | 70 (56.9%) | 1118 (55.4%) |
| Medium | 82 (34.3%) | 59 (36.9%) | 706 (36.3%) | 74 (35.4%) | 43 (35.0%) | 714 (35.4%) |
| High |
49 (20.5%) |
29 (18.1%) |
384 (19.8%) |
18 (8.6%) |
11 (8.1%) |
186 (9.2%) |
| Anti hypertensive medication (%) |
39 (16.3%) |
19 (11.9%) |
381 (19.6%) |
46 (22.0%) |
19 (15.5%) |
455 (22.6%) |
| Family history of pancreatic cancer (%) | 5 (2.1%) | 2 (1.3%) | 16 (0.8%) | 9 (4.3%) | 3 (2.4%) | 19 (0.9%) |
Abbreviations: BMI=body mass index; DM II=diabetes mellitus type II; SD=standard deviation.
Numbers of cases or subcohort members do not add up to the total number because of missing values on some observations of the main exposure variables.
Only for persons who reported diabetes diagnosed at or above the age of 30 years.
All cases excluded in whom a diagnosis of DM II was made within five years prior to pancreatic cancer diagnosis.
Only for ever smokers.
only for ex smokers.
The age- and sex-adjusted and multivariable-adjusted associations between medical conditions and pancreatic cancer risk are shown in Table 2. For participants reporting to have ever been diagnosed with DM II, we observed a statistically significantly increased pancreatic cancer risk (multivariable-adjusted HR: 1.79; 95% CI: 1.12–2.87; Table 2). When the NMCPC cases were excluded in additional analyses, similar results were observed (Table 2). A statistically non-significantly reduced pancreatic cancer risk was observed in the group that reported to have ever been diagnosed with hypertension. When we restricted this analysis to MCPC cases, the point estimate decreased and became statistically significant (multivariable-adjusted HR: 0.66; 95% CI: 0.49–0.90; Table 2). The sample size was too small to investigate the group that reported hypertension but no medication use or to study the risk of different types of antihypertensive medication, like diuretics and RAS blockers. Hepatitis/jaundice was positively associated with pancreatic cancer risk (HR: 1.37; 95% CI: 1.04–1.81; Table 2). This association became non-significant when we restricted the analyses to MCPC cases, although the risk estimate remained similar (Table 2). Null results were observed for the medical conditions cholecystectomy, gallstones and peptic ulcer (Table 2).
Table 2. Age-adjusted and multivariable-adjusted hazard ratios for pancreatic cancer according to medical conditions.
| |
|
All pancreatic cancer cases |
Microscopically confirmed pancreatic cancer cases |
||||
|---|---|---|---|---|---|---|---|
| Exposure | Person-years at risk | Number of cases | Age- and sex-adjusted HR (95% CI) | Multivariable-adjusteda HR (95% CI) | Number of cases | Age- and sex-adjusted HR (95% CI) | Multivariable-adjusteda HR (95% CI) |
|
DM IIb | |||||||
| No | 55.273 | 421 | 1.00 (reference) | 1.00 (reference) | 265 | 1.00 (reference) | 1.00 (reference) |
| Yes |
1.624 |
23 |
1.75 (1.10–2.78) |
1.79 (1.12–2.87) |
15 |
1.90 (1.09–3.32) |
1.87 (1.06–3.30) |
|
Hypertension | |||||||
| No | 41.984 | 333 | 1.00 ( reference) | 1.00 (reference)c | 227 | 1.00 (reference) | 1.00 (reference)c |
| Yes |
14.913 |
115 |
0.96 (0.77–1.21) |
0.94 (0.75–1.19) |
56 |
0.70 (0.52–0.95) |
0.66 (0.49–0.90) |
|
Cholecystectomy | |||||||
| No | 51.751 | 408 | 1.00 (reference) | 1.00 (reference)c | 263 | 1.00 (reference) | 1.00 (reference)c |
| Yes |
5.145 |
40 |
1.01 (0.71–1.43) |
0.97 (0.68–1.38) |
20 |
0.83 (0.52–1.33) |
0.79 (0.49–1.26) |
|
Gallstones | |||||||
| No | 51.358 | 403 | 1.00 (reference) | 1.00 (reference)c | 261 | 1.00 (reference) | 1.00 (reference)c |
| Yes |
5.539 |
45 |
1.06 (0.76–1.47) |
1.00 (0.72–1.41) |
22 |
0.85 (0.54–1.33) |
0.78 (0.50–1.24) |
|
Peptic ulcer | |||||||
| No | 52.405 | 409 | 1.00 (reference) | 1.00 (reference)c | 260 | 1.00 (reference) | 1.00 (reference)c |
| Yes |
4.491 |
39 |
1.02 (0.72–1.46) |
0.95 (0.66–1.37) |
23 |
0.94 (0.60–1.46) |
0.87 (0.55–1.37) |
|
Hepatitis | |||||||
| No | 49.607 | 378 | 1.00 (reference) | 1.00 (reference)c | 239 | 1.00 (reference) | 1.00 (reference)c |
| Yes | 7.289 | 70 | 1.32 (1.00–1.73) | 1.37 (1.04–1.81) | 44 | 1.30 (0.93–1.81) | 1.35 (0.96–1.90) |
Abbreviations: CI=confidence interval; DM II=diabetes mellitus type II; HR=hazard ratio.
Adjusted for: age (years), sex, smoking (current cigarette smoker status: yes/no, number of cigarettes/day, years of cigarette smoking), body mass index (kg/m2), level of education, (low/medium/high), alcohol (g/day), and family history of pancreatic cancer (yes/no).
All cases excluded in whom a diagnosis of DM II was made within five years prior to pancreatic cancer diagnosis.
Additionally adjusted for: DM II (yes/no).
In Table 3, results for pancreatic cancer risk and years since diagnosis of DM II, hypertension and hepatitis/jaundice are presented. Statistically significantly increased risks and clear dose-response effects were observed for increasing number of years since diagnosis of DM II (P for trend=0.04) and hepatitis/jaundice (P for trend=0.02). The results observed for MCPC cases were similar (Table 3). For hypertension, years since diagnosis was not associated with pancreatic cancer risk among all cases (Table 3). However, when restricted to MCPC cases, years since diagnosis significantly decreased pancreatic cancer risk (Table 3). Additional analyses with DMII and subsequently years since diagnosis of DMII as determinants in a model with all covariates included in model 1 completed with the medical conditions hypertension, cholecystectomy, gallstones and peptic ulcer yielded similar results (Results not shown).
Table 3. Age-adjusted and multivariable-adjusted hazard ratios for pancreatic cancer according to years since diagnosis DM IIa, hypertension and hepatitis.
| All pancreatic cancer cases | Microscopically confirmed pancreatic cancer cases | ||||||
|---|---|---|---|---|---|---|---|
|
Years since diagnosis |
Person-years at riskc |
Number of casesc |
Age- and sex-adjusted
HR (95% CI) |
Multivariable-adjustedb
HR (95% CI) |
Number of casesc |
Age- and sex-adjusted
HR (95% CI) |
Multivariable-adjustedb
HR (95% CI) |
|
DM II | |||||||
| No DM II | 55.273 | 421 | 1.00 (reference) | 1.00 (reference) | 265 | 1.00 (reference) | 1.00 (reference) |
| Diagnosed since <10 years | 1,110 | 13 | 1.46 (0.80–2.68) | 1.45 (0.78–2.70) | 8 | 1.50 (0.71–3.17) | 1.41 (0.66–3.02) |
| Diagnosed since ⩾10 years | 514 | 10 | 2.35 (1.16–4.72) | 2.56 (1.26–5.18) | 7 | 2.75 (1.23–6.15) | 2.97 (1.31–6.74) |
| P for linear trend |
|
|
0.008 |
0.004 |
|
0.008 |
0.006 |
|
Hypertension | |||||||
| No Hypertension | 41.984 | 333 | 1.00 (reference) | 1.00 (reference)d | 227 | 1.00 (reference) | 1.00 (reference)d |
| Diagnosed since <8.5 years | 7.126 | 53 | 0.94 (0.69–1.28) | 0.92 (0.68–1.26) | 29 | 0.76 (0.51–1.13) | 0.72 (0.48–1.08) |
| Diagnosed since ⩾8.5 years | 7.629 | 61 | 0.99 (0.74–1.32) | 0.97 (0.72–1.31) | 27 | 0.67 (0.44–1.01) | 0.62 (0.41–0.95) |
| P for linear trend |
|
|
0.91 |
0.79 |
|
0.04 |
0.02 |
|
Hepatitis | |||||||
| No Hepatitis | 49.607 | 378 | 1.00 (reference) | 1.00 (reference)d | 239 | 1.00 (reference) | 1.00 (reference)d |
| Diagnosed since <29 years | 3.442 | 24 | 1.11 (0.71–1.73) | 1.15 (0.74–1.80) | 16 | 1.05 (0.61–1.79) | 1.10 (0.65–1.89) |
| Diagnosed since ⩾29 years | 3.773 | 45 | 1.45 (1.03–2.04) | 1.51 (1.07–2.13) | 28 | 1.52 (1.00–2.32) | 1.57 (1.02–2.41) |
| P for linear trend | 0.04 | 0.02 | 0.09 | 0.06 | |||
Abbreviations: CI=confidence interval; DM II=diabetes mellitus type II; HR=hazard ratio.
All cases excluded in whom a diagnosis of DM II was made within five years prior to pancreatic cancer diagnosis.
Adjusted for: age (years), sex, smoking (current cigarette smoker status: yes/no, number of cigarettes/day, years of cigarette smoking), body mass index (kg/m2), level of education, alcohol (g/day), and family history of pancreatic cancer (yes/no).
Numbers of cases and person-years do not add up to the total number because of missing values on some observations of the main exposure variables.
Additionally adjusted for: DM II (yes/no).
When testing the multiplicative interaction between DM II and smoking, we observed a significant interaction between DM II and smoking (P for interaction=0.03). Compared to a non-smoker without DM II, a current smoker diagnosed with DM II had almost a 5 times higher risk of pancreatic cancer (multivariable-adjusted HR: 4.79; 95% CI: 1.96–11.75). After excluding the first 2 years of follow-up, the results did not differ (data not shown).
Discussion
In this study, we observed a significant positive association between both DM II and hepatitis/jaundice and pancreatic cancer risk. For hypertension, we observed an inverse association; however this was limited to MCPC cases. For cholecystectomy, peptic ulcer and gallstones, null results were observed. Furthermore, we observed a positive association between pancreatic cancer risk and time passed since diagnosis of DM II and of hepatitis/jaundice. Years since diagnosis of hypertension was inversely associated with pancreatic cancer risk, again, only for MCPC cases. The finding of a positive association between years since diagnosis of DM II and pancreatic cancer risk should be interpreted with some caution, however, as the number of cases in the group diagnosed with DM II for 10 years or more was limited to 10.
So far, the majority of reported risk estimates for DM II correspond to our findings (La Vecchia et al, 1994; Gullo et al, 1996; Silverman, 2001; Ghadirian et al, 2003; Huxley et al, 2005; Ansary Moghaddam et al, 2006; Wang et al, 2006; Hassan et al, 2007; Jamal et al, 2009; Ogunleye et al, 2009; Stevens et al, 2009). A recent meta-analysis, including 35 cohort studies, observed a pooled relative risk (RR) of 1.94 (95% CI: 1.66–2.27; Ben et al, 2011). Furthermore, this study showed an inverse association between the years since diagnosis of diabetes mellitus and pancreatic cancer risk. However, reverse causality was not taken into account in this meta-analysis because the highest risk was found among patients diagnosed within less than 1 year. In a meta-analysis performed by Ghadirian et al, a pooled RR of 2.6 (95% CI: 1.6–4.1; based on nine cohort studies) was observed (Ghadirian et al, 2003). In this meta-analysis, studies were only included that had excluded cases in which a diagnosis of diabetes mellitus was made within one year prior to the diagnosis of pancreatic cancer.
Regarding studies investigating the role of hypertension in the onset of pancreatic cancer, most reported null results (Grove et al, 1991; Lindgren et al, 2005; Ansary Moghaddam et al, 2006; Batty et al, 2009). To our best knowledge only one study reported a significantly protective effect of hypertension (Batty et al, 2003). Batty et al, attributed their observed inverse association to chance or residual confounding (Batty et al, 2003). Similarly, we cannot rule out these possibilities in our study. In contrast with the current study, all of the abovementioned studies had biomarkers at their disposal, although only Batty et al, adjusted their models for antihypertensive medication. Several studies discuss the potential role of antihypertensive medication in preventing pancreatic cancer. In addition to acting in an antihypertensive capacity, renin-angiotensin system (RAS) blockers (AT1R blockers or ACE inhibitors) may yield protective effects against pancreatic cancer (Nakai et al, 2010). Experimental data from studies using cell and animal models of pancreatic cancer suggest that the RAS regulates tumour growth, angiogenesis, and metastasis; and a convergence of such findings suggests that pharmacological RAS blockade could have therapeutic potential in the management of pancreatic cancer (Lau and Leung, 2011). In the current study we did not analyze the group who reported the use of RAS blockers or the group who reported hypertension and no antihypertensive medication use, due to their low numbers.
Our findings regarding hepatitis should be interpreted with caution, as the variable ‘hepatitis' included the condition ‘jaundice', which is not only a symptom of hepatitis but can also be a symptom of a variety of other diseases like inflammation of the bile ducts, gallstones, nonalcoholic steatohepatitis, and pancreatic cancer. However, findings were similar when excluding the first 2 years of follow-up. Furthermore, we observed an increased risk and a clear dose-response effect for increasing number of years since diagnosis of hepatitis/jaundice. Therefore, we conclude that jaundice as an early symptom of pancreatic cancer probably did not influence our results. Secondly, we were unable to differentiate between different subtypes of hepatitis in our dataset while most other studies had blood sample at their disposal and could therefore distinguish between different subtypes (Berrington de Gonzalez et al, 2008; Hassan et al, 2008; Iloeje et al, 2010; Wang et al, 2012a). In a cohort study, Uchenna et al, measured HBsAg at baseline and observed that chronic carriers of HBsAg had a almost twofold increased pancreatic cancer risk (RR=1.95; 95% CI: 1.01–3.78; Iloeje et al, 2010). Two case-control studies observed significantly increased pancreatic cancer risks as well for chronic carriers of HBsAg (Hassan et al, 2008; Wang et al, 2012a), whereas one of these studies failed to observe an association with hepatitis C (Odds Ratio=0.9; 95% CI: 0.3–2.8; Hassan et al, 2008). These results indicate that without distinction in subtypes, hepatitis as a determinant may be less informative. In the Netherlands, hepatitis A virus infection is the most common form of hepatitis infection, followed by HBV and HCV (National Institute for Public Health and the Environment, 2011). In western countries, the prevalence of hepatitis B core antibody (HbcAb) and/or hepatitis B surface antibody (HbsAb) positivity is higher than prevalence of HBsAg (Ott et al, 2012). Further research in low HBV endemic countries, like USA or Northern Europe, on the basis of biomarkers, including the assessment of HBsAg-/HBcAb+/HBsAb- and HBsAg-/HBcAb+/HBsAb+ patterns may contribute to the understanding of the etiology of pancreatic cancer.
We observed effect modification by smoking on the relation between DM II and pancreatic cancer risk. This finding should be interpreted with some caution, due to the low number of cases in some of the subgroups. Probably due to low sample sizes in several studies, this subgroup analysis has been reported only once to our knowledge (Hassan et al, 2007). It is possible that in people who smoke and are diagnosed with DM II smoking-induced oxidative stress appears, which results in the production of free radicals and peroxides. These substances might increase susceptibility to chronic inflammation, DNA damage and pancreatic cancer (Hassan et al, 2007). More research is needed to investigate whether and how smoking modifies the relation between DM II and pancreatic cancer risk.
Our findings showed no relationship between the conditions cholecystectomy and gallstones and pancreatic cancer risk. In some case-control studies, positive associations were observed for cholecystectomy and gallstones (Lee et al, 1996; Silverman, 2001; Ghadirian et al, 2003; Ko et al, 2007), whereas other cohort studies and case-control studies reported null results (Gullo et al, 1996; Ye et al, 2001; Schernhammer et al, 2002; Bosetti et al, 2003; Hassan et al, 2007). We also did not observe an association between peptic ulcer and pancreatic cancer risk. However, some studies have identified an increased pancreatic cancer risk for gastric ulcer (Ghadirian et al, 2003; Bao et al, 2010) and peptic ulcer (Ghadirian et al, 2003; Bao et al, 2010).
Some limitations in the current study need to be discussed. First, a validity issue might occur because we made use of self-reported medical conditions. Most researchers have biomarkers available at baseline (Lee et al, 1996; Gapstur et al, 2000; Zendehdel et al, 2003; Batty et al, 2003, 2009; Berrington de Gonzalez et al, 2008; Hassan et al, 2008; Ogunleye et al, 2009). Molenaar et al, compared self-reported data and biomarkers on hypertension and diabetes, observing an overall sensitivity of 34.5% for hypertension and 58.9% for diabetes and a high overall specificity of 96.4% and 99.4%, respectively (Molenaar et al, 2007). Molenaar et al, therefore concluded that making use of self-reported data on diabetes and hypertension underestimates the prevalence of these two conditions (Molenaar et al, 2007). Non-differential measurement error in our self-reported data is therefore likely. However, a meta-analysis conducted by Huxley et al, demonstrated no risk differences between studies that made use of self-reported diabetes and studies that used medical records or the oral glucose test (Huxley et al, 2005). The self-reported condition hepatitis/jaundice might not only represent hepatitis A, B, or C, but also a variety of other diseases; therefore it would be best for future researchers to use biomarkers over self-reported physician diagnosed data. Secondly, by using the cut-off point of age at diagnosis of 30 years as a surrogate for DM II, we inevitably introduced some measurement error. However, a study performed by Berger et al, showed that the mean age of diagnosis of diabetes mellitus type I and type II in a Swedish population in the early nineties was 24.1 and 66.6 years, respectively (Berger et al, 1999). Therefore, using the age of 30 as a cut-off point seems justified. Furthermore, it is not clear whether the observed inverse association between self-reported hypertension and pancreatic cancer risk is caused by hypertension itself or by the use of antihypertensive medication. Residual confounding by unmeasured variables could well have influenced our results. Finally, it should be noted that because of the relatively large number of analyses performed, some of the statistical significant findings might have occurred due to chance. This study has a number of strengths, which includes the fact that we could further restrict the analyses to MCPC cases. Moreover, selection bias caused by differential follow-up is unlikely to have made a substantial contribution to the findings, since there was very little loss to follow-up (Goldbohm et al, 1994b). Other strengths include a large sample size and detailed information on potential risk factors for pancreatic cancer. Furthermore, the prospective design avoided recall bias and the need to use next-of-kin respondents.
In summary, we observed a positive association between self-reported physician diagnosed DM II and hepatitis/jaundice and pancreatic cancer risk, whereas an inverse association was observed for hypertension. No association was observed for cholecystectomy, peptic ulcer and gallstones.
Acknowledgments
We are indebted to the participants of this study and further wish to thank the Netherlands Cancer Registry and the Netherlands nationwide registry of pathology (PALGA). We also thank Dr RA Goldbohm for designing and developing the NLCS, Dr A Volovics and Dr A Kester for statistical advice; S van de Crommert, H Brants, J Nelissen, C de Zwart, M Moll, and A Pisters for assistance; and H van Montfort, T van Moergastel, L van den Bosch, E Dutman, R Schmeitz, J Berben, R. Meijer for programming assistance. No funding source had a role in the research or preparation of this report.
Author contributions
Pieter Eijgenraam analyzed the data, and drafted and revised the paper. Mirjam M Heinen analyzed the data and revised the draft paper. Bas AJ Verhage and Leo J Schouten participated in the coordination of the study, and critically reviewed the draft paper. Yolande C Keulemans critically reviewed the draft paper. Piet A van den Brandt conceived the study, participated in its design and coordination, and critically reviewed the draft paper. All authors contributed to the interpretation of the data, and all authors approved the final draft of the paper.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Anderson LN, Cotterchio M, Gallinger S. Lifestyle, dietary, and medical history factors associated with pancreatic cancer risk in Ontario, Canada. Cancer causes & control: CCC. 2009;20 (6:825–834. doi: 10.1007/s10552-009-9303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansary Moghaddam A, Huxley R, Barzi F, Lawes C, Ohkubo T, Fang X, Jee SH, Woodward M. The effect of modifiable risk factors on pancreatic cancer mortality in populations of the Asia-Pacific region. Cancer Epidemiol Biomarkers Prev. 2006;15 (12:2435–2440. doi: 10.1158/1055-9965.EPI-06-0368. [DOI] [PubMed] [Google Scholar]
- Apte M, Pirola R, Wilson J. New insights into alcoholic pancreatitis and pancreatic cancer. J Gastroenterol Hepatol. 2009;24 (Suppl 3:S51–S56. doi: 10.1111/j.1440-1746.2009.06071.x. [DOI] [PubMed] [Google Scholar]
- Bao Y, Spiegelman D, Li R, Giovannucci E, Fuchs CS, Michaud DS. History of peptic ulcer disease and pancreatic cancer risk in men. Gastroenterology. 2010;138 (2:541–549. doi: 10.1053/j.gastro.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52 (12:1165–1172. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- Batty GD, Kivimaki M, Morrison D, Huxley R, Smith GD, Clarke R, Marmot MG, Shipley MJ. Risk factors for pancreatic cancer mortality: extended follow-up of the original Whitehall Study. Cancer Epidemiol Biomarkers Prev. 2009;18 (2:673–675. doi: 10.1158/1055-9965.EPI-08-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty GD, Shipley MJ, Marmot MG, Davey Smith G. Blood pressure and site-specific cancer mortality: evidence from the original Whitehall study. Br J Cancer. 2003;89 (7:1243–1247. doi: 10.1038/sj.bjc.6601255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Q, Li Z, Liu C, Cai Q, Yuan Y, Wang K, Xiao L, Gao J, Zhang H. Hepatitis B virus status and risk of pancreatic ductal adenocarcinoma: a case-control study from China. Pancreas. 2012;41 (3:435–440. doi: 10.1097/MPA.0b013e31822ca176. [DOI] [PubMed] [Google Scholar]
- Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W, Zhang H, Li Z. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer. 2011;47 (13:1928–1937. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Berger B, Stenstrom G, Sundkvist G. Incidence, prevalence, and mortality of diabetes in a large population. A report from the Skaraborg Diabetes Registry. Diabetes Care. 1999;22 (5:773–778. doi: 10.2337/diacare.22.5.773. [DOI] [PubMed] [Google Scholar]
- Berrington de Gonzalez A, Spencer EA, Bueno-de-Mesquita HB, Roddam A, Stolzenberg-Solomon R, Halkjaer J, Tjonneland A, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Boeing H, Pischon T, Linseisen J, Rohrmann S, Trichopoulou A, Benetou V, Papadimitriou A, Pala V, Palli D, Panico S, Tumino R, Vineis P, Boshuizen HC, Ocke MC, Peeters PH, Lund E, Gonzalez CA, Larranaga N, Martinez-Garcia C, Mendez M, Navarro C, Quiros JR, Tormo MJ, Hallmans G, Ye W, Bingham SA, Khaw KT, Allen N, Key TJ, Jenab M, Norat T, Ferrari P, Riboli E. Anthropometry, physical activity, and the risk of pancreatic cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2006;15 (5:879–885. doi: 10.1158/1055-9965.EPI-05-0800. [DOI] [PubMed] [Google Scholar]
- Berrington de Gonzalez A, Yun JE, Lee SY, Klein AP, Jee SH. Pancreatic cancer and factors associated with the insulin resistance syndrome in the Korean cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2008;17 (2:359–364. doi: 10.1158/1055-9965.EPI-07-0507. [DOI] [PubMed] [Google Scholar]
- Bosetti C, Negri E, Franceschi S, La Vecchia C. Reply: Gallstones, cholecystectomy, and the risk for developing pancreatic cancer. Br J Cancer. 2003;88 (1:159–160. doi: 10.1038/sj.bjc.6600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casparie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken JH, Meijer GA. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29 (1:19–24. doi: 10.1155/2007/971816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodick G, Heymann AD, Rosenmann L, Green MS, Flash S, Porath A, Kokia E, Shalev V. Diabetes and risk of incident cancer: a large population-based cohort study in Israel. Cancer Causes Control. 2010;21 (6:879–887. doi: 10.1007/s10552-010-9515-8. [DOI] [PubMed] [Google Scholar]
- Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283 (19:2552–2558. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- Ghadirian P, Lynch HT, Krewski D. Epidemiology of pancreatic cancer: an overview. Cancer Detect Prev. 2003;27 (2:87–93. doi: 10.1016/s0361-090x(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Goldbohm RA, van den Brandt PA, Brants HA, van't Veer P, Al M, Sturmans F, Hermus RJ. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. European journal of clinical nutrition. 1994;48 (4:253–265. [PubMed] [Google Scholar]
- Goldbohm RA, van den Brandt PA, Dorant E. Estimation of the coverage of Dutch municipalities by cancer registries and PALGA based on hospital discharge data. Tijdschr Soc Gezondheidsz. 1994;72:80–84. [Google Scholar]
- Greer JB, Whitcomb DC. Inflammation and pancreatic cancer: an evidence-based review. Curr Opin Pharmacol. 2009;9 (4:411–418. doi: 10.1016/j.coph.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Grove JS, Nomura A, Severson RK, Stemmermann GN. The association of blood pressure with cancer incidence in a prospective study. Am J Epidemiol. 1991;134 (9:942–947. doi: 10.1093/oxfordjournals.aje.a116178. [DOI] [PubMed] [Google Scholar]
- Gullo L, Pezzilli R, Morselli Labate AM. Risk of pancreatic cancer associated with cholelithiasis, cholecystectomy, or gastrectomy. Dig Dis Sci. 1996;41 (6:1065–1068. doi: 10.1007/BF02088220. [DOI] [PubMed] [Google Scholar]
- Hardt PD, Bretz L, Krauss A, Schnell-Kretschmer H, Wusten O, Nalop J, Zekorn T, Klor HU. Pathological pancreatic exocrine function and duct morphology in patients with cholelithiasis. Dig Dis Sci. 2001;46 (3:536–539. doi: 10.1023/a:1005690930325. [DOI] [PubMed] [Google Scholar]
- Hassan MM, Bondy ML, Wolff RA, Abbruzzese JL, Vauthey JN, Pisters PW, Evans DB, Khan R, Chou TH, Lenzi R, Jiao L, Li D. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol. 2007;102 (12:2696–2707. doi: 10.1111/j.1572-0241.2007.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MM, Li D, El Deeb AS, Wolff RA, Bondy ML, Davila M, Abbruzzese JL. Association between hepatitis B virus and pancreatic cancer. J Clin Oncol. 2008;26 (28:4557–4562. doi: 10.1200/JCO.2008.17.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howatson AG, Carter DC. Pancreatic carcinogenesis-enhancement by cholecystokinin in the hamster-nitrosamine model. Br J Cancer. 1985;51 (1:107–114. doi: 10.1038/bjc.1985.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley R, Ansary Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92 (11:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iloeje UH, Yang HI, Jen CL, Su J, Wang LY, You SL, Lu SN, Chen CJ. Risk of pancreatic cancer in chronic hepatitis B virus infection: data from the REVEAL-HBV cohort study. Liver international: official journal of the International Association for the Study of the Liver. 2010;30 (3:423–429. doi: 10.1111/j.1478-3231.2009.02147.x. [DOI] [PubMed] [Google Scholar]
- Inoue M, Noda M, Kurahashi N, Iwasaki M, Sasazuki S, Iso H, Tsugane S. Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev. 2009;18 (3:240–247. doi: 10.1097/CEJ.0b013e3283240460. [DOI] [PubMed] [Google Scholar]
- Jamal MM, Yoon EJ, Vega KJ, Hashemzadeh M, Chang KJ. Diabetes mellitus as a risk factor for gastrointestinal cancer among American veterans. World J Gastroenterol. 2009;15 (42:5274–5278. doi: 10.3748/wjg.15.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59 (4:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Ko AH, Wang F, Holly EA. Pancreatic cancer and medical history in a population-based case-control study in the San Francisco Bay Area, California. Cancer Causes Control. 2007;18 (8:809–819. doi: 10.1007/s10552-007-9024-6. [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Negri E, Franceschi S, D'Avanzo B, Boyle P. A case-control study of diabetes mellitus and cancer risk. Br J Cancer. 1994;70 (5:950–953. doi: 10.1038/bjc.1994.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai GY, Park Y, Hartge P, Hollenbeck AR, Freedman ND. The association between self-reported diabetes and cancer incidence in the NIH-AARP Diet and Health Study. J Clin Endocrinol Metab. 2013;98 (3:E497–E502. doi: 10.1210/jc.2012-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau ST, Leung PS. Role of the RAS in pancreatic cancer. Curr Cancer Drug Targets. 2011;11 (4:412–420. doi: 10.2174/156800911795538110. [DOI] [PubMed] [Google Scholar]
- Lee CT, Chang FY, Lee SD. Risk factors for pancreatic cancer in orientals. J Gastroenterol Hepatol. 1996;11 (5:491–495. doi: 10.1111/j.1440-1746.1996.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- Lin G, Zeng Z, Wang X, Wu Z, Wang J, Wang C, Sun Q, Chen Y, Quan H. Cholecystectomy and risk of pancreatic cancer: a meta-analysis of observational studies. Cancer Causes Control. 2012;23 (1:59–67. doi: 10.1007/s10552-011-9856-y. [DOI] [PubMed] [Google Scholar]
- Lindgren AM, Nissinen AM, Tuomilehto JO, Pukkala E. Cancer pattern among hypertensive patients in North Karelia, Finland. J Hum Hypertens. 2005;19 (5:373–379. doi: 10.1038/sj.jhh.1001834. [DOI] [PubMed] [Google Scholar]
- Luo G, Hao NB, Hu CJ, Yong X, Lu MH, Cheng BJ, Zhang Y, Yang SM. HBV infection increases the risk of pancreatic cancer: a meta-analysis. Cancer Causes Control. 2013;24 (3:529–537. doi: 10.1007/s10552-012-0144-2. [DOI] [PubMed] [Google Scholar]
- Luo J, Iwasaki M, Inoue M, Sasazuki S, Otani T, Ye W, Tsugane S. Body mass index, physical activity and the risk of pancreatic cancer in relation to smoking status and history of diabetes: a large-scale population-based cohort study in Japan—the JPHC study. Cancer Causes Control. 2007;18 (6:603–612. doi: 10.1007/s10552-007-9002-z. [DOI] [PubMed] [Google Scholar]
- Mhaskar RS, Ricardo I, Azliyati A, Laxminarayan R, Amol B, Santosh W, Boo K. Assessment of risk factors of helicobacter pylori infection and peptic ulcer disease. J Glob Infect Dis. 2013;5 (2:60–67. doi: 10.4103/0974-777X.112288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar EA, Van Ameijden EJ, Grobbee DE, Numans ME. Comparison of routine care self-reported and biometrical data on hypertension and diabetes: results of the Utrecht Health Project. Eur J Public Health. 2007;17 (2:199–205. doi: 10.1093/eurpub/ckl113. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Isayama H, Ijichi H, Sasaki T, Sasahira N, Hirano K, Kogure H, Kawakubo K, Yagioka H, Yashima Y, Mizuno S, Yamamoto K, Arizumi T, Togawa O, Matsubara S, Tsujino T, Tateishi K, Tada M, Omata M, Koike K. Inhibition of renin-angiotensin system affects prognosis of advanced pancreatic cancer receiving gemcitabine. Br J Cancer. 2010;103 (11:1644–1648. doi: 10.1038/sj.bjc.6605955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Public Health and the Environment 2011Hepatitis A, B, C [in Dutch] Vol. 2013available at www.rivm.nl . [Google Scholar]
- Ogunleye AA, Ogston SA, Morris AD, Evans JM. A cohort study of the risk of cancer associated with type 2 diabetes. Br J Cancer. 2009;101 (7:1199–1201. doi: 10.1038/sj.bjc.6605240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30 (12:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- Prentice R. A case-cohort design for epidemiologic studies and disease prevention. Biometrika. 1986;71:1–11. [Google Scholar]
- Schernhammer ES, Michaud DS, Leitzmann MF, Giovannucci E, Colditz GA, Fuchs CS. Gallstones, cholecystectomy, and the risk for developing pancreatic cancer. Br J Cancer. 2002;86 (7:1081–1084. doi: 10.1038/sj.bjc.6600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- Silverman DT. Risk factors for pancreatic cancer: a case-control study based on direct interviews. Teratog Carcinog Mutagen. 2001;21 (1:7–25. doi: 10.1002/1520-6866(2001)21:1<7::aid-tcm3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Stevens RJ, Roddam AW, Beral V. Pancreatic cancer in type 1 and young-onset diabetes: systematic review and meta-analysis. Br J Cancer. 2007;96 (3:507–509. doi: 10.1038/sj.bjc.6603571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RJ, Roddam AW, Spencer EA, Pirie KL, Reeves GK, Green J, Beral V. Factors associated with incident and fatal pancreatic cancer in a cohort of middle-aged women. Int J Cancer. 2009;124 (10:2400–2405. doi: 10.1002/ijc.24196. [DOI] [PubMed] [Google Scholar]
- Trikudanathan G, Philip A, Dasanu CA, Baker WL. Association between Helicobacter pylori infection and pancreatic cancer. A cumulative meta-analysis. JOP. 2011;12 (1:26–31. [PubMed] [Google Scholar]
- van den Brandt PA, Goldbohm RA, van 't Veer P, Volovics A, Hermus RJ, Sturmans F. A large-scale prospective cohort study on diet and cancer in The Netherlands. J Clin Epidemiol. 1990;43 (3:285–295. doi: 10.1016/0895-4356(90)90009-e. [DOI] [PubMed] [Google Scholar]
- van den Brandt PA, Schouten LJ, Goldbohm RA, Dorant E, Hunen PM. Development of a record linkage protocol for use in the Dutch Cancer Registry for Epidemiological Research. Int J Epidemiol. 1990;19 (3:553–558. doi: 10.1093/ije/19.3.553. [DOI] [PubMed] [Google Scholar]
- van der Sanden GA, Coebergh JW, Schouten LJ, Visser O, van Leeuwen FE. Cancer incidence in The Netherlands in 1989 and 1990: first results of the nationwide Netherlands cancer registry. Coordinating Committee for Regional Cancer Registries. Eur J Cancer. 1995;31A (11:1822–1829. doi: 10.1016/0959-8049(95)00355-m. [DOI] [PubMed] [Google Scholar]
- Wang DS, Chen DL, Ren C, Wang ZQ, Qiu MZ, Luo HY, Zhang DS, Wang FH, Li YH, Xu RH. ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int J Cancer. 2012;131 (2:461–468. doi: 10.1002/ijc.26376. [DOI] [PubMed] [Google Scholar]
- Wang DS, Wang ZQ, Zhang L, Qiu MZ, Luo HY, Ren C, Zhang DS, Wang FH, Li YH, Xu RH. Are risk factors associated with outcomes in pancreatic cancer. PLoS One. 2012;7 (7:e41984. doi: 10.1371/journal.pone.0041984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gupta S, Holly EA. Diabetes mellitus and pancreatic cancer in a population-based case-control study in the San Francisco Bay Area, California. Cancer Epidemiol Biomarkers Prev. 2006;15 (8:1458–1463. doi: 10.1158/1055-9965.EPI-06-0188. [DOI] [PubMed] [Google Scholar]
- Ye W, Lagergren J, Nyren O, Ekbom A. Risk of pancreatic cancer after cholecystectomy: a cohort study in Sweden. Gut. 2001;49 (5:678–681. doi: 10.1136/gut.49.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zendehdel K, Nyren O, Ostenson CG, Adami HO, Ekbom A, Ye W. Cancer incidence in patients with type 1 diabetes mellitus: a population-based cohort study in Sweden. J Natl Cancer Inst. 2003;95 (23:1797–1800. doi: 10.1093/jnci/djg105. [DOI] [PubMed] [Google Scholar]