Abstract
Background:
Besides tobacco and alcohol, dietary habits may have a relevant role in oral cavity and pharyngeal (OCP) cancer.
Methods:
We analysed the role of selected food groups and nutrients on OCP cancer in a case–control study carried out between 1997 and 2009 in Italy and Switzerland. This included 768 incident, histologically confirmed squamous cell carcinoma cases and 2078 hospital controls. Odds ratios (ORs) were estimated using logistic regression models including terms for tobacco, alcohol and other relevant covariates.
Results:
Significant inverse trends in risk were observed for all vegetables (OR=0.19, for the highest vs the lowest consumption) and all fruits (OR=0.39), whereas significant direct associations were found for milk and dairy products (OR=1.50), eggs (OR=1.71), red meat (OR=1.55), potatoes (OR=1.85) and desserts (OR=1.68), although trends in risk were significant only for potatoes and desserts. With reference to nutrients, significant inverse relations were observed for vegetable protein (OR=0.45, for the highest vs the lowest quintile), vegetable fat (OR=0.54), polyunsaturated fatty acids (OR=0.53), α-carotene (OR=0.51), β-carotene (OR=0.28), β-cryptoxanthin (OR=0.37), lutein and zeazanthin (OR=0.34), vitamin E (OR=0.26), vitamin C (OR=0.40) and total folate (OR=0.34), whereas direct ones were observed for animal protein (OR=1.57), animal fat (OR=2.47), saturated fatty acids (OR=2.18), cholesterol (OR=2.29) and retinol (OR=1.88). Combinations of low consumption of fruits and vegetables, and high consumption of meat with high tobacco and alcohol, led to 10- to over 20-fold excess risk of OCP cancer.
Conclusion:
Our study confirms and further quantifies that a diet rich in fruits and vegetables and poor in meat and products of animal origin has a favourable role against OCP cancer.
Keywords: case-control, diet, food, nutrient, oropharyngeal cancer, risk factor
Oral cavity and pharyngeal (OCP) cancer is the eighth most common neoplasm and the eleventh leading cause of cancer in Europe, with a five-fold higher incidence in men as compared with women (Ferlay et al, 2010; Bosetti et al, 2013).
Tobacco and alcohol are the major recognised risk factors for OCP cancer, with relative risks in the order of 5–10 for smokers as compared with nonsmokers (IARC, 2004) and for heavy drinkers as compared with abstainers or moderate drinkers (Bagnardi et al, 2001; IARC, 2010).
Dietary and nutritional habits have also been reported to have a relevant role in the development of this neoplasm. In particular, a diet rich in vegetables, fruits, carotenoids and other vitamins has been associated with a reduced risk of OCP cancer, whereas the evidence for other foods or nutrients is less convincing (World Cancer Research Fund and American Institute for Cancer Research, 2007; Lucenteforte et al, 2009; Bradshaw et al, 2012; Bravi et al, 2012; Chuang et al, 2012).
In a multicentric case–control study from Italy and Switzerland conducted in the 1990s, the odds ratio (OR) of OCP cancer for the highest vs the lowest level of consumption was 0.4 for raw vegetables, 0.5 for cooked vegetables, 0.5 for citrus fruits and 0.7 for other fruits (Franceschi et al, 1999a), and ORs were between 0.3 and 0.7 for various antioxidant vitamins, including carotene, vitamin C and E, thiamine, vitamin B6, folic acid and niacin (Negri et al, 2000). Significant inverse associations were also reported for high consumption of bread, poultry, fish, proteins and monounsaturated fats, whereas direct associations were observed for eggs, processed meat, sweets and desserts and saturated fatty acids (Franceschi et al, 1999a, 1999b; Negri et al, 2000).
In the present work, we analysed the role of selected food groups, macronutrients and micronutrients on OCP cancer in a case–control study carried out in Italy and Switzerland since the late 1990s.
Materials and methods
A case–control study on OCP cancer was conducted between 1997 and 2009 in the greater Milan area, Italy and in the canton of Vaud, Switzerland. Cases were 768 patients (593 men and 175 women) under age 79 years (median 58 years, range: 22–79 years) with incident, histologically confirmed squamous cell cancers of OCP (excluding cancers of the lip, salivary glands and nasopharynx), admitted to major teaching or general hospitals in the areas under investigation. Controls were 2078 subjects (1368 men and 710 women, median age 59 years, range: 19–79) with no previous history of cancer, admitted to the same hospitals for acute, nonneoplastic conditions unrelated to tobacco smoking, alcohol drinking or long-term dietary modifications. Among controls, 19% were admitted for traumas, 21% for other orthopaedic conditions, 51% for acute surgical conditions and 9% for other miscellaneous conditions. In Italy, less than 5% of the cases and controls approached refused to participate in the study; in Switzerland, the proportion of refunds was about 15%.
Trained personnel interviewed both cases and controls during their hospital stay using a structured questionnaire, including information on sociodemographic characteristics, anthropometric measures and selected lifestyle habits (including tobacco smoking and alcohol drinking). Subjects' dietary habits during the 2 years before cancer diagnosis or hospitalisation (for controls) were assessed through a valid (Decarli et al, 1996) and reproducible (Franceschi et al, 1993, 1995) food frequency questionnaire (FFQ), including information on weekly consumption of 78 foods, recipes and beverages. For a few vegetables and fruits, seasonal consumptions and corresponding durations were elicited. Food items were combined into 18 food groups: milk and yoghurt; cereals; soups; eggs; poultry; red meat; processed meat; fish; cheese; raw vegetables; cooked vegetables; all vegetables; potatoes; citrus fruits; other fruits; all fruits; desserts; and sugars. To estimate the daily intake of nutrients and total energy, we used an Italian food composition database (Gnagnarella et al, 2004). To evaluate the role of macronutrients independently from total energy intake, we derived energy-adjusted nutrients according to the residual method (Willett and Stampfer, 1986).
We categorised food groups, micronutrients and energy-adjusted macronutrients into quintiles (or quartiles/tertiles for a few foods with low frequency of consumption), according to the distribution among the control population. The ORs and corresponding confidence intervals for quantiles of intakes were estimated using multiple logistic regression models, including terms for age (5-year groups), sex, centre (Italy, Switzerland), education (<7, 7 to <12, ⩾12 years), year of interview (continuous), body mass index (BMI, <20, 20 to <25, 25 to <30 kg m−2), tobacco smoking (never smoker, ex-smoker, current smoker of: <15, 15–24, ⩾25 cigarettes per day), duration of smoking (<30, 30–39, ⩾40 years), total alcohol drinking (<2, 2 to <4, 4 to <8, ⩾8 drinks per day), duration of alcohol (<30, 30–39, ⩾40 years) and nonalcohol energy intake (quintiles) (Breslow and Day, 1980).
We also estimated the ORs for combinations of selected food groups (i.e., fruit, vegetable and meat) and lifestyle habits (i.e., tobacco smoking and alcohol drinking), and we tested the corresponding interaction by likelihood ratio tests.
Results
Table 1 shows the distribution of 768 OCP cancer cases and 2078 controls according to centre, sex, age and other selected characteristics. Cases and controls had similar distributions for age and education; cases had a lower BMI and were more frequently heavily exposed to tobacco smoking and alcohol drinking.
Table 1. Distribution of 768 cases of oral and pharyngeal cancer and 2078 controls according to sex, age, education and other selected variables (Italy and Switzerland, 1997–2009).
| |
Cases |
Controls |
||
|---|---|---|---|---|
| Characteristic | No. | % | No. | % |
|
Centre | ||||
| Italy | 348 | 45.3 | 1001 | 48.2 |
| Switzerland |
420 |
54.7 |
1077 |
51.8 |
|
Sex | ||||
| Men | 593 | 77.2 | 1368 | 65.8 |
| Women |
175 |
22.8 |
710 |
34.2 |
|
Age (years) | ||||
| <50 | 120 | 15.6 | 483 | 23.2 |
| 50 to <60 | 311 | 40.5 | 607 | 29.2 |
| 60 to <70 | 238 | 31.0 | 645 | 31.0 |
| ⩾70 |
99 |
12.9 |
343 |
16.5 |
|
Educationa (years) | ||||
| <7 | 143 | 18.6 | 379 | 18.3 |
| 7 to <12 | 278 | 36.2 | 641 | 31.0 |
| ⩾12 |
347 |
45.2 |
1048 |
50.7 |
|
Body mass index (kg m−2) | ||||
| <20 | 98 | 12.8 | 107 | 5.2 |
| 20 to <25 | 356 | 46.4 | 780 | 37.5 |
| 25 to <30 | 248 | 32.3 | 944 | 45.4 |
| ⩾30 |
66 |
8.6 |
247 |
11.9 |
|
Smoking statusa | ||||
| Never smokers | 115 | 15.1 | 1031 | 49.7 |
| Ex-smokers | 134 | 17.6 | 478 | 23.0 |
| Current smokers | ||||
| <15 cigarettes per day | 41 | 5.4 | 211 | 10.2 |
| 15–24 cigarettes per day | 181 | 23.7 | 288 | 13.9 |
| ⩾25 cigarettes per day |
292 |
38.3 |
67 |
3.2 |
|
Alcohol consumption (drinks per day) | ||||
| <2 | 168 | 21.9 | 1464 | 70.5 |
| 2–4 | 165 | 21.5 | 450 | 21.7 |
| 4–8 | 205 | 26.7 | 141 | 6.8 |
| ⩾8 | 230 | 30.0 | 23 | 1.1 |
The sum does not add up to the total because of some missing values.
Table 2 gives the median weekly intake of selected food groups, and the corresponding ORs of OCP cancer. Significant inverse trends in risk were observed for raw vegetables (OR=0.25, for the highest vs the lowest quintile of consumption), cooked vegetables (OR=0.50), citrus fruits (OR=0.50) and other fruits (OR=0.49). The ORs were 0.19 for all vegetables and 0.39 for all fruits. Significant trends of increasing risk were found with increasing consumption of potatoes (OR=1.85, for the highest vs the lowest quintile of consumption) and desserts (OR=1.68). Significant direct associations were also observed for high vs low intake of milk and yoghurt (OR=1.50), eggs (OR=1.71) and red meat (OR=1.55), though in the absence of a linear trend in risk.
Table 2. Odds ratiosa (ORs) and corresponding 95% confidence intervals (CIs) according to weekly consumption of selected food groups among 768 cases of oral and pharyngeal cancer and 2078 controls (Italy and Switzerland, 1997–2009).
| |
|
Quintiles of intake, OR (95% CI) |
|
|||
|---|---|---|---|---|---|---|
| Food groups | Median (IQR)b | II | III | IV | V | χ2trend (P-value) |
| Milk and yoghurt |
5.0 (0.0–9.0) |
1.21 (0.80–1.83) |
0.92 (0.62–1.37) |
0.98 (0.70–1.38) |
1.50 (1.05–2.13) |
2.26 (0.132) |
| Cereals |
19.0 (12.3–25.8) |
1.20 (0.79–1.82) |
1.09 (0.70–1.68) |
1.16 (0.74–1.83) |
0.78 (0.48–1.26) |
1.65 (0.200) |
| Soups |
1.0 (0.5–2.3) |
0.93 (0.57–1.49) |
1.58 (1.08–2.30) |
1.21 (0.78–1.86) |
1.49 (0.97–2.29) |
4.62 (0.032) |
| Eggs |
2.0 (1.0–2.5) |
1.26 (0.79–2.03) |
1.35 (0.93–1.98) |
0.94 (0.62–1.43) |
1.71 (1.14–2.58) |
2.81 (0.094) |
| Poultryc |
1.3 (0.6–2.0) |
0.86 (0.55–1.34) |
1.80 (1.23–2.63) |
0.76 (0.56–1.03) |
|
3.31 (0.069) |
| Red meat |
4.5 (3.3–5.9) |
0.91 (0.63–1.32) |
1.02 (0.69–1.51) |
0.95 (0.62–1.43) |
1.55 (1.04–2.31) |
3.55 (0.060) |
| Processed meat |
2.4 (1.5–4.0) |
1.03 (0.64–1.67) |
1.16 (0.77–1.73) |
1.08 (0.70–1.67) |
1.28 (0.84–1.93) |
1.48 (0.224) |
| Fishd |
1.7 (1.2–2.3) |
0.83 (0.59–1.18) |
0.81 (0.60–1.09) |
|
|
1.68 (0.195) |
| Cheese |
3.8 (2.6–5.0) |
0.92 (0.63–1.34) |
0.95 (0.64–1.39) |
0.89 (0.61–1.31) |
1.26 (0.86–1.85) |
1.16 (0.282) |
| Raw vegetables |
7.0 (4.5–9.0) |
0.51 (0.37–0.71) |
0.35 (0.24–0.52) |
0.33 (0.23–0.47) |
0.25 (0.17–0.36) |
60.46 (<0.0001) |
| Cooked vegetables |
3.5 (2.2–5.0) |
0.90 (0.63–1.30) |
0.80 (0.55–1.16) |
0.69 (0.47–0.99) |
0.50 (0.33–0.75) |
12.48 (0.0004) |
| All vegetables |
10.5 (7.5–13.5) |
0.60 (0.43–0.84) |
0.35 (0.24–0.50) |
0.45 (0.32–0.64) |
0.19 (0.13–0.29) |
62.46 (<0.0001) |
| Potatoes |
1.5 (1.0–2.5) |
0.89 (0.59–1.35) |
1.68 (1.10–2.56) |
1.06 (0.71–1.58) |
1.85 (1.19–2.86) |
7.00 (0.0081) |
| Citrus fruits |
3.5 (1.0–7.0) |
0.74 (0.53–1.05) |
0.56 (0.39–0.80) |
0.66 (0.46–0.95) |
0.50 (0.34–0.73) |
12.32 (0.0004) |
| Other fruits |
13.0 (6.0–21.1) |
0.88 (0.62–1.24) |
0.74 (0.52–1.05) |
0.63 (0.43–0.92) |
0.49 (0.32–0.73) |
13.80 (0.0002) |
| All fruits |
17 (8.7–27.8) |
0.71 (0.50–1.00) |
0.74 (0.52–1.05) |
0.62 (0.43–0.91) |
0.39 (0.26–0.59) |
17.04 (<0.0001) |
| Desserts |
3.0 (1.0–7.0) |
1.19 (0.77–1.84) |
1.52 (0.98–2.36) |
1.53 (1.00–2.35) |
1.68 (1.06–2.64) |
5.76 (0.0164) |
| Sugars | 29.0 (14.0–59.0) | 0.81 (0.55–1.19) | 0.92 (0.63–1.34) | 0.83 (0.56–1.23) | 1.44 (0.97–2.16) | 3.12 (0.0774) |
Estimated from unconditional logistic regression models adjusted for age, sex, centre, education, year of interview, body mass index, tobacco smoking, alcohol drinking and nonalcohol energy intake. Reference category: first (lowest) quintile.
Portions per week, interquartile range (IQR) among controls.
Approximate quartile of intake.
Approximate tertile of intake.
Table 3 shows the mean daily intake of selected macronutrients, fatty acids and cholesterol, and the corresponding ORs of OCP cancer. Significant inverse trends in risk were observed for vegetable protein (OR=0.45, for the highest vs the lowest quintile of intake), vegetable fat (OR=0.54) and polyunsaturated fatty acids (OR=0.53), whereas significant increased trends in risk were observed for animal protein (OR=1.57), animal fat (OR=2.47), saturated fatty acids (OR=2.18) and cholesterol (OR=2.29).
Table 3. Odds ratios (ORs)a and corresponding 95% confidence intervals (CIs) according to daily intake of selected macronutrients, fatty acids and cholesterol among 768 cases of oral and pharyngeal cancer and 2078 controls (Italy and Switzerland, 1997–2009).
| |
Quintiles of intake, OR (95% CI) |
|
||||
|---|---|---|---|---|---|---|
| Mean (s.d.)b | II | III | IV | V | χ2trend (P-value) | |
| Total protein (g) |
80.1 (23.4) |
0.84 (0.59–1.18) |
0.70 (0.49–1.02) |
0.65 (0.45–0.95) |
1.15 (0.82–1.63) |
0.00 (0.985) |
| Vegetable protein (g) | 25.6 (10.2) | 0.56 (0.39–0.81) | 0.43 (0.28–0.65) | 0.49 (0.32–0.74) | 0.45 (0.29–0.70) | 10.88 (0.001) |
| Animal protein (g) |
54.5 (16.8) |
0.89 (0.63–1.26) |
0.84 (0.58–1.23) |
1.17 (0.82–1.67) |
1.57 (1.11–2.22) |
7.75 (0.005) |
| Starch (g) |
136.6 (63.4) |
0.77 (0.53–1.11) |
0.75 (0.51–1.10) |
0.77 (0.52–1.15) |
0.77 (0.51–1.17) |
1.15 (0.284) |
| Sugars (g) |
103.8 (52.6) |
0.80 (0.57–1.11) |
0.64 (0.45–0.91) |
0.62 (0.43–0.90) |
1.04 (0.72–1.48) |
0.65 (0.422) |
| Total fat (g) |
80.2 (27.6) |
1.21 (0.88–1.67) |
0.96 (0.67–1.39) |
0.95 (0.64–1.41) |
1.23 (0.84–1.82) |
0.18 (0.676) |
| Vegetable fat (g) | 40.5 (16.7) | 0.80 (0.59–1.10) | 0.79 (0.56–1.11) | 0.40 (0.27–0.61) | 0.54 (0.37–0.78) | 18.89 (<0.0001) |
| Animal fat (g) |
39.7 (16.4) |
1.11 (0.77–1.61) |
1.26 (0.85–1.85) |
1.45 (1.00–2.10) |
2.47 (1.71–3.57) |
23.18 (<0.0001) |
| Saturated fatty acids (g) |
26.6 (10.5) |
1.31 (0.92–1.85) |
1.32 (0.91–1.92) |
1.38 (0.95–2.01) |
2.18 (1.49–3.20) |
13.04 (0.0003) |
| Monounsaturated fatty acids (g) |
34.8 (14.3) |
0.75 (0.52–1.06) |
0.89 (0.63–1.26) |
0.89 (0.62–1.27) |
0.79 (0.56–1.12) |
0.91 (0.340) |
| Polyunsaturated fatty acids (g) |
14.3 (6.8) |
1.52 (1.09–2.10) |
0.83 (0.54–1.26) |
0.85 (0.52–1.38) |
0.53 (0.30–0.91) |
4.03 (0.045) |
| Cholesterol (mg) | 307.4 (116.4) | 1.41 (0.99–2.01) | 1.13 (0.77–1.64) | 1.59 (1.09–2.33) | 2.29 (1.53–3.45) | 13.49 (0.0002) |
Estimated from unconditional logistic regression models adjusted for age, sex, centre, education, year of interview, body mass index, tobacco smoking and alcohol drinking. Nonalcoholic energy adjusted according to the residual model. Reference category: first (lowest) quintile.
Mean portions per week and s.d. among controls.
The mean daily intake of selected micronutrients, and the corresponding ORs of OCP cancer, are given in Table 4. Significant inverse trends in risk were observed for α-carotene (OR=0.51, for the highest vs the lowest quintile of intake), β-carotene (OR=0.28), β-cryptoxanthin (OR=0.37), lutein and zeazanthin (OR=0.34), vitamin E (OR=0.26), vitamin C (OR=0.40) and total folate (OR=0.34). A significant direct trend in risk was observed for retinol (OR=1.88).
Table 4. Odds ratios (ORs)a and corresponding 95% confidence intervals (CIs) according to daily intake of selected micronutrients among 768 cases of oral and pharyngeal cancer and 2078 controls (Italy and Switzerland, 1997–2009).
| |
Quintile of intake, OR (95% CI) |
|
||||
|---|---|---|---|---|---|---|
| Mean (s.d.)b | II | III | IV | V | χ2trend (P-value) | |
|
Fat-soluble vitamins | ||||||
| Retinol (μg) | 1100.8 (1224.4) | 0.90 (0.60–1.36) | 0.99 (0.66–1.50) | 0.87 (0.59–1.29) | 1.88 (1.22–2.90) | 5.99 (0.014) |
| α-Carotene (μg) | 696.6 (607.3) | 1.11 (0.80–1.55) | 1.02 (0.72–1.45) | 0.65 (0.45–0.95) | 0.51 (0.34–0.76) | 15.50 (0.0001) |
| β-Carotene (μg) | 4457.1 (2303.3) | 0.70 (0.49–0.98) | 0.54 (0.37–0.78) | 0.33 (0.22–0.49) | 0.28 (0.18–0.43) | 41.91 (<0.0001) |
| β-Cryptoxanthin (μg) | 463.0 (559.1) | 0.81 (0.58–1.13) | 0.74 (0.53–1.06) | 0.65 (0.45–0.93) | 0.37 (0.24–0.56) | 19.75 (<0.0001) |
| Lycopene (μg) | 4873.2 (3418.4) | 1.33 (0.88–2.02) | 1.15 (0.74–1.80) | 1.39 (0.87–2.22) | 0.92 (0.54–1.55) | 0.24 (0.627) |
| Lutein and zeaxanthin (μg) | 4381.5 (2286.3) | 0.97 (0.70–1.35) | 0.76 (0.54–1.08) | 0.55 (0.38–0.79) | 0.34 (0.23–0.51) | 32.85 (<0.0001) |
| Vitamin D (μg) | 2.6 (1.2) | 0.91 (0.60–1.37) | 1.13 (0.75–1.71) | 1.34 (0.89–2.03) | 1.28 (0.84–1.96) | 2.90 (0.088) |
| Vitamin E (mg) |
13.3 (4.7) |
0.77 (0.53–1.11) |
0.47 (0.31–0.72) |
0.41 (0.26–0.64) |
0.26 (0.16–0.43) |
28.87 (<0.0001) |
|
Water-soluble vitamins | ||||||
| Vitamin C (mg) | 166.5 (110.3) | 0.87 (0.61–1.24) | 0.80 (0.55–1.17) | 0.57 (0.38–0.85) | 0.40 (0.25–0.63) | 18.08 (<0.0001) |
| Thiamin (vit B1) (mg) | 0.8 (0.3) | 0.77 (0.48–1.25) | 0.70 (0.40–1.20) | 0.78 (0.43–1.41) | 0.67 (0.35–1.29) | 0.84 (0.359) |
| Riboflavin (vit B2) (mg) | 1.6 (0.6) | 0.83 (0.55–1.26) | 0.79 (0.50–1.24) | 0.77 (0.47–1.24) | 1.09 (0.65–1.84) | 0.22 (0.641) |
| Niacin (mg) | 17.2 (5.1) | 0.83 (0.53–1.29) | 0.63 (0.39–1.01) | 0.73 (0.44–1.20) | 0.77 (0.45–1.32) | 0.67 (0.413) |
| Vitamin B6 (mg) | 1.9 (0.6) | 0.91 (0.60–1.37) | 0.79 (0.50–1.27) | 0.67 (0.40–1.12) | 0.64 (0.36–1.13) | 2.94 (0.086) |
| Total folate (μg) | 278.7 (97.7) | 0.66 (0.44–0.98) | 0.45 (0.29–0.71) | 0.44 (0.27–0.72) | 0.34 (0.20–0.59) | 14.59 (0.0001) |
Estimated from unconditional logistic regression models adjusted for age, sex, centre, education, year of interview, body mass index, tobacco smoking, alcohol drinking and nonalcohol energy intake.
Mean daily intake and s.d. among controls.
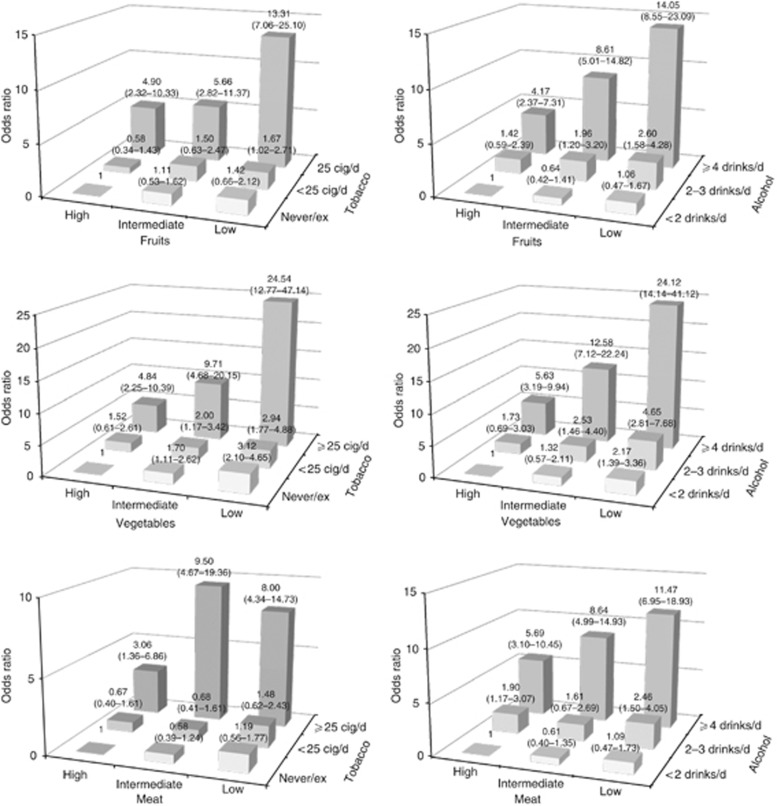
Figure 1 shows the ORs of OCP cancer for combinations of fruits, vegetables and meat and lifestyle habits (tobacco smoking and alcohol drinking). Compared with the never/ex-smokers in the highest intake tertile, current smokers of ⩾25 cigarettes per day in the lowest intake tertile had an OR of 13.31 for fruits and 24.54 for vegetables; as compared with never/ex-smokers in the lowest intake tertile, current smokers of ⩾25 cigarettes per day in the highest intake tertile had an OR of 8.00 for red meat. Compared with drinkers of <2 drinks per day in the highest tertile of intake, drinkers of ⩾4 drinks per day in the lowest intake tertile had an OR of 14.05 for fruits and of 24.12 for vegetables; as compared with drinkers of <2 drinks per day in the lowest tertile of intake, drinkers of ⩾4 drinks per day in the highest intake tertile had an OR of 11.47 for meat.
Figure 1.
Odds ratios (ORs) and 95% confidence intervals (CIs) for combinations of vegetables, fruit or meat with tobacco smoking and alcohol drinking among 768 cases of oral and pharyngeal cancer and 2078 controls. Italy and Switzerland, 1997–2009. Cig/d=cigarettes per day; drinks/d=drinks per day.
Discussion
Our study provides further evidence on the role of diet on OCP cancer risk. We observed a beneficial effect of vegetables and fruits, whereas we found that a diet rich in red meat, eggs, dairy products, potatoes and desserts increased the risk of OCP cancer. Consistently, we found inverse associations with vegetable protein, vegetable fats, polyunsaturated fatty acids and various antioxidant vitamins (such as carotenoids, vitamin C and E and folate), whereas direct associations were observed with animal protein, animal fat, saturated fatty acids, cholesterol and retinol.
Fruits and vegetables have been consistently associated with a reduced risk of OCP cancer in several case–control and cohort studies (Boeing et al, 2006; Lucenteforte et al, 2009; Bradshaw et al, 2012; Bravi et al, 2012), although recent evidence appears less strong and the conclusions of the second WCRF report (World Cancer Research Fund and American Institute for Cancer Research, 2007) were weaker as compared with those of the first one (World Cancer Research Fund and American Institute for Cancer Research, 1997). More recently, the International Head and Neck Cancer Epidemiology (INHANCE) Consortium including 22 case–control studies reported an OR of 0.52 for high fruit consumption and of 0.66 for high vegetable consumption (Chuang et al, 2012).
The protective role of vegetables and fruits on OCP cancer has been attributed to several micronutrients, including carotenoids, vitamin C and E, found to be inversely related to OCP cancer in our study, as well as in many other investigations (Negri et al, 2000; World Cancer Research Fund and American Institute for Cancer Research, 2007). These components display both complementary and overlapping mechanisms of action, including antioxidant effects, binding and dilution of carcinogens in the digestive tract (Potter and Steinmetz, 1996; IARC, 2003). However, data on the relation with single nutrients or food components are more limited and less consistent than those for fruits and vegetables (World Cancer Research Fund and American Institute for Cancer Research, 2007). Thus, the protective effect of plant foods may results from a combination of different nutrients, and it is also possible that a more frequent consumption of fruits and vegetables is a nonspecific indicator of a more affluent and healthy diet (Garavello et al, 2008).
We found a positive association between red meat and OCP risk, but no meaningful association with poultry and processed meat. Meat consumption has been related to an increased risk of OCP cancer, although not all studies provided consistent results (Sapkota et al, 2008; Aune et al, 2009a; Lucenteforte et al, 2009). The INHANCE consortium reported a significant OR of 1.40 for meat consumption (OR=1.37 for processed meat) (Chuang et al, 2012). Similarly, the European Prospective Investigation into Cancer and Nutrition (EPIC) including 682 upper aerodigestive tract cancers reported a positive association for total meat intake that was, however, mainly attributable to processed meat (RR=1.41) (Steffen et al, 2012). The unfavourable role of meat on cancers of the upper aerodigestive tract may be attributed to its content of animal fat and cholesterol, which were positively associated with OCP cancer in our study. Other possible mechanisms include the carcinogenity of nitrites and N-nitroso compounds contained particularly in processed meat in the past (Grosse et al, 2006; Keszei et al, 2013) and the mutagen role of heterocyclic amines and polycyclic aromatic hydrocarbons developed during cooking (Phillips, 1999; Sinha, 2002; Zheng and Lee, 2009). An analysis of meat and cancer risk – including part of the data of the present study – according to cooking methods showed stronger excess risk of OCP cancer for fried meat as compared with roasted/grilled or boiled/stewed meat (Di Maso et al, 2013).
We found increased risks of OCP cancer for eggs and milk and dairy products, whereas we found no association with fish consumption. Several studies suggested a possible increased risk relation with eggs, in the absence, however, of consistent results (World Cancer Research Fund and American Institute for Cancer Research, 2007; Aune et al, 2009b; Lucenteforte et al, 2009). Similarly, the evidence on the role of milk and other dairy products on OCP cancer is inconsistent, with several studies reporting both direct and inverse relationships (World Cancer Research Fund and American Institute for Cancer Research, 2007; Peters et al, 2008; Sapkota et al, 2008; Lucenteforte et al, 2009). A beneficial effect of fish on OCP cancer has been suggested by several studies (particularly case–control ones), but a few other studies provided direct or null associations (World Cancer Research Fund and American Institute for Cancer Research, 2007; Lucenteforte et al, 2009).
We also found positive associations with soups, potatoes and desserts, on which, however, data are scanty and inconsistent (Lucenteforte et al, 2009). A significant increased risk of OCP cancer in relation to soup consumption has also been reported in another Italian case–control study, and can be explained by thermal injury if soups are eaten hot (Chyou et al, 1995; Franceschi et al, 1999a); frequent consumption of soups may, however, also be an indicator of poor dentition. The positive relation with desserts (and sugars) may be related to their high glycaemic index and load (Augustin et al, 2003). However, in our study no association was evident with cereals, sugars and starch. Thus, these positive associations should be cautiously interpreted.
Limitations of our study are possible bias of case–control studies (Breslow and Day, 1980). Bias in the recall of dietary information should be limited, as the awareness of dietary hypothesis on OCP cancer is limited in our population. Moreover, this bias is less relevant in hospital-based studies (D'Avanzo et al, 1997). Dietary habits of hospital controls may be not representative of the general population, although we selected controls among patients with acute conditions not associated with long-term dietary modifications, and we excluded all diagnoses that might have been associated with tobacco smoking and alcohol drinking. This, together with the almost complete participation rate among cases and controls, is reassuring against a major role of selection bias. Among the strengths of our study are the large data set, the similar catchment areas of cases and controls and the satisfactory validity and reproducibility of information collected on dietary habits (Franceschi et al, 1993, 1995; Decarli et al, 1996).
In conclusion, there is possible evidence that a diet rich in fruit and vegetables and poor in meat and products of animal origin has a favourable role against OCP cancer. This is also in line with information from a few studies that analysed the overall impact of diet in relation to OCP using a priori-defined scores (including the Mediterranean diet score) (Bosetti et al, 2003) or a posteriori dietary patterns (Bravi et al, 2012). Moreover, our data further quantify the strong detrimental effect of the combination of low consumption of fruit and vegetables or high consumption of meat with high exposure to tobacco and alcohol (Toporcov et al, 2012), with 10- to over 20-fold excess risks of OCP cancer.
Acknowledgments
This work was conducted with the contribution of the Italian Association for Cancer Research (Grant No. 10068), and the Swiss League Against Cancer and the Swiss Research Against Cancer/OncoSuisse (KFS-700 and OCS-1633). We thank Mrs Ivana Garimoldi for editorial assistance.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Augustin LS, Gallus S, Franceschi S, Negri E, Jenkins DJ, Kendall CW, Dal Maso L, Talamini R, La Vecchia C. Glycemic index and load and risk of upper aero-digestive tract neoplasms (Italy) Cancer Causes Control. 2003;14:657–662. doi: 10.1023/a:1025676907942. [DOI] [PubMed] [Google Scholar]
- Aune D, De Stefani E, Ronco A, Boffetta P, Deneo-Pellegrini H, Acosta G, Mendilaharsu M. Meat consumption and cancer risk: a case-control study in Uruguay. Asian Pac J Cancer Prev. 2009;10:429–436. [PubMed] [Google Scholar]
- Aune D, De Stefani E, Ronco AL, Boffetta P, Deneo-Pellegrini H, Acosta G, Mendilaharsu M. Egg consumption and the risk of cancer: a multisite case-control study in Uruguay. Asian Pac J Cancer Prev. 2009;10:869–876. [PubMed] [Google Scholar]
- Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer. 2001;85:1700–1705. doi: 10.1054/bjoc.2001.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeing H, Dietrich T, Hoffmann K, Pischon T, Ferrari P, Lahmann PH, Boutron-Ruault MC, Clavel-Chapelon F, Allen N, Key T, Skeie G, Lund E, Olsen A, Tjonneland A, Overvad K, Jensen MK, Rohrmann S, Linseisen J, Trichopoulou A, Bamia C, Psaltopoulou T, Weinehall L, Johansson I, Sanchez MJ, Jakszyn P, Ardanaz E, Amiano P, Chirlaque MD, Quiros JR, Wirfalt E, Berglund G, Peeters PH, van Gils CH, Bueno-de-Mesquita HB, Buchner FL, Berrino F, Palli D, Sacerdote C, Tumino R, Panico S, Bingham S, Khaw KT, Slimani N, Norat T, Jenab M, Riboli E. Intake of fruits and vegetables and risk of cancer of the upper aero-digestive tract: the prospective EPIC-study. Cancer Causes Control. 2006;17:957–969. doi: 10.1007/s10552-006-0036-4. [DOI] [PubMed] [Google Scholar]
- Bosetti C, Bertuccio P, Malvezzi M, Levi F, Chatenoud L, Negri E, La Vecchia C. Cancer mortality in Europe, 2005–2009, and an overview of trends since 1980. Ann Oncol. 2013;24:2657–2671. doi: 10.1093/annonc/mdt301. [DOI] [PubMed] [Google Scholar]
- Bosetti C, Gallus S, Trichopoulou A, Talamini R, Franceschi S, Negri E, La Vecchia C. Influence of the Mediterranean diet on the risk of cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 2003;12:1091–1094. [PubMed] [Google Scholar]
- Bradshaw PT, Siega-Riz AM, Campbell M, Weissler MC, Funkhouser WK, Olshan AF. Associations between dietary patterns and head and neck cancer: the Carolina head and neck cancer epidemiology study. Am J Epidemiol. 2012;175:1225–1233. doi: 10.1093/aje/kwr468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravi F, Edefonti V, Randi G, Ferraroni M, La Vecchia C, Decarli A. Dietary patterns and upper aerodigestive tract cancers: an overview and review. Ann Oncol. 2012;23:3024–3039. doi: 10.1093/annonc/mds197. [DOI] [PubMed] [Google Scholar]
- Breslow NE, Day NE. Statistical Methods in Cancer Research. Vol. I. The Analysis of Case-Control Studies. IARC Sci Publ No. 32. IARC: Lyon, France; 1980. [PubMed] [Google Scholar]
- Chuang SC, Jenab M, Heck JE, Bosetti C, Talamini R, Matsuo K, Castellsague X, Franceschi S, Herrero R, Winn DM, La Vecchia C, Morgenstern H, Zhang ZF, Levi F, Dal Maso L, Kelsey K, McClean MD, Vaughan T, Lazarus P, Muscat J, Ramroth H, Chen C, Schwartz SM, Eluf-Neto J, Hayes RB, Purdue M, Boccia S, Cadoni G, Zaridze D, Koifman S, Curado MP, Ahrens W, Benhamou S, Matos E, Lagiou P, Szeszenia-Dabrowska N, Olshan AF, Fernandez L, Menezes A, Agudo A, Daudt AW, Merletti F, Macfarlane GJ, Kjaerheim K, Mates D, Holcatova I, Schantz S, Yu GP, Simonato L, Brenner H, Mueller H, Conway DI, Thomson P, Fabianova E, Znaor A, Rudnai P, Healy CM, Ferro G, Brennan P, Boffetta P, Hashibe M. Diet and the risk of head and neck cancer: a pooled analysis in the INHANCE consortium. Cancer Causes Control. 2012;23:69–88. doi: 10.1007/s10552-011-9857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyou PH, Nomura AM, Stemmermann GN. Diet, alcohol, smoking and cancer of the upper aerodigestive tract: a prospective study among Hawaii Japanese men. Int J Cancer. 1995;60:616–621. doi: 10.1002/ijc.2910600508. [DOI] [PubMed] [Google Scholar]
- D'Avanzo B, La Vecchia C, Katsouyanni K, Negri E, Trichopoulos D. An assessment, and reproducibility of food frequency data provided by hospital controls. Eur J Cancer Prev. 1997;6:288–293. doi: 10.1097/00008469-199706000-00006. [DOI] [PubMed] [Google Scholar]
- Decarli A, Franceschi S, Ferraroni M, Gnagnarella P, Parpinel MT, La Vecchia C, Negri E, Salvini S, Falcini F, Giacosa A. Validation of a food-frequency questionnaire to assess dietary intakes in cancer studies in Italy. Results for specific nutrients. Ann Epidemiol. 1996;6:110–118. doi: 10.1016/1047-2797(95)00129-8. [DOI] [PubMed] [Google Scholar]
- Di Maso M, Talamini R, Bosetti C, Montella M, Zucchetto A, Libra M, Negri E, Levi F, La Vecchia C, Franceschi S, Serraino D, Polesel J.2013Red meat and cancer risk in a network of case–control studies focusing on cooking practices Ann Oncole-pub ahead of print 11 October 2013doi: 10.1093/annonc/mdt392 [DOI] [PubMed]
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Franceschi S, Barbone F, Negri E, Decarli A, Ferraroni M, Filiberti R, Giacosa A, Gnagnarella P, Nanni O, Salvini S, La Vecchia C. Reproducibility of an Italian food frequency questionnaire for cancer studies. Results for specific nutrients. Ann Epidemiol. 1995;5:69–75. doi: 10.1016/1047-2797(95)92893-d. [DOI] [PubMed] [Google Scholar]
- Franceschi S, Favero A, Conti E, Talamini R, Volpe R, Negri E, Barzan L, La Vecchia C. Food groups, oils and butter, and cancer of the oral cavity and pharynx. Br J Cancer. 1999;80:614–620. doi: 10.1038/sj.bjc.6690400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi S, Levi F, Conti E, Talamini R, Negri E, Dal Maso L, Boyle P, Decarli A, La Vecchia C. Energy intake and dietary pattern in cancer of the oral cavity and pharynx. Cancer Causes Control. 1999;10:439–444. doi: 10.1023/a:1008918104757. [DOI] [PubMed] [Google Scholar]
- Franceschi S, Negri E, Salvini S, Decarli A, Ferraroni M, Filiberti R, Giacosa A, Talamini R, Nanni O, Panarello G, La Vecchia C. Reproducibility of an Italian food frequency questionnaire for cancer studies: results for specific food items. Eur J Cancer. 1993;29:2298–2305. doi: 10.1016/0959-8049(93)90225-5. [DOI] [PubMed] [Google Scholar]
- Garavello W, Giordano L, Bosetti C, Talamini R, Negri E, Tavani A, Maisonneuve P, Franceschi S, La Vecchia C. Diet diversity and the risk of oral and pharyngeal cancer. Eur J Nutr. 2008;47:280–284. doi: 10.1007/s00394-008-0722-y. [DOI] [PubMed] [Google Scholar]
- Gnagnarella P, Parpinel M, Salvini S, Franceschi S, Palli D, Boyle P. The update of the Italian food composition database. J Food Comp Analysis. 2004;17:509–522. [Google Scholar]
- Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of nitrate, nitrite, and cyanobacterial peptide toxins. Lancet Oncol. 2006;7:628–629. doi: 10.1016/s1470-2045(06)70789-6. [DOI] [PubMed] [Google Scholar]
- IARC IARC Handbooks of Cancer Prevention. IARC: Lyon; Fruit and Vegetables. 2003;Vol. 8 [Google Scholar]
- IARC IARC monographs on the evaluation of carcinogenic risk in humans. International Agency for Research on Cancer: Lyon; Tobacco Smoke and Involuntary Smoking. 2004;Vol. 83 [PMC free article] [PubMed] [Google Scholar]
- IARC IARC Monographs on the evaluation of carcinogenic risks to humans. International Agency for Research on Cancer: Lyon; Alcohol Consumption and Ethylcarbamate. 2010;Vol. 96 [Google Scholar]
- Keszei AP, Goldbohm RA, Schouten LJ, Jakszyn P, van den Brandt PA. Dietary N-nitroso compounds, endogenous nitrosation, and the risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Am J Clin Nutr. 2013;97:135–146. doi: 10.3945/ajcn.112.043885. [DOI] [PubMed] [Google Scholar]
- Lucenteforte E, Garavello W, Bosetti C, La Vecchia C. Dietary factors and oral and pharyngeal cancer risk. Oral Oncol. 2009;45:461–467. doi: 10.1016/j.oraloncology.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Negri E, Franceschi S, Bosetti C, Levi F, Conti E, Parpinel M, La Vecchia C. Selected micronutrients and oral and pharyngeal cancer. Int J Cancer. 2000;86:122–127. doi: 10.1002/(sici)1097-0215(20000401)86:1<122::aid-ijc19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Peters ES, Luckett BG, Applebaum KM, Marsit CJ, McClean MD, Kelsey KT. Dairy products, leanness, and head and neck squamous cell carcinoma. Head Neck. 2008;30:1193–1205. doi: 10.1002/hed.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res. 1999;443:139–147. doi: 10.1016/s1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Potter JD, Steinmetz K. Vegetables, fruit and phytoestrogens as preventive agents. IARC Sci Publ No. 139. 1996. pp. 61–90. [PubMed]
- Sapkota A, Hsu CC, Zaridze D, Shangina O, Szeszenia-Dabrowska N, Mates D, Fabianova E, Rudnai P, Janout V, Holcatova I, Brennan P, Boffetta P, Hashibe M. Dietary risk factors for squamous cell carcinoma of the upper aerodigestive tract in central and eastern Europe. Cancer Causes Control. 2008;19:1161–1170. doi: 10.1007/s10552-008-9183-0. [DOI] [PubMed] [Google Scholar]
- Sinha R. An epidemiologic approach to studying heterocyclic amines. Mutat Res. 2002;506-507:197–204. doi: 10.1016/s0027-5107(02)00166-5. [DOI] [PubMed] [Google Scholar]
- Steffen A, Bergmann MM, Sanchez MJ, Chirlaque MD, Jakszyn P, Amiano P, Quiros JR, Barricarte Gurrea A, Ferrari P, Romieu I, Fedirko V, Bueno-de-Mesquita HB, Siersema PD, Peeters PH, Khaw KT, Wareham N, Allen NE, Crowe FL, Skeie G, Hallmanns G, Johansson I, Borgquist S, Ericson U, Egeberg R, Tjonneland A, Overvad K, Grote V, Li K, Trichopoulou A, Oikonomidou D, Pantzalis M, Tumino R, Panico S, Palli D, Krogh V, Naccarati A, Mouw T, Vergnaud AC, Norat T, Boeing H. Meat and heme iron intake and risk of squamous cell carcinoma of the upper aero-digestive tract in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Epidemiol Biomarkers Prev. 2012;21:2138–2148. doi: 10.1158/1055-9965.EPI-12-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toporcov TN, Tavares GE, Rotundo LD, Vaccarezza GF, Biazevic MG, Brasileiro RS, De Carvalho MB, JMichaluart unior P, Kowalski LP, Antunes JL. Do tobacco and alcohol modify protective effects of diet on oral carcinogenesis. Nutr Cancer. 2012;64:1182–1189. doi: 10.1080/01635581.2012.721155. [DOI] [PubMed] [Google Scholar]
- Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund and American Institute for Cancer Research . Food, Nutrition and the Prevention of Cancer: A Global Perspective. American Institute for Cancer Research: Washington, DC; 1997. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund and American Institute for Cancer Research . Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. American Institute for Cancer Research: Washington, DC; 2007. [Google Scholar]
- Zheng W, Lee SA. Well-done meat intake, heterocyclic amine exposure, and cancer risk. Nutr Cancer. 2009;61:437–446. doi: 10.1080/01635580802710741. [DOI] [PMC free article] [PubMed] [Google Scholar]