Abstract
Background:
We previously showed that inhibitor of growth family member 4 (ING4) inhibits melanoma angiogenesis, and JWA suppresses the metastasis of melanoma cells. As angiogenesis is essential for tumour metastasis, further investigation of the function of ING4 and JWA in melanoma angiogenesis is needed, and their prognostic value are of great interest.
Methods:
Western blot, tube-formation assays and luciferase assays were used to investigate the correlation between ING4 and JWA in melanoma angiogenesis. JWA and integrin-linked kinase (ILK) expression was determined on a tissue microarray constructed from 175 biopsies.
Results:
ING4 promoted JWA expression by activating JWA promoter. Furthermore, the regulation of growth and tube formation of endothelial cells by ING4 was partially JWA dependent. Also, ING4 inhibited the ILK-induced angiogenesis signalling pathway via JWA. Moreover, reduced JWA, or increased ILK, expression was closely associated with 5-year disease-specific survival of melanoma patients (P=0.001 and 0.007, respectively). There was also a positive correlation between ING4 and JWA yet a negative correlation between ING4 and ILK. Importantly, their concomitant expressions were significantly related to 5-year survival of melanoma patients (P=0.002 and 0.003, respectively).
Conclusion:
JWA has an important role in ING4-regulated melanoma angiogenesis, and ING4/JWA/ILK are promising prognostic markers and may be used as anti-angiogenic therapeutic targets for melanoma.
Keywords: ING4, JWA, melanoma, angiogenesis, prognosis, ILK
Melanoma is the most lethal form of skin cancer, with a high capacity for metastasis. The incidence of melanoma has significantly increased over the last 30 years (Siegel et al, 2012). Once metastasis occurs, the 5-year survival rate of patients drops to <10% (Siegel et al, 2012). The underlying mechanisms that regulate melanoma progression and metastasis are still poorly understood. However, angiogenesis is known to be essential for tumour growth and metastasis and the factors driving this process may be important candidates for therapeutic exploitation.
ING4 is a member of the inhibitor of growth (ING) family of tumour suppressors, which comprises 5 members (ING1–ING5; Wang and Li, 2006). The ING4 gene is located on 12p13.31 and has been shown to have an important role in inhibiting tumour cell proliferation (Garkavtsev et al, 2004; Gunduz et al, 2005; Kim, 2005; Li et al, 2008; Moreno et al, 2010). ING4 also modulates melanoma angiogenesis by inhibiting the activity of HIF1a via binding to HIF prolyl hydroxylase (Ozer and Bruick, 2005; Ozer et al, 2005). Meanwhile, ING4 is frequently mutated and its expression is decreased in various types of human cancers (Colla et al, 2007; Lou et al, 2012). Our previous study demonstrated that ING4 expression was significantly decreased when dysplastic nevi transformed to malignant melanomas. We also illustrated that ING4 overexpression suppressed melanoma cell migration and invasion (Li et al, 2008). Recently, we have also confirmed that ING4-inhibited melanoma angiogenesis by suppressing NF-κB activity and IL-6 expression (Li and Li, 2010). However, the exact function of ING4 in melanoma angiogenesis still deserves further investigation.
JWA, also known as ADP-ribosylation-like factor 6 interacting protein 5, was initially cloned from human tracheal bronchial epithelial cells (Zhou et al, 1999). JWA is a structurally novel microtubule-associated protein, regulating cancer cell migration by the MAPK signalling pathway (Chen et al, 2007a). Our previous data showed that JWA inhibited the metastasis of melanoma cells by inhibiting integrin αVβ3 signalling (Bai et al, 2010). Angiogenesis is a hallmark for tumour metastasis, yet the potential role of JWA in tumour angiogenesis has not been elucidated, and the role of JWA in melanoma progression also remains unknown.
Integrin-linked kinase (ILK) is a multifunctional intracellular serine/threonine kinase that induces anchorage-independent cell growth, cell proliferation, metastasis and angiogenesis (Hannigan et al, 2005; Legate et al, 2006). We have previously confirmed that ILK upregulation is closely correlated with melanoma invasion and progression, and inversely correlated with 5-year survival of melanoma patients (Dai et al, 2003). We have also demonstrated that ILK promoted melanoma angiogenesis by upregulating NF-κB/IL-6/STAT3/VEGF signalling pathways (Wani et al, 2011). However, the molecular mechanism of elevated ILK expression in melanoma is not clear, and the regulation of ILK in melanoma angiogenesis is still not well understood.
Here, we investigated the correlation between ING4 and JWA in melanoma angiogenesis and evaluated their prognostic value in melanoma patients. We found that ING4 is the upstream regulator of JWA; promoting JWA expression by activating its promoter. We also demonstrate that ING4 regulated cell growth and tube formation of endothelial cells was partially JWA dependent. Furthermore, the ILK angiogenesis signalling was inhibited by ING4 through JWA. Moreover, the upregulation of JWA, or the downregulation of ILK expression, was closely associated with melanoma patient survival. As a result, the concomitant expressions of ING4 and JWA, or ING4 and ILK, were significantly related to the 5-year survival of melanoma patients. These findings suggested that ING4/JWA/ILK might be used as promising prognostic markers and may be anti-angiogenic therapeutic targets for melanoma patients.
Materials and methods
Study approval
The use of human skin tissues in this study was approved by the Clinical Research Ethics Board of University of British Columbia (CREB study ID H09-01321). The study was conducted according to the principles expressed in the Declaration of Helsinki.
Cell lines, plasmids and transfection
The human melanoma cell lines MMRU and MMLH, and 293T cells were cultured in Dulbecco's Modified Eagle's Medium (Hyclone, South Logan, UT, USA) supplemented with 10% fetal bovine serum (HyClone). Human umbilical vein endothelial cells (HUVECs) were cultured in endothelial cell medium (ScienCell, Carlsbad, CA, USA). HA-ING4, miING4 vector, 3 × Flag-JWA and shJWA were generated as described previously (Bai et al, 2010; Li and Li, 2010). pcDNA3.1-ILK plasmid was kindly provided by Dr Shoukat Dedhar (University of British Columbia). Four different truncated human JWA luciferase reporter promoter plasmids (−595/+107, −194/+107, −107/+107, −27/+107) were previously described (Chen et al, 2007b). For plasmid transfection, MMRU or MMLH cells were grown to approximately 60% confluency then transfected with the above plasmids with Effectene reagent (Qiagen, Mississauga, ON, Canada). After transfection plus 48 h, cells were collected for use.
Quantitative real-time RT–PCR assay
Total RNA of cells was extracted using the QIAzol lysis reagent (Qiagen). In all, 1 μg RNA was used for the reverse transcription reaction with a cDNA Synthesis Kit (Roche, Basel, Switzerland). qPCR was carried out in triplicate with SYBR Green PCR Master Mix (Roche) using a 7900HT qPCR system thermal cycler (Applied Biosystems, Foster, CA, USA) as recommended by the manufacturer. GAPDH was used as an internal control. Primer sequences are listed below: 5′-CACAGACCTGGCCCGTTTT-3′ (forward) and 5′-AGTCCGGCCTTTCTTTTTGC-3′ (reverse) for ING4; 5′-CGCCTGGGACGATTTCTT-3′ (forward) and 5′-TGGAAATGTCCCTGAAGT-3′ (reverse) for JWA; 5′-GACATTGTCGTGAAGGTGCTGAA-3′ (forward) and 5′-GCACTGGGAGCACATTTGGA-3′ (reverse) for ILK; 5′-TGAGGAACAGGGCAATAGTATGATG-3′ (forward) and 5′-GGTCTTACGCCCAAAAGTTAAAAGT-3′ (reverse) for IKK; 5′-TAGTGAGGAACAAGCCAGAGC-3′ (forward) and 5′-TGGCATTTGTGGTTGGGTCA-3′ (reverse) for IL-6; 5′-CCTCCGAAACCATGAACTTT-3′ (forward) and 5′-CCACTTCGTGATGATTCTGC-3′ (reverse) for VEGF; 5′-GAAGGCTGGGGCTCATTT-3′ (forward) and 5′-CAGGAGGCATTGCTGATGAT-3′ (reverse) for GAPDH.
Western blot
Protein extracts for western blot were prepared with lysis buffer (10 mM HEPES pH 8.0, 10 mM KCl, 1.5 mM MgCl2, 0.5 mM DTT, 0.2 mM EDTA) and complete protease inhibitor cocktail tablets. Protein concentration was checked by Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). Western blot was performed as previously described (Karst et al, 2009). The following antibodies were used in this study: anti-ING4 (Novus Biologicals, Littleton, CO, USA), anti-ILK, anti-STAT3 (Cell Signaling Technology, Boston, MA, USA), anti-p-Ser32-Ser36 IkBα, and anti-IkBα (Novus Biologicals), anti-p-S536 p65, and anti-p65 (Abcam Inc., Toronto, ON, Canada), anti-p50, and anti-VEGF (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-JWA (Abmax Biotechnology, Beijing, China), anti-Flag-tag (Applied Biological Materials Inc., Vancouver, BC, Canada), and anti-actin (Sigma, St Louis, MO, USA). Signals were detected with the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA).
HUVECs growth and tube formation assays
MMRU and MMLH cells were cultured in six-well plates to approximately 70% confluency with fresh, serum-free medium for 24 h and 1 ml of conditioned medium (CM) was collected. For HUVECs growth assay, HUVECs were seeded in 96-well plates at 5 × 103 per well and cultured in fresh ECM for 24 h, and then in 100 μl CM for another 24 h before a sulforhodamine B (SRB) assay was performed as previously described (Li et al, 1998). Tube formation assay was performed as previously described (Wani et al, 2011). Matrigel (BD Biosciences, Mississauga, ON, Canada) was coated on 96-well plates and kept at 37 °C for 2 h. Then, 2 × 104 HUVECs were suspended in 100 μl CM from MMRU and MMLH cells and added to the pre-coated 96-well plates. HUVECs tube formation was examined after incubation at 37 °C for 10 h. The cells were photographed by microscope and the tubular structures were counted in randomly selected fields.
Luciferase reporter assays
293T cells were seeded in triplicate in 24-well plates (1 × 105 per well) and transiently transfected with JWA promoter plasmids (−595/+107, −194/+107, −107/+107, −27/+107) and ING4 overexpression plasmid. To normalise for varying transfection efficiency, cells were co-transfected with the renilla plasmid pRL-SV40. Luciferase activities were determined 48 h later using the Dual-Luciferase Reporter assay system (Promega, Madison, WI, USA).
Immunofluorescence
Cells were fixed with 1% paraformaldehyde in 1 × PBS for 10 min at room temperature and permeabilised with methanol/acetone (1 : 1) at −20 °C for 10 min, then blocked with 5% bovine serum albumin in 1 × phosphate-buffered saline tween-20 or 1 × tris-buffered saline tween-20. The slides were incubated with anti-p65 or p-p65 antibody diluted 1 : 100 and incubated 1 h at room temperature, followed by incubation with Alexa Fluor 488 anti-mouse and Alexa Fluor 568 anti-rabbit secondary antibodies (Invitrogen, Carlsbad, CA, USA). The images were obtained by a LSM 780 laser scanning confocal microscope equipped with the ZEN software (Carl Zeiss, Toronto, ON, Canada).
Tissue microarray (TMA) construction
To compare with previous TMA data for ING4, we performed immunostaining of both JWA and ILK using the same TMA, which was used to examine ING4 expression in melanocytic lesions (Li et al, 2008). For the TMA study, formalin-fixed and paraffin-embedded biopsies were obtained from the 1990–1998 archives of the Department of Pathology at Vancouver General Hospital. A total of 237 biopsies, including 66 dysplastic nevi, 118 primary melanomas and 53 metastatic melanomas, were used for TMA construction. Owing to loss of biopsy cores, 45 dysplastic nevi, 81 primary melanomas and 49 metastatic melanomas could be evaluated for JWA and ILK staining. Clinicopathological data were available for all melanoma cases.
Immunohistochemistry
Immunohistochemistry procedure was performed as described previously (Jafarnejad et al, 2010). TMA slides were dewaxed at 55 °C for 30 min and washed three times with xylene. Tissues were rehydrated by a series of washes in 100%, 95%, and 80% ethanol, followed by two washes in distilled water. Antigen retrieval was performed by heating the samples at 95 °C for 30 min in 10 mmol l–1 sodium citrate (pH 6.0). After inactivating the endogenous peroxidase by incubating in 3% H2O2 for 30 min and blocking with universal blocking serum for 30 min, slides were incubated with a primary monoclonal mouse anti-JWA antibody (1 : 30, Abmax Biotechnology), or a polyclonal rabbit anti-ILK antibody (1 : 100, Cell Signaling) at 4 °C overnight. The slides were then incubated with biotin-labelled secondary antibody and streptavidin-peroxidase for 30 min each, followed by developing with diaminobenzidine substrate kit (DAKO Diagnostics, Glostrup, Denmark) and counterstained with haematoxylin.
Evaluation of immunostaining
The evaluation of JWA and ILK staining was blindly and independently examined by two observers, including one dermatopathologist. In the few cases with discrepancy between the two observers, the immunostained slides were reviewed in a double viewing microscope so that the discrepancy was settled. JWA or ILK staining intensity was scored as 0, 1+, 2+ and 3+. The percentage of JWA or ILK-positive cells was also scored into four categories: 1 (0–25%), 2 (26–50%), 3 (51–75%) and 4 (76–100%). On the basis of the immunoreactive score, the staining pattern was defined as: negative (0), weak (1), moderate (2–6) and strong (8–12). We grouped negative and weak staining as low expression, and moderate and strong staining as high expression because the optimal value of cutoff points for the JWA scores was identified as 2 based on the predictive value of this cutoff point for patient survival.
Statistical analysis
In cell-based models, the data represent mean±s.d. from three independent experiments. Statistical analysis was performed by Student's t-test. A P-value of <0.05 was considered significant. Differences in the demographic and clinical characteristics and expression levels of JWA and ILK were evaluated by χ2 tests between patient subgroups. The Kaplan–Meier method and log-rank test were used to evaluate the correlation of ING4, JWA and ILK expression with the survival of patients. SPSS version 16 (SPSS Inc., Chicago, IL, USA) software was used for all analyses.
Results
ING4 positively regulates JWA via JWA promoter
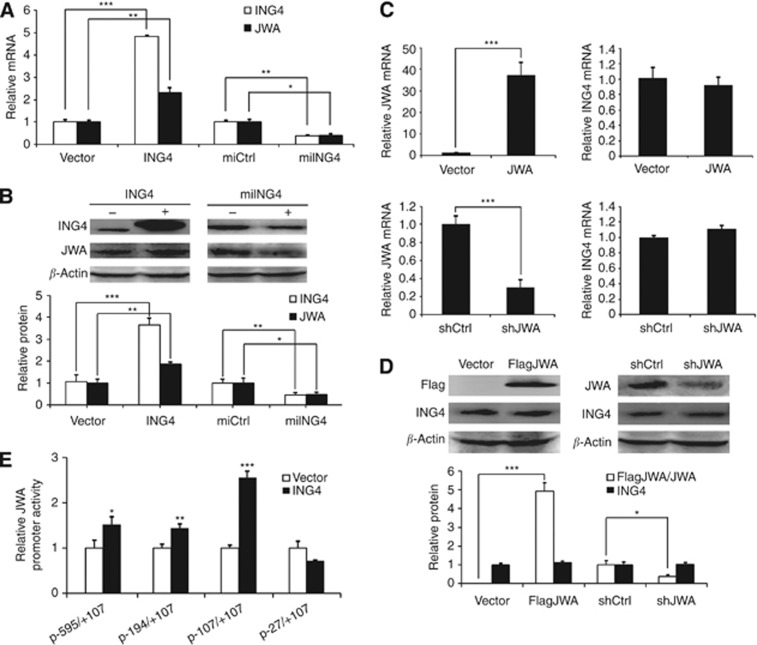
To check the correlation between ING4 and JWA, we overexpressed or knocked down ING4 in MMRU cells and found that ING4 overexpression clearly promoted, whereas ING4 knockdown (KD) inhibited, JWA expression at both mRNA and protein levels (Figure 1A and B). In contrast, both JWA overexpression and JWA KD did not affect ING4 expression at mRNA and protein levels (Figure 1C and D).
Figure 1.
ING4 is upstream of JWA, and regulates JWA through the JWA promoter. (A) mRNA levels of ING4 and JWA in ING4 overexpression or KD MMRU cells were detected by real-time PCR. (B) Western blot analysis of the protein levels of ING4 and JWA in ING4 overexpression or KD MMRU cells. (C) mRNA levels of JWA and ING4 in JWA overexpression or KD MMRU cells were detected by real-time PCR. (D) Western blot analysis of the protein levels of JWA and ING4 in JWA overexpression or KD MMRU cells. (E) 293T cells were transiently transfected with HA-ING4 plasmid and transfected with four different truncated human JWA luciferase reporter promoter plasmids respectively (−595/+107, −194/+107, −107/+107, −27/+107). Whole-cell extracts were prepared 48 h after transfection, and luciferase activity was measured by luminometry. *P<0.05; **P<0.01; ***P<0.001, compared with vector control group. Student's t-test.
To further investigate the mechanism how ING4 regulates JWA at the transcriptional level, we performed a dual-luciferase reporter assay to test whether the activity of the JWA promoter is regulated by ING4. ING4 overexpressing 293T cells were transfected with JWA promoter fragments −595/+107, −194/+107, −107/+107, −27/+107. The data showed that ING4 overexpression significantly enhanced the activity of JWA promoter fragments −595/+107, −194/+107, −107/+107 (P<0.05, P<0.01, P<0.001, respectively; Figure 1E) compared with controls. Specifically, ING4 overexpression exhibited a 2.56-fold increase in the activity of fragment −107/+107 compared with the control. However, this promotion was abolished in fragment −27/+107, suggesting that JWA promoter fragment −107/−27 had an important role for transcriptional activity of the JWA promoter induced by ING4.
ING4-regulated cell growth and tube formation of HUVECs is partially JWA dependent
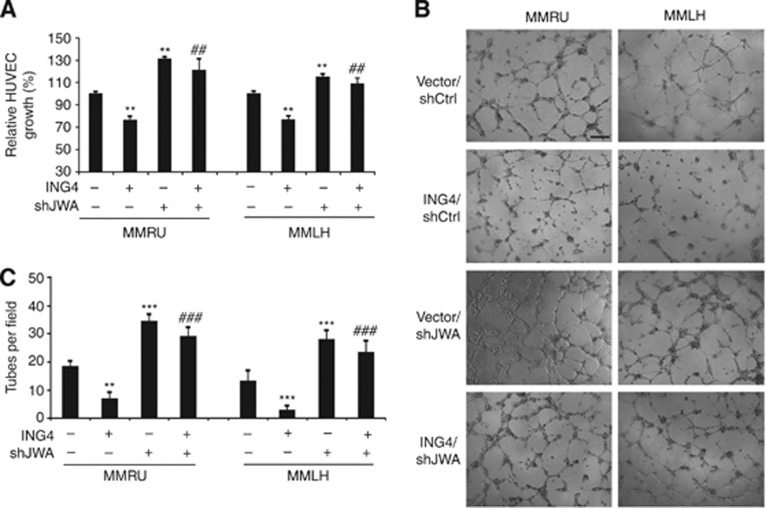
Angiogenesis is a multistep process, which includes endothelial cell proliferation, migration and the formation of blood vessels. We previously reported that ING4 overexpression strongly suppressed the cell growth and tube formation of HUVECs (Li and Li, 2010). In this study, we illustrated that JWA was the downstream target of ING4. We further investigated the relationship between the regulation of HUVEC cell growth by ING4 and JWA expression. We conducted the SRB assay by using the CM from with ING4 overexpression, JWA KD, a simultaneous treatment of ING4 overexpression and JWA KD, and control vector, in MMRU and MMLH cells. The results showed that ING4 overexpression significantly inhibited the growth of HUVECs (P<0.01 for both; Figure 2A), and this inhibition could be rescued by JWA KD to some extent (P<0.01 for both; Figure 2A), suggesting that ING4-inhibited HUVEC growth was partially JWA dependent.
Figure 2.
ING4 regulates cell growth and tube formation of HUVECs partially via JWA. The angiogenic potential of melanoma cells was determined by the cell growth and the tube formation assay of endothelial cells. (A) The CM were collected from MMRU and MMLH cells with ING4 overexpression, JWA KD, ING4 overexpression/JWA KD and added to HUVECs on 24-well plates to detect HUVECs growth by SRB assay. (B) In all, 2 × 104 HUVECs were suspended in 100 μl CM then plated on matrigel incubated 96-well plates to allow for capillary tube formation (scale bar 40 μm). (C) The number of tubes was counted in five random fields for each group. **P<0.01; ***P<0.001, compared with vector control group. ##P<0.01, ###P<0.001, compared with ING4 overexpression group. Student's t-test.
To further investigate whether JWA has an essential role in ING4-inhibited melanoma angiogenesis, we performed the tube formation assay with HUVECs in matrigel using the CM from MMRU and MMLH cells with overexpressing ING4, or JWA KD, or a simultaneous treatment of ING4 overexpression and JWA KD, or control vectors. We found that the CM from JWA KD melanoma cells clearly promoted the tube formation of HUVECs (P<0.001 for both; Figure 2B and C). In contrast, the CM from ING4 overexpressing melanoma cells significantly reduced the tube formation of HUVECs (P<0.01, P<0.001; respectively; Figure 2B and C). This inhibition, on the other hand, could be rescued by JWA KD (P<0.001 for both; Figure 2B and C). Based on these data, we conclude that ING4 regulates cell growth and tube formation of HUVECs that is partially dependent on JWA expression.
ING4-regulated cell growth and tube formation of HUVECs is partially ILK dependent
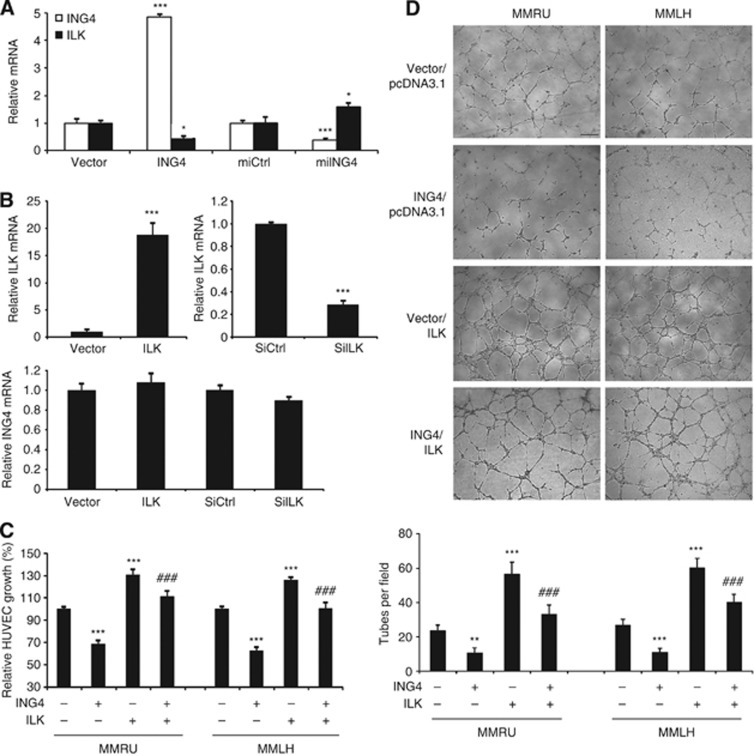
Our previous research showed that ILK expression was enhanced in melanoma cells that promoted melanoma angiogenesis (Wani et al, 2011). However, the regulation of ILK is still not well understood. In this study, we found that ING4 overexpression inhibited, whereas ING4 KD increased, ILK at mRNA level (Figure 3A). We also found that neither ILK overexpression nor ILK KD had any effect on ING4 mRNA levels (Figure 3B). These findings indicated that ING4 was the negative upstream regulator of ILK.
Figure 3.
ING4-regulated cell growth and tube formation of HUVECs is partially ILK dependent. (A) Real-time PCR analysis of the expression levels of ING4 and ILK in ING4 overexpression or KD MMRU cells. *P<0.05; ***P<0.001, compared with the vector control group. (B) Real-time PCR analysis of the expression levels of ILK and ING4 in MMRU cells with ILK overexpression or KD. ***P<0.001, compared with the vector control group. The CM were collected from MMRU and MMLH cells with ING4 overexpression, ILK overexpression, ING4/ILK co-overexpression for detection of HUVECs growth by SRB assay (C) and the tube formation of HUVECs (D) (scale bar 40 μm). The number of tubes was counted in five random fields for each group. **P<0.01; ***P<0.001, compared with vector control group. ###P<0.001, compared with ING4 overexpression group. Student's t-test.
To investigate the function of ILK in ING4-inhibited HUVEC cell growth, we conducted the SRB assay by using the CM from MMRU and MMLH cells with overexpressing ING4, or ILK, or a simultaneous treatment of ING4 and ILK overexpression, or control vectors. The results showed that ILK overexpression promoted the growth of HUVECs (P<0.001 for both; Figure 3C). In contrast, ING4 overexpression inhibited the growth of HUVECs (P<0.001 for both; Figure 3C), and this inhibition could be rescued by ILK overexpression to some extent (P<0.001 for both; Figure 3C), suggesting that ING4-inhibited HUVEC growth was partially ILK dependent.
To further investigate the role of ILK in ING4-regulated melanoma angiogenesis, we performed the tube formation assay with HUVECs in matrigel and found that the CM from ILK overexpression melanoma cells significantly promoted the tube formation of HUVECs (P<0.001 for both; Figure 3D), and ING4 overexpression reduced the tube formation of HUVECs (P<0.01, P<0.001; respectively; Figure 3D). This inhibition could be restored by ILK overexpression (P<0.001 for both; Figure 3D). Based on these data, we conclude that ING4-regulated cell growth and tube formation of HUVECs is partially mediated through ILK.
ING4 regulates the ILK/NF-κB/STAT3/VEGF signalling pathway
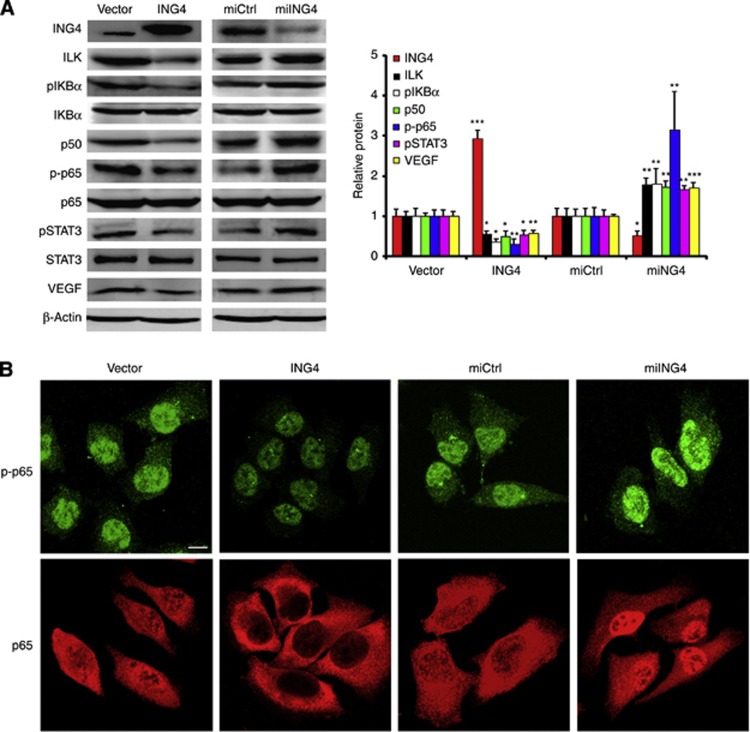
We have previously shown that ILK-regulated melanoma angiogenesis through the activation of NF-κB/IL-6/STAT3/VEGF signalling pathway (Wani et al, 2011), and IL-6 was inhibited by ING4 in melanoma angiogenesis through suppressing NF-κB activity (Li and Li, 2010). Combining these findings with the fact that ING4 is an upstream regulator of ILK, we asked whether ING4 has a regulatory function in the ILK/NF-κB/STAT3/VEGF signalling pathway. In our study, we found that ING4 overexpression decreased the protein level of ILK, p-IKBα, p50, p-p65, pSTAT3 and VEGF, whereas ING4 KD increased those protein levels, and both ING4 overexpression and ING4 KD did not affect the protein levels of total IKBα, total p65 and total STAT3 (Figure 4A). These results suggest that ING4 regulates the ILK/NF-κB/STAT3/VEGF signalling pathway.
Figure 4.
ING4 regulates ILK/NF-κB/STAT3/VEGF signalling pathway. (A) Western blot analysis of the protein levels of ING4, ILK, p-IKBα, IKBα, p50, p-p65, p65, pSTAT3, STAT3 and VEGF in MMRU cells with ING4 overexpression or KD. *P<0.05; **P<0.01; ***P<0.001, compared with the vector control group. Student's t-test. (B) The effect of ING4 overexpression or KD on p-p65 and total p65. After transfection 48 h, the change of p-p65 and the nucleus translocation of p65 in MMRU cells with ING4 overexpression or ING4 KD was checked by immunofluorescence by confocal microscopy (scale bar 10 μm).
To further investigate the effect of ING4 on p-p65 and total p65, we performed immunofluorescence by confocal microscopy to check the change of p-p65 and the nucleus translocation of p65 in MMRU cells with ING4 overexpression or ING4 KD. We found that p-p65 is mainly located in nucleus and ING4 overexpression inhibited the expression intensity of p-p65. In contrast, ING4 KD enhanced the expression intensity of p-p65 compared with the vector control group (Figure 4B). Meanwhile, we found that ING4 overexpression inhibited the nucleus translocation of p65, and ING4 KD promoted the nucleus translocation of p65 compared with the vector control group (Figure 4B). These results further demonstrated that ING4 regulates NF-κB signalling.
ING4 regulates the ILK/NF-κB/VEGF signalling pathway partially through JWA
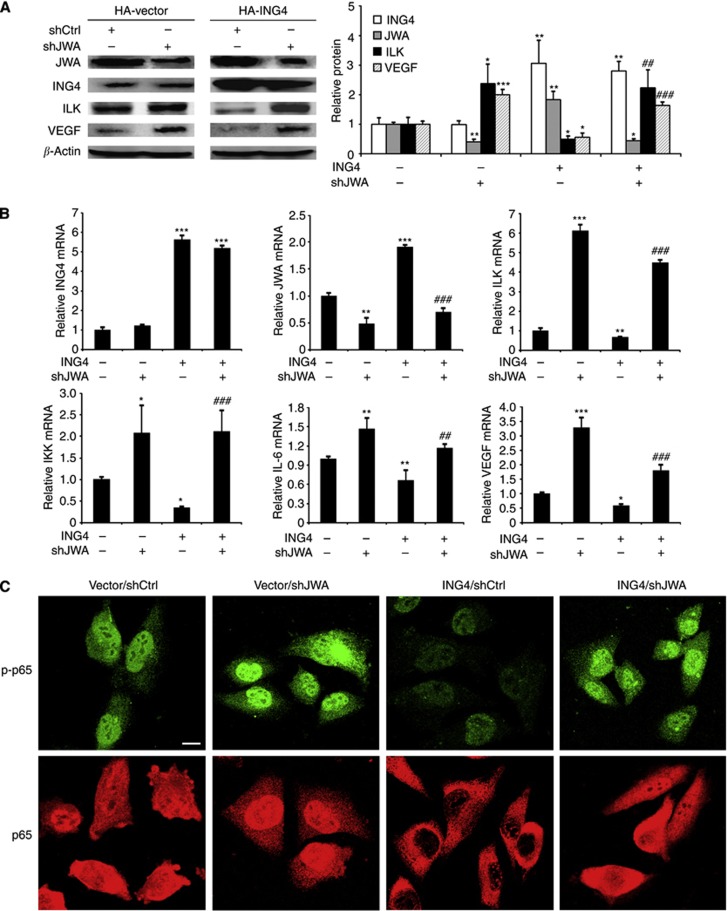
Based on the finding that ING4 is the upstream regulator of both JWA and ILK, we further found that ING4 overexpression increased JWA expression and decreased ILK expression at the protein level (Figure 5A). Moreover, JWA KD clearly promoted ILK expression (Figure 5A), and we also found that the inhibition from ING4 on ILK expression was abolished by JWA KD in MMRU cells to some extent (Figure 5A), suggesting that ILK expression was partially regulated by ING4 via JWA. Moreover, we also found VEGF and ILK have similar expression patterns in these groups, indicating that ING4/JWA/ILK/VEGF might be in the same signalling pathway.
Figure 5.
ING4 regulates ILK/NF-κB/VEGF signalling pathway via JWA. (A) The protein levels of JWA, ING4, ILK and VEGF in MMRU cells with ING4 overexpression, or JWA KD, or ING4 overexpression/JWA KD were detected by western blot. (B) Real-time PCR analysis of the mRNA expression levels of ING4, JWA, ILK, IKK, IL-6 and VEGF in MMRU cells with ING4 overexpression, or JWA KD, or ING4 overexpression/JWA KD. *P<0.05; **P<0.01; ***P<0.001, compared with vector control group. ##P<0.01; ###P<0.001, compared with ING4 overexpression group. (C) The effect of JWA on ING4-regulated p-p65 and total p65. After transfection 48 h, the change of p-p65 and the nucleus translocation of p65 in MMRU cells with ING4 overexpression, JWA KD, or ING4 overexpression/JWA KD were checked by immunofluorescence by confocal microscopy (scale bar 10 μm).
Then, we further investigated whether ING4 regulates the ILK/NF-κB/VEGF signalling pathway through JWA. We observed that ING4 overexpression inhibited the mRNA levels of ILK, IKK, IL-6 and VEGF compared with the control group (P<0.01, P<0.05, P<0.01, and P<0.05, respectively; Figure 5B). Excitingly, JWA KD rescued ING4-inhibited mRNA levels of these genes to some extent (Figure 5B), indicating that the ING4-regulated ILK angiogenesis signalling pathway was partially JWA dependent.
To further investigate the effect of JWA on ING4-regulated p-p65 and total p65, we performed immunofluorescence by confocal microscopy to check the change of p-p65 and the nucleus translocation of p65 in MMRU cells with ING4 overexpression, or JWA KD, or ING4 overexpression and JWA KD. We found that JWA KD enhanced, whereas ING4 overexpression inhibited, the expression intensity of p-p65, and JWA KD rescued the inhibition of p-p65 from ING4 overexpression to some extent (Figure 5C). Meanwhile, we found that JWA KD promoted, whereas ING4 overexpression inhibited, the nucleus translocation of p65, and JWA KD partially rescued the inhibition of the nucleus translocation of p65 from ING4 overexpression (Figure 5C). These results further showed that ING4 regulates NF-κB signalling partially via JWA.
We also demonstrated the role of ILK on ING4-regulated p-p65 and total p65 through immunofluorescence. The results showed that ILK overexpression enhanced, whereas ING4 overexpression inhibited, the expression intensity of p-p65, and ILK overexpression rescued the inhibition of p-p65 from ING4 overexpression to some extent (Supplementary Figure S1). On the other hand, we found that ILK overexpression promoted, whereas ING4 overexpression inhibited, the nucleus translocation of p65, and ILK overexpression partially rescued the inhibition on the nucleus translocation of p65 from ING4 overexpression (Supplementary Figure S1). These results further showed that ING4-regulated NF-κB signalling is partially through ILK.
Reduced JWA expression or increased ILK expression correlates with poor melanoma patient survival
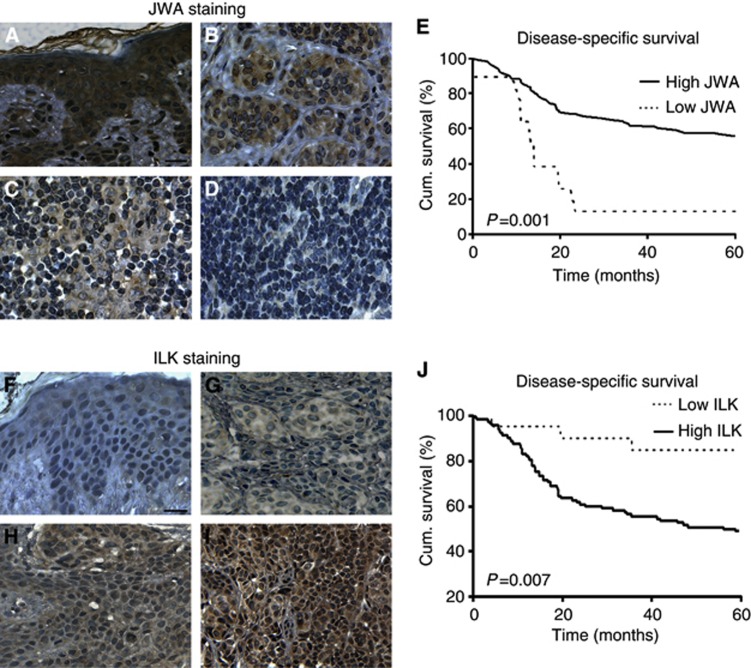
Our previous animal study showed that JWA-inhibited melanoma metastasis (Bai et al, 2010). Furthermore, our present study clarified the important role of JWA on ING4-regulated melanoma angiogenesis. Therefore, we next asked if JWA expression is associated with melanoma progression and patient survival. Our TMA results revealed that JWA immunohistochemical staining was strong in dysplastic nevi (Figure 6A), with a moderate-to-weak staining in primary melanoma (Figure 6B and C), and a negative staining in metastatic melanoma (Figure 6D). Next, the Kaplan–Meier analyses demonstrated that the melanoma patients with high JWA expression had better disease-specific 5-year survival than those with low JWA expression in melanoma patients (P=0.001; Figure 6E). These results indicated that reduced JWA expression closely correlated with poor 5-year survival of melanoma patients.
Figure 6.
JWA or ILK expression is correlated with 5-year survival of melanoma patients. (A–D) Representative images of JWA immunohistochemical staining in human melanocytic lesions. (A) Strong JWA staining in dysplastic nevi. (B) Moderate JWA staining in primary melanoma. (C) Weak JWA staining in primary melanoma. (D) Negative JWA staining in metastatic melanoma (scale bar 20 μm). (E) Reduced JWA expression was correlated with poor disease-specific 5-year survival of melanoma patients (n=130, P=0.001, log-rank test). (F–I) Representative images of ILK immunohistochemical staining in human melanocytic lesions. (F) Negative ILK staining in dysplastic nevi. (G) Weak ILK staining in primary melanoma. (H) Moderate ILK staining in primary melanoma. (I) Strong ILK staining in metastatic melanoma (scale bar 20 μm). (J) Increased ILK expression was correlated with poor disease-specific 5-year survival (n=130, P=0.007, log-rank test) of melanoma patients.
We also found that ILK staining increased from dysplastic nevi to primary and metastatic melanoma (Figure 6F–I). In addition, the increased ILK expression was also correlated with a poor 5-year survival of melanoma patients (P=0.007; Figure 6J), which was consistent with our previous report showing that ILK upregulation correlated with melanoma progression and poor patient survival in a small number of cases (Dai et al, 2003).
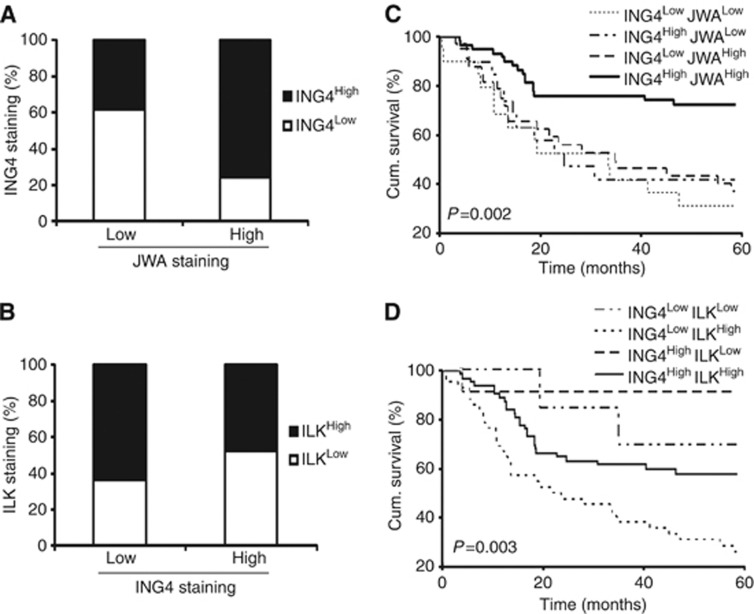
Correlation between ING4 and JWA, ING4 and ILK in melanoma samples, and the influence of their different expression pattern on patient survival
In this study, we have observed that ING4 is the upstream regulator of JWA, and ING4 regulates melanoma angiogenesis through JWA. We have also revealed that ING4 regulates ILK expression through JWA. Based on these findings, we next asked whether there is a correlation among these proteins in melanoma tissue and a relationship between their different expression patterns and the 5-year survival of melanoma patients. We used our previous data for ING4 TMA and obtained the JWA and ILK expression for the corresponding cases from the current TMA data set to compare the expression patterns between ING4 and JWA, as well as between ING4 and ILK in the same melanoma tissues (n=130). We found that ING4 expression was considerably higher in cases with high JWA staining than those with low JWA staining (P=0.049; Figure 7A), showing a positive correlation between ING4 and JWA expression in melanoma tissue. We also found that ILK expression was considerably higher in cases with low ING4 staining than those with high ING4 staining (P=0.032; Figure 7B), showing a negative correlation between ING4 and ILK expression in melanoma tissue. Importantly, our results revealed that the patients with high expression of ING4 and JWA, or high ING4 and low ILK expression showed a significantly increased 5-year survival rate, compared with patients with low expression of ING4 and JWA, or low ING4 and high ILK expression using Kaplan–Meier survival curves (P=0.002, P=0.003, respectively; Figure 7C and D).
Figure 7.
Correlation between ING4 and JWA, ING4 and ILK protein expression, and the influence of their concomitant expression on patient disease-specific 5-year survival. (A) Positive correlation between ING4 and JWA expression in melanoma. ING4 expression was compared between low and high JWA-expressing cases (n=130, P=0.049). (B) Negative correlation between ING4 and ILK expression in melanoma. ILK expression was compared between low and high ING4-expressing cases (n=130, P=0.032). (C) Log-rank test of the association between combined expression of ING4/JWA and patient survival (n=130, P=0.002). (D) Correlation of combined ING4/ILK expression on patient survival (n=130, P=0.003).
Discussion
High tendency to metastasise to other organs is the leading cause of melanoma patient deaths. Angiogenesis is one of the important hallmarks of tumour development, invasion and metastasis (Folkman, 1971; Weis and Cheresh, 2011). Rapid angiogenesis of cutaneous melanomas markedly enhances the risk of lethality and contributes to the progression of melanoma in young adults (Mahabeleshwar and Byzova, 2007). Therefore, it is of outstanding priority to identify the factors that are involved in the regulation of melanoma angiogenesis.
ING4 has been recently implicated in solid tumours as a repressor of angiogenesis (Garkavtsev et al, 2004; Colla et al, 2007; Liu et al, 2012). ING4 exerts an inhibitory effect on the production of proangiogenic molecules IL-8 and osteopontin and consequently on multiple myeloma-induced angiogenesis (Colla et al, 2007). In addition, ING4-mediated regulation of IL-8 significantly influences brain tumour growth and angiogenesis (Garkavtsev et al, 2004). Intratumoural injection of Ad-ING4 significantly suppressed the growth of A549 human lung carcinoma and reduced the tumour microvessel formation (Xie et al, 2008). Meanwhile, the JWA gene is located on chromosome 3p, a region associated with various cancers (Kok et al, 1997). We previously reported that JWA suppressed cell migration of cervical carcinoma, melanoma and liver cancer through MAPK cascades activation and F-actin cytoskeleton rearrangement (Chen et al, 2007a). Furthermore, our recent study demonstrated that JWA-inhibited melanoma metastasis in an animal model (Bai et al, 2010). But, the potential role of JWA in tumour angiogenesis and the molecular mechanisms underlying the regulation of JWA have not yet been elucidated.
In this study, we demonstrated that ING4 is upstream of JWA and influenced JWA expression through enhancing JWA promoter activity. Promoter deletion analysis showed that the region spanning −107 to −27 of the JWA promoter mediated a potent response to ING4 overexpression, indicating that the region has an important function for transcriptional activity of the JWA promoter induced by ING4, which is consistent with our previous report that the JWA proximal promoter region responds to oxidative stress (Chen et al, 2007b). Furthermore, ING4 overexpression in melanoma cells significantly inhibited the growth and tube formation of HUVECs, and this inhibition could be rescued by JWA knockdown to some extent, suggesting that ING4-inhibited melanoma angiogenesis was partially JWA dependent. In this study, JWA knockdown alone showed a significant effect on melanoma angiogenesis and targeted gene expression, and JWA knockdown rescued the inhibitory effect on these cases from ING4 overexpression. Combining the data, ING4 is an upstream regulator of JWA and activates JWA through enhancing JWA promoter activity; we concluded that the inhibition effect of ING4 in melanoma angiogenesis is mediated partially by JWA. However, it is also possible that the strong effect shown from shJWA could be dominant over the more limited negative effect of ING4 in all the cases, even if both proteins act to some extent through independent pathways.
ILK has also been identified as promoting vascular development and angiogenesis (Tan et al, 2004; Xie et al, 2011). When ILK was deleted in mice and zebrafish, the vasculature formation was markedly decreased (Friedrich et al, 2004). On the other hand, VEGF was shown to be the downstream target of ILK in regulating angiogenesis (Tan et al, 2004). Our recent research showed that ILK overexpression in melanoma cells promoted angiogenesis in vitro and in vivo (Wani et al, 2011). However, the regulation for ILK in melanoma angiogenesis is still unknown. In this study, the opposite properties of ILK and ING4 in melanoma angiogenesis led us to explore their relationship in melanoma. We found that ING4 was able to downregulate ILK through the regulation of JWA. ING4 also has a transcription factor function, it is possible ING4 directly regulates the ILK promoter. However, to our knowledge, until now no published paper showed the correlation between ING4 and ILK. Whether there is an ING4-binding site in the ILK promoter deserves to be investigated in the future.
NF-κB is involved in regulating cell survival, angiogenesis, tumourigenesis and metastasis (Ghosh and Karin, 2002; Karin and Lin, 2002). Our previous research demonstrated that ILK promoted melanoma angiogenesis by upregulating the NF-κB/IL-6/STAT3/VEGF signalling pathway (Wani et al, 2011). It was also consistent with a report where VEGF was shown as the downstream target of ILK regulating angiogenesis (Tan et al, 2004). On the other hand, ING4 can suppress brain tumour growth and angiogenesis by associating with the p65 (RelA) subunit of NF-kB (Garkavtsev et al, 2004). A recent study also reported that downregulation of ING4 led to activation of NF-κB in breast cancer, contributing to tumour progression and reduced disease-free patient survival (Byron et al, 2012), which is consistent with our recent study that ING4-inhibited melanoma angiogenesis in vitro and in vivo as a result of suppressing NF-kB activity and IL-6 expression (Li and Li, 2010). In this study, we further confirmed the function of JWA and ILK in ING4-regulated p-p65 and total p65. We found that JWA KD, or ILK overexpression, can rescue the inhibition of ING4 overexpression on p-p65 and the nucleus translocation of p65, suggesting ING4 regulates NF-κB signalling through JWA and ILK to some extent.
Recent research has also shown that low ING4 protein expression in gastrointestinal stromal tumours is associated with poor prognosis in untreated patients (Nanding et al, 2013). In addition, downregulation of nuclear ING4 is correlated with tumourigenesis and progression in head and neck squamous cell carcinoma (Li et al, 2011). Our previous study showed that ING4 expression significantly decreased from dysplastic nevi to malignant melanoma. At the same time, reduced ING4 was associated with melanoma thickness, ulceration and a poorer 5-year survival of melanoma patients (Li et al, 2008). To compare with previous TMA data for ING4, we performed the immunostaining for both JWA and ILK using the TMA, which was used to examine ING4 expression in melanocytic lesions (Li et al, 2008). According to the result, we found that JWA expression was decreased from dysplastic nevi to melanoma, indicating that decreased JWA expression might be a common requirement in melanoma progression. As the main cause of melanoma patient deaths is tumour metastasis, we analysed the correlation between JWA expression and patient survival. We revealed that low JWA expression was significantly correlated with a poor 5-year survival of melanoma patients. In contrast, we found that increased ILK staining in melanoma was also correlated with a poor 5-year survival of melanoma patients. Based on these findings and the fact that metastasis is the leading cause of melanoma patient death (Jemal et al, 2008), it is not surprising to see that reduced JWA could lead to increased melanoma angiogenesis through the JWA/ILK signalling pathway, which in turn results in tumour metastasis, and eventually worse survival of melanoma patients.
Furthermore, our results revealed that there was a positive correlation between expression of ING4 and JWA, and a negative correlation between ING4 and ILK expression. Importantly, the patients with high ING4/JWA expression, or high ING4/low ILK expression, showed significantly better 5-year survival than patients with low ING4/JWA expression or low ING4/high ILK expression. These results further confirm the prognostic value of ING4/JWA/ILK. The use of small-molecule inhibitors to abrogate ILK activity has been shown to reverse ILK oncogenic effects in animal model studies and clinical trials (Muranyi et al, 2009; Cox et al, 2010). Therefore, the ING4/JWA/ILK signalling in melanoma might provide new opportunities for therapeutic intervention.
Taken together, the data presented here, for the first time, reveal that ING4 is upstream of JWA and ING4 positively regulates JWA by enhancing JWA promoter activity. Furthermore, ING4-regulated cell growth and tube formation of HUVECs is partially JWA dependent. Also, ING4 inhibits the ILK angiogenesis signalling pathway through JWA. At the same time, our TMA results reveal that there is a positive correlation between ING4 and JWA expression, and a negative correlation between ING4 and ILK expression. Importantly, their concomitant expression is closely related to the 5-year survival of melanoma patients. These findings highlight the fact that JWA has an important role in ING4-regulated melanoma angiogenesis. As a result, ING4/JWA/ILK signalling molecules could be used as prognostic markers for melanoma and may be promising anti-angiogenic therapeutic targets.
Acknowledgments
We thank Dr Kevin McElwee for manuscript revision. We thank Drs Ronald PC Wong, Madhuri Bhandaru, Anand Rotte, Seyed Mehdi Jafarnejad, Reza Safaee Ardekani, Jun Li, Aijaz Wani, Jin Bai, Rui Chen and Liangliang Liu for valuable suggestions and technical assistance. This study was supported by the Canadian Institutes of Health Research (CCI-117958), the Cancer Research Society and the Canadian Dermatology Foundation. Dr Jing Lu is a recipient of a Postdoctoral Scholarship from the Canadian Institutes of Health Research-Skin Research Training Centre and is supported by the National Natural Science Foundation of China (81101731).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Bai J, Zhang J, Wu J, Shen L, Zeng J, Ding J, Wu Y, Gong Z, Li A, Xu S, Zhou J, Li G. JWA regulates melanoma metastasis by integrin alphaVbeta3 signaling. Oncogene. 2010;29:1227–1237. doi: 10.1038/onc.2009.408. [DOI] [PubMed] [Google Scholar]
- Byron SA, Min E, Thal TS, Hostetter G, Watanabe AT, Azorsa DO, Little TH, Tapia C, Kim S. Negative regulation of NF-kappaB by the ING4 tumor suppressor in breast cancer. PLoS One. 2012;7:e46823. doi: 10.1371/journal.pone.0046823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Bai J, Ye J, Liu Z, Chen R, Mao W, Li A, Zhou J. JWA as a functional molecule to regulate cancer cells migration via MAPK cascades and F-actin cytoskeleton. Cell Signal. 2007;19:1315–1327. doi: 10.1016/j.cellsig.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Chen R, Qiu W, Liu Z, Cao X, Zhu T, Li A, Wei Q, Zhou J. Identification of JWA as a novel functional gene responsive to environmental oxidative stress induced by benzo[a]pyrene and hydrogen peroxide. Free Radic Biol Med. 2007;42:1704–1714. doi: 10.1016/j.freeradbiomed.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Colla S, Tagliaferri S, Morandi F, Lunghi P, Donofrio G, Martorana D, Mancini C, Lazzaretti M, Mazzera L, Ravanetti L, Bonomini S, Ferrari L, Miranda C, Ladetto M, Neri TM, Neri A, Greco A, Mangoni M, Bonati A, Rizzoli V, Giuliani N. The new tumor-suppressor gene inhibitor of growth family member 4 (ING4) regulates the production of proangiogenic molecules by myeloma cells and suppresses hypoxia-inducible factor-1 alpha (HIF-1alpha) activity: involvement in myeloma-induced angiogenesis. Blood. 2007;110:4464–4475. doi: 10.1182/blood-2007-02-074617. [DOI] [PubMed] [Google Scholar]
- Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010;9:804–820. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- Dai DL, Makretsov N, Campos EI, Huang C, Zhou Y, Huntsman D, Martinka M, Li G. Increased expression of integrin-linked kinase is correlated with melanoma progression and poor patient survival. Clin Cancer Res. 2003;9:4409–4414. [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Friedrich EB, Liu E, Sinha S, Cook S, Milstone DS, MacRae CA, Mariotti M, Kuhlencordt PJ, Force T, Rosenzweig A, St-Arnaud R, Dedhar S, Gerszten RE. Integrin-linked kinase regulates endothelial cell survival and vascular development. Mol Cell Biol. 2004;24:8134–8144. doi: 10.1128/MCB.24.18.8134-8144.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garkavtsev I, Kozin SV, Chernova O, Xu L, Winkler F, Brown E, Barnett GH, Jain RK. The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogenesis. Nature. 2004;428:328–332. doi: 10.1038/nature02329. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M.2002Missing pieces in the NF-kappaB puzzle Cell 109SupplS81–S96. [DOI] [PubMed] [Google Scholar]
- Gunduz M, Nagatsuka H, Demircan K, Gunduz E, Cengiz B, Ouchida M, Tsujigiwa H, Yamachika E, Fukushima K, Beder L, Hirohata S, Ninomiya Y, Nishizaki K, Shimizu K, Nagai N. Frequent deletion and down-regulation of ING4, a candidate tumor suppressor gene at 12p13, in head and neck squamous cell carcinomas. Gene. 2005;356:109–117. doi: 10.1016/j.gene.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- Jafarnejad SM, Wani AA, Martinka M, Li G. Prognostic significance of Sox4 expression in human cutaneous melanoma and its role in cell migration and invasion. Am J Pathol. 2010;177:2741–2752. doi: 10.2353/ajpath.2010.100377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Karst AM, Gao K, Nelson CC, Li G. Nuclear factor kappa B subunit p50 promotes melanoma angiogenesis by upregulating interleukin-6 expression. Int J Cancer. 2009;124:494–501. doi: 10.1002/ijc.23973. [DOI] [PubMed] [Google Scholar]
- Kim S. HuntING4 new tumor suppressors. Cell Cycle. 2005;4:516–517. doi: 10.4161/cc.4.4.1584. [DOI] [PubMed] [Google Scholar]
- Kok K, Naylor SL, Buys CH. Deletions of the short arm of chromosome 3 in solid tumors and the search for suppressor genes. Adv Cancer Res. 1997;71:27–92. doi: 10.1016/s0065-230x(08)60096-2. [DOI] [PubMed] [Google Scholar]
- Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- Li G, Tang L, Zhou X, Tron V, Ho V. Chemotherapy-induced apoptosis in melanoma cells is p53 dependent. Melanoma Res. 1998;8:17–23. doi: 10.1097/00008390-199802000-00004. [DOI] [PubMed] [Google Scholar]
- Li J, Li G. Cell cycle regulator ING4 is a suppressor of melanoma angiogenesis that is regulated by the metastasis suppressor BRMS1. Cancer Res. 2010;70:10445–10453. doi: 10.1158/0008-5472.CAN-10-3040. [DOI] [PubMed] [Google Scholar]
- Li J, Martinka M, Li G. Role of ING4 in human melanoma cell migration, invasion and patient survival. Carcinogenesis. 2008;29:1373–1379. doi: 10.1093/carcin/bgn086. [DOI] [PubMed] [Google Scholar]
- Li XH, Kikuchi K, Zheng Y, Noguchi A, Takahashi H, Nishida T, Masuda S, Yang XH, Takano Y. Downregulation and translocation of nuclear ING4 is correlated with tumorigenesis and progression of head and neck squamous cell carcinoma. Oral Oncol. 2011;47:217–223. doi: 10.1016/j.oraloncology.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yu L, Wang Y, Zhang Y, Zhang G. Expression of tumor suppressor gene ING4 in ovarian carcinoma is correlated with microvessel density. J Cancer Res Clin Oncol. 2012;138:647–655. doi: 10.1007/s00432-011-1099-5. [DOI] [PubMed] [Google Scholar]
- Lou C, Jiang S, Guo X, Dong XS. ING4 is negatively correlated with microvessel density in colon cancer. Tumour Biol. 2012;6:2357–2364. doi: 10.1007/s13277-012-0498-9. [DOI] [PubMed] [Google Scholar]
- Mahabeleshwar GH, Byzova TV. Angiogenesis in melanoma. Semin Oncol. 2007;34:555–565. doi: 10.1053/j.seminoncol.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A, Palacios A, Orgaz JL, Jimenez B, Blanco FJ, Palmero I. Functional impact of cancer-associated mutations in the tumor suppressor protein ING4. Carcinogenesis. 2010;31:1932–1938. doi: 10.1093/carcin/bgq171. [DOI] [PubMed] [Google Scholar]
- Muranyi AL, Dedhar S, Hogge DE. Combined inhibition of integrin linked kinase and FMS-like tyrosine kinase 3 is cytotoxic to acute myeloid leukemia progenitor cells. Exp Hematol. 2009;37:450–460. doi: 10.1016/j.exphem.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Nanding A, Tang L, Cai L, Chen H, Geng J, Liu X, Ning X, Li X, Zhang Q.2013Low ING4 protein expression detected by paraffin-section immunohistochemistry is associated with poor prognosis in untreated patients with gastrointestinal stromal tumors Gastric CancerEpub ahead of print 16 March 2013. [DOI] [PubMed]
- Ozer A, Bruick RK. Regulation of HIF by prolyl hydroxylases: recruitment of the candidate tumor suppressor protein ING4. Cell Cycle. 2005;4:1153–1156. doi: 10.4161/cc.4.9.2040. [DOI] [PubMed] [Google Scholar]
- Ozer A, Wu LC, Bruick RK. The candidate tumor suppressor ING4 represses activation of the hypoxia inducible factor (HIF) Proc Natl Acad Sci USA. 2005;102:7481–7486. doi: 10.1073/pnas.0502716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- Tan C, Cruet-Hennequart S, Troussard A, Fazli L, Costello P, Sutton K, Wheeler J, Gleave M, Sanghera J, Dedhar S. Regulation of tumor angiogenesis by integrin-linked kinase (ILK) Cancer Cell. 2004;5:79–90. doi: 10.1016/s1535-6108(03)00281-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li G. ING3 promotes UV-induced apoptosis via Fas/caspase-8 pathway in melanoma cells. J Biol Chem. 2006;281:11887–11893. doi: 10.1074/jbc.M511309200. [DOI] [PubMed] [Google Scholar]
- Wani AA, Jafarnejad SM, Zhou J, Li G. Integrin-linked kinase regulates melanoma angiogenesis by activating NF-kappaB/interleukin-6 signaling pathway. Oncogene. 2011;30:2778–2788. doi: 10.1038/onc.2010.644. [DOI] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- Xie J, Lu W, Gu R, Dai Q, Zong B, Ling L, Xu B. The impairment of ILK related angiogenesis involved in cardiac maladaptation after infarction. PLoS One. 2011;6:e24115. doi: 10.1371/journal.pone.0024115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Zhang H, Sheng W, Xiang J, Ye Z, Yang J. Adenovirus-mediated ING4 expression suppresses lung carcinoma cell growth via induction of cell cycle alteration and apoptosis and inhibition of tumor invasion and angiogenesis. Cancer Lett. 2008;271:105–116. doi: 10.1016/j.canlet.2008.05.050. [DOI] [PubMed] [Google Scholar]
- Zhou JW, Di YP, Zhao YH, Wu R. Investigation on Cell Modulation: Signal Transduction, Apoptosis and Gene Expression. Military Medical Sciences Press: Beijing; 1999. pp. 110–119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.