Abstract
Inequities in the incidence of HIV infection and AIDS with associated continued persistence of HIV-associated neurocognitive disorders (HAND) exist in populations in Hawaii (HI) and PR. We previously reported that peripheral monocyte HIV DNA levels are high in patients in Hawaii with HAND and we now hypothesize that similar findings would be observed in the cerebrospinal fluid (CSF) cellular subsets. CSF cells were obtained from patients undergoing neurocognitive testing from PR and HI and sorted into monocytes (CD14+) and lymphocytes (CD14−) and HIV DNA measured. From 6 PR subjects (3 HAND, 3 normal cognition, NC) and 6 HI subjects (3 HAND, 3 NC), HIV DNA burden in CD14+ cells was higher in HAND than NC patients; NC patients had higher HIV DNA burden in CD14− cells versus HAND. Differences in HIV DNA burden in particular CSF cellular subsets suggest that HIV DNA burden may play a role in HAND neuropathogenesis.
Keywords: HIV DNA, CSF, HAND, CHOROID PLEXUS
In the United States, inequities in the incidence and prevalence of human immunodeficiency virus (HIV)-infection amongst ethnic minorities are well recognized [1, 2]. Because there are potential differences in access and adherence to combined antiretroviral therapy (cART) [3], there may be differences in clinical status regarding neurocognition that might be observed in segments of the population [4–6]. In spite of advancements made in improving the prognosis of HIV/AIDS, prevalence of HAND continues to persist [7]. This continued presence of HAND suggests that even with effective cART, other factors may be influencing neuropathogenesis.
Increasing reports support the hypothesis that chronic neuroinflammation may be present throughout the course of HIV infection even with effective cART [8–10]. Early in the HIV epidemic, percentages of a unique population of activated monocytes (CD14+/16+ phenotype) were noted to be elevated in subjects with HIV-associated neurocognitive disorder (HAND) [11]. Monocytes were known to be a site of latent HIV-infection and activated monocytes were known to be permissive to HIV-infection [12–14], but Neuenburg et al also observed increased percentages and numbers of activated monocytes in cerebrospinal fluid (CSF) from HIV-infected patients receiving cART [15]. In other studies, where CSF production is known to originate from the choroid plexus, monocytes were shown to traffic to the choroid plexus stroma and perivascular spaces, where many of the infiltrating monocytes in the choroid plexus were activated [16–19]. CSF monocytes are known to have slower CSF-compartmentalized variant viral decay in patients with HAND, therefore we hypothesized that CSF monocytes may be important in neuropathogenesis of HAND [20]. Recently cART penetration into the CSF compartment has been suggested as a factor in HAND persistence described as the CNS penetration effect (CPE) score [21]. We examined HIV DNA levels in CSF cells to determine if differences exist between the CSF cellular subsets, which could potentially provide insight into the role of viral DNA in the neuropathogenesis of HAND.
Methods
Patient Cohorts and Specimens
The study was designed based on convenience samples of two existing cohorts characterizing HAND in Hawaii (HI) and Puerto Rico (PR). The Hispanic/Latino Longitudinal NeuroAIDS Cohort of HIV-seropositive women from the University of Puerto Rico Medical Sciences Campus (PR) enrolled HIV-1-infected Puerto Rican women as per guidelines approved by the local Institutional Review Board (IRB). These women were at least 18 years of age, had CD4+ T lymphocyte counts of ≤500, with at least a 9th grade education, and no evidence of either active systemic infection or neurodegenerative disease [6]. Patients from the Hawaii Aging with HIV Cohort from the University of Hawaii (HI) were living in Hawaii, were between ages of 20–30 years old or ≥ 50 years old without any of the following criteria: history of head injury, learning disability, major neurologic/psychiatric disease, or opportunistic brain disease and enrolled in the cohort as per guidelines by the local IRB [22]. PR and HI subjects underwent a macroneurological exam and neurocognitive testing as previously described [6, 22]. They each had baseline evaluations that included medical history, risk behavior inventory, plasma and CSF viral levels (CLIA-certified clinical laboratory), CD4 count, nadir CD4 count, medication history, and co-morbid illnesses [6, 22]. Upon entry into the cohort, subjects who consented to have a lumbar puncture (LP) performed had CSF (10 mL) processed for viably-preserved cells and stored in PBS containing DMSO. The current study was designed as a cross-sectional study to assess HIV DNA in CSF cellular subsets.
HIV-Associated Neurocognitive Disorder Assessment
Cognitive function was determined using the American Academy of Neurology HIV dementia criteria (1991) as previously described [6, 22–24]. For the current study, subjects were divided into normal cognition (NC) and HIV-associated neurocognitive disorder (HAND), which included minor cognitive motor disorder and HIV-associated dementia [6, 22–24].
Fluorescent Activated Cell Sorting (FACS)
Frozen CSF cells were thawed in 100% fetal bovine serum (FBS), treated for 10 minutes with Accumax (Innovative Cell Technologies, Inc., San Diego, CA) and filtered through a 30 um CellTrics filter (Partec, Germany). Cells were then immediately fluorescently labeled with the following antibodies: CD3-PerCp/Cy5.5 and CD14-FITC (BD Biosciences, San Jose, CA) for 20 minutes at room temperature, washed with 2% FBS, PBS and fixed with a 2% paraformaldehyde (PFA) solution. After fixation, the cells were diluted with PBS and underwent cell sorting using the FACSAria (BD Biosciences, San Diego, CA) into the cell subsets: non-monocytes (CD14−) and monocytes (CD14+). The acquisition protocol gated on CD14+ and CD14−, Figure 1. The cells were sorted directly into lysis buffer in preparation for DNA extraction.
Figure 1.
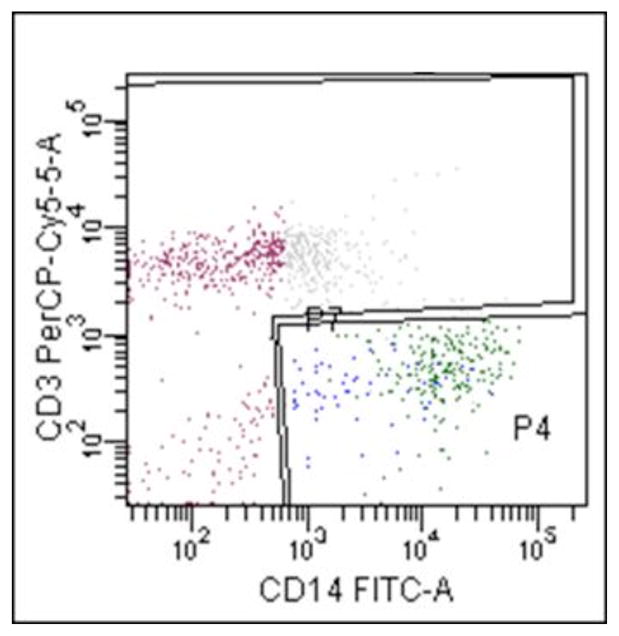
Gating Strategy for CSF Cells. Representative dot graph depicting gating of the CSF cell populations showing the CD14 and CD3 staining of monocytes (P4) and non-monocytes (P7), respectively, showing the two distinct CSF cellular subsets used for the analysis and HIV DNA assessments.
HIV DNA Quantification
DNA was extracted from the CSF cellular subsets using the QIAamp DNA Micro Kit (Qiagen, Valencia, CA) as per guidelines by the manufacturer and eluted in 20 μL TE buffer. HIV DNA copy numbers in each of the CSF cellular subsets were assessed as previously described [25] to obtain the amount of HIV DNA copies per cell. The total CSF HIV DNA burden was determined by multiplying the HIV DNA per cell by the total CSF subset cell count based on the number of events during the sort and isolated for each CSF cell fraction. CPE score was calculated based on guidelines set by Letendre et al [21, 26] to determine how effective the drug regimen was in crossing the blood brain barrier.
Statistics
Median total HIV DNA/CSF cell fraction was calculated using SAS software. Copyright, SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA. Spearman’s correlations for the CPE scores and total amount of HIV DNA in the CSF cellular subsets were determined using SPSS 13.0. A p value <0.05 was considered significant. Graphs were generated using GraphPad Prism 5 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com.
Results
Subject Demographics
Gender, age, and clinical characteristics are summarized in Table 1. The 6 PR subjects were diagnosed with HAND (n=3) or NC (n=3); the 6 HI subjects were diagnosed with HAND (n=3) or NC (n=3). With the exception of patients PR6, all patients were on cART at the time of the LP. No significant differences were observed among the cohort characteristics except that the PR cohort contained all women due to its design (p=0.02). The majority of the CSF specimens had relatively low CSF WBC (9 of 12) with only 3 (PR4, PR6, H6) subjects having mild CSF pleocytosis, defined as CSF WBC ≤ 50 cells/mm3. Overall, most subjects (9 of the 12 subjects) had concordant undetectable plasma and CSF viral levels.
Table 1.
Clinical Parameters of Subjects
| Patient | Cognitive Status | Gender | Age (yrs) | cART | CPE | CD4 Nadir (cells/mm3) | CD4 (cells/mm3) | CSF WBC | Viral Load (copies/mL) | |
|---|---|---|---|---|---|---|---|---|---|---|
| CSF | Plasma | |||||||||
| PR1 | NC | F | 44 | EFV, TDF+FTC | 6 | 28 | 194 | 2 | UND | UND |
| PR2 | NC | F | 40 | ABC+3TC, ATV | 7 | 69 | 134 | 3 | 11,871 | 100,000 |
| PR3 | NC | F | 25 | AZT+3TC, NFV | 7 | 379 | 379 | 1 | UND | 76,563 |
| PR4 | HAND | F | 34 | SQV, LPV/r, TDF+FTC | 8 | 111 | 197 | 6 | UND | UND |
| PR5 | HAND | F | 42 | ATV, ABC+3TC | 7 | 111 | 314 | 4 | UND | UND |
| PR6 | HAND | F | 51 | None | 300 | 337 | 10 | UND | 413 | |
| HI1 | NC | M | 53 | LPV/r, EFV | 5 | 137 | 742 | 0 | UND | UND |
| HI2 | NC | M | 61 | ABC, IDV, EFV, RTV | 9 | 151 | 331 | 2 | UND | UND |
| HI3 | NC | M | 37 | AZT+3TC, EFV | 8 | 302 | 628 | 1 | UND | UND |
| HI4 | HAND | M | 35 | TDF, EFV, ddI | 5 | 72 | 98 | 0 | 299 | 85,386 |
| HI5 | HAND | M | 38 | 3TC, d4T, NFV | 5 | 197 | 262 | 1 | UND | 137 |
| HI6 | HAND | F | 37 | TDF, d4T, LPV/r | 6 | 34 | 40 | 9 | 2,561 | 125,872 |
| Median (tertiles) | 39 (37, 43) | 7 (6, 7) | 124.0 (83.3, 177.7) | 288.0 (194.9, 334.5) | 2 (1, 4) | |||||
cART: combined antiretroviral treatment; CPE: CNS penetration effect [21, 26]; White blood cell (WBC) values are counts per mL; 2Viral Load (VL) were considered undetectable (UND) if they contained copies ≤ 50 copies/mL; EFV: Efavirenz, TDF: Tenofovir, FTC: Emtricitabine, ABC: Abacavir, 3TC: Lamivudine, ATV: Atazanavir, AZT: Zidovudine, NFV: Nelfinavir, SQV: Saquinavir, LPV/r: Lopinavir/Ritonavir, IDV: Indinavir, RTV: Ritonavir, ddI: Didanosine, d4T: Stavudine
HIV DNA
The HIV DNA results from the patients demonstrated an overall total median HIV DNA burden that was higher in CSF CD14+ cells in HAND individuals compared to those with NC; 141.1 versus 100.1 HIV DNA copies, Table 2. In contrast, individuals with NC had higher median HIV DNA burden in CSF 14− cells in comparison to those with HAND; 543.3 versus 79.1 copies, Table 2.
Table 2.
CSF Results
| Cognitive Status | Patient # | Total HIV DNA/CSF Cell Fraction (Total Cells) | Median (tertiles) Total HIV DNA/CSF Cell Fraction by Cognitive status | ||
|---|---|---|---|---|---|
| CD14− | CD14+ | CD14− | CD14+ | ||
| NC | PR1 | 13.2 (33) | 0 (18) | 543.3 (119.0, 918.0) | 100.1 (4.9, 201.6) |
| NC | PR2 | 119.0 (595) | 24.1 (5) | ||
| NC | PR3 | 742.5 (825) | 4.9 (7) | ||
| NC | HI1 | 344.0 (40) | 176.0 (15) | ||
| NC | HI2 | 918.0 (612) | 201.6 (110) | ||
| NC | HI3 | 2347.4 (2134) | 237.4 (149) | ||
| HAND | PR4 | 329.4 (1648) | 442.3 (161) | 79.1 (0, 329.6) | 141.6 (0, 442.3) |
| HAND | PR5 | 146.4 (488) | 264.7 (267) | ||
| HAND | PR6 | 6930.0 (1050) | 3438.9 (301) | ||
| HAND | HI4 | 0 (222) | 18.4 (13) | ||
| HAND | HI5 | 0 (720) | 0 (12) | ||
| HAND | HI6 | 11.7 (13) | 0 (0) | ||
PR: Puerto Rico; HI: Hawaii; NC: Normal Cognition; HAND: HIV-associated neurocognitive disorder; Total HIV DNA was determined by multiplying HIV DNA by the total CSF cell count isolated from each CSF cell fraction.
Positive correlations were observed between total HIV DNA and CNS penetration effect (CPE) [21, 26] of cART in both CD14+ and CD14− cells (R2=0.291; p=0.034 and R2=0.892; p=0.010 respectively, Figure 2). A positive correlation was also observed between total HIV DNA and CD4 cell count (p=0.003). No correlations were observed between total HIV DNA and age, CD4 nadir cell count, use of protease inhibitors (data not shown), CSF WBCs, and HIV viral loads in plasma and CSF. No correlation was observed with HAND. The total median HIV DNA on the combined CD14+ and CD14− subsets was higher in the PR patients in comparison to HI patients (data not shown), suggesting that differences may exist between the cohorts; however, the small sample size precludes any significant conclusion.
Figure 2.
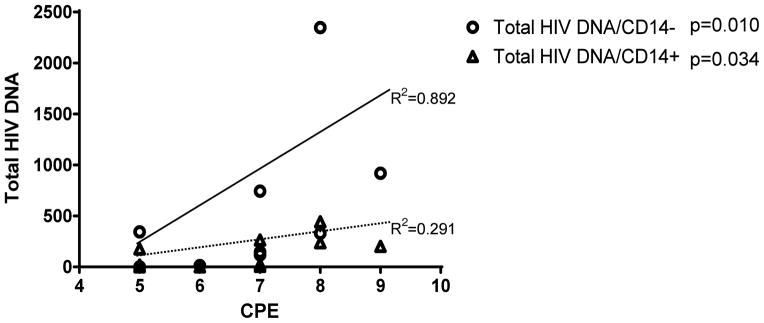
Total HIV DNA in CD14+ & CD14− Subsets Versus CPE. Correlation between CNS penetration effect (CPE) score and the total HIV DNA in CD14 negative (p=0.010) and positive cells (p=0.034); suggesting that an antiviral regimen consisting of drugs that penetrate the CNS (high CPE score) may not necessarily impact the HIV DNA copy numbers in the CSF cells.
Discussion
We report for the first time differences in HIV DNA copy numbers from CSF monocytes compared to CSF lymphocytes from two cohorts of patients from the HI and PR. However, because the data are based on a limited number of specimens, conclusions related to HIV DNA and CSF cellular subsets cannot be made. Our findings provide a foundation and feasibility data that HIV DNA can be measured from cellular subsets, which could be used as a tool for address mechanisms for HAND in the future. Previously, we demonstrated that HIV DNA levels were high in monocytes and peripheral blood mononuclear cells in patients with HAND and remained high while on cART. Because these circulating monocytes can traffic to the BBB and infiltrate the choroid plexus where CSF production occurs, the implication that these cellular subsets might also have different HIV DNA copy numbers might suggest a role in neuropathogenesis [16–19]. Adhesion molecules (E-selectin and P-selectin) and increased cytokine production (MCP-1) are thought to be involved in leukocyte recruitment into the choroid plexus [18, 27, 28]. Once through the blood-brain barrier via monocytes, HIV-1 is then transported into the choroid plexus and into the CSF. Studies on viral evolution suggest that cellular compartmentalization within the CSF provides an environment for the CSF to serve as a unique reservoir for cells with latent virus which may contribute to viral persistence in the CNS [20, 29–31]. These reservoirs may be unaffected by cART as demonstrated by the positive correlation between cART CPE and total HIV DNA which may be a mechanism by which the virus evades eradication. The identification of T cell-tropic and macrophage-tropic HIV-1 variants in the CSF of individuals with HAND may provide an explanation to the differences observed in CD14− and CD14+ HIV DNA levels in HAND and NC individuals.
A limitation of the study was the small number of specimens available however the high HIV DNA copies in CSF monocytes in HAND patients from both cohorts suggests that CSF monocytes may represent a viral reservoir in the CSF leading to viral persistence. Future studies with larger sample sizes are planned to verify our preliminary findings and to look into contributing factors for the possible differences that occur between the two cohorts. The efficacy and mechanisms of controlling HIV infection in the brain remains unknown, but likely the combination of cART and immune function are involved in restricting viral replication particularly with patients with undetectable CSF viral loads.
CSF viral reservoirs may play a role in HAND pathogenesis because latent cells upon activation are able to maintain low levels of viral replication. Because these reservoirs exist in the CSF and harbor HIV variants, others have suggested that CSF compartmentalization develops [20]. Therefore, we hypothesize that infiltrating monocytes, particularly activated monocytes (CD14+/16+) from the choroid plexus could contribute to the inflammatory milieu. In parallel, HIV DNA levels in the non-activated monocytes (CD14+/16−) may be represent latent viral reservoirs that allows the virus to evolve. Further characterization of the CSF cellular subsets is necessary to determine the significance of HIV DNA in this compartment as it relates to neuropathogenesis.
Acknowledgments
Supported by F31AI081450 (MA), NS053345 (BS, MA), MD007584 (BS), S11NS046278 (VW), U54NS43011 (VW, LM), P20RR11126 & U54MD007587 (VW); R21MH095524 (VW); R25MH080661; R01MH083516 (LM) Authors acknowledge the support and guidance from Drs. Avindra Nath and Amanda Brown; and training support through R25MH080661 (MA); as well as the University of Hawaii John A. Burns School of Medicine Flow Cytometry Core Facility P20GM103516.
References
- 1.Geographic differences in HIV infection among Hispanics or Latinos--46 states and Puerto Rico, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(40):805–10. [PubMed] [Google Scholar]
- 2.Control, C.f.D. HIV Surveillance Report: Diagnoses of HIV Infection and AIDS in the United States and Dependent Areas. 2010. [Google Scholar]
- 3.Thames AD, et al. Differential predictors of medication adherence in HIV: findings from a sample of African American and Caucasian HIV-positive drug-using adults. AIDS Patient Care STDS. 2012;26(10):621–30. doi: 10.1089/apc.2012.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maki PM, Martin-Thormeyer E. HIV, cognition and women. Neuropsychol Rev. 2009;19(2):204–14. doi: 10.1007/s11065-009-9093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson MA, et al. Utility of the HIV dementia scale in assessing risk for significant HIV-related cognitive-motor deficits in a high-risk urban adult sample. AIDS Care. 2005;17(8):1013–21. doi: 10.1080/09540120500100858. [DOI] [PubMed] [Google Scholar]
- 6.Wojna V, et al. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol. 2006;12(5):356–64. doi: 10.1080/13550280600964576. [DOI] [PubMed] [Google Scholar]
- 7.Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine AJ, et al. Systems analysis of human brain gene expression: mechanisms for HIV-associated neurocognitive impairment and common pathways with Alzheimer’s disease. BMC Med Genomics. 2013;6(1):4. doi: 10.1186/1755-8794-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valcour V, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206(2):275–82. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. Accumulation of nuclear and mitochondrial DNA damage in the frontal cortex cells of patients with HIV-associated neurocognitive disorders. Brain Res. 2012;1458:1–11. doi: 10.1016/j.brainres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Pulliam L, et al. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349(9053):692–5. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 12.Ellery PJ, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178(10):6581–9. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 13.González-Scarano F, Martín-García J. The neuropathogenesis of AIDS. Nature Reviews Immunology. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 14.Jaworowski A, et al. CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with Plasmodium falciparum malaria and HIV-1 infection. J Infect Dis. 2007;196(1):38–42. doi: 10.1086/518443. [DOI] [PubMed] [Google Scholar]
- 15.Neuenburg JK, et al. Enrichment of activated monocytes in cerebrospinal fluid during antiretroviral therapy. AIDS. 2005;19(13):1351–9. doi: 10.1097/01.aids.0000181008.39514.ee. [DOI] [PubMed] [Google Scholar]
- 16.Alexaki A, Wigdahl B. HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination. PLoS Pathog. 2008;4(12):e1000215. doi: 10.1371/journal.ppat.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bering EA., Jr Circulation of the cerebrospinal fluid. Demonstration of the choroid plexuses as the generator of the force for flow of fluid and ventricular enlargement. J Neurosurg. 1962;19:405–13. doi: 10.3171/jns.1962.19.5.0405. [DOI] [PubMed] [Google Scholar]
- 18.Clay CC, et al. Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection. J Virol. 2007;81(21):12040–8. doi: 10.1128/JVI.00133-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Symss NP, Oi S. Theories of cerebrospinal fluid dynamics and hydrocephalus: historical trend. J Neurosurg Pediatr. 2013;11(2):170–7. doi: 10.3171/2012.3.PEDS0934. [DOI] [PubMed] [Google Scholar]
- 20.Schnell G, et al. Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia. PLoS Pathog. 2009;5(4):e1000395. doi: 10.1371/journal.ppat.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letendre S, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65(1):65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valcour V, et al. Higher frequency of dementia in older HIV+ individuals. The Hawaii Aging with HIV Cohort. Neurology. 2004;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen RS, Cornblath DR, Epstein LG. Nomenclature and research case definitions for neurological manifestations of human immunodeficiency virus type 1 (HIV-1) infection. Neurology. 1991;41:778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- 24.Wojna V, Nath A. Challenges to the diagnosis and management of HIV dementia. AIDS Read. 2006;16(11):615–6. 621–4, 626, 629–32. [PubMed] [Google Scholar]
- 25.Kusao I, et al. Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci. 2012;24(1):71–80. doi: 10.1176/appi.neuropsych.11050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letendre SL, et al. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010;18(2):45–55. [PMC free article] [PubMed] [Google Scholar]
- 27.Kivisakk P, et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100(14):8389–94. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell K, et al. Monocyte chemoattractant protein-1 in the choroid plexus: a potential link between vascular pro-inflammatory mediators and the CNS during peripheral tissue inflammation. Neuroscience. 2009;158(2):885–95. doi: 10.1016/j.neuroscience.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burkala EJ, et al. Compartmentalization of HIV-1 in the central nervous system: role of the choroid plexus. AIDS. 2005;19(7):675–84. doi: 10.1097/01.aids.0000166090.31693.aa. [DOI] [PubMed] [Google Scholar]
- 30.Caragounis EC, et al. Comparison of HIV-1 pol and env sequences of blood, CSF, brain and spleen isolates collected ante-mortem and post-mortem. Acta Neurol Scand. 2008;117(2):108–16. doi: 10.1111/j.1600-0404.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- 31.Harrington PR, et al. Cross-sectional characterization of HIV-1 env compartmentalization in cerebrospinal fluid over the full disease course. AIDS. 2009;23(8):907–15. doi: 10.1097/QAD.0b013e3283299129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnell G, et al. HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog. 2011;7(10):e1002286. doi: 10.1371/journal.ppat.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clements JE, et al. The central nervous system is a viral reservoir in simian immunodeficiency virus--infected macaques on combined antiretroviral therapy: a model for human immunodeficiency virus patients on highly active antiretroviral therapy. J Neurovirol. 2005;11(2):180–9. doi: 10.1080/13550280590922748-1. [DOI] [PubMed] [Google Scholar]
- 34.Churchill M, Nath A. Where does HIV hide? A focus on the central nervous system. Curr Opin HIV AIDS. 2013 doi: 10.1097/COH.0b013e32835fc601. [DOI] [PMC free article] [PubMed] [Google Scholar]


