Abstract
Centriolar satellites are small, granular structures that cluster around centrosomes, but whose biological function and regulation are poorly understood. We show that centriolar satellites undergo striking reorganization in response to cellular stresses such as UV radiation, heat shock, and transcription blocks, invoking acute and selective displacement of the factors AZI1/CEP131, PCM1, and CEP290 from this compartment triggered by activation of the stress-responsive kinase p38/MAPK14. We demonstrate that the E3 ubiquitin ligase MIB1 is a new component of centriolar satellites, which interacts with and ubiquitylates AZI1 and PCM1 and suppresses primary cilium formation. In response to cell stress, MIB1 is abruptly inactivated in a p38-independent manner, leading to loss of AZI1, PCM1, and CEP290 ubiquitylation and concomitant stimulation of ciliogenesis, even in proliferating cells. Collectively, our findings uncover a new two-pronged signalling response, which by coupling p38-dependent phosphorylation with MIB1-catalysed ubiquitylation of ciliogenesis-promoting factors plays an important role in controlling centriolar satellite status and key centrosomal functions in a cell stress-regulated manner.
Keywords: cell stress, centriolar satellites, ciliogenesis, post-translational modifications, signalling pathways
Introduction
Centrioles are microtubule-based structures that are essential for the formation of centrosomes and cilia in vertebrate cells (Nigg and Raff, 2009). Centriolar satellites (not to be confused with mother centriole-associated structures that are now referred to as subdistal appendages) are small, 70–100 nm in size, granular structures that cluster around the centrosome throughout interphase of the cell cycle (Kubo et al, 1999; Barenz et al, 2011). They are readily visible by electron microscopy as electron-dense structures, formed by the coalescence of smaller particles that are transported towards the centrosome along the microtubule network by dynein motor proteins (Dammermann and Merdes, 2002; Kodani et al, 2010). Because they contain a range of centrosomal proteins, centriolar satellites are generally thought to function as storage sites that allow for the continuous influx of cargo to the centrosome (Barenz et al, 2011; Lopes et al, 2011). Accordingly, the integrity of centriolar satellites is required to support key cellular processes that impinge on specialized functions of the centrosome, such as cell division, primary cilium formation, and dendritic outgrowth in neurons (Barenz et al, 2011; Puram et al, 2011a). In particular, the process of ciliogenesis has been linked to centriolar satellites. These structures serve as assembly platforms for a range of factors that are mutated in ciliopathies, a heterogeneous group of inherited human syndromes characterized by defects in primary cilium formation (Hildebrandt et al, 2011; Lopes et al, 2011). The PCM1 protein is generally thought to function as a central molecular scaffold for centriolar satellite organization. It interacts with and recruits a number of factors to these structures, including CEP290, CEP90, CEP72, CEP70, HOOK3, PAR6α, BBS4, AZI1/CEP131, and OFD1 (Barenz et al, 2011; Staples et al, 2012). In the absence of PCM1, and hence of centriolar satellites per se, these factors are instead recruited directly to centrosomes, suggesting that centriolar satellites may also serve to restrict the direct centrosomal engagement of such proteins and thus contribute to regulation of centrosomal functions (Lopes et al, 2011).
Cells are continuously exposed to a variety of stresses such as DNA damage, hypoxia, and heat shock that arise from changing conditions in their environment. To mitigate the potential threats to overall cell homeostasis and fitness that such perturbations may present, cells are equipped with a diverse range of signalling pathways that sense different stress-induced cues and whose activation facilitates appropriate, protective downstream responses. To a large extent, cellular signalling responses to stress are driven by post-translational modifications (PTMs) such as phosphorylation, ubiquitylation, and SUMOylation, and the range of cellular proteins that are modified by stress-regulated PTMs is substantially large. For instance, proteome-wide analysis of stress-regulated ubiquitylation in human cells has shown that numerous ubiquitin-dependent signalling processes are modulated in response to perturbations such as proteasome inhibition and genotoxic stress (Kim et al, 2011; Wagner et al, 2011; Povlsen et al, 2012). Until now, studies of how PTMs regulate cellular stress responses have been mostly focussed on nuclear processes, while considerably less is known about stress-dependent signalling in other subcellular compartments.
Although centriolar satellites are increasingly being recognized as important subcellular structures that are tightly linked to centrosome functions, human disease, and cell differentiation, little is known about their precise functions, let alone whether these structures are subject to any form of regulatory control. In this study, we describe a striking new cellular signalling response involving the stress-activated p38 kinase and the MIB1 ubiquitin ligase, which regulates the composition and function of centriolar satellites in response to a range of cellular stresses.
Results
UV induces rapid displacement of AZI1, PCM1, and CEP290 from centriolar satellites
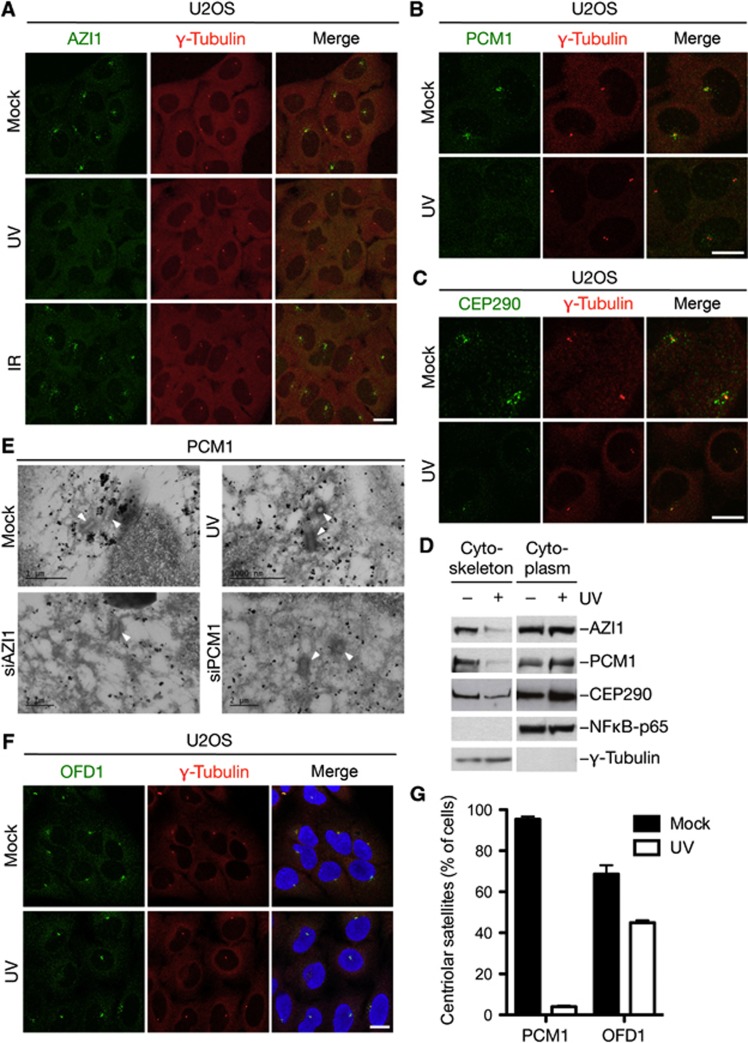
In the course of studying global PTM changes in response to different genotoxic agents, we noticed that several components of centriolar satellites are highly regulated by ubiquitylation- and phosphorylation-dependent signalling elicited in response to ultraviolet (UV) but not to ionizing radiation (IR) (see below). Prompted by such observations, we set out to investigate whether and how DNA damage affects the status of centriolar satellites. Strikingly, we found that the centriolar satellite factors AZI1/CEP131, PCM1, and CEP290 underwent acute and complete displacement from centriolar satellites, their primary site of localization in unperturbed U2OS cells, in response to UV or UV-mimetic agents such as 4NQO (Figure 1A–C; Supplementary Figure S1A and B). In contrast, the localization of these proteins to centriolar satellites was largely unaffected by IR, as well as a range of other genotoxic agents (Figure 1A; data not shown). We observed similar UV-induced dissociation of AZI1, PCM1, and CEP290 from centriolar satellites in other cell types, including RPE1 cells (see Supplementary Figure S4A and B; data not shown). Following UV-induced displacement of centriolar satellite components, both AZI1 and CEP290 remained associated with centrosomes, while PCM1 did not (Supplementary Figure 1C). A similar behaviour has been observed for a number of other centriolar satellite components upon depletion of PCM1 (Lopes et al, 2011). In biochemical fractionation experiments, we observed a marked decrease in cytoskeleton-associated AZI1, PCM1, and CEP290 following UV radiation (Figure 1D; data not shown), most likely reflecting the dissociation of these factors from centriolar satellites. Consistent with these observations, electron microscopy combined with gold labelling of PCM1 showed that PCM1 granules in the vicinity of centrioles become markedly smaller following UV, comparable to the effect of depleting AZI1, which impairs PCM1 association with centriolar satellites (Figure 1E; Supplementary Figure S1D). We observed similar pan-cytoplasmic distribution of AZI1 and PCM1 granules after UV using wide-field fluorescence microscopy combined with image deconvolution (Supplementary Figure S1E). Importantly, in contrast to AZI1, PCM1, and CEP290, other centriolar satellite factors including OFD1 remained associated with these structures following UV radiation (Figure 1F and G; Supplementary Figure S2). This suggests that centriolar satellites per se are not disassembled in response to UV and related agents, but that only selected factors, including AZI1, PCM1, and CEP290, are acutely expelled from this structure in response to such insults. We conclude from these experiments that centriolar satellites undergo prominent reorganization of their normal composition, involving the selective displacement of AZI1, PCM1, and CEP290, in response to perturbations such as UV radiation.
Figure 1.
Rapid displacement of AZI1, PCM1, and CEP290 from centriolar satellites in response to UV radiation. (A) U2OS cells were mock treated or exposed to UV or IR, fixed 1 h later and co-immunostained with AZI1 and γ-tubulin antibodies. Scale bar, 10 μm. (B) U2OS cells subjected to UV or not and treated as in (A) were co-immunostained with PCM1 and γ-tubulin antibodies. Scale bar, 10 μm. (C) As in (B), except that cells were co-immunostained with CEP290 and γ-tubulin antibodies. Scale bar, 10 μm. (D) U2OS cells were exposed or not to UV, collected 1 h later and separated into subcellular fractions. Cytoplasm- and cytoskeleton-enriched fractions were immunoblotted with indicated antibodies. (E) U2OS cells were left untreated, transfected with siRNAs against AZI1 or PCM1, or exposed to UV and subsequently prepared for electron microscopy combined with PCM1 immunogold labelling (black dots). Centrioles (rod shaped) are indicated by arrows. Scale bars, 1000, nm. (F) As in (B), except that cells were co-immunostained with OFD1 and γ-tubulin antibodies. Scale bar, 10 μm. High-magnification images are shown in Supplementary Figure S2. (G) Quantification of centriolar satellite localization of PCM1 and OFD1, analysed as in (B) and (F). At least 100 cells per condition were counted. Results depict the mean (±s.d.) of three independent experiments.
Stress-induced displacement of centriolar satellite factors is mediated by the p38 kinase
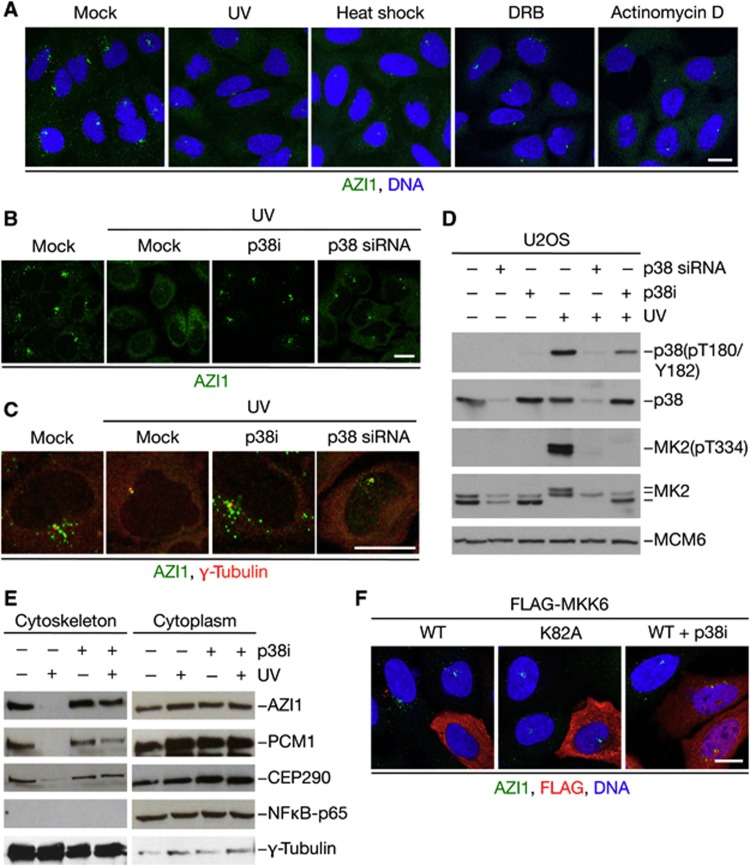
As only a minor subset of genotoxic agents triggered dissociation of AZI1, PCM1, and CEP290 from centriolar satellites, we wondered whether the displacement of these factors reflected a response to cellular stress rather than a genuine DNA damage response (DDR). To test this, we analysed how a variety of perturbations to normal cell physiology impacted centriolar satellite status. Interestingly, a number of stresses, such as heat shock, transcription blocks, and proteotoxic stress induced by proteasome inhibition, led to profound dislodgement of AZI1 and PCM1 from centriolar satellites comparable to the effect of UV (Figure 2A; Supplementary Figure S3A and B; data not shown), indicating that remodelling of normal centriolar satellite architecture occurs in response to a broad range of cellular stresses. Insults such as UV and heat shock are known to trigger activation of the stress-responsive kinase p38/MAPK14 (Nebreda and Porras, 2000), and we therefore asked whether the stress-induced reorganization of centriolar satellite composition was mediated by this kinase. Indeed, unlike inhibition of major DDR-associated kinases such as ATM/ATR and Chk1, we found that treatment of U2OS cells with a small molecule p38 inhibitor fully abrogated the UV-induced displacement of AZI1 and PCM1 from centriolar satellites (Figure 2B–D; data not shown). We observed a similar effect of siRNA-mediated depletion of p38 (Figure 2B–D), indicating that the suppression of UV-induced dispersal of centriolar satellite factors was a direct consequence of p38 inhibition. These effects could be fully reproduced in RPE1 cells, and treatment with p38 inhibitor did not affect the appearance of centriolar satellites in the absence of UV exposure (Supplementary Figure S4A and B). Consistent with these findings, only genotoxic agents that promoted dissociation of AZI1 and PCM1 from centriolar satellites led to activation of p38 (Supplementary Figure S4C). Inhibition of p38 activity also prevented the selective depletion of AZI1, PCM1, and CEP290 from the cytoskeletal fraction in response to UV (Figure 2E). Moreover, p38 inhibition impaired the loss of AZI1 from centriolar satellites in response to other stresses, including heat shock and inhibition of transcription (Supplementary Figure S4D). To further confirm that the stress-dependent dispersal of centriolar satellite components was mediated by p38, we analysed the effect of elevating cellular p38 activity by overexpression of the MKK6 kinase, which activates p38 by direct phosphorylation (Raingeaud et al, 1996). Indeed, overexpression of wild-type (WT) but not catalytically inactive (CI) MKK6 induced dispersal of centriolar satellites in unperturbed U2OS and RPE1 cells, in a manner that was fully dependent on p38 activity (Figure 2F; Supplementary Figure S4E), further supporting the notion that this process is mediated by p38-dependent signalling. Together, these data demonstrate that cellular stresses such as UV radiation and heat shock trigger p38-dependent reorganization of centriolar satellites.
Figure 2.
The stress-responsive kinase p38 is required for dispersal of centriolar satellite factors in response to a range of cell stresses. (A) U2OS cells were exposed to UV, heat shock, or the transcriptional inhibitors DRB or Actinomycin D, fixed and immunostained with AZI1 antibody. Scale bar, 10 μm. (B) U2OS cells were incubated with p38 inhibitor (p38i) for 1 h or transfected with p38 siRNA for 72 h. Subsequently, cells were irradiated or not with UV, fixed 1 h later and stained with AZI1 antibody. Scale bar, 10 μm. (C) U2OS cells treated as in (B) were stained with AZI1 and γ-tubulin antibodies. Scale bar, 10 μm. (D) U2OS cells treated as in (B) were immunoblotted with indicated antibodies. (E) Cytoskeletal and cytoplasmic fractions of cells treated with p38 inhibitor and/or UV for 1 h prior to harvest were analysed with the indicated antibodies. (F) U2OS cells transfected with WT or kinase-dead (K82A) versions of FLAG-tagged MKK6 were fixed and immunostained with AZI1 and FLAG antibodies. Scale bars, 10 μm.
The E3 ubiquitin ligase MIB1 is a new centriolar satellite factor that interacts with AZI1 and PCM1
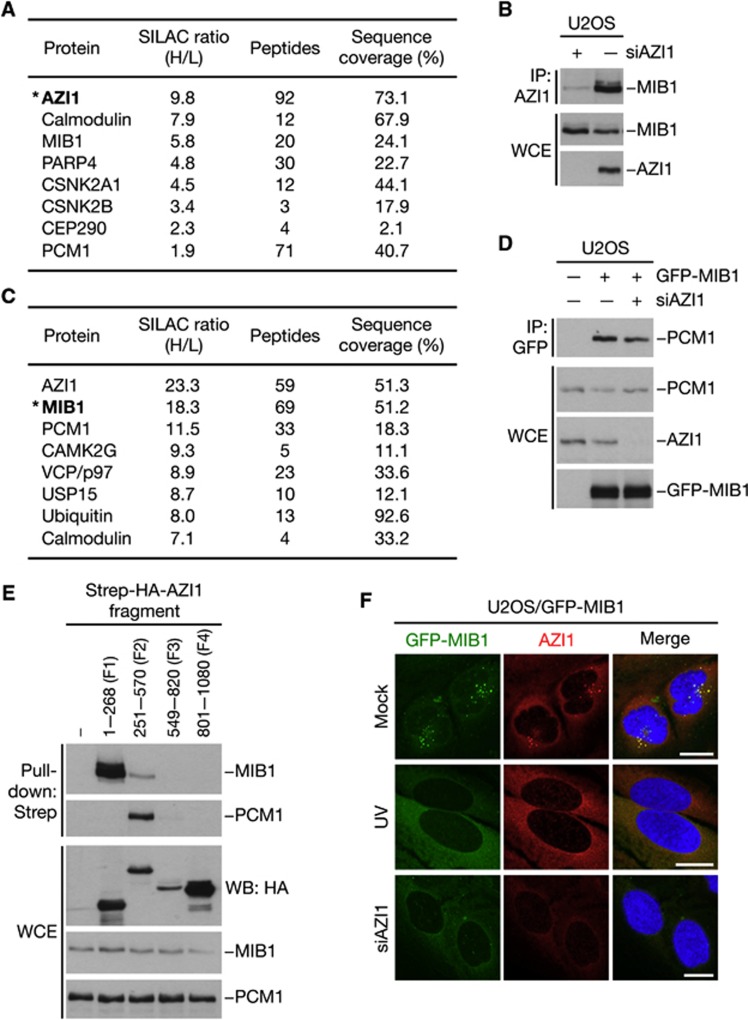
To gain further insight into the stress-responsive pathway(s) underlying centriolar satellite reorganization, we performed quantitative mass spectrometry (MS)-based mapping of cellular AZI1-interacting proteins. As expected, we identified PCM1 and several other centrosomal and centriolar satellite components in GFP-AZI1 pull-downs (Figure 3A; data not shown), confirming the validity of our approach. We also found substantial amounts of the E3 ubiquitin ligase MIB1 in AZI1 pull-downs (Figure 3A), consistent with previous observations (Akimov et al, 2011), and we confirmed a prominent interaction between endogeneous AZI1 and MIB1 proteins in co-immunoprecipitation experiments (Figure 3B). Reciprocal MS-based analysis of cellular MIB1-interacting proteins revealed that both AZI1 and PCM1 were among the proteins most prominently associated with MIB1 (Figure 3C). Subsequent analyses showed that binding of MIB1 to PCM1 was independent of AZI1 and vice versa (Figure 3D; data not shown), and MIB1 and PCM1 interacted with non-overlapping regions within the N-terminal half of AZI1 (Figure 3E). In support of a tight relationship between MIB1 and centriolar satellites, GFP-tagged MIB1 could also be clearly observed in these structures (Figure 3F). The presence of MIB1 at centriolar satellites was dependent on both AZI1 and PCM1, and like these factors, MIB1 was also quantitatively displaced from the satellites in response to UV radiation (Figure 3F; data not shown). Collectively, these data suggest that MIB1 is a novel component of centriolar satellites in unperturbed cells, and that AZI1 and PCM1 are primary MIB1 binding partners at these structures.
Figure 3.
The E3 ubiquitin ligase MIB1 is a centriolar satellite-associated protein that interacts with AZI1 and PCM1. (A) Mass spectrometry (MS)-based analysis of proteins interacting with GFP-AZI1. U2OS cells grown in light (L) or heavy (H) SILAC media were transfected with empty vector or GFP-AZI1 plasmid, respectively. GFP-AZI1 and its associated proteins were enriched using GFP-Trap resin and analysed by MS. Selected proteins with high SILAC (H/L) ratios and/or sequence coverage are indicated. Asterisk indicates the bait protein. (B) Interaction between AZI1 and MIB1 was analysed by immunoblotting AZI1 immunoprecipitates (IPs) from whole-cell extracts (WCEs) of U2OS cells transfected with AZI1 or control (−) siRNAs. (C) As in (A), except that cells labelled with H medium were transfected with GFP-MIB1 plasmid. (D) Extracts of U2OS cells transfected with indicated combinations of GFP-MIB1 plasmid and AZI1 siRNA were subjected to GFP IP followed by immunoblotting with PCM1, AZI1, and GFP antibodies. (E) U2OS cells were transfected with constructs encoding Strep-HA-tagged AZI1 fragments spanning the indicated amino acids. Interactions between AZI1 and MIB1 or PCM1 were analysed by immunoblotting Strep-Tactin pull-downs with MIB1 and PCM1 antibodies. (F) U2OS cells stably expressing GFP-MIB1 were mock treated or transfected with AZI1 siRNA for 48 h, exposed to UV or not and fixed 1 h later. Cells were immunostained with AZI1 antibody. Scale bars, 10 μm.
MIB1 ubiquitylates AZI1 and PCM1 and is inactivated in response to cell stress
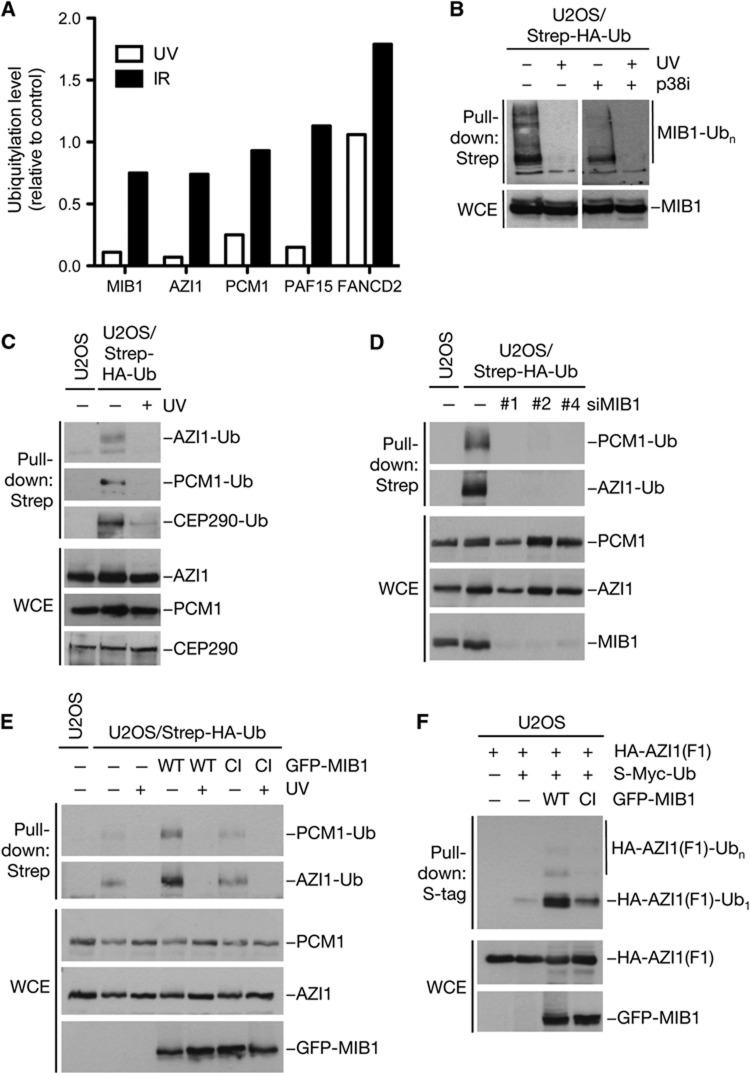
Given that MIB1 is released from centriolar satellites in response to UV, we interrogated a potential involvement of this ubiquitin ligase in the cell stress response leading to alteration of centriolar satellite status. Interestingly, from parallel ongoing work on DNA damage-regulated ubiquitylation, we noted that the ubiquitylation of MIB1 was strongly suppressed by exposure to UV but not to IR (Figure 4A; Supplementary Figure S5A; Supplementary Table S1). Because the ubiquitin-dependent modification of MIB1 largely reflects its intrinsic autoubiquitylation (Supplementary Figure S5B), we reasoned that concomitantly with its UV-induced release from centriolar satellites, the E3 ligase activity of MIB1 might be acutely inactivated. Indeed, an abrupt loss of MIB1 activity, as reflected by its autoubiquitylation, was clearly evident in biochemical experiments (Figure 4B). Notably, however, this effect was independent of p38 activity, as treatment of cells with p38 inhibitor or p38 siRNA did not compromise UV-induced MIB1 inactivation (Figure 4B; Supplementary Figure S5C). Intriguingly, our MS-based analysis of DNA damage-regulated ubiquitylation also revealed that like MIB1, both AZI1 and PCM1 were subjected to rapid ubiquitylation loss in response to UV but not to IR, a regulatory pattern resembling that for PAF15, which we have previously reported (Povlsen et al, 2012) (Figure 4A; Supplementary Table S1). We confirmed biochemically that the ubiquitylation of AZI1 and PCM1 was strongly and rapidly diminished by UV but not by IR (Figure 4C; Supplementary Figure S5D–G). Moreover, the ability to cause loss of these ubiquitylations was exclusive to genotoxic insults that promoted centriolar satellite reorganization (Supplementary Figure S5G). Other cell stresses that induce AZI1 and PCM1 dissociation from centriolar satellites, such as heat shock, also reduced AZI1 and PCM1 ubiquitylation (Supplementary Figure S5H; data not shown). Subsequent inspection of additional known centriolar satellite factors showed that CEP290, but not other proteins, was similarly regulated by ubiquitylation that was lost in response to UV radiation (Figure 4C; data not shown). Hence, there is a notable correlation between the centriolar satellite factors that are regulated by stress-induced ubiquitylation loss and those that undergo displacement from the satellites. In SDS–PAGE, the bulk of ubiquitylated AZI1, PCM1, and CEP290 migrated as distinct bands, the mobility of which were slightly shifted compared to the unmodified proteins, suggesting that they corresponded to monoubiquitylated forms (Figure 4C; Supplementary Figure S5D; data not shown).
Figure 4.
MIB1 promotes ubiquitylation of centriolar satellite factors and is inactivated by cell stress. (A) SILAC-labelled U2OS/Strep-HA-ubiquitin cells were mock treated (Light (L) medium) or subjected to UV radiation (25 J/m2) or ionizing radiation (IR, 10 Gy) (Heavy (H) medium) and collected 1 h later. Ubiquitylated proteins were purified on Strep-Tactin resin under denaturing conditions and analysed by mass spectrometry (MS) for determination of SILAC ratios for individual proteins (Supplementary Figure S4A; Supplementary Table S1). SILAC ratios (H/L), indicating relative DNA damage-induced change in ubiquitylation level, for selected proteins are shown. (B) U2OS/Strep-HA-ubiquitin cells left untreated or exposed to UV were collected 1 h later. Where indicated, p38 inhibitor (p38i) was added to the medium 30 min before UV radiation. Cell extracts were subjected to Strep-Tactin pull-down followed by immunoblotting with MIB1 antibody. (C) U2OS or U2OS/Strep-HA-ubiquitin cells were mock treated or subjected to UV, collected 1 h later, and lysed under denaturing conditions. Ubiquitylation of centriolar satellite proteins was analysed by immunoblotting Strep-Tactin pull-downs from whole-cell extracts (WCEs) with AZI1, PCM1, and CEP290 antibodies. (D) U2OS or U2OS/Strep-HA-ubiquitin cells were transfected for 72 h with control siRNA or independent siRNAs targeting MIB1. Ubiquitylation of AZI1 and PCM1 in WCEs was analysed by immunoblotting Strep-Tactin pull-downs with the indicated antibodies. (E) U2OS or U2OS/Strep-HA-ubiquitin cells were transfected for 24 h with WT or catalytically inactive (CI) versions of GFP-MIB1 and treated or not with UV. Ubiquitylation of AZI1 and PCM1 was analysed as in (C). (F) Extracts of U2OS cells transfected with indicated combinations of plasmids were analysed for ubiquitylation of AZI1(F1) (amino acids 1–268) by immunoblotting S-tag pull-downs with HA antibody.
On the basis of the tight relationship between MIB1, AZI1, and PCM1, we reasoned that MIB1 might be the E3 ubiquitin ligase responsible for ubiquitylation of AZI1 and PCM1. Indeed, depletion of MIB1 by any of several independent siRNAs strongly impaired both AZI1 and PCM1 ubiquitylation (Figure 4D). Moreover, overexpression of WT but not catalytically inactive (CI) MIB1 increased the ubiquitylation of endogeneous AZI1 and PCM1 (Figure 4E), and MIB1 WT but not CI ubiquitylated an N-terminal AZI1 fragment containing the MIB1 binding site (Figures 3E and 4F). Importantly, knockdown of MIB1 did not cause displacement of AZI1 and PCM1 from centriolar satellites, as determined by both immunofluorescence and subcellular fractionation analysis (Supplementary Figure S6A–C), indicating that the loss of AZI1 and PCM1 ubiquitylation upon MIB1 depletion did not result indirectly from their removal from this locale. These data strongly indicate that AZI1 and MIB1 are targeted by MIB1-dependent ubiquitylation during normal cell proliferation. Notably, MIB1 depletion did not impair the UV-induced dispersal of AZI1 and PCM1 from centriolar satellites (Supplementary Figure S6A and B). Knockdown of the E3 ligase WWP2, which has been suggested to interact with AZI1 (Akimov et al, 2011), did not affect AZI1 and PCM1 ubiquitylation and their UV-induced removal from centriolar satellites (Supplementary Figure S6D and E). These data indicate that MIB1 and its ubiquitylation of centriolar satellite-associated proteins including AZI1 and PCM1 are dispensable for stress-induced, p38-dependent remodelling of centriolar satellite composition.
MIB1-dependent ubiquitylation suppresses primary cilium formation
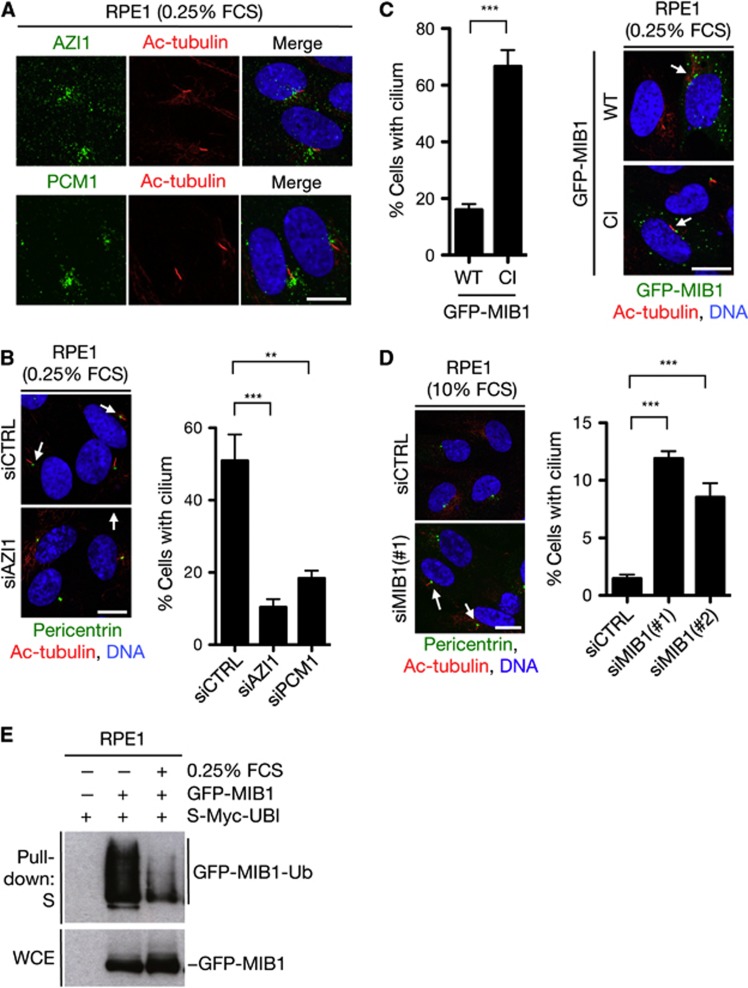
We set out to investigate the physiological significance of cell stress- and MIB1-dependent regulation of centriolar satellite factors. A number of centriolar satellite-associated proteins, including PCM1, are required for primary cilium formation (Barenz et al, 2011), and we found that AZI1, like PCM1, localized to the ciliary base and was required for ciliogenesis in human serum-starved RPE1 cells (Figure 5A and B), in accordance with recent findings on AZI1 orthologues from different vertebrate species (Graser et al, 2007; Wilkinson et al, 2009; Ma and Jarman, 2011). Interestingly, overexpression of WT MIB1, but not a CI mutant, prominently suppressed ciliogenesis in serum-starved cells (Figure 5C). Depletion of MIB1 from proliferating cells had an inverse effect, causing a marked increase in the proportion of cells that formed primary cilia, despite ciliogenesis is robustly repressed during normal cell-cycle progression (Kim and Tsiokas, 2011) (Figure 5D). On the other hand, knockdown of MIB1 did not affect cilium regression upon cell-cycle re-entry (Supplementary Figure S7A), indicating that the increased rate of ciliogenesis in proliferating cells lacking MIB1 did not result from impaired cilium disassembly. Moreover, depletion of MIB1 had little effect on overall cell-cycle distribution (Supplementary Figure S7B). These data suggest that MIB1-dependent ubiquitylation of AZI1 and PCM1 may help to suppress primary cilium formation during the cell cycle. To further explore the potential links between MIB1-mediated ubiquitylation of centriolar satellite components and primary cilium formation, we asked whether induction of ciliogenesis upon exit from the cell cycle was accompanied by an alteration of satellite-associated ubiquitylations. In agreement with our findings that MIB1 E3 ligase activity suppresses ciliogenesis, we found that MIB1 activity was robustly attenuated upon serum starvation (Figure 5E). The MIB1-dependent ubiquitylation of an N-terminal AZI1 fragment was also diminished in serum-starved cells (Supplementary Figure S7C). Together, these findings suggest that MIB1-dependent ubiquitylation of target proteins including AZI1 and PCM1 suppresses primary cilium formation during cell proliferation, and that repression of its E3 ligase activity upon exit from the cell cycle facilitates activation of the ciliation programme.
Figure 5.
MIB1 ubiquitin ligase activity suppresses primary cilium formation. (A) Human RPE1 cells were serum starved by culturing in medium containing 0.25% FCS, fixed and immunostained with antibodies against AZI1 and acetylated tubulin (Ac-tubulin). Scale bar, 10 μm. (B) Effect of AZI1 or PCM1 knockdown on primary cilium formation in cells treated as in (A) was analysed by immunostaining with antibodies against pericentrin and acetylated tubulin (left). Data from three independent experiments were quantified (right). At least 300 cells were counted in each experiment. Scale bar, 10 μm. (C) RPE1 cells were transfected with GFP-MIB1 WT or CI for 24 h and serum starved for 16 h. Cells were then fixed, immunostained with antibody to acetylated tubulin, and the fraction of GFP-positive cells with a cilium was determined. The experiment was performed in triplicates and at least 150 GFP-positive cells were counted for each condition. (D) RPE1 cells were transfected with control (CTRL) or MIB1 siRNAs for 72 h and cultured in normal medium containing 10% serum (10% FCS). Spontaneous cilium formation was visualized and quantified as in (B). Scale bar, 10 μm. Data in (B–D) were analysed by pairwise two-tailed t-test. **P<0.01, ***P<0.001. (E) GFP-MIB1 autoubiquitylation was analysed by transfecting RPE1 cells with GFP-MIB1 and S-Myc-ubiquitin plasmids. Cells were then grown in medium containing high (10% FCS) or low (0.25% FCS) serum concentrations for 24 h and collected. Whole-cell extracts (WCEs) were subjected to pull-down with S-tag agarose and immunoblotted with GFP antibody.
Cell stresses induce p38-dependent ciliogenesis
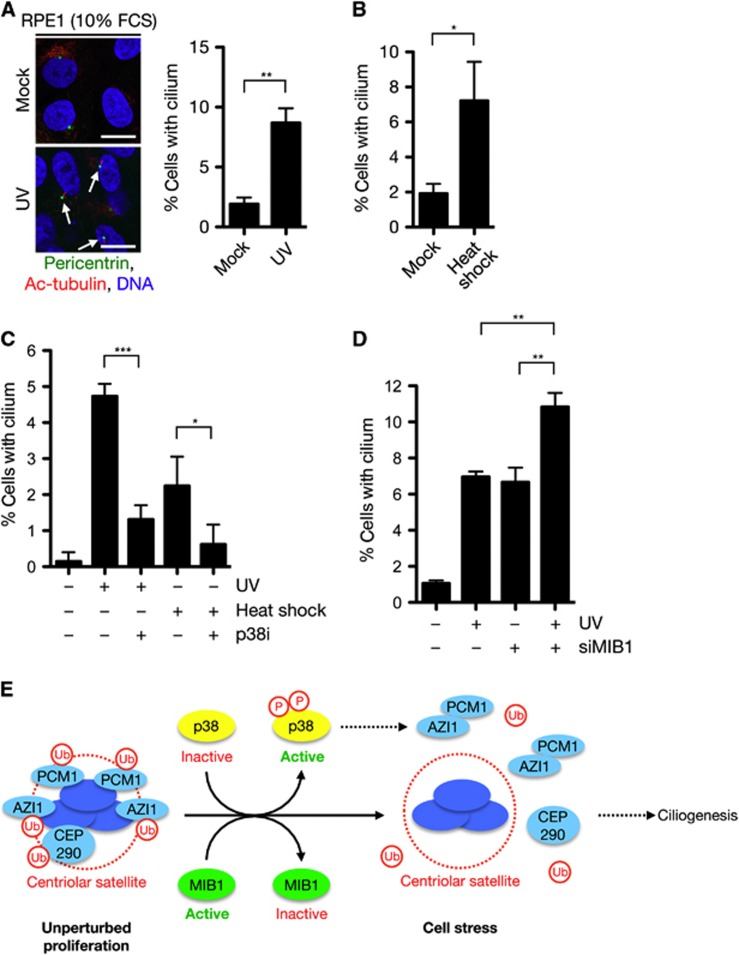
Prompted by our observations that UV radiation and other cell stresses trigger striking displacement of centriolar satellite factors accompanied by loss of their MIB1-dependent ubiquitylation, we asked whether such stress conditions would also induce primary cilium formation in proliferating RPE1 cells. Indeed, we reproducibly observed a marked increase in the proportion of ciliated cells following exposure to low doses of UV or heat shock (Figure 6A and B). Treatment with p38 inhibitor largely prevented the increase in ciliated cells observed in response to such stresses (Figure 6C), suggesting that p38-dependent reorganization of centriolar satellite structure is important for supporting ciliogenesis under these conditions. Moreover, consistent with the role of MIB1 in suppressing ciliogenesis, UV radiation and MIB1 inactivation had an additive effect on primary cilium formation, the combination of which led to an almost 10-fold stimulation of ciliogenesis in proliferating cells (Figure 6D). These data suggest that two parallel stress-inducible pathways, one involving p38-dependent dissociation of selected factors from centriolar satellites and one that triggers p38-independent MIB1 inactivation and concomitant loss of ubiquitylation of these factors, converge on regulation of centriolar satellites and their components, cooperatively stimulating ciliogenesis (Figure 6E). Although the precise mechanism by which displacement and deubiquitylation of centriolar satellite factors such as AZI1 and PCM1 promote stress-induced ciliogenesis remains to be resolved, MS-based quantification of AZI1-interacting proteins and immunoprecipitation experiments revealed that UV induced a striking, ∼8-fold increase in the association of AZI1 with PCM1 that was unparalled by other AZI1-interacting proteins, and which was dependent on p38 activity (Supplementary Figure S8A–C). It seems likely that this enhanced interaction between AZI1 and PCM1, both of which are necessary for primary cilium formation, may at least partially underlie the stimulation of ciliogenesis observed in cells exposed to cellular stresses.
Figure 6.
Cell stresses induce p38-dependent primary cilium formation in proliferating cells. (A) RPE1 cells were exposed or not to UV (10 J/m2), kept at normal serum concentrations (10% FCS), and fixed 18 h later. Spontaneous cilium formation was analysed by immunostaining with antibodies against pericentrin and acetylated tubulin (left). Data from three independent experiments were quantified (right). At least 300 cells were counted in each experiment. Scale bars, 10 μm. (B) As in (A), except that cells were mock treated or subjected to heat shock for 30 min and fixed 18 h later. (C) Exponentially growing RPE1 cells were exposed to UV (10 J/m2) or subjected to heat shock. Where indicated, p38 inhibitor (p38i) was added to the cultures 30 min before treatment. After an additional 16 h, cells were fixed, co-immunostained with antibodies to acetylated tubulin and pericentrin, and the proportion of cilium-positive cells was determined. The experiment was performed in triplicates and at least 300 cells were counted for each condition. (D) RPE1 cells were transfected with control (−) or MIB1 siRNAs for 48 h, exposed to UV (10 J/m2) and fixed 16 h later. Cells were then immunostained and analysed as in (C). Data in (A–D) were analysed by pairwise two-tailed t-test. *P<0.05, **P<0.01, ***P<0.001. (E) Model for stress-induced reorganization of centriolar satellites and ciliogenesis. During unperturbed cell proliferation, AZI1, PCM1, and CEP290 are associated with centriolar satellites and ubiquitylated by the MIB1 E3 ubiquitin ligase, which is itself a component of centriolar satellites. In response to cell stresses such as UV radiation, heat shock, and transcription blocks, activation of the p38 kinase triggers acute dissociation of these proteins from centriolar satellites, leading to enhanced AZI1–PCM1 interaction. In parallel, cell stress also causes p38-independent inactivation of MIB1 and concomitant loss of AZI1, PCM1, and CEP290 ubiquitylation. Cooperatively, these stress-induced pathways cooperate to relieve inhibitory constraints to primary cilium formation in proliferating cells.
Discussion
In this study, we have identified and characterized an all-new cellular stress response that impinges on the composition and functions of centriolar satellites, poorly studied subcellular structures whose components and functional integrity have not previously been known to be subject to signalling-dependent regulation. A broad range of cell stresses, such as UV radiation, heat shock, proteasome inhibition, and transcription blocks triggers selective displacement of a number of centriolar satellite factors that includes, but may not be limited to, AZI1, PCM1, CEP290, and MIB1. This stress-induced centriolar satellite response is centrally dependent on the activity of the stress-responsive kinase p38, and hyperactivation of p38 by overexpression of its upstream kinase MKK6 is sufficient to trigger dissolution of centriolar satellites even in the absence of cell stress. This suggests that p38-mediated signalling to centriolar satellites may be a common feature of cellular stress responses that directly impact on centrosomal functions. Several cellular differentiation processes impinge on specialized functions of the centrosome, including primary cilium outgrowth and dendritic patterning in neurons, and p38 activity is generally found to be associated with cell differentiation, in particular skeletal muscle differentiation and myogenesis (Nebreda and Porras, 2000; Nigg and Raff, 2009; Puram et al, 2011a). Thus, the links between activation of cellular stress pathways and cell differentiation may, at least in part, involve p38-mediated regulation of centriolar satellite organization.
Our findings suggest that MIB1-dependent ubiquitylation of centriolar satellite components restrains centrosomes from differentiating into more specialized structures such as basal bodies, from which primary cilia can grow. Upon stimuli such as serum starvation that generate permissive conditions for cilium outgrowth, MIB1 E3 ligase activity is diminished, relieving inhibitory constraints on its targets. Such negative regulation of ciliation is not unprecedented, as the centrosomal proteins CP110 and CEP97 were previously shown to be key constituents of a pathway that suppresses cilium formation during interphase (Spektor et al, 2007). Whereas these proteins are degraded prior to the ciliation process, MIB1 appears to maintain its targets in a latent state through inhibitory ubiquitylation that is reversed during ciliogenesis and in response to cell stress. We did not find CP110 and CEP97 among the candidate AZI1- and MIB1-interacting proteins mapped by our MS-based approach (data not shown), suggesting that the signalling response involving MIB1-dependent ubiquitylation of centriolar satellite components including AZI1 and PCM1 forms part of a distinct programme that suppresses cilia assembly in proliferating cells. The precise mechanism by which MIB1 inhibits primary cilium formation through ubiquitylation of ciliogenesis-promoting target proteins such as AZI1 and PCM1 remains to be determined. However, together with the observation that both AZI1 and PCM1 localize to the ciliary base and are required for primary cilium formation, the strong and specific increase in the interaction between these proteins occurring concomitantly with their cell stress-induced release from centriolar satellites and deubiquitylation suggest that MIB1-mediated ubiquitylation and p38-dependent signalling may help to prevent productive interactions between AZI1 and PCM1, which might otherwise contribute to triggering ciliogenesis. Collectively, our data suggest that two parallel signalling pathways cooperatively promote the cell stress-induced centriolar satellite response leading to stimulation of primary cilium formation (Figure 6E). One pathway invokes the p38-dependent dispersal of AZI1 and PCM1 from centriolar satellites and a concomitant increase in the interaction between these proteins. Another stress-activated signalling response triggers strong inactivation of MIB1 E3 ligase activity in a p38-independent manner, leading to loss of ubiquitylation of AZI1, PCM1, CEP290, and perhaps other proteins, under such conditions. The convergent effect of these pathways on the status of centriolar satellites and its constituents brings about a marked stimulation of primary cilium formation even in proliferating cells. The biological significance and ramifications of stress-induced ciliogenesis have yet to be determined.
Besides imposing constraints on primary cilium formation, the signalling pathway involving MIB1-dependent ubiquitylation of centriolar satellite components may well have a broader impact on centrosome functions. For instance, it has recently been shown that a calcium-dependent signalling pathway operating at the centrosome orchestrates dendrite patterning in neurons. This pathway requires calcium influx through the TRPC5 calcium channel, calcium/calmodulin-dependent kinase IIβ (CaMKIIβ) activity, as well as targeting of this kinase to the vicinity of centrosomes by interaction with PCM1 (Puram et al, 2011a, b, 24). Of note, AZI1 contains a calmodulin-binding IQ domain in its N-terminus, and we found that AZI1 avidly interacts with calmodulin as well as most of the major calmodulin kinase isoforms (Figure 3A; data not shown). Thus, it seems likely that AZI1, a prominent PCM1 interactor, also participates in the dendrite patterning pathway operating at the centrosome, and that this may be regulated by MIB1-dependent ubiquitylation and stress-induced disassembly of centriolar satellites.
Intriguing links between cilium formation and the DDR have been uncovered recently, as mutation of established as well as new DDR factors were shown to be the genetic determinants of a subset of ciliopathies (Chaki et al, 2012; Zhou et al, 2012). Here, we have shown that DNA damaging insults per se can induce cilium formation, and we have uncovered the first molecular links that connect DNA damage and other stresses with centriolar satellites and ciliogenesis. Future studies should shed more light on these emerging connections and uncover additional mechanisms by which the DDR and other stress-inducible signalling pathways impact on centrosome functions.
Materials and methods
Plasmids and siRNA
Full-length AZI1 and MIB1 cDNAs were amplified by PCR and inserted into pEGFP-C1 (Clontech) to generate mammalian expression plasmids for GFP-tagged AZI1 and MIB1. HA-tagged AZI1 fragments were generated by PCR cloning of amino acids (aa) 1–268 (F1), aa 251–570 (F2), aa 549–820 (F3), and aa 801–1080 (F4) into the previously described vector pcDNA4/TO/STREP-HA. S-Myc-ubiquitin cDNA was produced as a synthetic gene (Eurofins, MWG) and cloned into pcDNA4/TO (Invitrogen). The C985S point mutation in the MIB1 RING3 domain to generate CI MIB1 was introduced using the QuikChange Site Directed Mutagenesis Kit (Stratagene) according to the manufacturer’s instructions. All constructs were verified by sequencing. Plasmids expressing WT and kinase dead (K82A) versions of FLAG-tagged MKK6 were obtained from Addgene (Raingeaud et al, 1996). Plasmid transfections were performed using FuGene 6 (Promega) and siRNA transfections were done with Lipofectamine RNAiMAX (Invitrogen) as described. siRNA target sequences (Eurofins) used in this study were Control (CTRL) (5′-GGGAUACCUAGACGUUCUA-3′), AZI1 (5'-CUGACAAACUUGGAGAAAUUTT-3′), PCM1 (5′-GGUUUUAACUAAUUAUGGATT-3′), p38 (5′-GUGAAAUGUCAGAAGCUUATT-3′), CEP290 (5′-GAACAAACGUCUAAAGAAATT-3′), MIB1(#1) (5′-UCAUGGGCAUUCGAUGGAATT-3′), MIB1(#2) (5′-GGAUAAAGAUGGUGAUAGATT-3′), MIB1(#4) (5′-AGAUCAAGAUGGAGGAAAUTT-3′), and MIB1(3′UTR) (5′-GAUCUAAGGUCAUGGUAAATT-3′).
Cell culture, drugs, and treatments
Human U2OS cells were cultured in DMEM containing 10% fetal bovine serum. To generate cell lines stably expressing GFP-tagged MIB1, U2OS cells were co-transfected with pEGFP-C1-MIB1 and pBABE.puro and selected with 1 μg/ml puromycin (Sigma) for 14 days. Individual clones were picked and analysed for GFP-MIB1 expression by fluorescence microscopy. The U2OS/Strep-HA-ubiquitin cell line was described previously (Danielsen et al, 2011). RPE1 (hTERT immortalized human Retinal Pigment Epithelial cells) was obtained from ATCC and maintained in DMEM/F12 containing 10% fetal bovine serum. UV irradiation was delivered in a BS-02 irradiation chamber equipped with 254 nm bulbs (Gröbel Elektronik, Germany). The dose used was 50 J/m2 unless otherwise stated. To induce DNA double-strand breaks, cells were exposed to 10 Gy of X-rays using a Y.SMART tube (YXLON A/S, Denmark) at 6 mA and 160 kV through a 3-mM aluminium filter. Other genotoxic agents used were 4NQO (25 μg/ml, Sigma), campthotecin (10 μM, Sigma), and hydroxyurea (2 mM, Sigma). For heat shock, cell cultures were sealed with parafilm and submerged in a 42°C water bath for 30 min and allowed to recover for 1 h. For transcriptional inhibition, Actinomycin D (Sigma) was administered at 5 μg/ml for 1 h and DRB (Sigma) at 50 μg/ml for 6 h. Additional drugs used were p38 inhibitor SB203580 (10 μM, Cell Signaling) and proteasomal inhibitor MG132 (20 μM, AH Diagnostics).
MS-based analysis of UV-regulated protein ubiquitylation and AZI1- and MIB1-interacting proteins
For SILAC labelling, cells were cultured in medium containing either L-arginine and L-lysine or L-arginine-U-13C6-15N4 and L-lysine-U-13C6-15N2 (Cambridge Isotope Laboratories) as described previously (Ong et al, 2002). To analyse global changes in protein ubiquitylation in response to UV radiation, U2OS/Strep-HA-ubiquitin cells were subjected to SILAC labelling in Heavy (H) or Light (L) medium, treated with UV (25 J/m2) (SILAC Heavy) or mock treated (SILAC Light), and harvested 1 h later. Cells were lysed under denaturing conditions, and extracts were incubated with Strep-Tactin agarose to isolate Strep-HA-ubiquitin-conjugated proteins. Bound proteins were resolved by SDS–PAGE and processed for MS-based determination of SILAC ratios (H/L) for individual proteins. For identification of AZI1- and MIB1-interacting proteins, U2OS cells were transfected with GFP-tagged AZI1 or MIB1 constructs or empty vector and lysed in modified RIPA buffer (50 mM Tris, pH 7.5; 150 mM NaCl; 1 mM EDTA; 1% NP-40; 0.1% sodium deoxycholate) supplemented with protease inhibitor cocktail (Roche) 48 h later. Lysates were cleared by centrifugation at 17 000 g for 15 min at 4°C, and the GFP-tagged bait protein and its interacting proteins were enriched using GFP-Trap resin (Chromotek). Proteins were resolved by SDS–PAGE and in-gel digested with trypsin. Peptide fractions were analysed on a quadrupole Orbitrap mass spectrometer (Q-Exactive, Thermo Scientific) equipped with a nanoflow HPLC system (Thermo Scientific) as described (Michalski et al, 2011). Raw data files were analysed using the MaxQuant software (version 1.2.2.9) as described (Cox and Mann, 2008).
Immunochemical methods and subcellular fractionation
Immunoblotting, immunoprecipitation, and Strep-Tactin pull-downs were done as described (Mailand et al, 2006, 2007). GFP immunoprecipitation was performed with GFP-Trap agarose beads (Chromotek). Highly enriched fractions of ubiquitylated proteins from U2OS/Strep-HA-ubiquitin cells were obtained as described (Danielsen et al, 2011). Cytoplasmic and cytoskeleton fractions were obtained from 1.4 × 106 U2OS cells (corresponding to ∼20 μl packed cell volume) using a subcellular fractionation kit (Thermo Scientific), according to the manufacturer’s instructions. Antibodies used in this study included: rabbit polyclonals to AZI1 (A301-415A, Bethyl; ab84864, Abcam), PCM1 (A301-150A, Bethyl), MIB1 (NBP1-95846, Novus), p38 (9212S, Cell Signaling), MK2 (3042S, Cell Signaling), CEP290 (ab84870, Abcam), NF-κB-p65 (Cell Signaling), and Histone H3-pSer10 (06-570, Millipore); mouse monoclonals to p-p38 (T180/Y182) (9216S, Cell Signaling), p-MK2 (T334) (3007S, Cell Signaling), GFP (sc-9996, Santa Cruz), γ-tubulin (T5326, Sigma) and Acetylated tubulin (ab24610, Abcam); rat monoclonal to HA tag (11867423991, Roche). Rabbit polyclonal OFD1 antibody was a kind gift from Andrew Fry (University of Leicester, UK).
Immunofluorescence staining and microscopy
For immunofluorescence staining of centriolar satellites, cells were fixed in a 1:1 methanol/acetone mixture and incubated with primary antibodies diluted in DMEM for 1 h at room temperature. Following staining with secondary antibodies (Alexa Fluor 488 and 568; Life Technologies) for 30 min, coverslips were mounted in Vectashield mounting medium (Vector Laboratories) containing nuclear stain DAPI. To visualize primary cilia, RPE1 cells were fixed in 4% formaldehyde, permeabilized with PBS containing 0.2% Triton X-100 for 5 min and immunostained as above. Images were acquired with an LSM 780 confocal microscope (Carl Zeiss Microimaging Inc.) mounted on Zeiss-Axiovert 100M equipped with Plan-Apochromat x40/1.3 oil immersion objective, using standard settings. Image acquisition and analysis was carried out with the ZEN2010 software.
For electron microscopy and immunogold labelling, cells were grown on coverslips and fixed for 30 min in 4% paraformaldehyde in PEM (80 mM PIPES, pH 6.5; 5 mM EGTA; 2 mM MgCl2). Following rinsing in PEM, cells were processed for antibody staining with rabbit anti-PCM1, followed by secondary antibody coupled to Ultra-Small Immunogold (Aurion), and silver lactate treatment, as described (Merdes and De Mey, 1990). The original immunostaining and embedding protocol was altered by omitting any treatment with osmium tetroxide. Araldite-embedded cells were cut at a thickness of 150 nm, and contrasted with lead citrate for 1 min (Reynolds, 1963). Sections were analysed using a Jeol JEM-1400 electron microscope operated at 80 kV.
Supplementary Material
Acknowledgments
We thank Andrew Fry for providing OFD1 antibody and members of the Ubiquitin Signaling Group for helpful discussions. This work was supported by grants from the Novo Nordisk Foundation, Danish Medical Research Council, the Danish Cancer Society, and the Lundbeck Foundation.
Author contributions: NM and SB-J conceived and designed the experiments. BHV, JRD, LP, AM, and SB-J performed most of the experiments. BHV, JRD, Y-GY, NM, and SB-J analysed the data. KBS, PB, CC, and MLN performed mass spectrometry-based experiments and ensuing data analysis. NM wrote the paper, assisted by SB-J.
Footnotes
The authors declare that they have no conflict of interest.
References
- Akimov V, Rigbolt KT, Nielsen MM, Blagoev B (2011) Characterization of ubiquitination dependent dynamics in growth factor receptor signaling by quantitative proteomics. Mol Biosyst 7: 3223–3233 [DOI] [PubMed] [Google Scholar]
- Barenz F, Mayilo D, Gruss OJ (2011) Centriolar satellites: busy orbits around the centrosome. Eur J Cell Biol 90: 983–989 [DOI] [PubMed] [Google Scholar]
- Chaki M, Airik R, Ghosh AK, Giles RH, Chen R, Slaats GG, Wang H, Hurd TW, Zhou W, Cluckey A, Gee HY, Ramaswami G, Hong CJ, Hamilton BA, Cervenka I, Ganji RS, Bryja V, Arts HH, van Reeuwijk J, Oud MM et al. (2012) Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell 150: 533–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Mann M (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26: 1367–1372 [DOI] [PubMed] [Google Scholar]
- Dammermann A, Merdes A (2002) Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J Cell Biol 159: 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen JM, Sylvestersen KB, Bekker-Jensen S, Szklarczyk D, Poulsen JW, Horn H, Jensen LJ, Mailand N, Nielsen ML (2011) Mass spectrometric analysis of lysine ubiquitylation reveals promiscuity at site level. Mol Cell Proteomics 10: M110.003590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graser S, Stierhof YD, Lavoie SB, Gassner OS, Lamla S, Le Clech M, Nigg EA (2007) Cep164, a novel centriole appendage protein required for primary cilium formation. J Cell Biol 179: 321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F, Benzing T, Katsanis N (2011) Ciliopathies. N Engl J Med 364: 1533–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Tsiokas L (2011) Cilia and cell cycle re-entry: more than a coincidence. Cell Cycle 10: 2683–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, Harper JW, Gygi SP (2011) Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell 44: 325–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodani A, Tonthat V, Wu B, Sutterlin C (2010) Par6 alpha interacts with the dynactin subunit p150 Glued and is a critical regulator of centrosomal protein recruitment. Mol Biol Cell 21: 3376–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Sasaki H, Yuba-Kubo A, Tsukita S, Shiina N (1999) Centriolar satellites: molecular characterization, ATP-dependent movement toward centrioles and possible involvement in ciliogenesis. J Cell Biol 147: 969–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes CA, Prosser SL, Romio L, Hirst RA, O'Callaghan C, Woolf AS, Fry AM (2011) Centriolar satellites are assembly points for proteins implicated in human ciliopathies, including oral-facial-digital syndrome 1. J Cell Sci 124: 600–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Jarman AP (2011) Dilatory is a Drosophila protein related to AZI1 (CEP131) that is located at the ciliary base and required for cilium formation. J Cell Sci 124: 2622–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailand N, Bekker-Jensen S, Bartek J, Lukas J (2006) Destruction of Claspin by SCFbetaTrCP restrains Chk1 activation and facilitates recovery from genotoxic stress. Mol Cell 23: 307–318 [DOI] [PubMed] [Google Scholar]
- Mailand N, Bekker-Jensen S, Faustrup H, Melander F, Bartek J, Lukas C, Lukas J (2007) RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell 131: 887–900 [DOI] [PubMed] [Google Scholar]
- Merdes A, De Mey J (1990) The mechanism of kinetochore-spindle attachment and polewards movement analyzed in PtK2 cells at the prophase-prometaphase transition. Eur J Cell Biol 53: 313–325 [PubMed] [Google Scholar]
- Michalski A, Damoc E, Hauschild JP, Lange O, Wieghaus A, Makarov A, Nagaraj N, Cox J, Mann M, Horning S (2011) Mass spectrometry-based proteomics using Q Exactive, a high-performance benchtop quadrupole Orbitrap mass spectrometer. Mol Cell Proteomics 10: M111.011015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda AR, Porras A (2000) p38 MAP kinases: beyond the stress response. Trends Biochem Sci 25: 257–260 [DOI] [PubMed] [Google Scholar]
- Nigg EA, Raff JW (2009) Centrioles, centrosomes, and cilia in health and disease. Cell 139: 663–678 [DOI] [PubMed] [Google Scholar]
- Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M (2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics 1: 376–386 [DOI] [PubMed] [Google Scholar]
- Povlsen LK, Beli P, Wagner SA, Poulsen SL, Sylvestersen KB, Poulsen JW, Nielsen ML, Bekker-Jensen S, Mailand N, Choudhary C (2012) Systems-wide analysis of ubiquitylation dynamics reveals a key role for PAF15 ubiquitylation in DNA-damage bypass. Nat Cell Biol 14: 1089–1098 [DOI] [PubMed] [Google Scholar]
- Puram SV, Kim AH, Ikeuchi Y, Wilson-Grady JT, Merdes A, Gygi SP, Bonni A (2011a) A CaMKIIbeta signaling pathway at the centrosome regulates dendrite patterning in the brain. Nat Neurosci 14: 973–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puram SV, Riccio A, Koirala S, Ikeuchi Y, Kim AH, Corfas G, Bonni A (2011b) A TRPC5-regulated calcium signaling pathway controls dendrite patterning in the mammalian brain. Genes Dev 25: 2659–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ (1996) MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 16: 1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17: 208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spektor A, Tsang WY, Khoo D, Dynlacht BD (2007) Cep97 and CP110 suppress a cilia assembly program. Cell 130: 678–690 [DOI] [PubMed] [Google Scholar]
- Staples CJ, Myers KN, Beveridge RD, Patil AA, Lee AJ, Swanton C, Howell M, Boulton SJ, Collis SJ (2012) The centriolar satellite protein Cep131 is important for genome stability. J Cell Sci 125(Pt 20): 4770–4779 [DOI] [PubMed] [Google Scholar]
- Wagner SA, Beli P, Weinert BT, Nielsen ML, Cox J, Mann M, Choudhary C (2011) A proteome-wide, quantitative survey of in vivo ubiquitylation sites reveals widespread regulatory roles. Mol Cell Proteomics 10: M111.013284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson CJ, Carl M, Harris WA (2009) Cep70 and Cep131 contribute to ciliogenesis in zebrafish embryos. BMC Cell Biol 10: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Otto EA, Cluckey A, Airik R, Hurd TW, Chaki M, Diaz K, Lach FP, Bennett GR, Gee HY, Ghosh AK, Natarajan S, Thongthip S, Veturi U, Allen SJ, Janssen S, Ramaswami G, Dixon J, Burkhalter F, Spoendlin M et al. (2012) FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat Genet 44: 910–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.