Abstract
EMBO J (2013) 32 23, 3041–3054 ; DOI: 10.1038/emboj.2013.228; published online October 15 2013
Parkinson’s disease (PD) is characterised by the loss of dopaminergic neurons in the substantia nigra and accumulation of pathological inclusions containing α-synuclein termed Lewy bodies. However, to date the precise function of α-synuclein has remained elusive along with the mechanism of neuronal death. A paper published in this issue of EMBO Journal provides insight into the mechanism of α-synuclein-induced neuronal death and thereby reveals potential new targets for therapeutic intervention. Büttner et al (2013) highlight Endonuclease-G as a key executioner in α-synuclein toxicity and show that inhibiting mitochondrial release of Endonuclease-G can protect against α-synuclein toxicity.
Endonuclease-G (Endo-G) was originally purified as a DNase with preference for cleaving (dG)n.(dC)n tracts (Ruiz-Carrillo and Renaud, 1987). Endo-G is genomically encoded, however the first 48 amino acids contain a mitochondrial localisation sequence which directs the protein to mitochondria (Cote et al, 1989). Endo-G displays DNase and RNase activity and is thought to cleave DNA–RNA hybrids, which are formed at the mitochondrial DNA (mtDNA) origin of replication (Cote and Ruiz-Carrillo, 1993). This generates the RNA primers that are required by DNA polymerase-γ to initiate replication of mtDNA. Thus, Endo-G is intrinsically linked to mitochondrial physiology via promoting mtDNA replication, and overexpression of Endo-G has been shown to increase mitochondrial mass and respiration (McDermott-Roe et al, 2011; Figure 1A). In a landmark paper from Xiadong Wang’s laboratory, Endo-G has subsequently been shown to also act as a proapoptotic factor that is released in a Bcl-2-dependent manner from mitochondria during apoptosis and induces DNA fragmentation (Li et al, 2001).
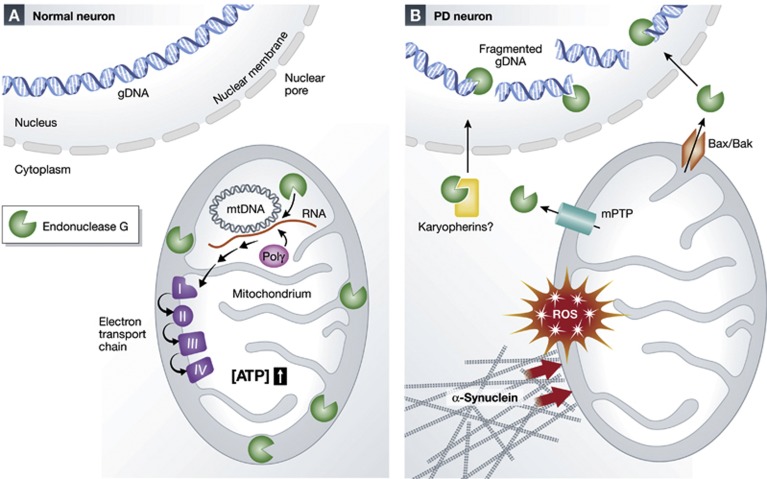
Figure 1.
Physiological roles of Endo-G (A) and during dopaminergic neurodegeneration (B). (A) Under physiological conditions Endo-G is located in the mitochondria where it regulates mitochondrial bioenergetics via cleaving DNA–RNA hybrids generated at the mtDNA origin of replication to yield the RNA primers necessary for mtDNA replication. This leads to increased mtDNA transcription and increased respiratory complex activity. (B) In PD neurons aggregated α-synuclein generates increased levels of ROS, which together lead to release of Endo-G from the mitochondria. Endo-G is then translocated to the nucleus, possibly via interactions with the Karyopherin family of nuclear import factors, where it digests genomic DNA leading to cell death.
Building on previous work that showed that functional mitochondria are essential for α-synuclein-induced cell death (Büttner et al, 2008), Madeo’s group now go on to demonstrate that Endo-G is a key downstream effector of α-synuclein-induced toxicity (Figure 1B). What’s more, knockdown of Endo-G in yeast, worms, flies and human cells abolishes α-synuclein toxicity at both the cellular and organismal level making Endo-G an exciting target in the battle to prevent the degeneration of dopaminergic neurons.
Oligonucleosomal DNA fragmentation is one of the hallmarks of apoptotic (type I) programmed cell death (PCD), along with nuclear condensation and the formation of apoptotic bodies (Leist and Jaattela, 2001). During initiation of apoptosis, the caspase-activated DNase (CAD) is activated via caspase 3-mediated cleavage of its inhibitor (ICAD; Enari et al, 1998). However in many cell types such as mature neurons, caspase activation is submaximal and nuclear DNA cleavage is achieved through alternative, caspase-independent pathways. These include the mitochondrial release of apoptosis-inducing factor (AIF) and Endo-G, which are able to induce DNA fragmentation in the absence of caspase activation (Susin et al, 1999; Li et al, 2001; Cregan et al, 2002). As nuclear condensation rather than fragmentation is often triggered under these circumstances, this type of apoptosis is referred to as type II apoptosis (Leist and Jaattela, 2001).
In a strategy to identify novel cell death pathways, Büttner et al (2013) turned to yeast as a model system and initially measured ROS production in response to α-synuclein in a range of mitochondrial-associated gene deletions. While many deletions had no effect on α-synuclein-induced ROS production, deletion of the homologous yeast protein to Endo-G (Nuc1p) substantially suppressed ROS formation. Further investigations in yeast revealed that deletion of several distinct regulators of the mitochondrial permeability transition pore (PTP) could also inhibit α-synuclein-induced toxicity through preventing the mitochondrial release and nuclear translocation of Endo-G, while having no effect on α-synuclein levels. Deletion of the Yeast Karyopherin Kap123p, which interacts with Endo-G and functions in nuclear import, also abrogated the α-synuclein-induced toxicity, hinting at the mechanism of Endo-G nuclear translocation.
To further dissect the pathways involved in α-synuclein-induced toxicity in yeast, the authors used the Stable Isotype Labelling with Amino acids in Cell culture (SILAC) technique, which can analyse changes in total protein composition. This approach identified two proteins Bna3p and Ecm33p, which are required for α-synuclein-induced toxicity. Further experiments demonstrated that these two proteins were necessary for the mitochondrial release of Endo-G/Nuc1p, however the function of Ecm33p is unknown and Bna3p is thought to function in the Kynurenine pathway, so it remains unclear how they may affect α-synuclein-induced mitochondrial release of Endo-G.
Moving on from yeast models the group then confirmed their findings in worms, flies and human cells. Endo-G-deficient C. elegans were significantly more resistant to α-synuclein-induced dopaminergic neuron cell death than wild-type worms, showing a decrease from 50% neuron loss in wild-type worms to 10% cell death in those lacking Endo-G. Drosophila proved more resistant to α-synuclein toxicity leading the authors to combine α-synuclein overexpression with manganese exposure, which increases cellular stress by generating additional ROS. In combination, these manipulations led to impaired movement of the flies and subsequent death. However, flies that co-express an RNAi hairpin, which decreases Endo-G levels by around 40%, were protected against α-synuclein-induced toxicity. Conversely, flies that overexpress both α-synuclein and Endo-G showed increased levels of death in response to manganese exposure. Furthermore, the α-synuclein toxicity was shown to selectively affect tyrosine hydroxylase-positive dopaminergic neurons, and this neurotoxicity could be abolished by RNAi-induced depletion of Endo-G. Interestingly, Endo-G has previously been shown to be crucial for cell death initiated by oxidative stress and Ca2+ overloading (Ishihara and Shimamoto, 2006; Higgins et al, 2009), processes that are also key to dopaminergic neurodegeneration.
The final model chosen to validate this finding was a previously established human neuronal cell culture model, whereby SH-SY5Y cells overexpressing α-synuclein are subjected to oxidative stress via exposure to hydrogen peroxide and iron chloride (Gerard et al, 2010). Stable knockdown of Endo-G via lentiviral shRNA reduced the levels of apoptosis in both stressed and unstressed cells; however, aggregated α-synuclein was still present in the cells. Immunofluorescence analysis of cells overexpressing α-synuclein showed that elevated levels of Endo-G were present in the cytoplasm, indicating mitochondrial release; however, total Endo-G levels were also increased suggesting that Endo-G was also regulated at the transcriptional or translational level. Finally, elevated levels of nuclear Endo-G were also found in dopaminergic neurons from the substantia nigra in post mortem tissue of PD patients when compared to age-matched controls, indicating DNA fragmentation may be initiated in these neurons.
The authors have demonstrated in multiple model systems that α-synuclein toxicity towards dopaminergic neurons is dependent on the function of Endo-G. A cautionary note may be required here. Many mitochondrial proteins have been shown to have dual functions in both cellular bioenergetics and cell death signalling (Kilbride and Prehn, 2013). Similar to cytochrome c and AIF, Endo-G has an evolutionarily conserved function in the regulation of mitochondrial mass and mitochondrial respiration (Cote and Ruiz-Carrillo, 1993; McDermott-Roe et al, 2011). This may make cell death data from gene knockout or gene silencing studies difficult to interpret, as Endo-G-deficient cells may adapt to enhanced bioenergetics or oxidative stress. The importance of mitochondrial bioenergetics in PD is highlighted by the fact that several genes associated with familial PD have crucial roles associated with mitochondrial bioenergetics and mitochondrial quality control, including Parkin, PINK1 and DJ-1, reviewed in Exner et al (2012). Using elegant genetic manipulations in yeast, Büttner et al (2013) have also demonstrated that if Endo-G is prevented from exiting the mitochondria then α-synuclein toxicity is abrogated, however further work is required to clarify the mechanism of Endo-G release from mitochondria and which components of the mPTP (or the Bcl-2-dependent apoptotic machinery) are involved in mammalian cells. Initial investigations in yeast suggest that the Karyopherin family may be involved in nuclear import of Endo-G, but this also requires further investigation. If these findings can be confirmed in mammalian systems, this research makes both Endo-G and potentially the factors that govern the subcellular localisation of Endo-G exciting new targets for therapeutic intervention.
Acknowledgments
Work in the laboratory was supported by grants from Science Foundation Ireland and the Health Research Board.
Footnotes
The authors declare that they have no conflict of interest.
References
- Büttner S, Habernig L, Broeskamp F, Ruli D, Nora Vögtle F, Vlachos M, Macchi F, Küttner V, Carmona-Gutierrez D, Eisenberg T, Ring J, Markaki M, Taskin AA, Benke S, Ruckenstuhl C, Braun R, Van den Haute C, Bammens T, van der Perren A, Fröhlich K-U et al. (2013) Endonuclease G mediates α-synuclein cytotoxicity during Parkinson’s disease. EMBO J 32: 3041–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner S, Bitto A, Ring J, Augsten M, Zabrocki P, Eisenberg T, Jungwirth H, Hutter S, Carmona-Gutierrez D, Kroemer G, Winderickx J, Madeo F (2008) Functional mitochondria are required for alpha-synuclein toxicity in aging yeast. J Biol Chem 283: 7554–7560 [DOI] [PubMed] [Google Scholar]
- Cote J, Renaud J, Ruiz-Carrillo A (1989) Recognition of (dG)n.(dC)n sequences by endonuclease G. Characterization of the calf thymus nuclease. J Biol Chem 264: 3301–3310 [PubMed] [Google Scholar]
- Cote J, Ruiz-Carrillo A (1993) Primers for mitochondrial DNA replication generated by endonuclease G. Science 261: 765–769 [DOI] [PubMed] [Google Scholar]
- Cregan SP, Fortin A, MacLaurin JG, Callaghan SM, Cecconi F, Yu SW, Dawson TM, Dawson VL, Park DS, Kroemer G, Slack RS (2002) Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J Cell Biol 158: 507–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391: 43–50 [DOI] [PubMed] [Google Scholar]
- Exner N, Lutz AK, Haass C, Winklhofer KF (2012) Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences. EMBO J 31: 3038–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard M, Deleersnijder A, Daniels V, Schreurs S, Munck S, Reumers V, Pottel H, Engelborghs Y, Van den Haute C, Taymans JM, Debyser Z, Baekelandt V (2010) Inhibition of FK506 binding proteins reduces alpha-synuclein aggregation and Parkinson’s disease-like pathology. J Neurosci 30: 2454–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GC, Beart PM, Nagley P (2009) Oxidative stress triggers neuronal caspase-independent death: endonuclease G involvement in programmed cell death-type III. Cell Mol Life Sci 66: 2773–2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara Y, Shimamoto N (2006) Involvement of endonuclease G in nucleosomal DNA fragmentation under sustained endogenous oxidative stress. J Biol Chem 281: 6726–6733 [DOI] [PubMed] [Google Scholar]
- Kilbride SM, Prehn JH (2013) Central roles of apoptotic proteins in mitochondrial function. Oncogene 32: 2703–2711 [DOI] [PubMed] [Google Scholar]
- Leist M, Jaattela M (2001) Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2: 589–598 [DOI] [PubMed] [Google Scholar]
- Li LY, Luo X, Wang X (2001) Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412: 95–99 [DOI] [PubMed] [Google Scholar]
- McDermott-Roe C, Ye J, Ahmed R, Sun XM, Serafin A, Ware J, Bottolo L, Muckett P, Canas X, Zhang J, Rowe GC, Buchan R, Lu H, Braithwaite A, Mancini M, Hauton D, Martí R, García-Arumí E, Hubner N (2011) Endonuclease G is a novel determinant of cardiac hypertrophy and mitochondrial function. Nature 478: 114–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Carrillo A, Renaud J (1987) Endonuclease G: a (dG)n X (dC)n-specific DNase from higher eukaryotes. EMBO J 6: 401–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397: 441–446 [DOI] [PubMed] [Google Scholar]