Abstract
Removal of the androgen receptor (AR) from bone-forming cells has been shown to reduce trabecular bone volume in male mice. In female mice, the role of AR in the regulation of bone homeostasis has been poorly understood. We generated a mouse strain in which the AR is completely inactivated only in mineralizing osteoblasts and osteocytes by breeding mice carrying osteocalcin promoter-regulated Cre-recombinase with mice possessing loxP recombination sites flanking exon 2 of the AR gene (ARΔOB/ΔOB mice). In female ARΔOB/ΔOB mice, the trabecular bone volume was reduced owing to a smaller number of trabeculae at 6 months of age compared with the control ARfl/fl animals. In male ARΔOB/ΔOB mice, an increase in trabecular bone separation could already be detected at 3.5 months of age, and at 6 months, the trabecular bone volume was significantly reduced compared with that of male ARfl/fl mice. No AR-dependent changes were observed in the cortical bone of either sex. On the basis of micro-computed tomography and histomorphometry, we conclude that in male mice, the AR is involved in the regulation of osteoclast number by osteoblasts, whereas in female mice, the lack of the AR in the bone-forming cells leads to a decreased number of trabeculae upon aging.
Introduction
Androgens are known to affect bone development, growth and maintenance in men.1,2 Reduction of serum androgen levels in aging men or in patients treated with anti-androgen therapy has been shown to be associated with the reduced bone mineral density (BMD) of the skeleton.3 In mice, the androgen receptor (AR) is expressed in osteoblasts, osteocytes4,5,6 and osteoclasts.7 Androgens have been suggested to regulate the periosteal expansion of long bones and to inhibit endosteal bone growth.6,8 The inhibition of endosteal bone growth has been considered to be due to androgen-mediated osteoblast apoptosis.8 Overexpression of the AR in premature osteoblasts of male mice using the 3.6 kb type I collagen promoter has been shown to lead to an increase in periosteal bone formation and a reduction in endosteal bone formation,9 whereas use of the 2.3 kb type I collagen promoter, expressed in mature osteoblasts, only reduced endosteal bone formation with no effect on the periosteum.10 These different effects can be attributed, in addition to different stages of osteoblast maturation at which these promoters are active, to various patterns of their expression in bone tissue. The 2.3 kb type I collagen promoter is strongly expressed in endosteal osteoblasts and osteocytes, but not in the periosteum,10,11 whereas the 3.6 kb type I collagen promoter has been shown to be active in the periosteum.9,12 A full AR knockout has been shown to exhibit increased bone turnover and reduced trabecular and cortical bone volume in male mice.13,14,15,16 Further elimination of estrogen receptor α (ERα) caused an additional reduction in cortical bone mass.14 In another full AR knockout model, the bone growth and composition of knockout female mice were indistinguishable from those of wild-type (WT) mice at 8 weeks of age,17 suggesting that the AR is not important during the early development or rapid growth phase of the female skeleton. In the study by MacLean et al.,18 using mice devoid of the AR DNA-binding capacity, trabecular bone structure, but not volume, was altered in 9-week-old female mice, with increased trabecular thickness and decreased trabecular number.
Inactivation of the AR DNA-binding activity in osteocalcin-expressing cells19 and in collagen-1-synthesizing osteoblasts20 has been shown to reduce trabecular bone volume in male mice. In cortical bone various effects have been reported. Some results have indicated a reduction in cortical bone thickness but no change in periosteal circumference when the osteocalcin promoter was used to target the exon 3 of the AR gene,19 whereas no effect on the cortical bone was observed in another study utilizing the type I collagen 2.3 promoter.20
In a recent study by Sinnesael et al.,21 osteocytic AR was shown to be crucial for the maintenance of trabecular bone volume in male mice. The role of AR in the regulation of the female skeleton is currently incompletely characterized. In this research, we show, for the first time, that the lack of AR in mineralizing osteoblast and osteocytes leads to a reduction of trabecular bone volume in mature female mice.
Results
Analysis of the general phenotype
Both ARfl/fl and ARΔOB/ΔOB mice were fertile and healthy in appearance. The average body weights and lengths of the tibiae of the two mouse lines did not differ significantly (Table 1). No histological abnormalities were observed in any of the soft tissues analyzed, including the lung, heart, spleen, kidney, striated muscle, reproductive organs and brain (data not shown). In a recent study, we analyzed the expression of the Cre protein in the same parental mice used for breeding, and the osteocalcin-promoter-regulated expression of Cre itself was not found to affect the bone phenotype.22
Table 1. Length and ash weight of tibiae, body weight.
| N: fl/fl, ΔOB/ΔOB |
Age, gender |
||||
|---|---|---|---|---|---|
| 3 months ♂ | 6 months ♂ | 3 months ♀ | 6 months ♀ | ||
| 8,9 | 8,10 | 6,9 | 6,12 | ||
| Body weight (g) | ARfl/fl | 27.5±1.1 | 32.2±0.6 | 23.1±0.9 | 24.8±1.4 |
| ARΔOB/ΔOB | 29.1±0.6 | 33.8±0.7 | 22.5±0.2 | 26.0±0.5 | |
| Tibia length (mm) | ARfl/fl | 17.96±0.10 | 18.33±0.08 | 17.67±0.17 | 18.08±0.07 |
| ARΔOB/ΔOB | 17.94±0.07 | 18.32±0.07 | 17.29±0.24 | 18.15±0.05 | |
| Ash weight (mg) | ARfl/fl | 20.66±0.72 | 24.31±0.67* | 19.80±0.59 | 21.70±0.48* |
| ARΔOB/ΔOB | 20.66±0.72 | 21.67±0.62 | 18.87±0.27 | 20.12±0.51 | |
Abbreviations: AR, androgen receptor.
Lengths and ash weights of tibiae and body weight of mice.
*P<0.05: At 6 months of age, the ash weight of the tibia was significantly reduced in the ARΔOB/ΔOB mice compared with the ARfl/fl mice of the same gender. Data represented as means±s.e.m.
Expression of AR and ERα mRNA in cortical bone
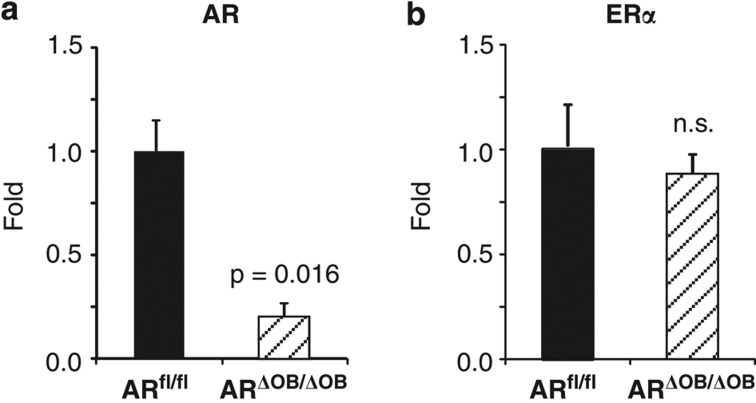
Total RNA was isolated from the purified tibial and femoral cortical bone of 7-week-old male mice and subjected to quantitative real-time polymerase chain reaction (RT-qPCR). The level of AR mRNA was reduced by 79% in ARΔOB/ΔOB mice (Figure 1a; P=0.016). There was no difference in the expression of ERα mRNA between the genotypes (Figure 1b), ruling out any possible compensatory upregulation of ERα upon AR inactivation.
Figure 1.
qPCR analysis of cortical bone. Messenger RNA levels of AR and ERα in the cortical bone of 7-week-old male mice analyzed with RT-qPCR. (a) The AR mRNA levels were reduced by 79% in ARΔOB/ΔOB mice compared with ARfl/fl mice. (b) There were no differences in the ERα mRNA levels (n=4 per group). Data are represented as means±s.e.m. NS, non-significant.
Analysis of the bone phenotypes at the age of 3.5 and 6 months
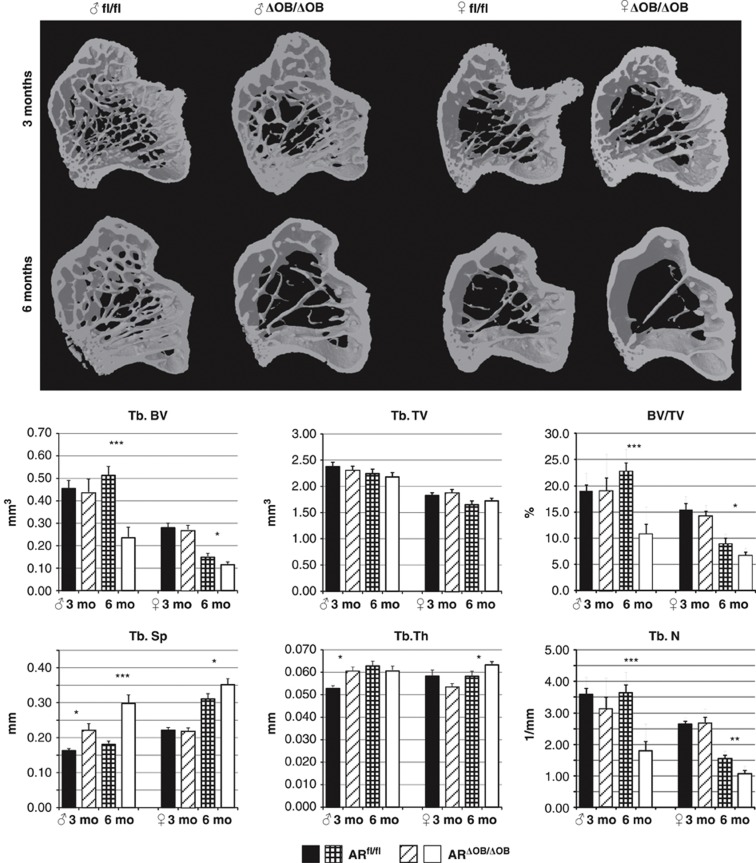
The lengths of the tibiae of ARfl/fl and ARΔOB/ΔOB did not differ significantly in either gender, but the tibial ash weights of ARΔOB/ΔOB mice were reduced compared with those of ARfl/fl mice at 6 months of age in both genders (Table 1). Imaging and volumetric analysis with micro-computed tomography (μCT) revealed an increase in the trabecular bone separation (Tb.Sp, P=0.02) and thickness (Tb.Th, P=0.02) in male ARΔOB/ΔOB mice compared with ARfl/fl males at the age of 3.5 months (Figure 2). The differences in the trabecular bone became larger with age, as the trabecular number (Tb.N) became smaller (P<0.001) and the Tb.Sp became wider (P<0.001) in ARΔOB/ΔOB male mice compared with the ARfl/fl male mice. Thus, a statistically significant difference in trabecular bone volume (Tb.BV) between these mice (P<0.001) was identified. However, the difference in the Tb.Th did not continue to demonstrate further changes. In female mice, there was no difference in the measured trabecular bone parameters at the age of 3.5 months (Figure 2). However, at the age of 6 months, the tibial Tb.N and Tb.BV were significantly reduced (P=0.002 and 0.036, respectively) and the mean Tb.Sp was increased (P=0.049) in ARΔOB/ΔOB female mice compared with the ARfl/fl mice. The Tb.Th was slightly but significantly increased in ARΔOB/ΔOB female mice (Figure 2; P=0.028). In contrast to the trabecular bone, no significant differences could be demonstrated in the μCT analysis of the cortical bone of tibial diaphysis in either males or females (Table 2).
Figure 2.
Modeling and volumetric analysis of the tibial metaphyses. Three-dimensional modeling of the tibial metaphysis and volumetric analysis of the trabecular bone with μCT. At the age of 3.5 months, no differences were detected between any of the parameters measured in female ARΔOB/ΔOB (n=8) and ARfl/fl (n=6) mice, whereas the Tb.Sp and Tb.Th were higher in ARΔOB/ΔOB males (n=8) compared with ARfl/fl males (n=9). At 6 months of age, the Tb.N was highly reduced, leading to a wider Tb.Sp and reduced Tb.BV in ARΔOB/ΔOB mice of both sexes (males: n=8; females: n=8) compared with ARfl/fl mice (males: n=7; females: n=6). *P<0.05; **P<0.01; ***P<0.001. Data are represented as means±s.e.m.
Table 2. μCT analysis of cortical bone of tibiae.
|
Age, gender |
|||||
|---|---|---|---|---|---|
| 3 months ♂ | 6 months ♂ | 3 months ♀ | 6 months ♀ | ||
| |
N: fl/fl, ΔOB/ΔOB |
8, 9 |
8, 8 |
6, 10 |
6, 12 |
| BV (mm3) | ARfl/fl | 0.24±0.007 | 0.26±0.007 | 0.24±0.005 | 0.23±0.006 |
| ARΔOB/ΔOB | 0.26±0.004 | 0.24±0.009 | 0.23±0.005 | 0.23±0.004 | |
| TV (mm3) | ARfl/fl | 0.48±0.01 | 0.50±0.02 | 0.42±0.01 | 0.39±0.01 |
| ARΔOB/ΔOB | 0.51±0.01 | 0.46±0.02 | 0.43±0.01 | 0.40±0.01 | |
| B.Pm (mm) | ARfl/fl | 13.33±0.53 | 12.95±0.32 | 11.27±0.24 | 10.34±0.27 |
| ARΔOB/ΔOB | 13.77±0.28 | 12.24±0.41 | 11.83±0.37 | 10.71±0.14 | |
| B.Ar (mm2) | ARfl/fl | 0.89±0.03 | 0.95±0.02 | 0.90±0.02 | 0.86±0.02 |
| ARΔOB/ΔOB | 0.95±0.02 | 0.89±0.03 | 0.86±0.02 | 0.86±0.01 | |
| Ec.Ar (mm2) | ARfl/fl | 0.86±0.02 | 0.90±0.04 | 0.64±0.03 | 0.57±0.02 |
| ARΔOB/ΔOB | 0.92±0.03 | 0.82±0.03 | 0.72±0.03 | 0.59±0.01 | |
| BMD (g cm−3) | ARfl/fl | 1.11±0.02 | 1.15±0.02 | 1.13±0.04 | 1.14±0.02 |
| ARΔOB/ΔOB | 1.17±0.03 | 1.17±0.02 | 1.09±0.02 | 1.14±0.02 | |
Abbreviations: AR, androgen receptor; BMD, bone mineral density; CT, computed tomography.
Analysis of the cortical bone of tibia with μCT. No differences were detected between the two genotypes within the same age group and a gender in BMD or in any of the volumetric (BV, TV) or two-dimensional (bone perimeter, B. Pm, bone area, B.Ar, and endocortical area, Ec.Ar) parameters analyzed. Data are represented as means±s.e.m.
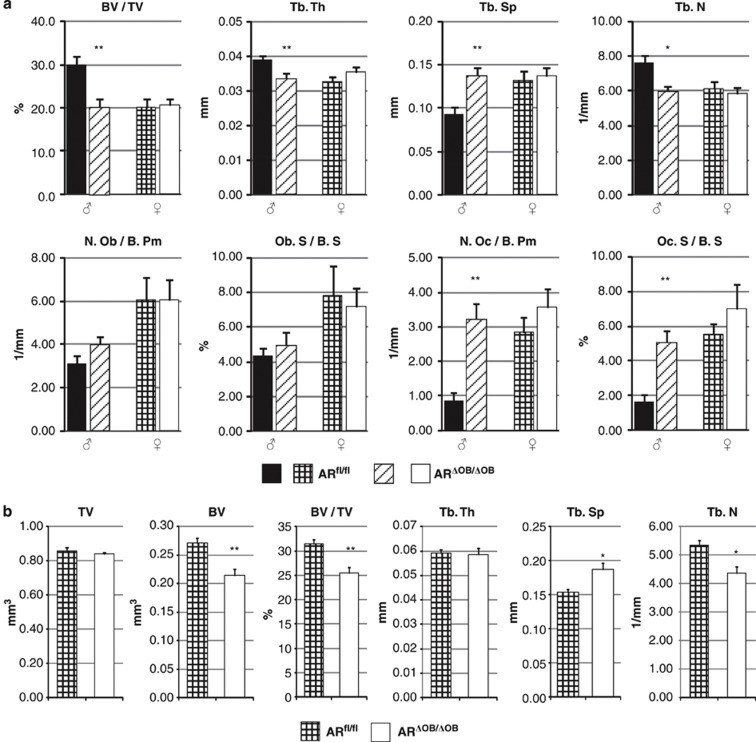
Determined with histomorphometry, the trabecular BV of the L3 lumbar vertebra of 3-month-old ARΔOB/ΔOB male mice was significantly reduced compared with that of ARfl/fl mice (Figure 3a). This change was accompanied by a reduced Tb.Th (P=0.007), reduced Tb.N (P=0.012) and increased Tb.Sp (P=0.005) (Figure 3a). The number of osteoblasts/bone perimeter (N.Ob/B.Pm) and osteoblast surface/bone surface (Ob.S/BS) did not differ significantly between the groups (Figure 3a). However, the number of osteoclasts/bone perimeter (N.Oc/B.Pm) and osteoclast surface/bone surface (Oc.S/BS) were significantly increased in ARΔOB/ΔOB male mice (Figure 3a; P=0.003 and 0.011, respectively). In female mice, no significant differences were detected (Figure 3a).
Figure 3.
Volumetric analysis of the vertebral body of the L3 lumbar vertebra. (a) Histomorphometry of the vertebrae. The trabecular bone content was reduced in 3-month- old ARΔOB/ΔOB male mice when compared with ARfl/fl mice. This change was indicated as significantly reduced BV/TV, Tb.Th and Tb.N and increased Tb.Sp. The N.Oc/B.Pm and Oc.S/BS values were significantly elevated in 3-month- old ARΔOB/ΔOB male mice when compared with ARfl/fl mice. N=6 mice per group. (b) Volumetric analysis of trabecular bone of L3 lumbar vertebra in 6-month-old female mice with μCT. The Tb.N was highly reduced leading to a wider Tb.Sp and reduced Tb.BV in ARΔOB/ΔOB mice compared with ARfl/fl mice (n=7 per group). *P<0.05; **P<0.01. Data are represented as means±s.e.m.
Differences in the trabecular bone phenotype of 6-month-old female mice were also found with the μCT analysis of the vertebral body. The marked reduction of Tb.N in ARΔOB/ΔOB mice compared with controls (P=0.010) was accompanied by a wider Tb.Sp (P=0.021), leading to a significantly reduced Tb.BV and BV/TV ratio (Figure 3b; P=0.005 and 0.004, respectively). There was no difference in Tb.Th (Figure 3b).
Serum markers
In 3-month-old male ARΔOB/ΔOB mice, the serum tartrate-resistant acidic phosphatase 5b (TRAcP5b) activity was significantly increased compared with male ARfl/fl mice (Table 3; P=0.001); this increase was accompanied by an increase in the serum procollagen 1 N-terminal peptide (PINP) concentration (Table 3; P=0.036). In 6-month-old male mice, no significant difference was found in the serum TRAcP5b activity or PINP concentration (Table 3). In females, the differences did not reach statistical significance.
Table 3. Serum TRAcP5b activity and PINP concentration.
| Age N: fl/fl, ΔOB/ΔOB |
Male mice |
Female mice |
|||
|---|---|---|---|---|---|
| 3 months | 6 months | 3 months | 6 months | ||
| |
|
8,8 |
8,7 |
6,9 |
6,12 |
| TRAcP (U ml−1) | ARfl/fl | 2.54±0.22 | 3.51±0.62 | 4.36±0.36 | 4.14±0.51 |
| ARΔOB/ΔOB | 3.84±0.24** | 2.49±0.33 | 5.07±0.72 | 3.31±0.43 | |
| PINP (ng ml−1) | ARfl/fl | 49.79±5.68 | 40.10±3.52 | 61.52±6.59 | 32.55±3.18 |
| ARΔOB/ΔOB | 65.62±3.45* | 35.90±2.03 | 50.73±5.44 | 25.71±2.27 | |
Abbreviations: AR, androgen receptor; PINP, procollagen 1 N-terminal peptide; TRAcP5b, tartrate-resistant acidic phosphatase 5b.
Analysis of serum TRAcP5b and PINP. In 3-month-old ARΔOB/ΔOB male mice the serum TRAcP5b activity was significantly elevated when compared with ARfl/fl mice. In addition, serum PINP concentrations were elevated in these mice.
*P<0.05, **P<0.01 when the values of the same age group were compared between the genotypes. Data represent means±s.e.m.
Discussion
Our study demonstrates a reduction in trabecular bone in male and female mice after deletion of the AR by osteocalcin promoter-driven Cre-recombinase. As a novel finding, we observed that, similarly to results in males, the Tb.BV and Tb.N were significantly reduced in 6-month-old female mice. Similar to the findings in the tibial metaphysis, trabecular bone volume was found to be reduced in the vertebral bodies of 6-month-old female ARΔOB/ΔOB mice. This observation indicates that the AR also has a substantial role in trabecular bone regulation in female mice. The genome-wide inactivation of the capacity of the AR to bind DNA has been shown to decrease the Tb.N (but not Tb.BV) in female mice.18 The total genome-wide inactivation of the AR did not have any effect on female bone during the rapid growth phase of young mice.17
In female mice, no significant differences were detected in histomorphometry or the serum markers analyzed. This result suggests that AR inactivation-induced differences in osteoblast and osteocyte function and possibly in osteoblast-regulated osteoclast function are more subtle in females than in males. However, even small changes, effected over a long period of time, can lead to an altered phenotype, such as the decreased trabecular bone volume observed in 6-month-old female mice. In the recent study by Sinnesael et al.,21 osteocytic AR expression was found to be pivotal for the maintenance of trabecular, but not cortical, bone volume in male mice upon aging.21 Our study shows that the AR is also involved in the maintenance of trabecular bone volume of female mice. This result has to be viewed in the light that although low levels of testosterone have been reported in fetal23 and neonate24 female mice, the serum testosterone concentration in adult female mice is actually 10-fold higher than the estradiol concentration,25 which suggests that testosterone can act as a ligand, driving AR-mediated effects in the trabecular bone maintenance of female mice.
Our results in male mice were in line with those of Chiang et al.,19 who also reported a reduction in Tb.BV in male mice with osteoblast-targeted AR inactivation. However, differing from their results, we did not detect significant differences in cortical bone thickness in male mice. In the work by Chiang et al.19 and Notini et al.20 exon 3 of the AR was excised upon osteocalcin promoter-regulated Cre activity. This deletion has been shown to retain the DNA-binding-independent activities of the AR.26,27,28 In our study, exon 2 was deleted instead; this change has been reported to lead to the complete abolition of AR activity,29 including DNA-binding-independent signaling.30 This distinction may have had a role in the small differences in the cortical bone phenotype reported by Chiang et al.,19 and us, but there is no clear evidence for this possibility. As the genetic background of mice has been shown to affect the bone response to the hormonal effects of gonadectomy,31 the different genetic backgrounds of mice used might also cause some variation to the results observed. In our study, the exon 2-targeted ARfl/fl mice used for breeding carried a 129 Sv × 129 cx/Sv background, whereas in the study by Chiang et al.,19 the exon 3-targeted ARfl/fl mice carried a C57BL/6J background.32 Finally, the recombination efficacy was not estimated in the study by Chiang et al.,19 leaving it unknown whether that value was better than ours due to the use of different loxP sites.
The deletion of the AR in mineralizing osteoblasts did not alter the number of osteoblasts in the bone, as determined by histomorphometry. Unfortunately, the use of decalcified bone samples did not allow measurement of osteoids or use of fluorochromes for dynamic histomorphometry in our study, thus limiting comparability of our results to those in the study by Chiang et al.,19 where the osteoid surface was shown to be increased in male mice devoid of functional AR in osteoblasts and osteocytes. However, an elevation of serum PINP in male ARΔOB/ΔOB mice may be an indication for recombination-related alteration of osteoblast function. More strikingly, N.Oc/B.Pm, Oc.S/BS values and serum TRAcP5b activity were remarkably elevated in male ARΔOB/ΔOB mice, suggesting an increased osteoclast number and accelerated bone turnover in these mice.
The trabecular bone phenotype of ARΔOB/ΔOB mice was largely complementary to the phenotype we recently reported for ERαΔOB/ΔOB mice.22 In young female ERαΔOB/ΔOB mice, Tb.BV was greatly reduced compared with ERαfl/fl mice, whereas the bone phenotype of young male mice was indistinguishable between the different genotypes. Instead, in 6-month-old male ERαΔOB/ΔOB mice, the Tb.BV was significantly reduced compared with ERαfl/fl mice.22 Taken together, these findings suggest that, for bone-forming cells in male mice, the AR is essential for trabecular bone formation during rapid growth, whereas in female mice, the same is true for ERα. However, with age, the AR becomes required to maintain Tb.BV in female mice, and ERα become required in male mice. Furthermore, at least in male mice, the AR signaling in osteoblasts seems to affect the capability of osteoblasts to regulate the osteoclast number, as Oc.N/B.Pm was elevated in male ARΔOB/ΔOB mice. The molecular mechanisms behind this difference remain to be studied.
Osteocalcin has been shown to be expressed in osteoblasts, osteocytes and hypertrophic chondrocytes.33,34 The osteocalcin promoter-regulated expression of Cre and the resulting recombination of the ARfl/fl allele were sufficient to alter the bone phenotype of the mice. It is unlikely that the phenotypic differences can be attributed to the toxicity of the Cre itself,35 as no bone phenotype has been demonstrated in this particular parental osteocalcin-Cre mouse used by us22 or others.36 No AR activity has been shown after the deletion of the exon 229 used in our mice, whereas the residual activity of the AR after exon 3 deletion complicates the analysis of other mice. The recombination efficacy measured as AR mRNA expression in the bone-forming cells of our mouse model was good (79% as determined in male mice), which is in line with the previous reports utilizing the osteocalcin-Cre construct.36 The remaining residual activity (which indicates full activity in a small subset of cells and none in the rest of them in this model) may have ameliorated the phenotypes observed to some extent.
In mouse models, the role of the AR in the regulation of cortical bone thickness and perimeter has been somewhat controversial. The expression of the AR has been reported to be higher in cortical bone osteocytes compared with those in the trabecular bone.6 Further, the removal of the DNA-binding-dependent activity of the AR in osteoblasts and osteocytes using the osteocalcin promoter19 has been shown to lead to a reduction in cortical bone thickness, but not perimeter among male mice. However, in the study by Notini et al.,20 in which an artificial 2.3-kb-long collagen-1 promoter was used to induce the Cre-mediated deletion of exon 3 of the AR gene in osteoblasts before mineralization, no changes in the cortical bone thickness could be detected, either. The use of the 3.6 kb type I collagen promoter to overexpress AR leads to the overexpression of the receptor in periosteal osteoblast precursors and an increase in cortical bone perimeter.9
However, in our mouse model, no significant effects were observed in tibial cortical bone or in the femoral cortical bone (not shown). This outcome differs from the results reported by Chiang et al.,19 who observed a small reduction in the femoral cortical bone thickness in mice in which the exon 3 of the AR gene was deleted by similar osteocalcin promoter-activated Cre recombination. Taken together, the osteoblast targeted models by Notini et al.,20 Chiang et al.19 and us as well as the overexpression models by Wiren et al.,9,10 indicate that osteoblast precursors, but not mature osteoblasts or osteocytes, are the cells implementing the AR-mediated effects on periosteal apposition.
Studies on male mice in which the AR, either alone or together with ERα, was inactivated in all tissues have shown that the complete abolition of the AR reduces cortical bone area and density, whereas the inactivation of ERα had an additional effect on these parameters.14 However, in these models, the circulating sex steroid levels and the growth hormone–insulin-like growth factor 1 axis were also affected, limiting their comparability to the targeted mouse models.
However, we propose that the model described in the present study provides a tool to identify the specific role of AR in bone-forming cells. Our results demonstrate that the AR has a role in the regulation of the trabecular bone volume not only in male but also in female mice. In human studies, serum testosterone, but not estradiol, has been shown to be positively correlated to lumbar BMD in elderly women.37 Further, women with polycystic ovary syndrome have varying degrees of hyperandrogenism, which is associated with increased body weight-adjusted BMD.38 Moreover, the combined treatment of androgen excess in women with antiandrogens spironolactone and lynestrenol leads to a decrease of the lumbar spine BMD.39 Taken together, these data suggest that AR-mediated signaling is essential for the maintenance of trabecular bone in adult female mice.
Materials and methods
Generation of the ARΔOB/ΔOB mouse strain
Mice carrying loxP (locus of crossing over in P1 bacteriophage) sequences flanking the second exon of the AR gene (ARfl/fl mice, genetic background 129 Sv × 129 cx/Sv)29 were provided by Professor Guido Verhoeven (Catholic University of Leuven, Leuven, Belgium). Removal of exon 2 in the AR gene has been shown to completely abolish AR activity.29 Osteocalcin-Cre mice (genetic background FVB-N) expressing the Cre-recombinase under the control of the human osteocalcin promoter36 were provided by Professor Thomas L Clemens (Johns Hopkins University School of Medicine, Baltimore, MD, USA). ARΔOB/ΔOB (ARfl/fl mice carrying an osteocalcin-Cre gene construct) and ARfl/fl mice were generated by breeding.
Analysis of the presence of the Cre transgene and the floxed AR allele was carried out by PCR as follows. Genomic DNA was isolated from lysed ear marks by silica adsorption (DNAeasy; Qiagen, Germantown, MD, USA), and the PCR primers for AR allele detection were as follows: AR-forward, 5′-AGCCTGTATACTCAGTTGGGG-3′; AR-reverse, 5′-AATGCATCACATTAAGTTGATACC-3′. This primer pair produces an 856 bp long fragment from the WT AR gene, an approximately 1000, bp fragment from the targeted AR gene with the two loxP sites and a 450 bp fragment from the recombined AR gene with one of the loxP sites. The PCR cycles were as follows: 2 min at 95 °C for initial denaturation; 35 cycles of 94 °C for 20 s, 58 °C for 20 s and 72 °C for 30 s; and final polymerization for 2 min at 72 °C using Phire polymerase (Finnzymes, Vantaa, Finland). The presence of the Cre transgene was detected using the following primers: Cre-forward, 5′-GCGGCATGGTGCAAGTTGAAT-3′, Cre-reverse; 5′-ACCCCCAGGCTAAGTGCCTT-3′. The PCR conditions using Dynazyme II polymerase (Finnzymes) were as follows: 2 min at 95 °C for initial denaturation; 31 cycles of 94 °C for 30 s, 57 °C for 30 s and 73 °C for 30 s; and final polymerization for 2 min at 72 °C.
Mice from the F1 and F2 generations were used in the experiments. As the AR is X-linked, two steps of breeding were required to generate female mice homozygous for the AR allele and heterozygous for Cre. Male mice hemizygous for the AR allele and heterozygous for Cre were obtained from the F1 generation. The mice were maintained at the Central Animal Laboratory of University of Turku (Turku, Finland). Animal studies were approved by the Finnish Animal Ethics Committee of the State Provincial Office. The experimental criteria fully meet the requirements defined in the NIH guide on animal experimentation. All the experiments were covered by the State Provincial Office permission nos. 30007/06, 1552/05, 1677/06 and 2008-06083.
μCT analysis and statistics
Left tibiae were used for analysis with Skyscan 1070 X-ray computed tomography (Bruker-microCT, Kontich, Belgium). Plastic tubes were used to seal the sample to ensure immobilization and constant positioning. The following parameters were applied for scanning (in air): pixel resolution of 5.33 μm, X-ray tube voltage 70 kV, tube current 200 μA and integration time 3.9 s. The tibiae were rotated in 0.45° degree steps (total angle, 182.45°) and an internal 0.25 mm aluminum filter was applied for beam hardening. Cross-sectional images were reconstructed with NRecon 1.4 software (Bruker-microCT) as follows: dynamic range 0.0025–0.130 attenuation coefficient units, smoothing level 3, beam hardening reduction 85% and ring artifact reduction level 7. CTan 1.10.10 software (Bruker-microCT) was used for analysis and 3D image generation. For the analysis of the trabecular bone in the tibia, a volumetric region of interest (VOI) excluding the cortical bone was defined at the metaphysis of the tibia starting 20 layers (106.6 μm) below the lower surface of the growth plate and extending 150 layers deep (800 μm). For the analysis of the cortical bone, a slice at the diaphysis of the tibia starting 4100 μm below the growth plate and extending for 51 layers (272 μm) was defined. For the analysis of the trabecular bone in L3 lumbar vertebra, a VOI excluding the cortical bone height of 0.53 mm was defined using detectable anatomic markers above the distal growth plate of the vertebral body.
Histomorphometry, serum markers and determination of ash weight
The L3 lumbar vertebra was fixed with 10% neutral formalin, decalcified with 14% ethylenediaminetetraacetic acid for 2 weeks and embedded in paraffin. Sections (5 μm thick) were histochemically stained for TRAcP using an Acid Phosphatase Leukocyte kit (Sigma-Aldrich, St Louis, MO, USA), according to the manufacturer's instructions. Histomorphometry was performed using light microscopy and the OsteomeasureXP 3.1.0.1 program (Osteometrics, Decatur, GA, USA). The lengths of left tibiae were measured with a caliper, and the ash weight was determined with analytical scales after 20 h of heating at 600 °C with a Thermolyne 62700 furnace (Thermo Scientific, West Palm Beach, FL, USA). Serum TRAcP5b activity was determined using an in-house assay as described previously.40 The PINP concentration was determined with a rat/mouse PINP ELISA assay (Immunodiagnostic Systems, Bolton, UK) according to the manufacturer's instructions.
RT-qPCR of cortical bone
Cortical bone was dissected from the diaphyses of tibiae and femora of 7-week-old male ARΔOB/ΔOB mice. The bone marrow was carefully removed, and the samples were stored immersed in RNA-Later (Qiagen) at −70 °C. For RNA isolation, the samples were pulverized by hammering in a steel piston/cylinder cooled with liquid nitrogen. The powdered bone was immediately suspended in RNA Lysis Buffer (Qigen). The viscosity of the suspension was reduced by pipette extrusion. After brief centrifugation, the RNA was purified with the RNAEasy kit (Qiagen) according to the manufacturer's instructions. The cDNA was synthesized using 12 μl of total RNA as the starting material, with SuperScript III Reverse Transcriptase (Invitrogen, Grand Island, NY, USA) according to the manufacturer's instructions. Quantification of the ERα and AR mRNA was performed by DyNAmo HS SYBR Green qPCR Kit (Finnzymes) using Bio-Rad's CFX96 qPCR device (Bio-Rad, Hercules, CA, USA). Messenger RNA levels were normalized to β-actin expression and the results were analyzed by the 2−ΔΔCT method. The primers used were as follows: ERα forward, 5′-CCGTGTGCAATGACTATGCC-3′; ERα reverse, 5′-GTGCTTCAACATTCTCCCTCCTC-3′; ERβ forward, 5′-GCCCTCCCAGCATGCCCTTC-3′; ERβ reverse, 5′-GAGCGCCACATCAGCCCCAC-3′; AR forward, 5′-GTCTCCGGAAATGTTATGAA-3′; AR reverse, 5′-AAGCTGCCTCTCTCCAAG-3′; β-actin forward, 5′-CGTGGGCCGCCCTAGGCACCA-3′; and β-actin reverse, 5′-TTGGCCTTAGGGTTCAGGGGG-3′
Statistical analysis
The data are presented as means and standard errors of means. The equality of variances was analyzed with Levene's test. Based on the results from Levene's test, independent samples t-tests with the unequal variances assumption were used to reveal the differences between groups. P-values <0.05 were considered statistically significant. All analyses were performed with IBM SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, NY, USA).
Acknowledgments
The osteocalcin-Cre mice were a generous gift from Professor Thomas Clemens (Johns Hopkins University School of Medicine, Baltimore, MD, USA) and the ARfl/fl mice from Professor Guido Verhoeven (Catholic University of Leuven, Leuven, Belgium). We thank Mrs Liudmila Shumskaya, Mrs Natalia Habilainen-Kirillov, Ms Soili Jussila, Mr Lauri Määttä and Mr Jani Seppänen for excellent technical help, as well as Professor Michael Courtney, University of Eastern Finland, for his critical reading of the manuscript. Furthermore, we also thank MSc Tommi Kauko (Department of Biostatistics, University of Turku, Turku, Finland) for expert opinion in statistics. This work was supported by grants from the Finnish Funding Agency for Technology and Innovation (TEKES), the Academy of Finland and Sigrid Jusélius Foundation.
Footnotes
The authors declare no conflict of interest.
References
- Frank GR. Role of estrogen and androgen in pubertal skeletal physiology. Med Pediatr Oncol 2003;41:217–221. [DOI] [PubMed] [Google Scholar]
- Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocrin Rev 2004;25:389–425. [DOI] [PubMed] [Google Scholar]
- Basaria S, Dobs AS. Hypogonadism and androgen replacement therapy in elderly men. Am J Med 2001;110:563–572. [DOI] [PubMed] [Google Scholar]
- Abu EO, Horner A, Kusec V, Triffitt JT, Compston JE. The localization of androgen receptors in human bone. J Clin Endocrinol Metab 1997;82:3493–3497. [DOI] [PubMed] [Google Scholar]
- Noble B, Routledge J, Stevens H, Hughes I, Jacobson W. Androgen receptors in bone-forming tissue. Horm Res 1999;51:31–36. [DOI] [PubMed] [Google Scholar]
- Wiren KM, Chapman Evans A, Zhang XW. Osteoblast differentiation influences androgen and estrogen receptor-alpha and -beta expression. J Endocrinol 2002;175:683–694. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Hosoi T, Inoue S, Ikegami A, Kaneki M, Akedo Y et al. Immunocytochemical identification of androgen receptor in mouse osteoclast-like multinucleated cells. Calcif Tissue Int 1994;54:325–326. [DOI] [PubMed] [Google Scholar]
- Wiren KM, Semirale AA, Hashimoto JG, Zhang XW. Signaling pathways implicated in androgen regulation of endocortical bone. Bone 2010;46:710–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiren KM, Zhang XW, Toombs AR, Kasparcova V, Gentile MA, Harada S et al. Targeted overexpression of androgen receptor in osteoblasts: unexpected complex bone phenotype in growing animals. Endocrinology 2004;145:3507–3522. [DOI] [PubMed] [Google Scholar]
- Wiren KM, Semirale AA, Zhang XW, Woo A, Tommasini SM, Price C et al. Targeting of androgen receptor in bone reveals a lack of androgen anabolic action and inhibition of osteogenesis: a model for compartment-specific androgen action in the skeleton. Bone 2008;43:440–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalajzic Z, Liu P, Kalajzic I, Du Z, Braut A, Mina M et al. Directing the expression of a green fluorescent protein transgene in differentiated osteoblasts: comparison between rat type i collagen and rat osteocalcin promoters. Bone 2002;31:654–660. [DOI] [PubMed] [Google Scholar]
- Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC et al. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res 2002;17:15–25. [DOI] [PubMed] [Google Scholar]
- Venken K, De Gendt K, Boonen S, Ophoff J, Bouillon R, Swinnen JV et al. Relative impact of androgen and estrogen receptor activation in the effects of androgens on trabecular and cortical bone in growing male mice: a study in the androgen receptor knockout mouse model. J Bone Miner Res 2006;21:576–585. [DOI] [PubMed] [Google Scholar]
- Callewaert F, Venken K, Ophoff J, De Gendt K, Torcasio A, van Lenthe GH et al. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-α. FASEB J 2009;23:232–240. [DOI] [PubMed] [Google Scholar]
- Callewaert F, Venken K, Kopchick J, Torcasio A, van Lenthe GH, Boonen S et al. Sexual dimorphism in cortical bone size and strength but not density is determinated by independent and time-specific actions of sex steroids and IGF-I: evidence from pubertal mouse models. J Bone Miner Res 2010;25:617–626. [DOI] [PubMed] [Google Scholar]
- Callewaert F, Sinnesael M, Gielen E, Boonen S, Vanderschueren D. Skeletal sexual dimorphism: relative contribution of sex steroids, GH-IGF1, and mechanical loading. J Endocrinol 2010;207:127–134. [DOI] [PubMed] [Google Scholar]
- Kawano H, Sato T, Yamada T, Matsumoto T, Sekine K, Watanabe T et al. Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci USA 2003;100:9416–9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean HE, Moore AJ, Sastra SA, Morris HA, Ghasem-Zadeh A, Rana K et al. DNA-binding-dependent androgen receptor signaling contributes to gender differences and has physiological actions in males and females. J Endocrinol 2010;206:93–103. [DOI] [PubMed] [Google Scholar]
- Chiang C, Chiu M, Moore AJ, Anderson PH, Ghasem-Zadeh A, McManus JF et al. Mineralization and bone resorption are regulated by the androgen receptor in male mice. J Bone Miner Res 2008;24:621–631. [DOI] [PubMed] [Google Scholar]
- Notini AJ, McManus JF, Moore A, Bouxsein M, Jimenez M, Chiu WSM et al. Osteoblast deletion of Exon 3 of the androgen receptor gene results in trabelular bone loss in adult male mice. J Bone Miner Res 2007;22:347–356. [DOI] [PubMed] [Google Scholar]
- Sinnesael M, Claessens F, Laurent M, Dubois V, Boonen S, Deboel L et al. Androgen receptor (AR) in osteocytes is important for the maintenance of male skeletal integrity: evidence from targeted AR disruption in mouse osteocytes. J Bone Miner Res 2012;27:2535–2543. [DOI] [PubMed] [Google Scholar]
- Määttä JA, Kalman BG, Gu G, Alanne MH, Vääräniemi J, Liljenbäck H et al. Inactivation of estrogen receptor alpha in bone forming cells induces bone loss in female mice. FASEB J 2013;27:478–488. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Bronson FH. Sexual characteristics of adult female mice are correlated with their blood testosterone levels during prenatal development. Science 1980;208:597–599. [DOI] [PubMed] [Google Scholar]
- Pang SF, Tang F. Sex differences in the serum concentrations of testosterone in mice and hamsters during their critical periods of neural sexual differentiation. J Endocrinol 1984;100:7–11. [DOI] [PubMed] [Google Scholar]
- Liew SH, Drummond AE, Jones ME, Findlay JK. The lack of estrogen and excess luteinizing hormone are responsible for the female ArKO mouse phenotype. Mol Cell Endocrinol 2010;327:56–64. [DOI] [PubMed] [Google Scholar]
- Lim P, Robson M, Spaliviero J, McTavish KJ, Jimenez, Zajac JD et al. Sertoli cell androgen receptor DNA binding domain is essential for the completion of spermatogenesis. Endocrinology 2009;15:4755–4765. [DOI] [PubMed] [Google Scholar]
- Walters KA, Allan CM, Jimenez M, Lim PR, Davey RA, Zajac JD et al. Female mice haploinsufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the ar null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocinology 2007;148:3674–3684. [DOI] [PubMed] [Google Scholar]
- Pang TP, Clarke MV, Ghasem-Zadeh A, Lee NK, Davey RA, MacLean HE. A physiological role for androgen actions in the absence of androgen receptor DNA binding activity. Mol Cell Endocrinol 2012;348:189–197. [DOI] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PTK, Schoonjans L, Dewerchin M, Devos A et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA 2004;101:1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T et al. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest 2003;111:1651–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinck J, Boyd SK. The magnitude and rate of bone loss in ovariectomized mice differs among inbred strains as determined by longitudinal in vivo micro-computed tomography. Calcif Tissue Int 2008;83:70–79. [DOI] [PubMed] [Google Scholar]
- Notini AJ, Davey RA, McManus JF, Bate KL, Zajac JD. Genomic actions of the androgen receptor are required for normal male sexual differentiation in a mouse model. J Mol Endocrinol 2005;35:547–555. [DOI] [PubMed] [Google Scholar]
- Lian JB, McKee MD, Todd AM, Gerstenfeld LC. Induction of bone-related proteins, osteocalcin and osteopontin, and their matrix ultrastructural localization with development of chondrocyte hypertrophy in vitro. J Cell Biochem 1993;52:206–219. [DOI] [PubMed] [Google Scholar]
- Mueller MB, Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arth Rheum 2008;58:1377–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol 2007;8:665–668. [DOI] [PubMed] [Google Scholar]
- Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem 2002;277:44005–44012. [DOI] [PubMed] [Google Scholar]
- Rariy CM, Ratcliffe SJ, Weinstein R, Bhasin S, Blackman MR, Cauley JA et al. Higher serum free testosterone concentration in older women is associated with greater bone mineral density, lean body mass, and total fat mass: the cardiovascular health study. J Clin Endocrinol Metab 2011;96:989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan JR, Hospodar P, Myers C, Leuenberger P, Demers LM. Effect of excess endogenous androgens on bone density in young women. J Clin Endocrinol Metab 1988;67:937–943. [DOI] [PubMed] [Google Scholar]
- Prezelj J, Kocijancic A. Antiandrogen treatment with spironolactone and linestrenol decreases bone mineral density in eumenorrhoeic women with androgen excess. Horm Metab Res 1994;26:46–48. [PubMed] [Google Scholar]
- Alatalo SL, Halleen JM, Hentunen TA, Mönkkönen J, Väänänen HK. Rapid screening method for osteoclast differentiation in vitro that measures tartrate-resistant acid phosphatase 5b activity secreted into the culture medium. Clin Chem 2000;46:1751–1754. [PubMed] [Google Scholar]