Abstract
The development of high-throughput sequencing and genome-wide association studies allows us to deduce the genetic factors underlying diseases much more rapidly than possible through classical genetics, but a true understanding of the molecular mechanisms of these diseases still relies on integrated approaches including in vitro and in vivo model systems. One such model that is particularly suitable for studying bone diseases is the zebrafish (Danio rerio), a small fresh-water teleost that is highly amenable to genetic manipulation and in vivo imaging. Zebrafish physiology and genome organization are in many aspects similar to those of humans, and the skeleton and mineralizing tissues are no exception. In this review, we highlight some of the contributions that have been made through the study of mutant zebrafish that feature bone and/or mineralization disorders homologous to human diseases, including osteogenesis imperfecta, fibrodysplasia ossificans progressiva and generalized arterial calcification of infancy. The genomic and phenotypic similarities between the zebrafish and human cases are illustrated. We show that, despite some systemic physiological differences between mammals and teleosts, and a relative lack of a history as a model for bone research, the zebrafish represents a useful complement to mouse and tissue culture systems in the investigation of genetic bone disorders.
Introduction
Zebrafish, and other small teleosts like medaka, have been widely used as a model in developmental biology for decades. However, their utility as a model for human bone disorders remains largely underappreciated. As with any other model system, there are advantages and limitations: on one hand, joints appear not to be synovial, and whereas zebrafish do contain cellular bone, this is not true of all teleosts.1,2 On the other hand, there are several aspects of zebrafish biology that offer significant advantages over other systems. Ethynitrosourea (ENU) mutagenesis-based forward genetic screens, perhaps the most informative technique for unbiased discovery of gene function, are greatly facilitated by the large clutch sizes and relatively simple husbandry of zebrafish (reviewed in Spoorendonk et al.3). The transparent, rapidly developing larvae are also highly amenable for live imaging of chondrocytes, osteoblasts and osteoclasts. Numerous transgenic lines expressing fluorescent proteins under the control of bone-related promoters have been produced, notably osterix (sp7),4 osteocalcin (bglap)5 and collagen types II and X6,7 (Figure 1; also reviewed in Hammond and Moro8). Mineralization occurs in a predictable and stereotypic manner beginning at 3 days post fertilization (d.p.f.), and craniofacial bones develop in a similar manner to those of higher vertebrates.9 Furthermore, there are elegant studies that use the fish tail fin as a model for regenerating bone.5
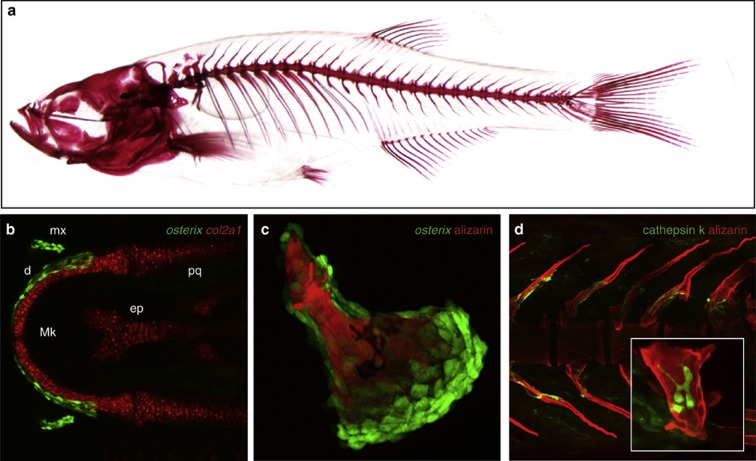
Figure 1.
Imaging osteogenesis in vivo: transgenic zebrafish lines highlighting chondrocytes and osteoblasts. (a) Skeletal structure of a zebrafish at 5 weeks post fertilization (w.p.f.). Scales were removed and the bone stained with alizarin red. (b) Ventral view of the Meckel's cartilage (red) and abutting osteoblasts (green) in a 6 d.p.f. embryo expressing col2a1:mCherry;osterix:GFP. Mk, Meckel's cartilage; mx, maxilla; d, dentary; pq, palatoquadrate; ep, ethmoid plate. (c) Magnification of the developing operculum in a 6 d.p.f embryo expressing osterix:gal4;UAS:GFP. Note that the osteoblasts (green) are placed distally to the mineralized matrix they produce. (d) Vertebral column of a 5 w.p.f. transgenic zebrafish showing expression of cathepsin K, a marker for osteoclasts, in green. Inset: magnification of a hypural bone with osteoclasts in close proximity to the bone surface.
Because historically zebrafish have started out as a model to study early development, it can be argued that they lack to some extent a ‘track record' in areas of later organogenesis, including osteogenesis. In recent years, however, a number of mutant zebrafish lines have been characterized that correlate to human pathological conditions, or that turned out to be otherwise instrumental in providing answers to open questions. In this review, we will discuss these cases in order to highlight how zebrafish can advance our knowledge about osteogenesis.
Osteogenesis imperfecta
Osteogenesis imperfecta (OI) (OMIM #166200) is a disease of the bone collagen matrix that results in skeletal deformities, fragile bones with high rates of fractures, and in most cases, reduced bone density (reviewed in Forlino et al.10). Most cases of OI are caused by autosomal dominant mutations in genes encoding collagen type I (COL1A1 and COL1A2), but more recently an atypical form of OI was identified and found to be caused by mutations in the collagen-processing enzyme Bmp1.11,12
One model of typical OI is the zebrafish mutant chihuahua, identified in a forward-genetics screen of adult fish using X-radiography.13 Heterozygous fish display normal cartilage formation but abnormal bone growth, with irregular vertebrae, uneven mineralization and frequent fractures, which resemble characteristics found in heterozygous OI patients. The chihuahua phenotype is caused by a heterozygous missense mutation in col1a1a, a homologue of human COL1A1, resulting in a G390D change that interrupts the conserved Gly-X-Y motif. The majority of OI-causing mutations in COL1A1 are in analogous Gly residues; the substitution impairs fibril assembly, reducing matrix density and impairing mineralization.13 Thus, the chihuahua zebrafish is a model of dominant human OI, both genetically and phenotypically.
A rarer form of recessive OI was recently identified independently in families in Turkey11 and Egypt.12 Both of these cases were linked to mutations in BMP1, which encodes a protease essential for the formation of mature collagen. Bmp1 cleaves the C-terminal cap from procollagen monomers, enabling their self-assembly into fibrils.14 The mutation in the Turkish case (G12R) resides within the signal peptide, whereas the mutation in the Egyptian case (F249L) lies in the protease domain. Significant advances were made through the use of a zebrafish mutant, frilly fins,15 carrying a missense mutation in the Bmp1a protease domain.11 Homozygous mutants showed greatly delayed ossification of the vertebrae, and when mineralization did occur the vertebrae were misshapen or fused. In situ hybridization on whole-mount zebrafish embryos, allowed direct visualisation of bmp1a expression in osteoblasts. A transgenic line expressing mCherry under the control of the osteoblast marker sp7 (osterix) revealed that osteoblasts were still present in frf−/− mutants yet, importantly, they did not become hyperactive when embryos were treated with retinoic acid (RA). RA has previously been shown to stimulate osteoblasts, increasing production of collagen and resulting in a dramatic over-ossification of the vertebral column.4 The inability of RA to increase ossification in frf−/− embryos despite the presence of sp7-positive osteoblasts suggests that osteoblasts require Bmp1 to produce mature osteoid.11 The osteoid was found to contain a disruption in the periodic fibrillar–collagen structure when examined using picrosirius red, indicative of a problem in collagen processing. Finally, in order to test whether the G12R mutation found in the Turkish family was responsible for the OI phenotype, human BMP1 mRNA was injected into frf−/− embryos; wild-type mRNA was able to rescue the frilly fins phenotype, whereas mRNA carrying the G12R mutation did not. The ability to express functional human proteins after injection of mRNA is one of the great strengths of the zebrafish model system.
Fibrodysplasia ossificans progressiva
Fibrodysplasia ossificans progressiva (FOP) (MIM #135100) is a serious genetic disorder causing heterotopic endochrondral ossification, which begins in utero and progresses throughout life (reviewed in Kaplan et al.16). Patients with FOP eventually develop a ‘second skeleton' as connective tissues are progressively mineralized. Mobility becomes severely restricted during adolescence and the median lifespan of patients is only 40 years. In 2006, Shore et al. found that FOP is caused by an R206H substitution in ACVR1, a BMP receptor.17 Protein modeling predicted that this mutation would lead to constitutive activation, rather than suppression, of downstream BMP signaling. To investigate this, zebrafish dorsal-ventral (DV) patterning was used as an assay for BMP signaling.18 In the acvr1l mutant lost-a-fin, BMP signaling is reduced causing an expansion of cells on the dorsal side. Injection of wild-type human ACVR1 mRNA rescued this phenotype resulting in relatively normal development, whereas ACVR1R206H mRNA induced a strongly ventralized phenotype showing that the R206H mutation does indeed cause hyperactive BMP signaling. However, an over-ossification phenotype was not seen in these embryos, possibly because transient ACVR1R206H expression declines before mineralization begins.
Nevertheless, the zebrafish DV assay is a useful tool for the investigation of compounds for the treatment of FOP. A screen of over 7500 small molecules using zebrafish embryos arrayed in 96-well plates identified a compound now known as dorsomorphin (DM),19 which inhibits BMP signaling in a dose-dependent manner, producing embryos that resemble lost-a-fin mutants. An assay for bone density using calcein staining on 10-day-old larvae showed that DM reduced mineralization when supplied to embryos at 24 h post-fertilization (once DV patterning had been established). However, DM exhibited severe off-target effects, most notably inhibition of vascular endothelial growth factor (VEGF) signaling, which prevents the development of blood vessels.20 To address this shortcoming, analogs of DM were systematically synthesized and tested in vivo for their effect on both the DV axis and intersegmental vessel development. This identified the novel compounds, DMH1 and DMH4, as selective inhibitors of the BMP and VEGF pathways, respectively.20 Because the screen was performed with live zebrafish larvae rather than a more traditional in vitro assay, the screen was considerably more ‘content rich': the screening technique allows compounds to be simultaneously tested for efficacy, bioavailability and whole-organism toxicity, whereas still retaining the possibility to test reasonably high numbers. Later refinements to the DMH1 structure improved selectivity for AVCR1 over other BMP receptors,21,22 possibly paving the way for a drug effective in the treatment of patients with FOP.
Craniosynostosis and other skeletal anomalies
Recently, Laue et al.23 describe three children from a consanguineous couple with craniosynostosis and multiple skeletal anomalies (OMIM #614416). All three died in utero, with large encephaloceles caused by hypoplasia, fusion of the elbows and missing toes. Genomic analysis revealed the siblings to be homozygous for a missense mutation, R363L, in the CYP26B1 gene encoding a cytochrome P450 enzyme responsible for metabolizing RA. By sequencing the CYP26B1 gene from other patients with cranial deformities, Laue et al. found an additional patient with a S146P substitution. This patient also had radiohumeral fusions, but had craniosynostoses rather than an encephaloceles, and survived until 5 months of age. This suggests the S146P mutation is less severe (hypomorphic) than R363L, a notion confirmed by Cyp26b1 activity tests in transfected cultured cells.23 Zebrafish were used to help resolve the apparent contradiction of the two primary symptoms in these patients: hypermineralization of the joints and hypomineralization of the cranium.
Three mutant alleles for the zebrafish ortholog of CYP26B1, termed stocksteif, were independently reported in 2008,4,24 displaying phenotypes of extensive over-ossification of the vertebral column, and a reduced (or absent) cartilaginous ethmoid plate. Craniosynostoses and encephaloceles are seen in stocksteif juveniles.23 These effects are recapitulated in zebrafish embryos treated with exogenous RA.4,23,24 Electron microscopy of sutural sections showed a marked difference in morphology: wild-type cells resembled osteoblasts, whereas stocksteif cells resembled preosteocytes that were embedded in a much less dense surrounding matrix.23 In situ hybridizations showed a reduction in col1a1 and col10a expression in stocksteif mutants, suggesting that excessive RA accelerates the transition from osteoblasts to preosteocytes, in turn causing a reduction in osteoid production as well as promoting premature mineralization. Thus, the different phenotypes seem to be manifested dependent on when and to which extent premature osteoblast–osteocyte transitioning occurs. In severe cases like the human R363L mutation, it affects earlier stages of skull formation, before frontal and parietal calvarial plates meet to form the coronal suture, leading to reduced calvarial growth and encephaloceles without suture formation. In more moderate cases like the S146P substitution or the zebrafish stocksteif, calvarial plates might be able to grow normally until suture formation, whereas enhanced preosteocytic activity within the sutures leads to their mineralization and craniosynostoses. This model adequately explains the craniofacial phenotypes seen in the human subjects and zebrafish mutants, but it does not explain the lack of a spinal defect in human subjects, whereas the over-ossification of the vertebral column is such a distinctive feature of the stocksteif allele. This may be attributed to differences in the population of osteoblasts present in the centra of fish and humans.4,24
Raine syndrome
Raine Syndrome (RNS) (OMIM #259775), also called osteosclerotic bone dysplasia, is a severe disorder characterized by increased bone density and cranial hypermineralization. Most cases result in death at the neonatal stage but some patients survive beyond childhood, albeit with mental retardation. RNS is caused by homozygous or compound heterozygous mutations in FAM20C.25,26 In vitro studies by Tagliabracci et al. found that FAM20C encodes a kinase that is secreted by mineralizing tissue and phosphorylates a class of mineralization inhibitors including osteopontin.27
Zebrafish homozygous for mutations in the paralogous gene fam20b were described by Eames et al. as having similar phenotypes to RNS patients: increased chondral bone density and accelerated onset of mineralization in craniofacial bones.28 Investigation of these zebrafish mutants showed a reduction in cartilage, suggesting a premature chrondrocyte maturation and conversion to osteoblasts. Eames et al. showed that Fam20b kinase activity aids in the synthesis of proteoglycans, important regulators of chondrocyte maturation. This mechanism provides an additional explanation for the etiology of RNS. It is not yet clear whether zebrafish fam20b has the role of FAM20C in humans, as no fam20c alleles have been reported. Similarly, no human mutations in FAM20B have been identified, although most patients have not been studied on a molecular basis.
Generalized arterial calcification of infancy
Recently, two zebrafish mutants were reported displaying defects in skeletal biomineralization, caused by changes in phosphate/pyrophosphate homeostasis of the embryos.29 One mutant, nob (no bone), displays complete lack of skeletal mineralization, whereas a second mutant, dragonfish (dgf), shows enhanced mineralization, most notably in the axial skeleton. The nob phenotype is caused by a mutation in the ectonucleotidase entpd5 gene, with the protein product having a crucial role in supplying sufficient levels of phosphate in the microenviroment of osteoblasts through hydrolyzing nucleoside tripsophates (NTPs) and diphosphates (NDPs). Dgf, in turn, encodes another ectonucleotidase, Enpp1, a well-known player in the regulation of biomineralization, which predominantly supplies pyrophosphate, a chemical inhibitor of hydroxyapatite formation.30 Most notably, recessive human ENPP1 mutations are associated with generalized arterial calcification of infancy syndrome. Generalized arterial calcification of infancy is characterized by calcification of large- and medium-sized arteries and is usually lethal due to heart failure. Furthermore, pseudoxanthomatous skin lesions, angoid streaks or hypophosphatemic rickets can be associated with human ENPP1 mutations.31 In zebrafish embryos, we fail to find arterial calcifications, probably due to the absence of arterial structures reminiscent of human medium- or large-sized arteries. At later stages, however, calcifications can be found within the bulbus arteriosus, the outflow tract of the zebrafish heart (Apschner et al., in preparation), which is rich in smooth muscle cells and thought to histomorphologically resemble arterial structures.32 Further, we observed ectopic mineralizations in the skin, and cartilaginous structures in early stages of development (4–8 d.p.f.) thus providing evidence for a conserved function of ENPP1 between mammals and zebrafish.
Conclusion
As all other vitally important organs, bone was only once introduced during the course of evolution. Hence, it comes as no surprise that many of the genetic regulatory networks that govern osteogenesis are evolutionary conserved and have identical or very similar functions in both teleosts and amniotes, including mammals. The above-mentioned examples of pathological situations clearly attest to this, as do the expression patterns of many osteoblast-specific genes across the vertebrate phyla.
The question remains what zebrafish and other teleosts have to offer in addition to the tools that mice provide. Mice are (and will remain) the most relevant model system in osteogenesis research, but as in any other model system, there are short comings and technical limitations that must be filled by other systems—and this is where zebrafish come in. The ability to carry out forward genetic screens3 has led to the identification and appreciation of new gene functions relevant for bone formation. The optical transparency of fish larvae permits the visualization of osteoblasts and osteoclasts in vivo, and although the lack of classical osteocytes in some teleosts is often used as an argument that fish are not a relevant model, this notion does not pertain to zebrafish. Furthermore, zebrafish have been widely used for chemical screens,33 and particularly for osteoclast research this appears an attractive opportunity: osteoclasts are readily visible in fish larvae34 (Figure 1d), and do respond to pathologically mineralized tissue (Apschner et al., in preparation). Hence, if compounds need to be tested for their effect on osteoclasts, this could be accomplished much quicker and more economically in fish than in mice. Finally, there are numerous genome-wide association studies that provide candidate gene lists without having sufficient resolution for unambiguous identification of the responsible gene. Here, because of the ease of generating stable mutant lines in fish through the TALEN35 or CRISPR36 technology, it will be possible to knock out 5 or 10 candidate genes within a few months, in order to single out the gene that mutates to an osteogenesis phenotype.
Of course, issues remain, and there are further developments required to make optimal use of teleosts as an additional experimental system. For example, measuring bone density has been difficult because of the small size of fish larvae. This will probably not remain a limitation for long, with the technical advances in the field of nanoCT measurements. Furthermore, although (as discussed above) many of the genetic regulatory networks are evolutionary conserved, we need to obtain a better understanding of phosphate and pyrophosphate homeostasis of zebrafish, and of endocrinological parameters influencing bone homeostasis in general. These points notwithstanding, zebrafish and rodent models have complementary strengths, and will in many cases allow faster progress if used in combination.
Acknowledgments
SS-M acknowledges support from the Smart Mix Programme of the Netherlands Ministry of Economic Affairs, the European Space Agency and TreatOA (Translational Research in Europe Applied Technologies for OsteoArthritis, FP7). AA is a recipient of a DOC Fellowship (Austrian Academy of Sciences).
Footnotes
The authors declare no conflict of interest.
References
- Witten PE, Huysseune A. A comparative view on mechanisms and functions of skeletal remodelling in teleost fish, with special emphasis on osteoclasts and their function. Biol Rev 2009; 84:315–346. [DOI] [PubMed] [Google Scholar]
- Apschner A, Schulte-Merker S, Witten PE. Chapter 10 - Not All Bones are Created Equal – Using Zebrafish and Other Teleost Species in Osteogenesis Research. In: Detrich HW, Westerfield M, Zon LI (eds).Methods in Cell Biology Massachusetts: Academic Press, 2011; pp 239–255. [DOI] [PubMed] [Google Scholar]
- Spoorendonk KM, Hammond CL, Huitema LFA, Vanoevelen J, Schulte-Merker S. Zebrafish as a unique model system in bone research: the power of genetics and in vivo imaging. J Appl Ichthyol 2010; 26:219–224. [Google Scholar]
- Spoorendonk KM, Peterson-Maduro J, Renn J, Trowe T, Kranenbarg S, Winkler C et al. Retinoic acid and Cyp26b1 are critical regulators of osteogenesis in the axial skeleton. Development 2008; 135:3765–3774. [DOI] [PubMed] [Google Scholar]
- Knopf F, Hammond C, Chekuru A, Kurth T, Hans S, Weber Christopher W et al. Bone regenerates via dedifferentiation of osteoblasts in the zebrafish fin. Dev Cell 2011; 20:713–724. [DOI] [PubMed] [Google Scholar]
- Hammond CL, Schulte-Merker S. Two populations of endochondral osteoblasts with differential sensitivity to Hedgehog signalling. Development 2009; 136:3991–4000. [DOI] [PubMed] [Google Scholar]
- Mitchell RE, Huitema LF, Skinner RE, Brunt LH, Severn C, Schulte-Merker S et al. New tools for studying osteoarthritis genetics in zebrafish. Osteoarthritis Cartilage 2013; 21:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond CL, Moro E. Using transgenic reporters to visualize bone and cartilage signaling during development in vivo. Front Endocrinol 2012; 3:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling TF, Kimmel CB. Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development 1994; 120:483–494. [DOI] [PubMed] [Google Scholar]
- Forlino A, Cabral WA, Barnes AM, Marini JC. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol 2011; 7:540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asharani PV, Keupp K, Semler O, Wang W, Li Y, Thiele H et al. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am J Hum Genet 2012; 90:661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Glez V, Valencia M, Caparrós-Martín JA, Aglan M, Temtamy S, Tenorio J et al. Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Hum Mutat 2012; 33:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S, Jagadeeswaran P, Halpern ME. Radiographic analysis of zebrafish skeletal defects. Dev Biol 2003; 264:64–76. [DOI] [PubMed] [Google Scholar]
- Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci 2005; 118:1341–1353. [DOI] [PubMed] [Google Scholar]
- van Eeden FJ, Granato M, Schach U, Brand M, Furutani-Seiki M, Haffter P et al. Genetic analysis of fin formation in the zebrafish, Danio rerio. Development 1996; 123:255–262. [DOI] [PubMed] [Google Scholar]
- Kaplan FS, Chakkalakal SA, Shore EM. Fibrodysplasia ossificans progressiva: mechanisms and models of skeletal metamorphosis. Dis Model Mech 2012; 5:756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho T-J, Choi IH et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet 2006; 38:525–527. [DOI] [PubMed] [Google Scholar]
- Shen Q, Little SC, Xu M, Haupt J, Ast C, Katagiri T et al. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest 2009; 119:3462–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 2008; 4:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR et al. In vivo structure−activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol 2009; 5:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engers DW, Frist AY, Lindsley CW, Hong CC, Hopkins CR. Synthesis and structure–activity relationships of a novel and selective bone morphogenetic protein receptor (BMP) inhibitor derived from the pyrazolo[1.5-a]pyrimidine scaffold of Dorsomorphin: the discovery of ML347 as an ALK2 versus ALK3 selective MLPCN probe. Bioorg Med Chem Lett 2013; 23:3248–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanvitale CE, Kerr G, Chaikuad A, Ramel MC, Mohedas AH, Reichert S et al. A New Class of Small Molecule Inhibitor of BMP Signaling. PLoS ONE 2013; 8:e62721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue K, Pogoda H-M, Daniel Philip B, van Haeringen A, Alanay Y, von Ameln S et al. Craniosynostosis and multiple skeletal anomalies in humans and zebrafish result from a defect in the localized degradation of retinoic acid. Am J Hum Genet 2011; 89:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue K, Jänicke M, Plaster N, Sonntag C, Hammerschmidt M. Restriction of retinoic acid activity by Cyp26b1 is required for proper timing and patterning of osteogenesis during zebrafish development. Development 2008; 135:3775–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson MA, Hsu R, Keir LS, Hao J, Sivapalan G, Ernst LM et al. Mutations in FAM20C are associated with lethal osteosclerotic bone dysplasia (raine syndrome), highlighting a crucial molecule in bone development. Am J Hum Genet 2007; 81:906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson MA, Scheuerle A, Hurst J, Patton MA, Stewart H, Crosby AH. Mutations in FAM20C also identified in non-lethal osteosclerotic bone dysplasia. Clin Genet 2009; 75:271–276. [DOI] [PubMed] [Google Scholar]
- Tagliabracci VS, Engel JL, Wen J, Wiley SE, Worby CA, Kinch LN et al. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science 2012; 336:1150–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames BF, Yan YL, Swartz ME, Levic DS, Knapik EW, Postlethwait JH et al. Mutations in fam20b and xylt1 reveal that cartilage matrix controls timing of endochondral ossification by inhibiting chondrocyte maturation. PLoS Genet 2011; 7:e1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitema LFA, Apschner A, Logister I, Spoorendonk KM, Bussmann J, Hammond CL et al. Entpd5 is essential for skeletal mineralization and regulates phosphate homeostasis in zebrafish. Proc Natl Acad Sci USA 2012; 109:21372–21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millán J. The role of phosphatases in the initiation of skeletal mineralization. Calcif Tissue Int 2012; 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke Y, Rutsch F. Generalized arterial calcification of infancy and pseudoxanthoma elasticum: two sides of the same coin. Front Genet 2012; 3:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N, Yost HJ, Clark EB. Cardiac morphology and blood pressure in the adult zebrafish. Anat Rec 2001; 264:1–12. [DOI] [PubMed] [Google Scholar]
- Tan JL, Zon LI. Chemical screening in zebrafish for novel biological and therapeutic discovery. Methods Cell Biol 2011; 105:493–516. [DOI] [PubMed] [Google Scholar]
- To TT, Witten PE, Renn J, Bhattacharya D, Huysseune A, Winkler C. Rankl-induced osteoclastogenesis leads to loss of mineralization in a medaka osteoporosis model. Development 2012; 139:141–150. [DOI] [PubMed] [Google Scholar]
- Huang P, Xiao A, Zhou M, Zhu Z, Lin S, Zhang B. Heritable gene targeting in zebrafish using customized TALENs. Nat Biotech 2011; 29:699–700. [DOI] [PubMed] [Google Scholar]
- Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotech 2013; 31:227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]