Abstract
Recent advances have established connexin43 (Cx43) as a key regulator of osteoblast function and of bone response to mechanical stimuli. Work by independent laboratories has consistently demonstrated postnatal development of larger than normal cross-section of long bones after conditional ablation of the Cx43 gene, Gja1, selectively in osteoblasts and/or osteocytes. This phenotype is caused by excessive endocortical bone resorption associated with periosteal expansion and cortical thinning. Review of published data suggests that the earlier in the osteogenic lineage is Gja1 deleted, the more severe is the cortical phenotype, implying functional roles of Cx43 at different stages of the osteoblast differentiation program. Such cortical modeling abnormalities resemble the changes occurring in the cortex upon disuse or aging. Indeed, Cx43 deficiency desensitizes endocortical osteoclasts from activation induced by removal of mechanical load, thus preventing medullary area expansion. The action of Cx43 on cancellous bone is controversial. Furthermore, the absence of Cx43 in osteoblasts and osteocytes results in activation of periosteal bone formation at lower strains than in wild-type bones, suggesting that Cx43 deficiency increased cortical sensitivity to mechanical load. Thus, Cx43 modulates cortical bone modeling in homeostatic conditions and in response to mechanical load by restraining both endocortical bone resorption and periosteal bone formation. Cx43 may represent a novel pharmacologic target for improving cortical bone strength through modulation of mechano-responsiveness.
Introduction
Adult bone is a dynamic tissue, constantly being remodeled in response to mechanical, hormonal, nutritional and environmental cues.1 Remodeling is orchestrated through coordinated activities of bone-forming cells—osteoblasts—and bone resorbing cells—osteoclasts. Osteocytes, embedded in the bone matrix, are the most numerous of bone cells and are generally regarded as the sensor of mechanical load in bone.2 One mechanism by which bone cells can coordinate their activity is intercellular communication via gap junctions, a particular type of intercellular junction that does not provide cell–cell anchorage but allows direct cell-to-cell communication.3,4 Gap junctions are abundant among osteoblasts and osteocytes, and between osteocytes and surface osteoblasts and bone lining cells.5,6,7 Gap junctions are composed of connexins arranged in hexameric arrays called connexons. Connexins are integral membrane proteins with four transmembrane domains and both amino- and carboxyl termini in the cytoplasm. When two connexons from apposing cells align and dock, they form a transcellular channel that provides aqueous continuity between two cytosols. Connexons can also function as individual membrane channels, without docking to apposing connexons, in a conformation also called ‘hemichannels'.8 The most abundant connexin present in bone is connexin43 (Cx43), encoded by the Gja1 gene.9,10,11 Other connexins are present in bone, namely, Cx45, Cx40 and Cx37, which are expressed at various stages of development in the osteoblast lineage.9,12,13 Because the permeability and electrophysiological properties of gap junctions formed by different connexins vary,9,14 it is unclear whether any of these connexins can compensate for lack of Cx43 in bone. Nonetheless, studies on dominant Gja1 mutants, discussed later in more detail, suggest that some degree of redundancy may exist. Furthermore, new, still unpublished studies suggest that Cx37 may be involved in osteoclast regulation.15 This review, however, will focus on the role of Cx43 in bone homeostasis, as most of the knowledge has been accumulated on this connexin.
Cx43 in skeletal homeostasis
The importance of Cx43 in bone development emerged with the report of craniofacial abnormalities, delayed ossification of the axial and appendicular skeleton, and osteoblast defects in mice with germline ablation of Gja1.16 The discovery that the human disease oculodentodigital dysplasia (ODDD) is linked to GJA1 mutations confirmed that Cx43 has a major role in skeletal development and homeostasis.17 Patients with this autosomal dominant disease have skeletal abnormalities including craniofacial dysmorphism, syndactyly of hands and feet, dentition abnormalities and tubularization of long bones.17,18 Two mouse models of ODDD have been so far described, the first one carries a germline heterozygous G60S Gja1 mutation, not found in ODDD patients;19,20 the second was generated by conditional replacement of one wild-type (WT) allele with a Gja1G138R mutant allele, frequently seen in ODDD patients.21,22 Both mutants largely phenocopy the human disorder, with syndactyly, craniofacial and dentition abnormalities; they also disclose skeletal features not yet reported in the human disease, in particular, larger but thinner cortical bones, lower than normal bone mass and compromised bone strength.19,21,22
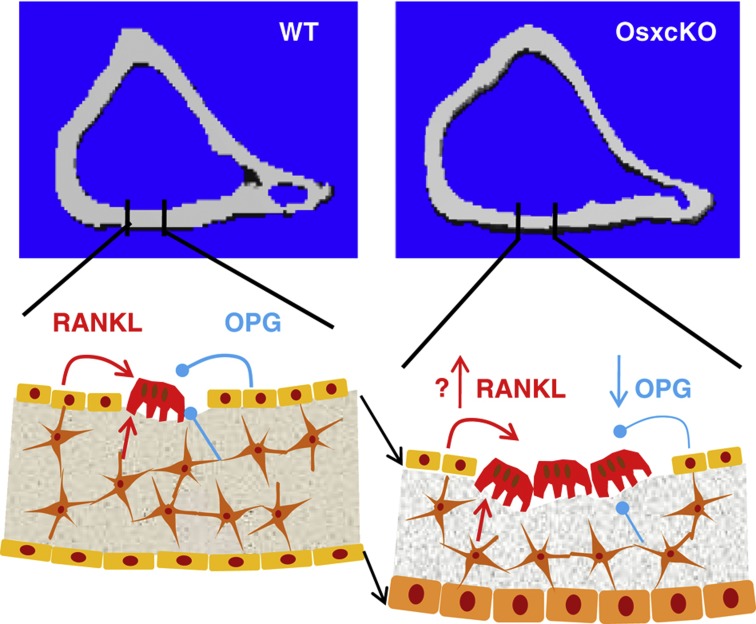
As germline Gja1 deletion is embryonic lethal,23 various models of conditional ablation of Gja1 at different stages of the osteogenic lineage have been developed over the past decade using different promoters to drive Cre-mediated Gja1 recombination; Dermo1/Twist2, for deletion in the entire chondro-osteogenic lineage starting early in embryogenesis (TW2cKO),22 Col1a1 for ablation in committed, bone-forming osteoblasts (Col1cKO),24 Bglap, for deletion in fully differentiated osteoblasts (OG2cKO),25,26,27 and Dmp1, which is believed to target primarily osteocytes but is also widely expressed in osteoblasts (Dmp1cKO).27 Common skeletal features have emerged in all these mouse mutants, consisting of abnormalities primarily affecting the cortex, which in the absence of Cx43 is larger but thinner in its cross-section, with larger marrow area.22,25,27 Preliminary data show that Gja1 ablation driven by the Osx/Sp7 promoter also results in similar cortical changes (Figure 1). Increased endocortical osteoclast resorption and periosteal bone formation are the fundamental mechanisms of the cortical modeling abnormalities of Cx43-deficient bones. In our hands, increased endocortical bone resorption is associated with decreased osteoprotegrin (OPG) production by Cx43-deficient osteoblasts or bone marrow stromal cells,22 probably via mechanisms analogous to those found for other Cx43-regulated genes.28,29,30,31,32 Decreased OPG mRNA was also observed in whole-bone extracts of Dmp1cKO model.27 In the same study, receptor activator of nuclear factor-κB ligand (RANKL) was increased in Cx43-deficient calvaria cells in addition to decreased OPG;27 and others have reported increased RANKL/OPG ratio in the osteocytic cell line, MLO-Y4.25,27 Despite these discrepancies, it is clear that Cx43 in cells of the osteogenic lineage inhibits osteoclastogenesis through paracrine effects. Indeed, increased osteoclast number on the endocortical surface is a common finding in all models of Gja1 deletion.22,25,27,33 It should be noted that osteoclasts also express Cx43,34 and gap junctions may be involved in osteoclastogenesis;35 however, direct communication among osteoclasts or between osteoclasts and other bone cells through Cx43 gap junctions has not been demonstrated. Increased endocortical bone resorption in Cx43-deficient bones expands the medullary cavity and the associated enhanced periosteal bone apposition results in a larger cortical cross-section (Figure 1). It remains to be elucidated whether such cortical expansion is centrifugal or eccentric.27 Notably, the increased periosteal bone apposition is sufficient to maintain a normal cortical thickness only when Gja1 ablation is restricted primarily to osteocytes;27 when osteoblast and/or precursors are targeted by Gja1 deletion, periosteal bone formation does not fully compensate for endocortical resorption, resulting in cortical thinning.22,25,33 Indeed, comparison of cortical parameters between the different stage-specific Gja1 mutants and their respective WT controls reveals that the severity of the cortical phenotype worsens with broader and earlier gene inactivation (Figure 2). That the increased endocortical bone resorption is the main pathophysiologic abnormality consequent to Cx43 deficiency in the osteoblastic lineage is demonstrated by normalization of cortical thickness and bone strength by treatment of Cx43-deficient animals with bisphosphonates, potent bone resorption inhibitors.36 Thus, Cx43 serves different functions at different stages of the osteoblast differentiation program, control of cell proliferation in osteogenic precursors,22,26 precursor maturation into a functional osteoblast via facilitation of Runx2-Osx transition,22,30,31 stimulation of matrix protein production by osteoblasts,22,28 regulation of osteoblast apoptosis,26,37 response to anabolic agents,24,30,32,38 support for osteoclastogenesis22,27 and modulation of mechanical responses via osteocytes and osteoblasts.25,27,39,40
Figure 1.
Cortical expansion in Cx43-deficient bones. Cross-sections of tibia at mid-diaphysis of WT and Osx-Cre::Gja1fl/− (OsxcKO) mice (upper panels); and representation of the homeostatic changes consequent to Gja1 ablation (lower panels). Black arrows indicate centrifugal expansion of the cortex.
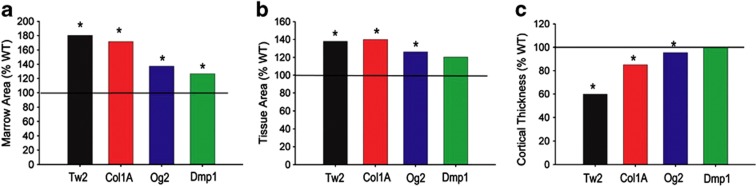
Figure 2.
Increased severity of the cortical phenotype in mice with broader Gja1 ablation along the osteogenic lineage. Data are derived from previously published results of independent studies using different stage-specific promoters to drive Cre-mediated Gja1 ablation; Dermo1/Twist 2 (Tw2);22 2.3 kb fragment of Col1A1 (Col1A);39 human osteocalcin (OC)27 and Dmp1.27 Percent difference relative to the respective wild-type (WT) controls in (a) marrow area; (b) total area and (c) cortical thickness. *Significantly different from respective WT littermates, P<0.05 (based on data reported in each original publication).
As indicated above, despite an increased geometric moment of inertia (reflecting resistance of bone to bending forces), cortical bone strength is decreased in Cx43-deficient bones, associated with decreased cortical bone mineral density, increased cortical porosity, production of disorganized fibrillar collagen matrix and woven bone appearance,22 underscoring an intrinsic defect of bone forming cells. This phenotype is quite similar to that described in the two ODDD mouse models,19,22 corroborating the notion that those ODDD mutations are dominant negative for Cx43. Furthermore, as the G138R Gja1 mutant is associated with increased intracellular ATP release,21 a function ascribed to Cx43 ‘hemichannel' activity,41 these genetic mouse models argue against a physiologic role of Cx43 hemichannels in bone modeling. Further details on these topics are critically discussed in recent reviews.15,42
Cx43 and mechanotransduction
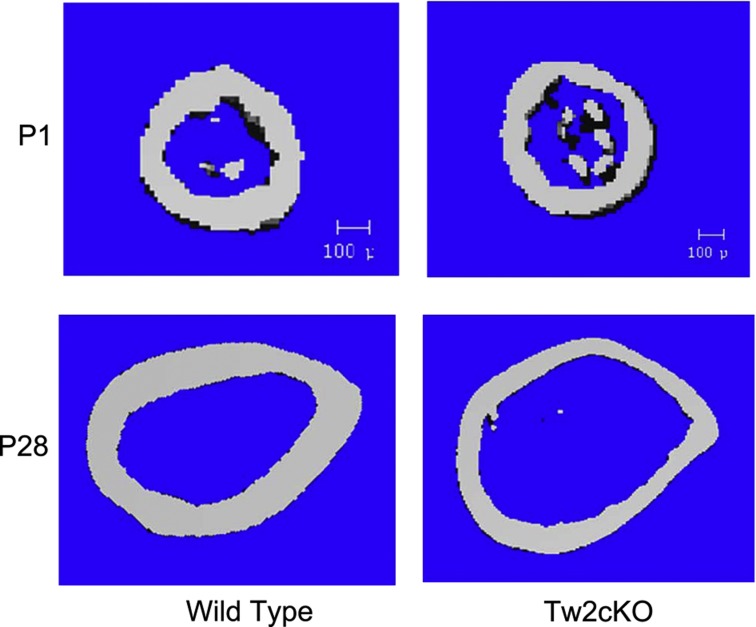
The cortical modeling abnormalities of Cx43-deficient bones are similar to those occurring as a result of skeletal mechanical unloading (for example, space flight, prolonged bed rest) and during skeletal aging. Although mice with a conditional Gja1 deletion in bone forming cells do not age prematurely, there is substantial evidence that Cx43 has an essential role in mechanotransduction. Recent in vivo work has shown that Gja1-deficient mice exhibit an abnormal response to mechanical loading and unloading, supporting the hypothesis that in the absence of Cx43 bone perceives normal ambulatory mechanical loading as a disuse scenario, resulting in abnormal activation of endocortical osteoclasts and eventual expansion of the marrow cavity.22,25,27,33 Further strengthening the notion that the cortical modeling abnormalities primarily reflect a mechano-sensing defect is the observation that such abnormalities develop post-natally. Newborn TW2cKO mice, having yet to experience the mechanical forces of normal ambulation, do not display the enlarged marrow cavity and cortical thinning evident in the more mature, fully ambulating (1 month and older) animals (Figure 3).
Figure 3.
Cross-sections of femoral shafts by μCT scans of wild-type and Gja1 conditionally deleted mice using the Dermo1/Twist 2 promoter (Tw2cKO). Postmortem scans were taken at ages P1 (upper panels) and P28 (lower panels). Cortical expansion and thinning evident in 1-month-old mice are not present at birth.
It is generally accepted that the osteocyte is ideally situated to be bone's mechanosensor, their dendritic processes providing for an intricate communication network through canaliculi, and that Cx43 gap junctions may provide the intercellular conduits for such communicating network. One widely held notion is that canalicular fluid flow may represent a key physiologic stimulus during normal physical activity. Fluid flow generates shear stress, thus creating drag forces on the dendritic processes through tethering filaments or primary cilia.2,43 These events would lead to generation of signaling molecules, which are either transmitted to adjacent cells through gap junction channels and/or ‘secreted' into the extracellular milieu via Cx43 hemichannels (connexons). In vivo evidence of these events is still lacking because of the inherent difficulties in imaging bone embedded osteocytes. On the other hand, direct osteocyte-to-osteoblast communication has been demonstrated in vitro using an ingenious co-culture system, whereby the two cells types are physically separated, but are still able to form gap junctions. Application of fluid flow on the osteocyte side led to increased alkaline phosphatase on the osteoblast side, whereas application of fluid flow directly on the osteoblasts did not.44 As inhibition of gap junction channel function prevented such response, this study provided in vitro proof of the concept that the osteocyte is the main mechanosensor that transmits the mechanical signal to osteoblasts via gap junctional communication. Another study provides evidence of cell-to-cell communication via Cx43 hemichannels in response to mechanical stimulation. Application of fluid flow to the osteocytic cell line, MLO-Y4 (also used in the co-culture study mentioned above), resulted in uptake of Cx43-permeable fluorescent dye—taken as evidence of hemichannel opening—associated with release of prostaglandins45,46 and ATP.47 In this model, Cx43 does not provide the means for direct cell-to-cell communication, but it allows activation of a paracrine system of intercellular communication. However, distinguishing between hemichannels and gap junctions is difficult, as chemical inhibitors are not selective for either function. Blocking antibodies or peptides46,48 may provide more specificity but in vivo genetic approaches are required to establish the physiologic relevance of Cx43 ‘hemichannels' in bone.
Cx43 alters cortical responses to mechanical loading
The development of skeletal-specific gene deletion models has allowed testing of Cx43 role in mechano-responsiveness in vivo. An early study from our group using Col1cKO mice demonstrated that application of mechanical load with a three-point bending protocol resulted in an attenuated endocortical bone formation response in mutant relative to WT littermates, assessed by reduced calcein labeling at sites of maximum tension and pressure.39 Periosteal bone formation was not assessed in this study because of an exuberant periosteal woven bone response observed in all mice, which was interpreted as a reaction to pressure on the periosteum. Notably, direct measurement of periosteal strain using strain gauges indicated, somewhat surprisingly, that greater loads were necessary to generate strains in the Col1cKO comparable to those obtained in WT littermates. Such discrepancy may be related to the different geometry and/or altered material properties of Cx43-deficient bones. Although these results pointed to a positive action of Cx43 on cortical bone response to mechanical load, an apparent opposite result was later reported by another group. Using cantilever bending to generate a bending moment at the midshaft of the tibia in vivo in OG2cKO mice—in which Gja1 is ablated in mature osteoblasts and osteocytes—these investigators found a larger bone formation response to mechanical loading on the periosteal surface compared with their WT littermates.25 Notably, endocortical bone formation was not reported. Aside the different loading regimes and strain applied, these two studies raised the intriguing hypothesis that the periosteal and endosteal surfaces may respond differently to mechanical loading, and that the absence of Cx43 amplifies such difference.
We recently tested this hypothesis in young (2-month old) Tw2cKO mice, using axial tibial compression, a more physiologically relevant loading regime. Strain gauge studies confirmed that as for Col1cKO39 and OG2cKO mice,25 Tw2cKO mice require a greater load to generate the same periosteal strain as in WT littermates.40 We applied a loading force that would generate the same strain (1200 μɛ) in both genotypes. In contrast to our earlier study using three-point bending in older mice, axial loading did not stimulate significant bone formation on the endocortical surface in either genotype. In fact, endocortical bone formation was paradoxically decreased by axial tibial loading, and this decrease was more pronounced in Tw2cKO mice, a finding consistent with our previous study.39 More importantly, axial loading stimulated periosteal bone formation in both genotypes; however, a larger strain (1900 μɛ) was necessary in WT mice to obtain the same degree of periosteal response obtained by applying 1200 μɛ to Tw2cKO mice. This finding supports the previous findings using cantilever bending,25 confirming that lack of Cx43 increases the sensitivity of periosteal bone formation to mechanical load. Such conclusion has been further corroborated by a recent study using ulna compression to apply mechanical load to Dmp1cKO mice (4-month old). In contrast to a previous study from the same group, showing no changes in cortical thickness at the femoral midshaft in mutant mice,27 marrow area of the ulna was increased in Dmp1cKO mice, although total tissue area and bone area were similar to WT, a discrepancy difficult to explain. Nonetheless, loading resulted in an enhanced periosteal bone formation response in the Dmp1cKO, whereas it did not affect endocortical bone formation in either genotype.49 Two groups have reported that osteocyte apoptosis may be involved in Cx43 modulation of periosteal and endocortical response to mechanical loading.27,50 Intriguingly, apoptotic osteocytes are only prevalent in the cortex of long bones and not in trabecular regions,50 suggesting that higher levels of Cx43 may be necessary to maintain cell viability in the cortex relative to cancellous bone.27 Osteocyte apoptosis has also been proposed as a mechanism for recruiting osteoclasts to the endocortical surface in Cx43-deficient mice.27 The precise relationship among osteocyte apoptosis and Cx43 expression in mechano-transduction is an emerging area of research.
The studies just reviewed provide consistent evidence that the endocortical and periosteal envelopes respond independently to mechanical load. In the absence of Cx43 responses at the endocortical and periosteal surfaces are not only independent but also, at least in one case,39,40 even opposite. These are novel concepts that strengthen the physiologic importance of cortical bone in the homeostatic response to mechanical stimuli. They also raise questions for future research. For example, is periosteal bone apposition directly coupled to enhanced endocortical osteoclast activity, thus representing an adaptive reaction, or are the two processes independently regulated? Are the different responses at the endocortical and periosteal surfaces driven by different sensitivities of endocortical or periosteal cells to mechanical stimuli, or are the cells on the two envelopes exposed to different paracrine regulators? Are the osteocytes the critical mechano-sensing cells, which, in the absence of Cx43, inappropriately perceive the loads of normal ambulation activities? Is Cx43 in osteoblasts and/or osteocytes directly involved in the sensing and/or transduction of mechanical signals in bone? In the absence of Cx43, is there increased osteocytic apoptosis and therefore decreased conduction of mechanical signals through bone? Finally, what is the molecular nature of the signals being communicated by these cells to regulate cortical bone modeling and remodeling? Addressing these questions may be challenging, but it would expand our knowledge of how the cortex is modeled under the effect of mechanical load and whether Cx43 can be used as a potential therapeutic target for maximizing the anabolic effect of load.
Cx43 sensitizes cortical bone to unloading
Substantial work has also been produced on the other end of the spectrum, that is, how Cx43 modulates response to skeletal unloading. Our laboratory applied a method consisting of temporary muscle paralysis in the right hind limb induced by one injection of Botulinum Toxin Type A (BtxA).33 In this study, 4-month-old male Col1cKO mice lost cancellous bone at the same rate and extent as did WT mice; this loss was followed by slow and partial recovery within 19 weeks. However, Col1cKO mice were insensitive to the effect of muscle paralysis on cortical bone compared with WT mice, in which loss of muscle function led to a significant expansion of the medullary cavity and cortical thinning associated with increased number of endocortical osteoclasts. Indeed, osteoclast number and cortical thickness 19 weeks after induction of muscle paralysis in WT mice were very similar to control, non-BtxA injected Col1cKO mice, further suggesting that one of the main functions of Cx43 in the cortex is to modulate responses to mechanical load.33
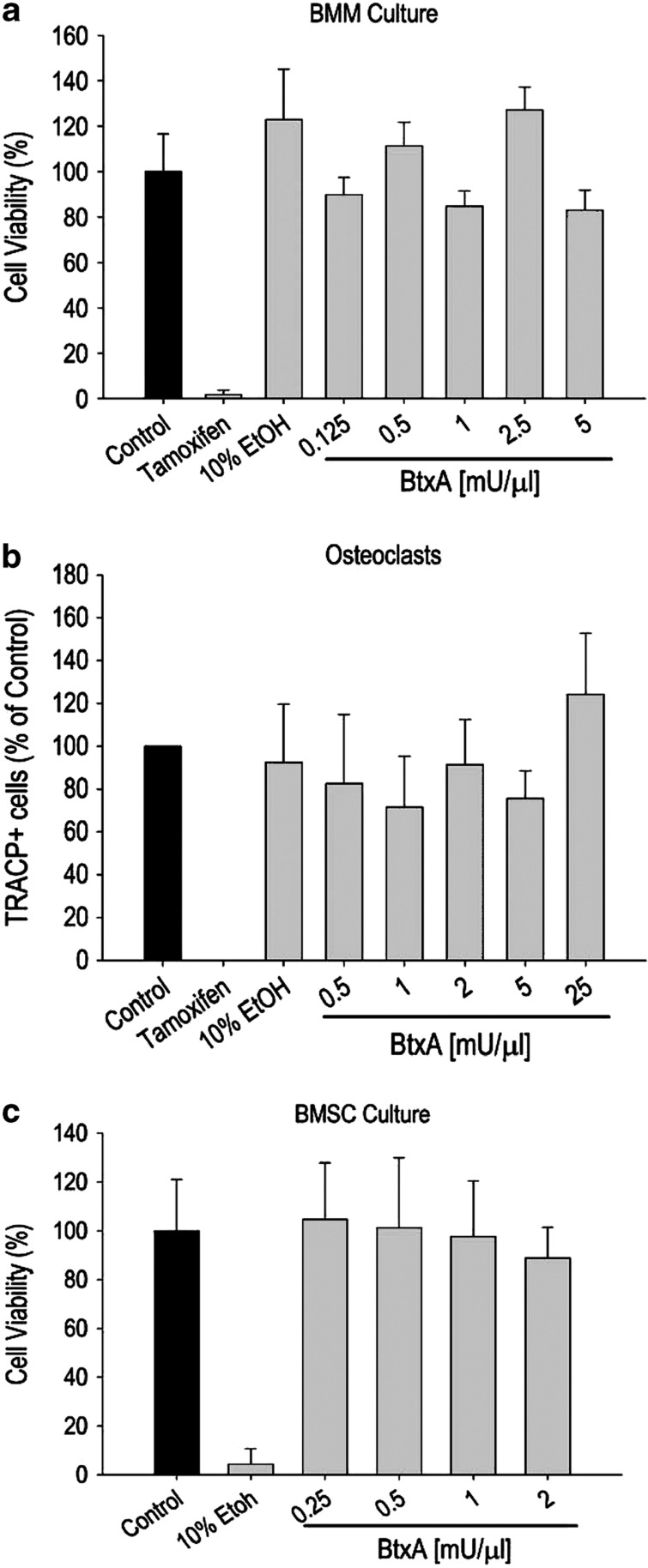
A potential limitation to the BtxA model is the possibility that BtxA may be toxic to bone cells, thus potentially contributing to bone loss. To directly address this issue, we determined that exposure of bone marrow-derived primary osteoblasts and osteoclasts to BtxA (0.125–25 mU μl−1) for 24 h did not alter their viability. Furthermore, the presence of BtxA in the culture medium did not stimulate differentiation of bone marrow macrophages into osteoclasts in the absence of macrophage colony stimulating factor (M-CSF) and RANKL; nor did it affect bone marrow stromal cell support of osteoclast differentiation in a co-culture system. Finally, BtxA had no effect on in vitro osteogenic differentiation of bone marrow stromal cells (Figure 4). Based on these results it would appear highly unlikely that the profound bone loss following BtxA-induced muscle paralysis is related to direct effects of the toxin on bone cells.
Figure 4.
Lack of a direct effect of Botulinum toxin type A (BtxA) on primary bone cells. Data from three independent experiments. (a) Bone marrow macrophages (BMM) cultured in osteoclastogenic medium were exposed to varying concentrations of BtxA for 24 h. Tamoxifen (10 μM) was used as an inhibitor of osteoclastogenesis. Cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl assay. (b) BMM were cultured as in a, and stained for TRAcP-positive osteoclasts (large multinucleated cells with a minimum of seven nuclei). (c) Bone marrow stromal cells (BMSCs) were cultured in osteoblastogenic medium and exposed to different concentrations of BtxA for 24 h. Ethanol was used as an inhibitor of osteoblastogenesis. Cell viability was assessed using the MTT assay. Data were analyzed using one-way analysis of variance. No statistical differences were detected in any of the BtxA-treated cultures relative to their respective controls.
Indeed, recent studies using hind limb unloading by tail suspension provides corroborating evidence to the role of Cx43 in cortical response to removal of skeletal mechanical load.50,51 Whereas the BtxA method addresses the role of muscle function on bone homeostasis, hind limb unloading models weightlessness, even though it does not reproduce loss of gravity.52 Consistent with our BtxA-based study, limb unloading caused both trabecular and cortical bone loss in WT animals, and cortical bone changes were attenuated in OG2cKO mice. At slight variance with our study, however, unloading-induced trabecular bone loss was also attenuated in the Cx43-deficient mice compared with WT.51 Interestingly, when Cx43-deficient mice were subjected to unloading, whole-bone Sost expression with decreased osteoclast indices was shown.50 Although these investigators did not follow the mice through a recovery period of normal ambulatory loading, their findings strengthen the notion that Cx43 deficiency decreases bone sensitivity to skeletal unloading.
Mechanisms of Cx43 modulation of mechano-responsiveness
Understanding how Cx43 alters cell function and gene expression in bone subjected to mechanical loading and unloading would greatly help clarify the complex actions of Cx43 in mechanotransduction. In our axial tibial compression study, mRNA products associated with bone formation and Cox-2, a known mechanically responsive gene, were upregulated to a significantly lesser degree in Tw2cKO compared with WT bones.40 Conversely, genes associated with bone resorption, for example, Rankl were downregulated by loading and Cx43 deficiency. Importantly, this study also showed that the abundance of Sost mRNA was lower in Tw2cKO than in WT bones in resting conditions. As sclerostin is a Wnt inhibitor, this observation may theoretically provide a mechanism for the increased periosteal bone apposition in mutant mice. Consistent with this hypothesis, others reported increased β-catenin abundance and upregulation of the Wnt target gene Axin-2 in Dmp1cKO mice.49 β-Catenin abundance was increased in ulnae subjected to compression loading relative to unloaded controls.49 This result is consistent with observations that mechanical loading leads to stabilization and accumulation of β-catenin in the cytoplasm,53,54 and with the finding that sensitivity to mechanical loading is related to cytoplasmic β-catenin levels.55 Although the accumulated data strongly suggest that Wnt/β-catenin signaling is involved in Cx43 modulation of mechano-responsiveness, the mechanisms of this modulatory action remain largely to be determined. For example, sclerostin is decreased in Cx43-deficient mice and it is further downregulated by mechanical stimulation.22,27,40 However, decreased sclerostin is inconsistent with defective osteoblast differentiation and function in Cx43-deficient mice,16,22 which would instead point to deficient Wnt signaling. Indeed, expression of several osteoblast genes is downregulated by interference with Cx43 expression or function.10,28,29,31 One possibility is that Cx43-deficient osteoblasts may develop a ‘resistance' to Wnt signals—and perhaps to other stimulators of differentiation—so that decreased sclerostin may represent a homeostatic response in an attempt to overcome such resistance. Although the increased Wnt stimulus does not fully overcome the resistance to full differentiation, it may generate a proliferative response in osteoblast precursors. Such a model would be consistent with increased periosteal bone apposition but production of an abnormal, under-mineralized organic matrix (Figure 1). Further complexity arises from the fact that Cx43 itself is a Wnt target gene.55,56 It is also unclear whether Cx43 directly senses mechanical load, or provides a mechanism to maximize skeletal response to mechanical load. Work from one group proposed that Cx43 hemichannels open in response to fluid flow, and that this requires a physical interaction between Cx43 and α5β1. In this model, the integrin heterodimer functions as the mechanical sensor of fluid flow-induced shear stress, and conformational changes open Cx43 hemichannels.57 This intriguing working model has yet to be corroborated by in vivo data and reconciled with the now established fact that lack of Cx43 leads to different bone formation responses at the endocortical and periosteal surfaces, as discussed above.
Hints as to how Cx43 modulates mechanical responsiveness in bone cells may be gleaned from the effect of Cx43 on osteoblast and osteocyte function and survival. For example, Cx43 has been shown to modulate transcription factors, such as Sp1,28 Runx230 and Osterix31 in osteoblasts, at least in part, by regulating signal transduction cascades that converge on protein kinase C-δ30,32 and/or extracellular signal regulated kinases.29,32 In the case of protein kinase C-δ/Runx2 signaling downstream of Cx43, a novel inositol pyrophosphate (diphosphoinositol pentakisphosphate, InsP7) has been implicated as a second messenger relaying signals downstream of Cx43 and converging upon Runx2 activity in osteoblasts.58 Whether or not this novel second messenger has a role in mechanosensing by osteocytes is unknown. In addition, Cx43 gap junction-dependent and Cx43 hemichannel-dependent calcium waves have also been implicated in mechanosensing by bone cells in culture.59,60,61,62 These long range, connexin-dependent calcium waves have been demonstrated ex vivo in bone.63,64 Another way that Cx43 may impact the mechanical responsiveness of osteocytes could be via the modulation of apoptotic processes. As noted earlier, it has been shown in vivo27,50 and in vitro that Cx43 can affect osteocyte survival by modulating integrins and the extracellular signal regulated kinase pathway.38,57,65,66 Undoubtedly, numerous second messenger systems are likely to relay through gap junctions among osteocytes, osteoblasts and progenitor cells to regulate the tissue response to mechanical stress. A better understanding of how Cx43 modulates mechanical responsiveness at the molecular level will help devise more effective approaches to potential interference with Cx43 action for therapeutic purposes.
Cx43 deficiency models aging of cortical bone
The cortical architecture of Cx43-deficient long bones is also reminiscent of aging bones, and in particular cortical expansion.67 Such similarity also extends to the bone matrix. Collagen crosslinking and lysyl oxidase activity decline with age.68,69 Similarly, TW2cKO bones have reduced abundance of Lox mRNA associated with abnormal collagen fiber organization and decreased mineralization;22 and the ratio of nonreducible/reducible collagen crosslinks is reduced in OG2cKO mice.27 This abnormality was not apparent in Dmp1cKO mice,70 consistent with the notion that the severity of the skeletal phenotype is attenuated with late stage Gja1 ablation. Nonetheless, these results strengthen the notion that Cx43 deficiency resembles bone aging. Further, it has been argued that as Cx43 is a Wnt target gene55,56 and Wnt signaling tends to decrease with age71,72 particularly in bone, any reduction in Cx43 occurring in the backdrop of decreased Wnt signaling may mediate some of the bone changes induced by aging. It is tempting to speculate that as a function of age, Cx43 expression and gap junctional communication decrease and bone becomes osteopenic and more sensitive to load as in Cx43-deficient mice. However, data are conflicting regarding skeletal mechano-responsiveness in aging, with increased,73,74 unchanged,75,76,77,78,79 but also decreased80,81 bone formation response being reported. This line of reasoning will have to be tested in vivo, but it is consistent with the notion put forth by H Frost many years ago that the minimum effective strain mechanostat set point for bone remodeling increases with age, such that loads previously perceived as being within the physiological range are perceived as below that set point in an aged system.82 As noted earlier, the same concept is applicable to Cx43-deficient bones. Hence, Cx43 may provide a unifying mechanism linking aging and mechano-sensing in cortical modeling.
The future: translating connexin biology to human disease
Connexin biology in the skeleton is a vibrant area of research. New developments in mouse genetics and skeletal imaging have propelled activity in this area for the past few years. The accumulated knowledge establishes Cx43 as a key molecule for skeletal homeostasis, and in particular for post-natal cortical modeling and for skeletal responsiveness to mechanical load (loading and unloading). Accordingly, it has been suggested that a transient decrease in Cx43 might serve as a therapeutic technique to enhance the anabolic response of bone to mechanical loading.25 This desirable effect will have to be reconciled with attenuated endocortical response to load and the increased endocortical bone resorption occurring in Cx43 deficiency. Conceivably, one could use short-term inhibition of Cx43 for preventing disuse bone loss in an acute or short-term setting; in the long-term, upregulation of Cx43 might be more desirable for facilitating bone formation.
Cx43 can communicate both osteoanabolic and osteocatabolic signals among bone cells, depending upon cellular context, anatomical location and the nature of the extracellular cue (for example, loading or unloading). Although in vivo validation of these findings is required, identifying the second messenger networks that are diffused among osteoblast and osteocytes will be a crucial element in developing therapies that use Cx43 to improve cortical bone in aging and disuse. We are still at an early stage in regards to developing connexin-based therapeutics. Nonetheless, the substantial knowledge accumulated over the past two decades has now reached a point where one can begin to develop strategies for translating this knowledge into clinically useful directions. This area of research is likely to offer novel therapeutic options to improve bone health in disuse and other forms of osteoporosis.
Acknowledgments
Part of the work reported here was supported by NIH grants AR041255 (to RC), AR052719, AR063631 (to JPS), by the Washington University Core Center for Musculoskeletal Biology and Medicine (P30 AR057235), and by grants from the Barnes-Jewish Foundation.
Footnotes
Roberto Civitelli receives research support from Pfizer, Inc. and Amgen, and holds stock of Amgen, Eli-Lilly and Merck & Co. The remainning authors declare no conflict of interest.
References
- Grimston SK. An application of mechanostat theory to research design: a theoretical model. Med Sci Sports Exerc 1993;25:1293–1297. [PubMed] [Google Scholar]
- Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone 2008;42:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough DA, Goliger JA, Connexins Paul DL. connexons, and intercellular communication. Ann Rev Biochem 1996;65:475–502. [DOI] [PubMed] [Google Scholar]
- Paul DL. New functions for gap junctions. [Review]. Curr Opin Cell Biol 1995;7:665–672. [DOI] [PubMed] [Google Scholar]
- Jones SJ, Gray C, Sakamaki H, Arora M, Boyde A, Gourdie R et al. The incidence and size of gap junctions between the bone cells in rat calvaria. Anat Embriol 1993;187:343–352. [DOI] [PubMed] [Google Scholar]
- Doty SB. Morphological evidence of gap junctions between bone cells. Calcif Tissue Int 1981;33:509–511. [DOI] [PubMed] [Google Scholar]
- Marotti G, Ferretti M, Muglia MA, Palumbo C, Palazzini S. A quantitative evaluation of osteoblast-osteocyte relationships on growing endosteal surface of rabbit tibiae. Bone 1992;13:363–368. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol 2003;4:285–294. [DOI] [PubMed] [Google Scholar]
- Steinberg TH, Civitelli R, Geist ST, Robertson AJ, Hick E, Veenstra RD et al. Connexin43 and connexin45 form gap junctions with different molecular permeabilities in osteoblastic cells. EMBO J 1994;13:744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitelli R, Stains JP, Shin CS, Jørgensen N. Intercellular junctions and cell-cell communication in the skeletal system. In: Bilezikian JP, Raisz LG, Martin TJ (eds).Principles of Bone Biology 3 edn. Academic Press: San Diego, 2008; pp425–444. [Google Scholar]
- Civitelli R, Beyer EC, Warlow PM, Robertson AJ, Geist ST, Steinberg TH. Connexin43 mediates direct intercellular communication in human osteoblastic cell networks. J Clin Invest 1993;91:1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizard A, Burgon PG, Paul DL, Bruneau BG, Seidman CE, Seidman JG. Connexin 40, a target of transcription factor Tbx5, patterns wrist, digits, and sternum. Mol Cell Biol 2005;25:5073–5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P et al. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone 2009;45:682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra RD, Wang HZ, Westphale EM, Beyer EC. Multiple connexins confer distinct regulatory and conductance properties of gap junctions in developing heart. Circ Res 1992;71:1277–1283. [DOI] [PubMed] [Google Scholar]
- Plotkin LI, Bellido T. Beyond gap junctions: Connexin43 and bone cell signaling. Bone 2013;52:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol 2000;151:931–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Gen 2003;72:408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama J, Paznekas W, Seki A, Taffet S, Jabs EW, Delmar M et al. Functional characterization of connexin43 mutations found in patients with oculodentodigital dysplasia. Circ Res 2005;96:e83–e91. [DOI] [PubMed] [Google Scholar]
- Flenniken AM, Osborne LR, Anderson N, Ciliberti N, Fleming C, Gittens JE et al. A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development 2005;132:4375–4386. [DOI] [PubMed] [Google Scholar]
- Churko JM, Chan J, Shao Q, Laird DW. The G60S connexin43 mutant regulates hair growth and hair fiber morphology in a mouse model of human oculodentodigital dysplasia. J Invest Dermatol 2011;131:2197–2204. [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M et al. The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum Mol Gen 2008;17:539–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins M, Grimston SK, Norris JY, Guillotin B, Shaw A, Beniash E et al. Osteoblast connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol Biol Cell 2011;22:1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume AG, De Sousa PA, Kulkarmi S, Langille BL, Zhu D, Davies TC et al. Cardiac malformation in neonatal mice lacking connexin43. Science 1995;267:1831–1834. [DOI] [PubMed] [Google Scholar]
- Chung DJ, Castro CH, Watkins M, Stains JP, Chung MY, Szejnfeld VL et al. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci 2006;119:4187–4198. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Paul EM, Sathyendra V, Davison A, Sharkey N, Bronson S et al. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PloS one 2011;6:e23516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Lezcano V, Thostenson J, Weinstein RS, Manolagas SC, Bellido T. Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. J Bone Miner Res 2008;23:1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivi N, Condon KW, Allen MR, Farlow N, Passeri G, Brun LR et al. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Miner Res 2012;27:374–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stains JP, Lecanda F, Screen J, Towler DA, Civitelli R. Gap junctional communication modulates gene transcription by altering the recruitment of Sp1 and Sp3 to connexin-response elements in osteoblast promoters. J Biol Chem 2003;278:24377–24387. [DOI] [PubMed] [Google Scholar]
- Stains JP, Civitelli R. Gap junctions regulate extracellular signal-regulated kinase signaling to affect gene transcription. Mol Biol Cell 2005;16:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima F, Niger C, Hebert C, Stains JP. Connexin43 potentiates osteoblast responsiveness to fibroblast growth factor 2 via a protein kinase C-delta/Runx2-dependent mechanism. Mol Biol Cell 2009;20:2697–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niger C, Lima F, Yoo DJ, Gupta RR, Buo AM, Hebert C et al. The transcriptional activity of osterix requires the recruitment of Sp1 to the osteocalcin proximal promoter. Bone 2011;49:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niger C, Buo AM, Hebert C, Duggan BT, Williams MS, Stains JP. ERK acts in parallel to PKC delta to mediate the connexin43-dependent potentiation of runx2 activity by FGF2 in MC3T3 osteoblasts. Am J Physiol Cell Physiol 2012;302:C1035–C1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimston SK, Goldberg DB, Watkins M, Brodt MD, Silva MJ, Civitelli R. Connexin43 deficiency reduces the sensitivity of cortical bone to the effects of muscle paralysis. J Bone Miner Res 2011;26:2151–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransjo M, Sahli J, Lie A. Expression of connexin 43 mRNA in microisolated murine osteoclasts and regulation of bone resorption in vitro by gap junction inhibitors. Biochem Biophy Res Comm 2003;303:1179–1185. [DOI] [PubMed] [Google Scholar]
- Matemba SF, Lie A, Ransjo M. Regulation of osteoclastogenesis by gap junction communication. J. Cell Biochem 2006;99:528–537. [DOI] [PubMed] [Google Scholar]
- Watkins MP, Norris JY, Grimston SK, Zhang X, Phipps RJ, Ebetino FH et al. Bisphosphonates improve trabecular bone mass and normalize cortical thickness in ovariectomized, osteoblast connexin43 deficient mice. Bone 2012;51:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Manolagas SC, Bellido T. Connexin43 hemichannel opening: A requirement for bisphosphonate-mediated prevention of osteocyte apoptosis. J Bone Miner Res 2000;15:S172. [Google Scholar]
- Bivi N, Lezcano V, Romanello M, Bellido T, Plotkin LI. Connexin43 interacts with βarrestin: a pre-requisite for osteoblast survival induced by parathyroid hormone. J Cell Biochem 2011;112:2920–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimston SK, Brodt MD, Silva MJ, Civitelli R. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1). J Bone Miner Res 2008;23:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimston SK, Watkins MP, Brodt MD, Silva MJ, Civitelli R. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin43 deficient mice. PLoS One 2012;7:e44222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JX, Gu S. Gap junction- and hemichannel-independent actions of connexins. Biochim Biophys Acta 2005;1711:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stains JP, Watkins M, Grimston S, Hebert C, Civitelli R. Molecular mechanisms of osteoblast/osteocyte regulation by connexin43. Calcif Tissue Int 2013; e-pub ahead of print 11 June 2013; 10.1007/s00223-013-9742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald LF. The amazing osteocyte. J Bone Miner Res 2011;26:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AF, Saunders MM, Shingle DL, Cimbala JM, Zhou Z, Donahue HJ. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am J Physiol Cell Physiol 2007;292:C545–C552. [DOI] [PubMed] [Google Scholar]
- Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E et al. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell 2005;16:3100–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller-Jackson AJ, Burra S, Gu S, Xia X, Bonewald LF, Sprague E et al. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem 2008;283:26374–26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Phys 2007;212:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burra S, Jiang JX. Connexin 43 hemichannel opening associated with Prostaglandin E(2) release is adaptively regulated by mechanical stimulation. Commun Integr Biol 2009;2:239–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivi N, Pacheco-Costa R, Brun LR, Murphy TR, Farlow NR, Robling AG et al. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J Orthop Res 2013;31:1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd SA, Loiselle AE, Zhang Y, Donahue HJ. Connexin 43 deficiency desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. Bone 2013;57:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd SA, Lewis GS, Zhang Y, Paul EM, Donahue HJ. Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. J Bone Miner Res 2012;27:2359–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol 2002;92:1367–1377. [DOI] [PubMed] [Google Scholar]
- Johnson ML. The high bone mass family--the role of Wnt/Lrp5 signaling in the regulation of bone mass. J Musculoskelet Neuronal Interact 2004;4:135–138. [PubMed] [Google Scholar]
- Norvell SM, Alvarez M, Bidwell JP, Pavalko FM. Fluid shear stress induces beta-catenin signaling in osteoblasts. Calcif Tissue Int 2004;75:396–404. [DOI] [PubMed] [Google Scholar]
- Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 2006;281:31720–31728. [DOI] [PubMed] [Google Scholar]
- van der Heyden MA, Rook MB, Hermans MM, Rijksen G, Boonstra J, Defize LH et al. Identification of connexin43 as a functional target for Wnt signalling. J Cell Sci 1998;111:(Pt 12): 1741–1749. [DOI] [PubMed] [Google Scholar]
- Batra N, Burra S, Siller-Jackson AJ, Gu S, Xia X, Weber GF et al. Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci USA 2012;109:3359–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niger C, Luciotti MA, Buo AM, Hebert C, Ma V, Stains JP. The regulation of runt-related transcription factor 2 by fibroblast growth factor-2 and connexin43 requires the inositol polyphosphate/protein kinase Cdelta cascade. J Bone Miner Res 2013;28:1468–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen NR, Geist ST, Civitelli R, Steinberg TH. ATP- and gap junction-dependent intercellular calcium signaling in osteoblastic cells. J Cell Biol 1997;139:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo B, Lu XL, Costa KD, Xu Q, Guo XE. An ATP-dependent mechanism mediates intercellular calcium signaling in bone cell network under single cell nanoindentation. Cell Calcium 2010;47:234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XL, Huo B, Chiang V, Guo XE. Osteocytic network is more responsive in calcium signaling than osteoblastic network under fluid flow. J Bone Miner Res 2012;27:563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellowley CE, Li Z, Zhou Z, Jacobs CR, Donahue HJ. Functional gap junctions between osteocytic and osteoblastic cells. J Bone Miner Res 2000;15:209–217. [DOI] [PubMed] [Google Scholar]
- Ishihara Y, Sugawara Y, Kamioka H, Kawanabe N, Hayano S, Balam TA et al. Ex vivo real-time observation of Ca(2+) signaling in living bone in response to shear stress applied on the bone surface. Bone 2013;53:204–215. [DOI] [PubMed] [Google Scholar]
- Ishihara Y, Sugawara Y, Kamioka H, Kawanabe N, Kurosaka H, Naruse K et al. In situ imaging of the autonomous intracellular Ca(2+) oscillations of osteoblasts and osteocytes in bone. Bone 2012;50:842–852. [DOI] [PubMed] [Google Scholar]
- Li X, Liu C, Li P, Li S, Zhao Z, Chen Y et al. Connexin 43 is a potential regulator in fluid shear stress-induced signal transduction in osteocytes. J Orthop Res 2013; 31:1959–1965. [DOI] [PubMed] [Google Scholar]
- Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol Cell Physiol 2005;289:C633–C643. [DOI] [PubMed] [Google Scholar]
- Ruff CB, Hayes WC. Subperiosteal expansion and cortical remodeling of the human femur and tibia with aging. Science 1982;217:945–948. [DOI] [PubMed] [Google Scholar]
- Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int 2006;17:319–336. [DOI] [PubMed] [Google Scholar]
- Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone 2002;31:1–7. [DOI] [PubMed] [Google Scholar]
- Bivi N, Nelson MT, Faillace ME, Li J, Miller LM, Plotkin LI. Deletion of Cx43 from osteocytes results in defective bone material properties but does not decrease extrinsic strength in cortical bone. Calcif Tissue Int 2012;91:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauner M, Sipos W, Pietschmann P. Age-dependent Wnt gene expression in bone and during the course of osteoblast differentiation. Age (Dordr) 2008;30:273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Bryant HU, MacDougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest 2006;116:1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl KM, Jacobs CR, Turner RT, Evans GL, Farrell PA, Donahue HJ. Aged bone displays an increased responsiveness to low-intensity resistance exercise. J Appl Physiol 2001;90:1359–1364. [DOI] [PubMed] [Google Scholar]
- Leppanen OV, Sievanen H, Jokihaara J, Pajamaki I, Kannus P, Jarvinen TL. Pathogenesis of age-related osteoporosis: impaired mechano-responsiveness of bone is not the culprit. PloS one 2008;3:e2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab DM, Smith EL, Crenshaw TD, Thomas DP. Bone mechanical properties after exercise training in young and old rats. J Appl Physiol 1990;68:130–134. [DOI] [PubMed] [Google Scholar]
- Umemura Y, Ishiko T, Tsujimoto H, Miura H, Mokushi N, Suzuki H. Effects of jump training on bone hypertrophy in young and old rats. Int J Sports Med 1995;16:364–367. [DOI] [PubMed] [Google Scholar]
- Jarvinen TL, Pajamaki I, Sievanen H, Vuohelainen T, Tuukkanen J, Jarvinen M et al. Femoral neck response to exercise and subsequent deconditioning in young and adult rats. J Bone Miner Res 2003;18:1292–1299. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Brodt MD, Lynch MA, Stephens AL, Wood DJ, Civitelli R. Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice. PloS One 2012;7:e34980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. J Bone Miner Res 2010;25:2006–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi A, Watanabe H, Chiba M, Inaba Y. Effects of exercise at different ages on bone density and mechanical properties of femoral bone of aged mice. Tohoku J Exp Med 1998;185:15–24. [DOI] [PubMed] [Google Scholar]
- Silbermann M, Bar-Shira-Maymon B, Coleman R, Reznick A, Weisman Y, Steinhagen-Thiessen E et al. Long-term physical exercise retards trabecular bone loss in lumbar vertebrae of aging female mice. Calcif Tissue Int 1990;46:80–93. [DOI] [PubMed] [Google Scholar]
- Frost HM. Perspectives: a proposed general model of the ‘mechanostat' (suggestions from a new skeletal-biologic paradigm). Anat Rec 1996;244:139–147. [DOI] [PubMed] [Google Scholar]