Abstract
Although the large stress/heat shock proteins (HSPs), i.e., Hsp110 and Grp170, were identified over 30 years ago, these abundant and highly conserved molecules have received much less attention compared to other conventional HSPs. Large stress proteins act as molecular chaperones with exceptional protein-holding capability and prevent the aggregation of proteins induced by thermal stress. The chaperoning properties of Hsp110 and Grp170 are integral to the ability of these molecules to modulate immune functions and are essential for developing large chaperone complex vaccines for cancer immunotherapy. The potent antitumor activity of the Hsp110/Grp170-tumor protein antigen complexes, demonstrated in preclinical studies, has led to a phase I clinical trial through the National Cancer Institute's RAID Program that is presently underway. Here we review aspects of the structure and function of these large stress proteins, their roles as molecular chaperones in the biology of cell stress, and prospects for their use in immune regulation and cancer immunotherapy. Lastly, we will discuss the recently revealed immunosuppressive activity of scavenger receptor A that binds to Hsp110 and Grp170, as well as the feasibility of targeting this receptor to promote T-cell activation and antitumor immunity induced by large HSP vaccines and other immunotherapies.
Introduction
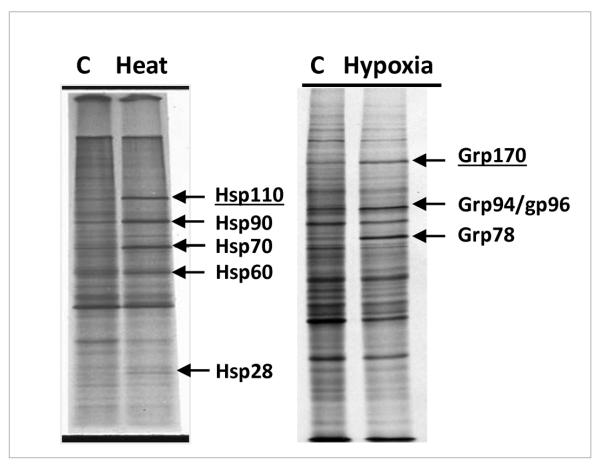
It is well understood that heat shock proteins (HSPs) are a set of highly conserved molecules that are highly inducible by various proteotoxic stresses, such as heat shock (reviewed in (1)). The mammalian HSPs are classified into several families of proteins based on their molecular weight, i.e., the Hsp25/Hsp27, the Hsp40, the Hsp60, the Hsp70, the Hsp90 and the Hsp110 (also called Hsp105) families (2). Analogous to these HSPs that are resident in the cytoplasm, nucleus, and mitochondria, there is a second set of the endoplasmic reticulum (ER)-resident stress proteins referred to as “glucose-regulated proteins” (GRPs) (3). The “GRP” nomenclature derives from the original finding that these molecules are induced by glucose deprivation (4, 5). The major proteins in this group include Grp78 (also called BiP), Grp94 (also called Gp96) and Grp170 (also called Orp150). While GRPs are functionally and structurally related to the HSPs, they are induced by different sets of stressors, such as chronic hypoxia, calcium ionophores, and inhibition of glycosylation (6–8) but not by heat shock. Indeed, induction of GRPs has been used as a marker for the `unfolded protein response' or UPR based on the disruption of ER homeostasis (9). Sequence related homologs of most, if not all, of these stress proteins are constitutively expressed in all living organisms and function as molecular chaperones that are key players in protein homeostasis, including protein synthesis and degradation, folding and assembling, preventing the irreversible aggregation of misfolded proteins and, in some instances, refolding denatured proteins (10–12). Figure 1 shows the effects of heat shock and chromic hypoxia on stress protein induction in Chinese hamster ovary (CHO) cells where the major HSPs and GRPs are evident.
Figure 1.
Induction of HSPs by heat shock and GRPs by chronic hypoxia in CHO cells. Protein synthesis was examined using 35S-methionine pulse assays, followed by SDS-Polyacrylamide gel electrophoresis and autoradiograph analysis. Left panel reproduced from Subjeck et al, British Journal of Cancer, Suppl 5, 1982. Right panel unpublished.
Hsp110
Some HSPs (e.g., Hsp70, Hsp90) have been intensely studied for over 30 years. While several studies also identified a HSP of approximately 110 kDa in the earliest studies of the mammalian heat shock response (13–18), it was largely ignored until the 1990s (19–21). Interestingly, some of the earliest studies of Hsp110 demonstrated that its expression in mammalian cells correlated with the expression of the highly protective phenomenon of thermotolerance in coordination with Hsp70 and Hsp90 (15, 16). A protective effect of a mild heat exposure to damage created by a later and more severe heat shock in non-mammalian and mammalian systems is a concept that goes back to the late 1950s and early 1960s, and includes protection from heat induced teratogenesis, inhibition of protein synthesis, as well as cell death (for a review of the early literature see (2)).
Hsp110 was finally cloned in our laboratory in the early 1990s (19). Subsequent sequence analysis combined with two homologous yeast sequences and the sea urchin egg receptor for sperm that were in the data base at that time showed that this limited group of genes represented a large and highly diverged set of Hsp70s. This contradicted a view at that time that the Hsp70 family was among the most highly conserved gene families in biology. It soon became evident that newly identified sequences from numerous organisms from yeast to fungi to plants also expressed a Hsp110-like protein, indicating that Hsp110 was representative of its own family of stress proteins (reviewed in (1)).
We then demonstrated that the overexpression of Hsp110 in Rat-1 and HeLa cells provided significantly greater cell survival to a severe heat shock compared to control cells (22), supporting the ability of Hsp110 to confer partial thermotolerance on its own as suggested in our initial correlative studies. It was suggested that this occurs due to the ability of Hsp110 to bind to proteins damaged by heat and prevent their irreversible aggregation (22). It was shown that Hsp110 was four times more efficient than Hsp70 in performing this important chaperoning function (22). In other words, it takes four Hsp70s to produce an equivalent protective effect as does one Hsp110. This property was later used to create `chaperone vaccines' for the treatment of cancer (discussed below).
Grp170 and identification of the Hsp70 superfamily
An early study of GRP induction by glucose starvation indicated an obvious GRP of approximately 170 kDa (6). Studies of additional potential inducing conditions soon identified chronic hypoxia as a physiologically important inducer of GRPs, including Grp170 (7, 23–26). Since an oxidative environment is required for the formation of disulfide bonds involved in protein folding in the ER, the removal of oxygen may be interpreted as a reductive stress interfering with this process. In these hypoxia studies it was also observed that reoxygenation of hypoxic cells resulted in the repression of GRP induction, as expected, but surprisingly also lead to the induction of HSPs. This observation forms a basis for the concept that reperfusion of hypoxic tissues can lead to a heat shock response and tissue damage, a concept central to many areas of medical research as discussed briefly below. Induction of HSPs during conventional fractionated radiation therapy due to reoxygenation is also a possibility and could conceivably inhibit tumor response to hyperthermia.
Years later, almost in parallel with the cloning of Hsp110, we undertook the cloning of Grp170. Analysis of the Grp170 sequence together with one related sequence then available in the database, an open reading frame from C. elegans, clearly indicated that Grp170 also represented a new stress protein family that was distantly related to, but different from, both Hsp70 and Hsp110 families (20). The cloning of Hsp110 and Grp170 therefore revealed a new and unexpected view of the Hsp70 gene family. We named these distantly related stress protein families, i.e. the Hsp70, Hsp110 and Grp170 families, the `Hsp70 Super-Family' (1). Later studies using numerous sequences that became available in the database further emphasized our conclusions regarding the interrelationships between these three families (1, 27).
In parallel to the protective properties of HSPs, we believed that GRPs, including Grp170, also possessed protective functions, perhaps protecting cells from damaging effects of long term hypoxia and ischemia. Indeed, our early studies showed that samples from nectrotic regions of mouse tumors expressed high (or induced) levels of GRPs. Unexpectedly, this necrotic material, which was presumed to be largely non-viable, was found to be highly clonogenic. While the clonogenicity of cells from necrotic material was less than that obtained from histologically viable tumor regions (that did not show induced levels of GRPs), the differences were small (24). More direct support for a role for GRPs in protection came from the observation that a cell line expressing anti-sense Grp78 exhibited reduced cell viability when exposed to long hypoxic exposures (28). Further, cultured neurons that overexpress Grp170 have also been shown to be resistant to hypoxic stress, whereas astrocytes with reduced Grp170 expression levels are more vulnerable (29). It was recently shown that upregulation of Grp170 effectively ameliorates hepatic ER stress and hypercholesterolemia-related liver damage (30), which underscores the functional significance of these large stress proteins in cellular protection during stress responses. The induction of GRPs, including Grp170, by conditions associated with the ischemic state, such as glucose starvation, low pH, and hypoxia, suggests important roles for these proteins in several areas of pathophysiology, e.g., myocardial infarction and stroke as well as tumor resistance to ischemic conditions and chemotherapeutic agents (26, 31, 32). Protective functions of GRPs to ischemic/reperfusion injury is a current major area of investigation (e.g. (33, 34) among numerous other excellent studies).
Grp170 associates with the other major GRPs in the ER and interacts with immunoglobulin chains (8, 35), implicating its potential involvement in folding/assembly of secretory proteins in concert with other ER chaperones. Interestingly, Grp170 was reported to be the most efficient ATP-binding protein in a microsomal extract (36), and to assist polypeptide translocation into the ER (37). Indeed, yeast Grp170 (Lhs1; Lumenal hsp seventy) is involved in signal recognition particle (SRP)-independent post-translational translocation of proteins into the ER (27, 38, 39). These studies provide supporting evidence that Grp170 may participate in polypeptide transport into the ER, which is pre-requisite not only for protein modification, but also for loading of peptide onto major histocompatibility complex (MHC) molecules for antigen presentation.
Chaperoning activity of Hsp110/Grp170
Hsp110 and Grp170 are significantly more effective in inhibiting the denaturation of heat damaged proteins than other stress proteins. Figure 2 shows the ability of Hsp110, Grp170, Hsc70 and Grp94 (Gp96) to prevent heat induced aggregation of luciferase, a reporter protein used for this purpose. It is seen that at a 1:1 molar ratio that Hsp110 or Grp170 are nearly totally effective in inhibiting heat induced aggregation. This is accomplished through the formation of chaperone complexes as discussed below.
Figure 2.
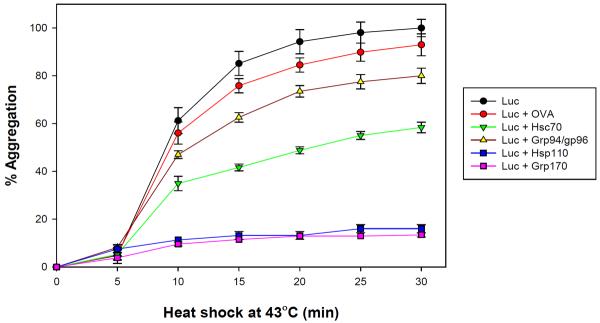
The chaperoning effects of various stress proteins as indicated by their ability to bind to and inhibit the heat induced aggregation of luciferase (Luc). It is seen that Hsp110 and Grp170 are almost totally effective at a 1:1 molar ratio in inhibiting Luc aggregation. Aggregation is determined by light scattering.
Predicted secondary structural models of Hsp110 and Grp170 (1, 40, 41) were generated based on the crystallographic structure of DnaK, the E. coli homolog of Hsp70 (42). The overall organization of Hsp110 and Grp170 appear similar to that of Hsp70/DnaK with the strongest sequence homology occurring in the N-terminal ATP binding domain with very little similarity observed in C-terminal regions (20, 27). These C-terminal domains are significantly larger in Hsp110 and Grp170 and account for much of the increase in size of these proteins. There are two highly conserved motifs in these enlarged regions of both Hsp110 and Grp170. These motifs can be found in many other proteins in the database such as malarial cell surface protein and viral poly proteins (reviewed in (1)). The purpose of these motifs is unknown. That both Hsp110 and Grp170 are significantly more efficient than Hsp70/Hsc70 in stabilizing heat-denatured protein (22, 41, 43–45) may be attributed to the large size of these two chaperones caused by the expansion of the C-terminal domains of each.
The regulatory mechanism for chaperoning activity of Hsp70 involves ATP binding and hydrolysis (42). While Grp170 binds ATP readily, Hsp110 displays a very weak ATP binding ability (20, 40). Neither Hsp110 nor Grp170 requires ATP for stabilizing unfolded protein in vitro and both chaperones require rabbit reticulocyte lysate (RRL) to promote refolding of the bound protein (22, 41). Similar results have been observed in yeast Hsp110 (referred to as Sse1) (46), suggesting that this chaperoning mechanism for these large HSPs may differ from that of Hsp70, where large HSPs can hold client protein in a folding competent state for subsequent refolding by other chaperones (specifically Hsp70).
It has long been recognized that Hsp110 exists in large multi-protein complexes that contain Hsp70/Hsc70 (22, 47, 48), suggesting a potential collaborative action of molecular chaperones in the maintenance of protein homeostasis. Hsp110's ability to function cooperatively with Hsc70 and Hdj-1 (a DnaJ homolog) in the folding of luciferase also supports interactions between chaperone molecules (22). Similar observations were made in yeast, where Hsp110 Sse1 forms heterodimeric complexes with Hsp70s Ssa and Ssb, and regulates Hsp70-substrate interactions (49, 50). These studies support the notion that the Hsp110 participates in many processes associated with Hsp70, and operates in concert with Hsp70 in cellular functions. Other evidence also comes from recent studies showing that Hsp110, Hsp70 and Hsp40 constitute a disaggregase machinery, and efficiently disaggregate and refold aggregates of chemically or thermally denatured protein in vitro and in cell extracts, emphasizing a crucial role of Hsp110 in counteracting protein unfolding stress (51, 52).
A recent study showed that the deficiency of the Hsp110 impairs the refolding of heat-denatured luciferase in mammalian cells, and the Hsp110-deficient cells appear to be more sensitive to various stresses. In contrast, the overexpression of Hsp110 enhances the recovery of heat-inactivated luciferase (53). Similarly, the expression of Hsp110 suppresses the protein aggregation and apoptosis induced by expanded polyQ tract (54). Intriguingly, Hsp110 facilitates cystic fibrosis transmembrane conductance regulator (CFTR) quality control at an early stage in its biosynthesis but promotes CFTR post-translational folding (55). Thus, Hsp110 plays a dynamic role in folding of newly synthesized protein, the refolding of denatured proteins, and protection against stress-induced cell death.
Protein folding by Hsp70 is tightly controlled by co-chaperones, including J-domain proteins that trigger ATP hydrolysis and nucleotide exchange factors (NEFs) that remove ADP from Hsp70. Recent studies revealed that Hsp110 (56–59) and Grp170 (60–62) can function as NEFs for Hsp70 or Grp78, respectively, and are important components of the eukaryotic Hsp70s machinery of protein folding. In light of the fact that Hsp110 and Grp170 exhibit much more efficient activity in holding and preventing aggregation of denatured proteins compared to Hsp70/Hsc70, it is reasonable to believe that the function of Hsp110 or Grp170 extends beyond acting as NEFs. A recent report showed that Hsp110 has a strong preference for aromatic residues in their substrates in contrast to Hsp70 that prefers aliphatic residues, and Hsp110 binds peptide substrates with exceptionally fast kinetics (63). It is possible that these large HSPs serve dual roles as chaperones of their own and as co-chaperones or NEFs for Hsp70s to assist protein folding in a cooperative process (64). So far, the relative contribution of these two functions to protein folding and translocation remains less defined.
In addition to assisting with protein folding, Hsp110 may have in vivo RNA chaperoning properties, regulating mRNA degradation and/or translation of lymphokine and other short-lived messages (65). Recently, we made a striking finding that extracellular Grp170 is highly efficient in binding CpG oligodeoxynucleotides (CpG-ODN), the microbial DNA mimetic sensed by toll-like receptor 9 (TLR9). This interaction potentiates the internalization of CpG-ODN by macrophages, resulting in the synergistic activation of the MyD88-dependent signaling. This CpG-ODN chaperone complex-promoted innate immunity confers increased resistance in Listeria monocytogenes. This work demonstrates not only the ability of Grp170 to facilitate the sensing of pathogen-associated “danger” signals by intracellular receptors, but also the dynamics of ancient chaperoning functions inside and outside the cell (66).
Hsp110/Grp170-based chaperone complex vaccines
In the later 1990s, with our experience in working with Hsp110 and Grp170, we turned our attention to a potential cancer related application for these stress proteins. Extensive studies over the last 20 years support a concept that tumor-derived HSP/GRPs are associated with the individually distinct array of antigenic peptides and can induce tumor-specific immune response (67–75). Although peptide interactions with molecular chaperones in vivo remain less defined (76, 77), the antitumor response generated by autologous tumor-derived HSP/GRPs (e.g., Hsp70, Hsp90, Grp94/gp96, and calreticulin) have been well documented (67, 78–80). Our studies also demonstrate that animals immunized with Hsp110 or Grp170 purified from various murine tumors (methylcholanthrene-induced fibrosarcoma, colon carcinoma, and melanoma) develop a robust antitumor immune response (81, 82). An interesting observation was made in our studies that Hsp70 and Hsp110, when purified from tumors obtained from hyperthermia treated mice, were more effective in inhibiting tumor growth compared to the same HSPs purified from untreated animals (81).
It has been well documented that a broad spectrum of cancers display increased expression of HSP/GRPs, possibly induced by the high levels of mutated and misfolded oncoproteins (83–86). The upregulation of HSP/GRPs in cancer cells may be required to chaperone these molecules essential for tumorigenesis. Therefore, HSP/GRP preparations from tumors are expected to carry tumor antigens and offer a personalized, polyvalent vaccine therapy. In addition to tumor-specific antigenic peptides, it is also anticipated that stress proteins like Hsp110 and Grp170 that possess the superior capacity to bind client proteins, would naturally associate in situ with tumor protein antigens. We transfected mouse colon cancer cells with a secretory form of Grp170 (discussed below) and purified Grp170 from the supernatants. Proteomic studies showed that the secreted Grp170 was associated with tumor protein antigen carried by the tumor model (87), suggesting that large stress proteins may interact with endogenous antigens and participate in the antigen-processing pathways. It is also supported by the finding that Grp170 is involved in polypeptide transportation into the ER through interaction with transporter-associated with antigen processing (TAP) (36, 37). Our findings are consistent with the recent reports that endogenous Hsp90 binds to truncated protein antigens or polypeptides (88, 89). Therefore, in addition to HSP/GRP-peptide vaccines, HSP/GRP-protein interactions can also occur inside the cell and possess anti-cancer vaccine activity.
Early phase trials of an autologous HSP/GRP vaccine, Oncophage (also known as gp96 or Grp94) showed the positive results in metastatic colorectal carcinoma (74, 90), metastatic melanoma (91, 92), non-Hodgkin Lymphoma (93), renal cell carcinoma (94, 95). However, the phase III trial failed to demonstrate survival benefits in Stage IV melanoma patients (92). In the introspective analysis, an overall survival benefit is seen within the early stage IV melanoma patients (M1a, distant skin, subcutaneous or nodal metastasis; M1b, lung metastasis) (92).
The clinical use of autologous HSP/GRP vaccines is often limited by requirement for a patient specimen and complex procedures of vaccine preparation (96, 97). As a result, some patients are unable to participate in this autologous vaccine trial (98). The lack of information on targeted antigens also limits immune monitoring. Taking advantage of the exceptional protein-holding capacity of Hsp110 and Grp170 as described above (22, 41), we developed a recombinant chaperone vaccine by reconstituting Hsp110/Grp170-tumor protein antigen complexes under heat shock conditions in vitro (43, 99–101). We show that complexes of Hsp110 and the intracellular domain (ICD) of human epidermal growth factor receptor 2 protein (HER-2)/neu elicited ICD-specific CD8+ and CD4+ T cell responses (99). This Hsp110-ICD vaccine was able to significantly delay or inhibit mammary tumor development in FVB-neu (FVBN202) transgenic mice (100), a high bar for evaluation of vaccine activity. Similar antitumor efficacy was seen in a parallel study, in which mice with established B16 melanoma were treated with the Hsp110 complexed with melanoma-associated antigen gp100. The immunogenicity of this chaperone complex is much higher than the Hsp110-native antigen mixture, and Hsp110 is more effective as an adjuvant in promoting antitumor immunity than is Complete Freud's Adjuvant (CFA) (43). Hsp110 complexed with Carbonic anhydrase IX (CA9) protein, a renal cell carcinoma (RCC) antigen, also generates a potent antitumor response against mouse RENCA tumors (102, 103). Like Hsp110, the reconstituted Grp170-gp100 complex exhibits similar vaccine potency against aggressive, poorly immunogenic B16 melanoma (101). This recombinant Grp170-based chaperone vaccine approach has also been validated by others in vaccinations targeting different tumor-associated antigens (104, 105).
Since these large chaperones efficiently complex with protein antigens at a 1:1 molar ratio, a highly concentrated recombinant HSP vaccine can be prepared. The protein antigen contains a large reservoir of potential peptides, which not only can stimulate polyepitope-directed T and B cells, but also can help circumvent HLA restriction. Since it does not require tumor specimens for vaccine production, this approach may be used as adjuvant therapy for patients with completely resected disease or those at high risk for cancer or for recurrence of cancer. Using well-defined antigens as opposed to whole tumor cells or lysates also facilitates immune monitoring in the clinic. Importantly, this synthetic approach can serve as a model to develop vaccines against many different antigen targets, either alone or in combination. We demonstrate that Hsp110-gp100 complex vaccine, when used in combination with Hsp110-Trp2 (i.e. tyrosinase-related protein 2) complex vaccine, significantly improved antitumor efficacy compared to either of the single vaccine alone (45). This `piggy backing' of additional melanoma proteins offers the opportunity to generate highly powerful multivalent anti-melanoma vaccines in a straight forward manner. We also show that Hsp110 or Grp170 readily forms complexes with antigenic peptides (e.g., TRP2175–192), and enhances CD8+ T-cells reactive with this MHC class I-restricted epitope. However, the protective antitumor effect elicited by the TRP2175–192 peptide vaccine is much weaker than that achieved by full-length TRP2 protein antigen chaperoned by Grp170 (45). These studies reinforce the concept of delivering tumor protein antigens using Hsp110 or Grp170 to augment multivalent T cell responses for tumor eradication. As a result, a phase I clinical trial of recombinant Hsp110-gp100 chaperone vaccine for treatment of human melanoma is underway at the Roswell Park Cancer Institute in collaboration with the National Cancer Institute.
Basis for Hsp110/Grp170 vaccine-promoted immune activation
One of key features of HSP/GRP function in promoting immune activation is their capability to shuttle and deliver antigens into the endogenous antigen-processing pathway of professional antigen-presenting cells (APCs) for cross-presentation (106–108). Capture of HSP-antigen complexes by APCs, such as dendritic cells (DCs), via receptor-mediated endocytosis appears to be essential for HSP-facilitated antigen cross-presentation (109–112). Search for receptors that mediate this process has not revealed a dedicated HSP/GRP receptor. Instead, several receptors that display a broad ligand binding specificity, e.g., CD91, scavenger receptor class A (SRA), LOX-1, and SRECI, have been identified (113–117), discussed in more detail below). In addition to enhancing antigen delivery and processing, it has been shown that APC-HSP/GRP interaction can promote phenotypic and functional maturation of APCs (118–122). Despite the controversy over the intrinsic stimulatory effect of stress proteins (123), an overwhelming amount of evidence indicates that upon release from injured or stressed cells, they can serve as immunological “danger signals” in the extracellular environment (124, 125). Recent studies also support the notion that Hsp70 triggers innate immunity through TLR signaling in vivo (126, 127). Hsp110 and Grp170 have been shown to induce upregulation of MHC class II, co-stimulatory molecules (CD40, CD86), and pro-inflammatory cytokines (IL-6, IL-12 and TNF-α) in DCs (44, 128, 129).
Effective vaccine function of Hsp110 or Grp170 requires three separate steps: 1) the ability to bind to a tumor protein antigen, 2) the ability to bind to receptors on APCs, and 3) the ability to cross-present antigen and initiate an antigen-specific immune response. We hypothesized that different regions of these proteins were responsible for each of these separate functions. We had already defined the contributions to chaperoning for each of the structural domains for both Hsp110 and Grp170 (40, 41), and selected Grp170 for this analysis since it has two separate, non-overlapping chaperoning domains. We examined which of the deletion mutants containing one or both or neither of the chaperoning domains was capable of binding to receptors on APCs. It was observed that only mutants with chaperone function exhibited APC binding properties. We then examined the immunological activities of these domain deletion mutants using melanoma antigen gp100 and the B16 melanoma model (101). All chaperoning competent mutants were able to generate a potent, antigen-specific antitumor immune response. Further, immunization with gp100 mixed with non-chaperone competent mutants showed no tumor-inhibitory activity. Interestingly, two of the Grp170 mutants contained no overlapping sequences, although both acted as molecular chaperones that avidly bound to heated gp100. Both bound to receptors on APCs and both were able to stimulate gp100-specific T cells and inhibit tumor growth. This indicates that it is not a specific domain or sequence that is responsible for these individual properties, but simply the ability to act as a molecular chaperone. Thus, we proposed that the ancient property of molecular chaperoning is the major common denominator underlying the immunoregulatory effects of Grp170 and most likely other HSPs as well (Figure 3).
Figure 3.
A schematic indicating the central role of molecular chaperoning by large stress proteins in many cellular functions, including thermotolerance and immune modulation, which are discussed in this report.
Modulation of tumor immunogenicity by Hsp110 and Grp170
Despite a cellular protective effect of Hsp110 to heat shock, CT26 colorectal tumor cells stably transfected with Hsp110 (CT26-Hsp110) show increased immunogenicity in vivo and administration of irradiated CT26-Hsp110 cells to mice results in an antitumor immune response (130), suggesting that manipulation of Hsp110 expression in tumor cells can facilitate immune recognition of tumor antigens that otherwise remain immunologically silent. It is also possible that Hsp110 induction or overexpression in cancer cells increases endogenous processing and presentation of tumor antigens. Interestingly, the Hsp110 isolated from thermally stressed tumor cells is more efficient in priming an antitumor immune response than those from untreated cells, suggesting that heat shock may alter antigenic profiles associated with autologous Hsp110 (81). Hsp110 itself could also serve as a potential tumor antigen since it is often overexpressed in various cancers and correlates with the stage of diseases (131–133). Indeed, an Hsp110 DNA vaccine inhibits both CT26 colorectal cancer and B16 melanoma in mice, accompanied with activation of Hsp110-specific CD4+ and CD8+ T cells (134). It should be noted that in our studies mice treated with recombinant Hsp110 chaperone complex vaccine only develop a robust immune response against associated protein antigen, not Hsp110 itself (99).
Genetic modification of poorly immunogenic murine B16 melanoma (44), TRAMP-C2 prostate cancer (135) or CT26 colorectal cancer (87) to express a secretable form of Grp170 (sGrp170) by removing its ER-retention sequence “KNDEL” results in significant suppression of tumor growth in vivo, which is associated with increased tumor-infiltrating of CD8+ T-cells. Although the Grp170-modified B16 tumor cells (B16-sgrp170) show no changes in their transformation phenotypes and growth rate in culture, these cells stimulate the production of proinflammatory cytokines by DCs (44), implying that released Grp170 may be involved in stimulation of DCs. Our studies show that natural killer (NK) cells are responsible for elimination of viable B16-sgrp170 tumor cells inoculated into mice, whereas both NK cells and CTLs are required for a long-term protection from secondary challenge with wild-type B16 tumors (44). Furthermore, these Grp170-secreting tumor cells, when used as a cell-based vaccine, exhibit therapeutic efficacy in treatment of mice with established tumors (44, 135). In this context, Grp170 appears to trigger tumor immunity via its ability to chaperone and deliver intracellular tumor antigens (44). These observations support the idea of manipulating cellular compartmentalization of Grp170 to restore immune recognition and protective tumor immunity.
Given a large repertoire of undefined tumor-associated antigens in cancer cells, Grp170 can also be exploited to improve molecular-targeted therapy by promoting tumor immunogenic death. We demonstrate that intratumoral administration of a nonreplicating adenovirus encoding Grp170 (Ad.sgrp170) enhances the antitumor efficacy of melanoma differentiation-associated gene-7 (mda-7), a cancer-specific, apoptosis-inducing gene with broad-spectrum antitumor activity (136, 137). This combinatorial treatment generates systemic antitumor immunity, indicated by an increased antigen-/tumor-specific CTL response, enhanced protection against subsequent tumor challenge, and improved control of distant tumors. These data provide rationale for optimizing tumor cytotoxic therapy by concurrently targeting the immune environment using Grp170 as novel immune modulators, which may help achieve more efficient tumor eradication and prevention of tumor recurrence.
Tumor escape may result from loss of immunogenicity of cancer cells and/or the establishment of an immunosuppressive state within the tumor microenvironment (TME) (138, 139). We recently showed that strategic incorporation of a pathogen-derived `danger' signal into the Grp170 sequence markedly enhances its potency in driving and/or restoring antitumor immunity in the TME (140). This chimeric chaperone created by fusing Grp170 with the defined NF-κB-activating domain of Flagellin (141), termed Flagrp170, not only maintains highly efficient antigen-holding ability, but also possesses a strong capability to activate DCs. Intratumoral administration of adenoviruses expressing Flagrp170 induces a superior antitumor response against B16 melanoma and its distant lung metastasis compared to either unmodified Grp170 or Flagellin. The enhanced tumor destruction is accompanied with significantly increased tumor infiltration by CD8+ cells as well as elevation of IFN-γ and IL-12 levels in the tumor sites. The mechanistic studies reveal that CD8+ DCs are required for the improved T-cell cross-priming. Studies are under way to define the molecular actions of this chimeric chaperone in subverting tumor-associated immunosuppressive mechanisms and promoting highly immunostimulatory presentation of tumor antigens in the TME (140).
Interestingly, certain HSPs, such as Hsp60 and Hsp70, have been reported to display immunoregulatory effects and induce anti-inflammatory, immunosuppressive Foxp3+ regulatory T cells (Treg) (142–145). It is possible that the immunostimulatory or immunoregulatory consequences induced by these HSPs are context dependent or may be determined by the conditions in which the HSPs are presented to the immune system. Whether these large HSP/GRPs may also stimulate Treg in the setting of therapeutic vaccination against tumor antigens is not clear, and needs to be examined in the future studies.
Hsp110 and Grp170-binding receptors and vaccine design
Scavenger receptors are a major set of receptors identified to mediate the binding and internalization of HSP/GRPs by APCs (115–117, 122, 127, 146). We showed that SRA, which is primarily expressed on myeloid DCs and macrophages, binds Hsp110 and Grp170 (147). As a prototype scavenger receptor, SRA is known to bind a broad range of polyanionic ligands, including oxidized lipoproteins, pathogen-associated molecules, and apoptotic cells. The observation of SRA interaction with Hsp110/Grp170 prompted us to investigate the effect of SRA on activities of these large stress protein vaccines in vivo. It was anticipated that removal of a receptor of these stress proteins would result in a partial reduction in immune activity. Surprisingly, genetic ablation of SRA was found to strongly enhance antitumor immune responses generated by vaccination with autologous tumor-derived Grp170 or recombinant Grp170-gp100 chaperone complex (148). SRA absence also improves the therapeutic efficacy of the Hsp110- or Grp170-gp100 vaccines in mice established with B16 melanoma (149). In addition, the lack of SRA restores the immune recognition of several poorly immunogenic mouse tumors, which is mediated by CD8+ CTLs and APCs with phagocytic activity (148). The enhanced antitumor immunity was similarly seen in SRA-deficient mice treated with vaccines formulated using monophosphoryl lipid A (MPL), a detoxified form of Lipopolysaccharide (LPS) that selectively activates the toll-like receptor 4 (TLR4) signaling pathway (150). In addition to suppressing the activation of CTLs, the presence of SRA inhibits MPL-induced T-helper 1 (Th1) response, indicated by enhanced production of IFN-γ, not IL-4 or IL-17, by antigen-specific CD4+ T cells in SRA-deficient mice (151). These findings suggest that SRA dampens the immunostimulatory activities of adjuvants of both foreign- or self-origins. Indeed, our recent studies of concanavalin A-induced hepatitis demonstrate that SRA loss increased mortality and liver pathology correlates with excessive production of IFN-γ and heightened activation of T cells (152), underscoring the role of SRA as an immunoregulator that may promote tolerance.
The presence of SRA reduces the immunostimulatory activity of DCs by decreasing expression of co-stimulatory molecules and production of proinflammatory cytokines or chemokines, which results in reduced antigen cross-presentation and attenuation of CTL response to tumors (149, 150, 153). Mechanistic studies reveal that SRA suppresses TLR4 ligation-induced NF-κB activation in DCs by interfering with the trimerization and ubiquitination of TNF receptor-associated factor 6 (154). NF-κB activation has been shown to be essential for the functional activation of DCs (155), and TLR4 signaling plays a pivotal role in the cross-presentation of tumor cell-associated antigens by DCs following cancer therapies, e.g., radiotherapy or chemotherapy (156).
The immunosuppressive activity of SRA in DC functions makes it an appealing target for cancer immunotherapy. Our proof-of-principle studies demonstrate that downregulation of SRA in DCs enhances the immunostimulatory activity of a DC vaccine (157). We show that SRA-silenced DCs carrying melanoma antigen gp100 are more efficient than controls in mobilizing antigen-specific CTLs, leading to improved control of established melanoma and its metastases. This enhanced antitumor efficacy correlates positively with greater tumor-infiltrating CD8+ T cells and NK cells, as well as increased intratumoral ratios of effector T cells to regulatory T cells (157). In addition, in situ administration of SRA-silenced DCs enhances the effectiveness of local radiotherapy by provoking a robust systemic antitumor immunity (158). SRA downregulation also profoundly increases the immunogenicity of DCs exposed to the Hsp110-gp100 complex vaccine (Qian et al., unpublished data). These promising results provide a rationale for developing novel SRA-targeting approaches to improve the antitumor potency of immunotherapy, including large HSP-based vaccines.
Summary
The large stress proteins, Hsp110 and Grp170, exhibit structural similarities as well as differences from Hsp70. Early studies suggest an important role for these large stress proteins in collaboration with smaller HSPs and GRPs in protection from heat shock and chronic ischemia. The most distinctive feature of these large stress proteins is their superior ability to hold denatured protein, which represents a crucial factor involved in their immunological functions and serves as an important basis for developing recombinant chaperone complex vaccines for potential treatment of cancer and infectious diseases. Further studies are needed to better understand the activities of these conserved large chaperones in biological and immunological responses, which should provide opportunities to design a new generation of large HSP/GRP-targeting approaches to enhancing immune-mediated tumor control. It is conceivable that Hsp110/Grp170-based vaccines for immunotherapy may be rationally combined with other cancer treatments (e.g., radiotherapy, chemotherapy) to achieve an improved, multivalent antitumor response. Other strategies to overcome cancer-induced immunosuppressive mechanisms should also be considered to enhance therapeutic outcomes.
Lastly, the potential involvement of multiple HSP/GRP-binding scavenger receptors and their distinct effects on Hsp110/Grp170 vaccine-induced immune responses reflects the complexity of the immune system and its regulatory mechanisms. In addition to the immune suppressive properties of SRA, other scavenger receptors, i.e., LOX-1 and SRECI, which are also known to bind to these large chaperones and have been reported to mediate Hsp70-assisted antigen cross-presentation, should be investigated in the future (115, 127, 159). It is possible that different scavenger receptors have different immune regulatory functions. Nonetheless, identification of SRA as an immune suppressor provides a novel target for intervention to optimize the current large chaperone complex vaccines and other cancer immunotherapies.
Acknowledgements
We are both greatly appreciative of the many colleagues, faculty and students that we had the privilege and enjoyment of interacting with in our laboratories over many years and who contributed significantly to many of the discussions presented in this work. We also would like to thank Dr. Stuart Calderwood and Dr. Michael Graner for helpful discussion and critical reading of the manuscript.
The work was supported in part by NIH research Grants (CA129111, CA154708, and CA099326), DOD/CDMRP W81XWH-10-PCRP-SIDA, and Harrison Scholarship.
Footnotes
Parts of this report were presented as the Twenty-Third Annual Robinson Award Lecture of the Society for Thermal Medicine, Portland, Oregon, April, 2012.
Declarations of interest: The authors report no declarations of interest.
References
- 1.Easton DP, Kaneko Y, Subjeck JR. The hsp110 and Grp1 70 stress proteins: newly recognized relatives of the Hsp70s. Cell Stress Chaperones. 2000;5(4):276–90. doi: 10.1379/1466-1268(2000)005<0276:thagsp>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subjeck JR, Shyy TT. Stress protein systems of mammalian cells. Am J Physiol. 1986;250(1 Pt 1):C1–17. doi: 10.1152/ajpcell.1986.250.1.C1. [DOI] [PubMed] [Google Scholar]
- 3.Lee AS. Mammalian stress response: induction of the glucose-regulated protein family. Curr Opin Cell Biol. 1992;4(2):267–73. doi: 10.1016/0955-0674(92)90042-b. Epub 1992/04/01. [DOI] [PubMed] [Google Scholar]
- 4.Shiu RP, Pouyssegur J, Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977;74(9):3840–4. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pouyssegur J, Shiu RP, Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977;11(4):941–7. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- 6.Sciandra JJ, Subjeck JR. The effects of glucose on protein synthesis and thermosensitivity in Chinese hamster ovary cells. J Biol Chem. 1983;258(20):12091–3. [PubMed] [Google Scholar]
- 7.Sciandra JJ, Subjeck JR, Hughes CS. Induction of glucose-regulated proteins during anaerobic exposure and of heat-shock proteins after reoxygenation. Proc Natl Acad Sci U S A. 1984;81(15):4843–7. doi: 10.1073/pnas.81.15.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin HY, Masso-Welch P, Di YP, Cai JW, Shen JW, Subjeck JR. The 170-kDa glucose-regulated stress protein is an endoplasmic reticulum protein that binds immunoglobulin. Mol Biol Cell. 1993;4(11):1109–19. doi: 10.1091/mbc.4.11.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13(10):1211–33. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 10.Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 11.Craig EA, Gambill BD, Nelson RJ. Heat shock proteins: molecular chaperones of protein biogenesis. Microbiol Rev. 1993;57(2):402–14. doi: 10.1128/mr.57.2.402-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381(6583):571–9. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 13.Levinson W, Oppermann H, Jackson J. Transition series metals and sulfhydryl reagents induce the synthesis of four proteins in eukaryotic cells. Biochim Biophys Acta. 1980;606(1):170–80. doi: 10.1016/0005-2787(80)90108-2. [DOI] [PubMed] [Google Scholar]
- 14.Hightower LE. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol. 1980;102(3):407–27. doi: 10.1002/jcp.1041020315. [DOI] [PubMed] [Google Scholar]
- 15.Subjeck JR, Sciandra JJ, Johnson RJ. Heat shock proteins and thermotolerance; a comparison of induction kinetics. Br J Radiol. 1982;55(656):579–84. doi: 10.1259/0007-1285-55-656-579. [DOI] [PubMed] [Google Scholar]
- 16.Landry J, Bernier D, Chretien P, Nicole LM, Tanguay RM, Marceau N. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 1982;42(6):2457–61. [PubMed] [Google Scholar]
- 17.Subjeck JR, Sciandra JJ, Chao CF, Johnson RJ. Heat shock proteins and biological response to hyperthermia. Br J Cancer Suppl. 1982;45(5):127–31. [PMC free article] [PubMed] [Google Scholar]
- 18.Tomasovic SP, Steck PA, Heitzman D. Heat-stress proteins and thermal resistance in rat mammary tumor cells. Radiat Res. 1983;95(2):399–413. [PubMed] [Google Scholar]
- 19.Lee-Yoon D, Easton D, Murawski M, Burd R, Subjeck JR. Identification of a major subfamily of large hsp70-like proteins through the cloning of the mammalian 110-kDa heat shock protein. J Biol Chem. 1995;270(26):15725–33. doi: 10.1074/jbc.270.26.15725. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Easton D, Oh HJ, Lee-Yoon DS, Liu X, Subjeck J. The 170 kDa glucose regulated stress protein is a large HSP70-, HSP110-like protein of the endoplasmic reticulum. FEBS Lett. 1996;380(1–2):68–72. doi: 10.1016/0014-5793(96)00011-7. [DOI] [PubMed] [Google Scholar]
- 21.Hylander BL, Chen X, Graf PC, Subjeck JR. The distribution and localization of hsp110 in brain. Brain Res. 2000;869(1–2):49–55. doi: 10.1016/s0006-8993(00)02346-5. [DOI] [PubMed] [Google Scholar]
- 22.Oh HJ, Chen X, Subjeck JR. Hsp110 protects heat-denatured proteins and confers cellular thermoresistance. J Biol Chem. 1997;272(50):31636–40. doi: 10.1074/jbc.272.50.31636. [DOI] [PubMed] [Google Scholar]
- 23.Whelan SA, Hightower LE. Differential induction of glucose-regulated and heat shock proteins: effects of pH and sulfhydryl-reducing agents on chicken embryo cells. J Cell Physiol. 1985;125(2):251–8. doi: 10.1002/jcp.1041250212. [DOI] [PubMed] [Google Scholar]
- 24.Cai JW, Henderson BW, Shen JW, Subjeck JR. Induction of glucose regulated proteins during growth of a murine tumor. J Cell Physiol. 1993;154(2):229–37. doi: 10.1002/jcp.1041540204. [DOI] [PubMed] [Google Scholar]
- 25.Kuwabara K, Matsumoto M, Ikeda J, Hori O, Ogawa S, Maeda Y, et al. Purification and characterization of a novel stress protein, the 150-kDa oxygen-regulated protein (ORP150), from cultured rat astrocytes and its expression in ischemic mouse brain. J Biol Chem. 1996;271(9):5025–32. doi: 10.1074/jbc.271.9.5025. [DOI] [PubMed] [Google Scholar]
- 26.Shen J, Hughes C, Chao C, Cai J, Bartels C, Gessner T, et al. Coinduction of glucose-regulated proteins and doxorubicin resistance in Chinese hamster cells. Proc Natl Acad Sci U S A. 1987;84(10):3278–82. doi: 10.1073/pnas.84.10.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craven RA, Tyson JR, Colin J, Stirling CJ. A novel subfamily of Hsp70s in the endoplasmic reticulum. Trends in Cell Biology. 1997;7(7):277–82. doi: 10.1016/S0962-8924(97)01079-9. [DOI] [PubMed] [Google Scholar]
- 28.Koong AC, Chen EY, Lee AS, Brown JM, Giaccia AJ. Increased cytotoxicity of chronic hypoxic cells by molecular inhibition of GRP78 induction. Int J Radiat Oncol Biol Phys. 1994;28(3):661–6. doi: 10.1016/0360-3016(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 29.Tamatani M, Matsuyama T, Yamaguchi A, Mitsuda N, Tsukamoto Y, Taniguchi M, et al. ORP150 protects against hypoxia/ischemia-induced neuronal death. Nat Med. 2001;7(3):317–23. doi: 10.1038/85463. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Wu Z, Li D, Wang D, Wang X, Feng X, et al. Involvement of oxygen-regulated protein 150 in AMP-activated protein kinase-mediated alleviation of lipid-induced endoplasmic reticulum stress. J Biol Chem. 2011;286(13):11119–31. doi: 10.1074/jbc.M110.203323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes CS, Shen JW, Subjeck JR. Resistance to etoposide induced by three glucose-regulated stresses in Chinese hamster ovary cells. Cancer Res. 1989;49(16):4452–4. [PubMed] [Google Scholar]
- 32.Mann MJ, Pereira ER, Liao N, Hendershot LM. UPR-induced resistance to etoposide is downstream of PERK and independent of changes in topoisomerase IIalpha levels. PLoS One. 2012;7(10):e47931. doi: 10.1371/journal.pone.0047931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye Z, Wang N, Xia P, Wang E, Liao J, Guo Q. Parecoxib Suppresses CHOP and Foxo1 Nuclear Translocation, but Increases GRP78 Levels in a Rat Model of Focal Ischemia. Neurochemical research. 2013;38(4):686–93. doi: 10.1007/s11064-012-0953-4. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Wang M, Chen H, Guo Y, Ma F, Shi F, et al. Hypothermia protects the brain from transient global ischemia/reperfusion by attenuating endoplasmic reticulum response-induced apoptosis through CHOP. PLoS One. 2013;8(1):e53431. doi: 10.1371/journal.pone.0053431. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Melnick J, Dul JL, Argon Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994;370(6488):373–5. doi: 10.1038/370373a0. [DOI] [PubMed] [Google Scholar]
- 36.Dierks T, Volkmer J, Schlenstedt G, Jung C, Sandholzer U, Zachmann K, et al. A microsomal ATP-binding protein involved in efficient protein transport into the mammalian endoplasmic reticulum. Embo J. 1996;15(24):6931–42. [PMC free article] [PubMed] [Google Scholar]
- 37.Spee P, Subjeck J, Neefjes J. Identification of novel peptide binding proteins in the endoplasmic reticulum: ERp72, calnexin, and grp170. Biochemistry. 1999;38(32):10559–66. doi: 10.1021/bi990321r. [DOI] [PubMed] [Google Scholar]
- 38.Tyson JR, Stirling CJ. LHS1 and SIL1 provide a lumenal function that is essential for protein translocation into the endoplasmic reticulum. Embo J. 2000;19(23):6440–52. doi: 10.1093/emboj/19.23.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Craven RA, Egerton M, Stirling CJ. A novel Hsp70 of the yeast ER lumen is required for the efficient translocation of a number of protein precursors. Embo J. 1996;15(11):2640–50. [PMC free article] [PubMed] [Google Scholar]
- 40.Oh HJ, Easton D, Murawski M, Kaneko Y, Subjeck JR. The chaperoning activity of hsp110. Identification of functional domains by use of targeted deletions. J Biol Chem. 1999;274(22):15712–8. doi: 10.1074/jbc.274.22.15712. [DOI] [PubMed] [Google Scholar]
- 41.Park J, Easton DP, Chen X, MacDonald IJ, Wang XY, Subjeck JR. The chaperoning properties of mouse grp170, a member of the third family of hsp70 related proteins. Biochemistry. 2003;42(50):14893–902. doi: 10.1021/bi030122e. [DOI] [PubMed] [Google Scholar]
- 42.Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, et al. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272(5268):1606–14. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang XY, Chen X, Manjili MH, Repasky E, Henderson R, Subjeck JR. Targeted immunotherapy using reconstituted chaperone complexes of heat shock protein 110 and melanoma-associated antigen gp100. Cancer Res. 2003;63(10):2553–60. [PubMed] [Google Scholar]
- 44.Wang XY, Arnouk H, Chen X, Kazim L, Repasky EA, Subjeck JR. Extracellular targeting of endoplasmic reticulum chaperone glucose-regulated protein 170 enhances tumor immunity to a poorly immunogenic melanoma. J Immunol. 2006;177(3):1543–51. doi: 10.4049/jimmunol.177.3.1543. [DOI] [PubMed] [Google Scholar]
- 45.Wang XY, Sun X, Chen X, Facciponte J, Repasky EA, Kane J, et al. Superior antitumor response induced by large stress protein chaperoned protein antigen compared with peptide antigen. J Immunol. 2010;184(11):6309–19. doi: 10.4049/jimmunol.0903891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brodsky JL, Werner ED, Dubas ME, Goeckeler JL, Kruse KB, McCracken AA. The requirement for molecular chaperones during endoplasmic reticulum-associated protein degradation demonstrates that protein export and import are mechanistically distinct. J Biol Chem. 1999;274(6):3453–60. doi: 10.1074/jbc.274.6.3453. [DOI] [PubMed] [Google Scholar]
- 47.Wang XY, Chen X, Oh HJ, Repasky E, Kazim L, Subjeck J. Characterization of native interaction of hsp110 with hsp25 and hsc70. FEBS Lett. 2000;465(2–3):98–102. doi: 10.1016/s0014-5793(99)01733-0. [DOI] [PubMed] [Google Scholar]
- 48.Ishihara K, Yasuda K, Hatayama T. Molecular cloning, expression and localization of human 105 kDa heat shock protein, hsp105. Biochim Biophys Acta. 1999;1444(1):138–42. doi: 10.1016/s0167-4781(98)00254-1. [DOI] [PubMed] [Google Scholar]
- 49.Yam AY, Albanese V, Lin HT, Frydman J. Hsp110 cooperates with different cytosolic HSP70 systems in a pathway for de novo folding. J Biol Chem. 2005;280(50):41252–61. doi: 10.1074/jbc.M503615200. [DOI] [PubMed] [Google Scholar]
- 50.Shaner L, Wegele H, Buchner J, Morano KA. The yeast Hsp110 Sse1 functionally interacts with the Hsp70 chaperones Ssa and Ssb. J Biol Chem. 2005;280(50):41262–9. doi: 10.1074/jbc.M503614200. [DOI] [PubMed] [Google Scholar]
- 51.Shorter J. The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PLoS One. 2011;6(10):e26319. doi: 10.1371/journal.pone.0026319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rampelt H, Kirstein-Miles J, Nillegoda NB, Chi K, Scholz SR, Morimoto RI, et al. Metazoan Hsp70 machines use Hsp110 to power protein disaggregation. Embo J. 2012;31(21):4221–35. doi: 10.1038/emboj.2012.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamagishi N, Yokota M, Yasuda K, Saito Y, Nagata K, Hatayama T. Characterization of stress sensitivity and chaperone activity of Hsp105 in mammalian cells. Biochem Biophys Res Commun. 2011;409(1):90–5. doi: 10.1016/j.bbrc.2011.04.114. [DOI] [PubMed] [Google Scholar]
- 54.Yamagishi N, Goto K, Nakagawa S, Saito Y, Hatayama T. Hsp105 reduces the protein aggregation and cytotoxicity by expanded-polyglutamine proteins through the induction of Hsp70. Exp Cell Res. 2010;316(15):2424–33. doi: 10.1016/j.yexcr.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Saxena A, Banasavadi-Siddegowda YK, Fan Y, Bhattacharya S, Roy G, Giovannucci DR, et al. Human heat shock protein 105/110 kDa (Hsp105/110) regulates biogenesis and quality control of misfolded cystic fibrosis transmembrane conductance regulator at multiple levels. J Biol Chem. 2012;287(23):19158–70. doi: 10.1074/jbc.M111.297580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raviol H, Sadlish H, Rodriguez F, Mayer MP, Bukau B. Chaperone network in the yeast cytosol: Hsp110 is revealed as an Hsp70 nucleotide exchange factor. Embo J. 2006;25(11):2510–8. doi: 10.1038/sj.emboj.7601139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dragovic Z, Broadley SA, Shomura Y, Bracher A, Hartl FU. Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s. Embo J. 2006;25(11):2519–28. doi: 10.1038/sj.emboj.7601138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andreasson C, Fiaux J, Rampelt H, Druffel-Augustin S, Bukau B. Insights into the structural dynamics of the Hsp110-Hsp70 interaction reveal the mechanism for nucleotide exchange activity. Proc Natl Acad Sci U S A. 2008;105(43):16519–24. doi: 10.1073/pnas.0804187105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polier S, Dragovic Z, Hartl FU, Bracher A. Structural basis for the cooperation of Hsp70 and Hsp110 chaperones in protein folding. Cell. 2008;133(6):1068–79. doi: 10.1016/j.cell.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 60.Weitzmann A, Volkmer J, Zimmermann R. The nucleotide exchange factor activity of Grp170 may explain the non-lethal phenotype of loss of Sil1 function in man and mouse. FEBS Lett. 2006;580(22):5237–40. doi: 10.1016/j.febslet.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 61.de Keyzer J, Steel GJ, Hale SJ, Humphries D, Stirling CJ. Nucleotide binding by Lhs1p is essential for its nucleotide exchange activity and for function in vivo. J Biol Chem. 2009;284(46):31564–71. doi: 10.1074/jbc.M109.055160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andreasson C, Rampelt H, Fiaux J, Druffel-Augustin S, Bukau B. The endoplasmic reticulum Grp170 acts as a nucleotide exchange factor of Hsp70 via a mechanism similar to that of the cytosolic Hsp110. J Biol Chem. 2010;285(16):12445–53. doi: 10.1074/jbc.M109.096735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu X, Sarbeng EB, Vorvis C, Kumar DP, Zhou L, Liu Q. Unique peptide substrate binding properties of 110-kDa heat-shock protein (Hsp110) determine its distinct chaperone activity. J Biol Chem. 2012;287(8):5661–72. doi: 10.1074/jbc.M111.275057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mandal AK, Gibney PA, Nillegoda NB, Theodoraki MA, Caplan AJ, Morano KA. Hsp110 chaperones control client fate determination in the hsp70-Hsp90 chaperone system. Mol Biol Cell. 2010;21(9):1439–48. doi: 10.1091/mbc.E09-09-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henics T, Nagy E, Oh HJ, Csermely P, von Gabain A, Subjeck JR. Mammalian Hsp70 and Hsp110 proteins bind to RNA motifs involved in mRNA stability. J Biol Chem. 1999;274(24):17318–24. doi: 10.1074/jbc.274.24.17318. [DOI] [PubMed] [Google Scholar]
- 66.Zuo D, Yu X, Guo C, Yi H, Chen X, Conrad DH, et al. Molecular chaperoning by glucose-regulated protein 170 in the extracellular milieu promotes macrophage-mediated pathogen sensing and innate immunity. Faseb J. 2012;26(4):1493–505. doi: 10.1096/fj.11-197707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srivastava PK, DeLeo AB, Old LJ. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci U S A. 1986;83(10):3407–11. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ullrich SJ, Robinson EA, Law LW, Willingham M, Appella E. A mouse tumor-specific transplantation antigen is a heat shock-related protein. Proc Natl Acad Sci U S A. 1986;83(10):3121–5. doi: 10.1073/pnas.83.10.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178(4):1391–6. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arnold D, Faath S, Rammensee H, Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med. 1995;182(3):885–9. doi: 10.1084/jem.182.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nieland TJ, Tan MC, Monne-van Muijen M, Koning F, Kruisbeek AM, van Bleek GM. Isolation of an immunodominant viral peptide that is endogenously bound to the stress protein GP96/GRP94. Proc Natl Acad Sci U S A. 1996;93(12):6135–9. doi: 10.1073/pnas.93.12.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishii T, Udono H, Yamano T, Ohta H, Uenaka A, Ono T, et al. Isolation of MHC class I-restricted tumor antigen peptide and its precursors associated with heat shock proteins hsp70, hsp90, and gp96. J Immunol. 1999;162(3):1303–9. [PubMed] [Google Scholar]
- 73.Castelli C, Ciupitu AM, Rini F, Rivoltini L, Mazzocchi A, Kiessling R, et al. Human heat shock protein 70 peptide complexes specifically activate antimelanoma T cells. Cancer Res. 2001;61(1):222–7. [PubMed] [Google Scholar]
- 74.Rivoltini L, Castelli C, Carrabba M, Mazzaferro V, Pilla L, Huber V, et al. Human tumor-derived heat shock protein 96 mediates in vitro activation and in vivo expansion of melanoma- and colon carcinoma-specific T cells. J Immunol. 2003;171(7):3467–74. doi: 10.4049/jimmunol.171.7.3467. [DOI] [PubMed] [Google Scholar]
- 75.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 76.Baker-LePain JC, Reed RC, Nicchitta CV. ISO: a critical evaluation of the role of peptides in heat shock/chaperone protein-mediated tumor rejection. Curr Opin Immunol. 2003;15(1):89–94. doi: 10.1016/s0952791502000067. [DOI] [PubMed] [Google Scholar]
- 77.Nicchitta CV, Carrick DM, Baker-Lepain JC. The messenger and the message: gp96 (GRP94)-peptide interactions in cellular immunity. Cell Stress Chaperones. 2004;9(4):325–31. doi: 10.1379/CSC-62.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278(5335):117–20. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 79.Vanaja DK, Grossmann ME, Celis E, Young CY. Tumor prevention and antitumor immunity with heat shock protein 70 induced by 15-deoxy-delta12,14-prostaglandin J2 in transgenic adenocarcinoma of mouse prostate cells. Cancer Res. 2000;60(17):4714–8. [PubMed] [Google Scholar]
- 80.Graner M, Raymond A, Romney D, He L, Whitesell L, Katsanis E. Immunoprotective activities of multiple chaperone proteins isolated from murine B-cell leukemia/lymphoma. Clin Cancer Res. 2000;6(3):909–15. [PubMed] [Google Scholar]
- 81.Wang XY, Kazim L, Repasky EA, Subjeck JR. Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity. J Immunol. 2001;166(1):490–7. doi: 10.4049/jimmunol.166.1.490. [DOI] [PubMed] [Google Scholar]
- 82.Wang XY, Kazim L, Repasky EA, Subjeck JR. Immunization with tumor-derived ER chaperone grp170 elicits tumor-specific CD8+ T-cell responses and reduces pulmonary metastatic disease. Int J Cancer. 2003;105(2):226–31. doi: 10.1002/ijc.11058. [DOI] [PubMed] [Google Scholar]
- 83.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10(2):86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends in Biochemical Sciences. 2006;31(3):164–72. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 85.Fu Y, Lee AS. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol Ther. 2006;5(7):741–4. doi: 10.4161/cbt.5.7.2970. [DOI] [PubMed] [Google Scholar]
- 86.Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32(7):805–18. doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arnouk H, Zynda ER, Wang XY, Hylander BL, Manjili MH, Repasky EA, et al. Tumour secreted grp170 chaperones full-length protein substrates and induces an adaptive anti-tumour immune response in vivo. Int J Hyperthermia. 2010;26(4):366–75. doi: 10.3109/02656730903485910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kunisawa J, Shastri N. The Group II Chaperonin TRiC Protects Proteolytic Intermediates from Degradation in the MHC Class I Antigen Processing Pathway. Molecular Cell. 2003;12(3):565–76. doi: 10.1016/j.molcel.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 89.Kunisawa J, Shastri N. Hsp90alpha chaperones large C-terminally extended proteolytic intermediates in the MHC class I antigen processing pathway. Immunity. 2006;24(5):523–34. doi: 10.1016/j.immuni.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 90.Mazzaferro V, Coppa J, Carrabba MG, Rivoltini L, Schiavo M, Regalia E, et al. Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res. 2003;9(9):3235–45. [PubMed] [Google Scholar]
- 91.Pilla L, Patuzzo R, Rivoltini L, Maio M, Pennacchioli E, Lamaj E, et al. A phase II trial of vaccination with autologous, tumor-derived heat-shock protein peptide complexes Gp96, in combination with GM-CSF and interferon-alpha in metastatic melanoma patients. Cancer Immunol Immunother. 2006;55(8):958–68. doi: 10.1007/s00262-005-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: the C-100-21 Study Group. J Clin Oncol. 2008;26(6):955–62. doi: 10.1200/JCO.2007.11.9941. [DOI] [PubMed] [Google Scholar]
- 93.Younes A. A phase II study of heat shock protein-peptide complex-96 vaccine therapy in patients with indolent non-Hodgkin's lymphoma. Clin Lymphoma. 2003;4(3):183–5. doi: 10.3816/clm.2003.n.029. [DOI] [PubMed] [Google Scholar]
- 94.Jonasch E, Wood C, Tamboli P, Pagliaro LC, Tu SM, Kim J, et al. Vaccination of metastatic renal cell carcinoma patients with autologous tumour-derived vitespen vaccine: clinical findings. Br J Cancer. 2008;98(8):1336–41. doi: 10.1038/sj.bjc.6604266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. The Lancet. 2008;372(9633):145–54. doi: 10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]
- 96.Oki Y, Younes A. Heat shock protein-based cancer vaccines. Expert Rev Vaccines. 2004;3(4):403–11. doi: 10.1586/14760584.3.4.403. [DOI] [PubMed] [Google Scholar]
- 97.Gordon NF, Clark BL. The challenges of bringing autologous HSP-based vaccines to commercial reality. Methods. 2004;32(1):63–9. doi: 10.1016/s1046-2023(03)00188-9. [DOI] [PubMed] [Google Scholar]
- 98.Belli F, Testori A, Rivoltini L, Maio M, Andreola G, Sertoli MR, et al. Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J Clin Oncol. 2002;20(20):4169–80. doi: 10.1200/JCO.2002.09.134. [DOI] [PubMed] [Google Scholar]
- 99.Manjili MH, Henderson R, Wang XY, Chen X, Li Y, Repasky E, et al. Development of a recombinant HSP110-HER-2/neu vaccine using the chaperoning properties of HSP110. Cancer Res. 2002;62(6):1737–42. [PubMed] [Google Scholar]
- 100.Manjili MH, Wang XY, Chen X, Martin T, Repasky EA, Henderson R, et al. HSP110-HER2/neu chaperone complex vaccine induces protective immunity against spontaneous mammary tumors in HER-2/neu transgenic mice. J Immunol. 2003;171(8):4054–61. doi: 10.4049/jimmunol.171.8.4054. [DOI] [PubMed] [Google Scholar]
- 101.Park JE, Facciponte J, Chen X, MacDonald I, Repasky EA, Manjili MH, et al. Chaperoning function of stress protein grp170, a member of the hsp70 superfamily, is responsible for its immunoadjuvant activity. Cancer Research. 2006;66(2):1161–8. doi: 10.1158/0008-5472.CAN-05-2609. [DOI] [PubMed] [Google Scholar]
- 102.Kim H, Sun X, Subjeck J, Wang X-Y. Evaluation of renal cell carcinoma vaccines targeting carbonic anhydrase IX using heat shock protein 110. Cancer Immunology, Immunotherapy. 2007;56(7):1097–105. doi: 10.1007/s00262-006-0258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br J Cancer. 2011;104(4):643–52. doi: 10.1038/bjc.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huo W, Ye J, Liu R, Chen J, Li Q. Vaccination with a chaperone complex based on PSCA and GRP170 adjuvant enhances the CTL response and inhibits the tumor growth in mice. Vaccine. 2010;28(38):6333–7. doi: 10.1016/j.vaccine.2010.06.093. [DOI] [PubMed] [Google Scholar]
- 105.Yuan B, Xian R, Wu X, Jing J, Chen K, Liu G, et al. Endoplasmic reticulum chaperone glucose regulated protein 170-Pokemon complexes elicit a robust antitumor immune response in vivo. Immunobiology. 2012;217(7):738–42. doi: 10.1016/j.imbio.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 106.Wang XY, Facciponte JG, Subjeck JR. Molecular chaperones and cancer immunotherapy. Handb Exp Pharmacol. 2006;172(172):305–29. doi: 10.1007/3-540-29717-0_13. [DOI] [PubMed] [Google Scholar]
- 107.Murshid A, Gong J, Calderwood SK. Heat-shock proteins in cancer vaccines: agents of antigen cross-presentation. Expert Rev Vaccines. 2008;7(7):1019–30. doi: 10.1586/14760584.7.7.1019. [DOI] [PubMed] [Google Scholar]
- 108.Murshid A, Gong J, Calderwood SK. The role of heat shock proteins in antigen cross presentation. Frontiers in immunology. 2012;3:63. doi: 10.3389/fimmu.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee HG, de la Salle H, et al. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162(7):3757–60. [PubMed] [Google Scholar]
- 110.Wassenberg JJ, Dezfulian C, Nicchitta CV. Receptor mediated and fluid phase pathways for internalization of the ER Hsp90 chaperone GRP94 in murine macrophages. J Cell Sci. 1999;112(Pt 13):2167–75. doi: 10.1242/jcs.112.13.2167. [DOI] [PubMed] [Google Scholar]
- 111.Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, Houghton AN, et al. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med. 2000;191(11):1957–64. doi: 10.1084/jem.191.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Singh-Jasuja H, Toes RE, Spee P, Munz C, Hilf N, Schoenberger SP, et al. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 2000;191(11):1965–74. doi: 10.1084/jem.191.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1(2):151–5. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 114.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14(3):303–13. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 115.Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17(3):353–62. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 116.Berwin B, Hart JP, Rice S, Gass C, Pizzo SV, Post SR, et al. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. Embo J. 2003;22(22):6127–36. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berwin B, Delneste Y, Lovingood RV, Post SR, Pizzo SV. SREC-I, a type F scavenger receptor, is an endocytic receptor for calreticulin. J Biol Chem. 2004;279(49):51250–7. doi: 10.1074/jbc.M406202200. [DOI] [PubMed] [Google Scholar]
- 118.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6(4):435–42. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 119.Kuppner MC, Gastpar R, Gelwer S, Nossner E, Ochmann O, Scharner A, et al. The role of heat shock protein (hsp70) in dendritic cell maturation: hsp70 induces the maturation of immature dendritic cells but reduces DC differentiation from monocyte precursors. Eur J Immunol. 2001;31(5):1602–9. doi: 10.1002/1521-4141(200105)31:5<1602::AID-IMMU1602>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 120.Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee HG, Toes RE, et al. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol. 2000;30(8):2211–5. doi: 10.1002/1521-4141(2000)30:8<2211::AID-IMMU2211>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 121.Wang Y, Kelly CG, Singh M, McGowan EG, Carrara AS, Bergmeier LA, et al. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J Immunol. 2002;169(5):2422–9. doi: 10.4049/jimmunol.169.5.2422. [DOI] [PubMed] [Google Scholar]
- 122.Theriault JR, Adachi H, Calderwood SK. Role of scavenger receptors in the binding and internalization of heat shock protein 70. J Immunol. 2006;177(12):8604–11. doi: 10.4049/jimmunol.177.12.8604. [DOI] [PubMed] [Google Scholar]
- 123.Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009;85(6):905–10. doi: 10.1189/jlb.0109005. [DOI] [PubMed] [Google Scholar]
- 124.Calderwood SK, Mambula SS, Gray PJ., Jr. Extracellular heat shock proteins in cell signaling and immunity. Ann N Y Acad Sci. 2007;1113:28–39. doi: 10.1196/annals.1391.019. Epub 2007/11/06. [DOI] [PubMed] [Google Scholar]
- 125.Henderson B, Calderwood SK, Coates AR, Cohen I, van Eden W, Lehner T, et al. Caught with their PAMPs down? The extracellular signalling actions of molecular chaperones are not due to microbial contaminants. Cell Stress Chaperones. 2010;15(2):123–41. doi: 10.1007/s12192-009-0137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen T, Guo J, Han C, Yang M, Cao X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol. 2009;182(3):1449–59. doi: 10.4049/jimmunol.182.3.1449. [DOI] [PubMed] [Google Scholar]
- 127.Gong J, Zhu B, Murshid A, Adachi H, Song B, Lee A, et al. T cell activation by heat shock protein 70 vaccine requires TLR signaling and scavenger receptor expressed by endothelial cells-1. J Immunol. 2009;183(5):3092–8. doi: 10.4049/jimmunol.0901235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Manjili MH, Park J, Facciponte JG, Subjeck JR. HSP110 induces “danger signals” upon interaction with antigen presenting cells and mouse mammary carcinoma. Immunobiology. 2005;210(5):295–303. doi: 10.1016/j.imbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 129.Manjili MH, Park JE, Facciponte JG, Wang XY, Subjeck JR. Immunoadjuvant chaperone, GRP170, induces `danger signals' upon interaction with dendritic cells. Immunol Cell Biol. 2006;84(2):203–8. doi: 10.1111/j.1440-1711.2006.01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang XY, Li Y, Manjili MH, Repasky EA, Pardoll DM, Subjeck JR. Hsp110 over-expression increases the immunogenicity of the murine CT26 colon tumor. Cancer Immunol Immunother. 2002;51(6):311–9. doi: 10.1007/s00262-002-0287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Muchemwa FC, Nakatsura T, Fukushima S, Nishimura Y, Kageshita T, Ihn H. Differential expression of heat shock protein 105 in melanoma and melanocytic naevi. Melanoma Res. 2008;18(3):166–71. doi: 10.1097/CMR.0b013e3282fe9a16. [DOI] [PubMed] [Google Scholar]
- 132.Oda T, Morii E, Inoue M, Ikeda J, Aozasa K, Okumura M. Prognostic significance of heat shock protein 105 in lung adenocarcinoma. Molecular medicine reports. 2009;2(4):603–7. doi: 10.3892/mmr_00000144. [DOI] [PubMed] [Google Scholar]
- 133.Zappasodi R, Bongarzone I, Ghedini GC, Castagnoli L, Cabras AD, Messina A, et al. Serological identification of HSP105 as a novel non-Hodgkin lymphoma therapeutic target. Blood. 2011;118(16):4421–30. doi: 10.1182/blood-2011-06-364570. [DOI] [PubMed] [Google Scholar]
- 134.Miyazaki M, Nakatsura T, Yokomine K, Senju S, Monji M, Hosaka S, et al. DNA vaccination of HSP105 leads to tumor rejection of colorectal cancer and melanoma in mice through activation of both CD4 T cells and CD8 T cells. Cancer Sci. 2005;96(10):695–705. doi: 10.1111/j.1349-7006.2005.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gao P, Sun X, Chen X, Subjeck J, Wang XY. Secretion of stress protein grp170 promotes immune-mediated inhibition of murine prostate tumor. Cancer Immunol Immunother. 2009;58(8):1319–28. doi: 10.1007/s00262-008-0647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao P, Sun X, Chen X, Wang Y, Foster BA, Subjeck J, et al. Secretable chaperone Grp170 enhances therapeutic activity of a novel tumor suppressor, mda-7/IL-24. Cancer Res. 2008;68(10):3890–8. doi: 10.1158/0008-5472.CAN-08-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dash R, Bhutia SK, Azab B, Su ZZ, Quinn BA, Kegelmen TP, et al. mda-7/IL-24: a unique member of the IL-10 gene family promoting cancer-targeted toxicity. Cytokine Growth Factor Rev. 2010;21(5):381–91. doi: 10.1016/j.cytogfr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 139.Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev. 2011;241(1):104–18. doi: 10.1111/j.1600-065X.2011.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yu X, Guo C, Yi H, Qian J, Fisher PB, Subjeck JR, et al. A multifunctional chimeric chaperone serves as a novel immune modulator inducing therapeutic antitumor immunity. Cancer Res. 2013;73(7):2093–103. doi: 10.1158/0008-5472.CAN-12-1740. Epub 2013/01/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Murthy KGK, Deb A, Goonesekera S, Szabó C, Salzman AL. Identification of Conserved Domains in Salmonella muenchen Flagellin That Are Essential for Its Ability to Activate TLR5 and to Induce an Inflammatory Response in Vitro. Journal of Biological Chemistry. 2004;279(7):5667–75. doi: 10.1074/jbc.M307759200. [DOI] [PubMed] [Google Scholar]
- 142.van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5(4):318–30. doi: 10.1038/nri1593. Epub 2005/04/02. [DOI] [PubMed] [Google Scholar]
- 143.de Kleer I, Vercoulen Y, Klein M, Meerding J, Albani S, van der Zee R, et al. CD30 discriminates heat shock protein 60-induced FOXP3+ CD4+ T cells with a regulatory phenotype. J Immunol. 2010;185(4):2071–9. doi: 10.4049/jimmunol.0901901. [DOI] [PubMed] [Google Scholar]
- 144.van Herwijnen MJ, Wieten L, van der Zee R, van Kooten PJ, Wagenaar-Hilbers JP, Hoek A, et al. Regulatory T cells that recognize a ubiquitous stress-inducible self-antigen are long-lived suppressors of autoimmune arthritis. Proc Natl Acad Sci U S A. 2012;109(35):14134–9. doi: 10.1073/pnas.1206803109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Keijzer C, Wieten L, van Herwijnen M, van der Zee R, Van Eden W, Broere F. Heat shock proteins are therapeutic targets in autoimmune diseases and other chronic inflammatory conditions. Expert opinion on therapeutic targets. 2012;16(9):849–57. doi: 10.1517/14728222.2012.706605. [DOI] [PubMed] [Google Scholar]
- 146.Murshid A, Gong J, Calderwood SK. Heat shock protein 90 mediates efficient antigen cross presentation through the scavenger receptor expressed by endothelial cells-I. J Immunol. 2010;185(5):2903–17. doi: 10.4049/jimmunol.0903635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Facciponte JG, Wang XY, Subjeck JR. Hsp110 and Grp170, members of the Hsp70 superfamily, bind to scavenger receptor-A and scavenger receptor expressed by endothelial cells-I. Eur J Immunol. 2007;37(8):2268–79. doi: 10.1002/eji.200737127. [DOI] [PubMed] [Google Scholar]
- 148.Wang XY, Facciponte J, Chen X, Subjeck JR, Repasky EA. Scavenger receptor-A negatively regulates antitumor immunity. Cancer Res. 2007;67(10):4996–5002. doi: 10.1158/0008-5472.CAN-06-3138. [DOI] [PubMed] [Google Scholar]
- 149.Qian J, Yi H, Guo C, Yu X, Zuo D, Chen X, et al. CD204 suppresses large heat shock protein-facilitated priming of tumor antigen gp100-specific T cells and chaperone vaccine activity against mouse melanoma. J Immunol. 2011;187(6):2905–14. doi: 10.4049/jimmunol.1100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yi H, Yu X, Gao P, Wang Y, Baek SH, Chen X, et al. Pattern recognition scavenger receptor SRA/CD204 down-regulates Toll-like receptor 4 signaling-dependent CD8 T-cell activation. Blood. 2009;113(23):5819–28. doi: 10.1182/blood-2008-11-190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yi H, Zuo D, Yu X, Hu F, Manjili MH, Chen Z, et al. Suppression of antigen-specific CD4(+) T cell activation by SRA/CD204 through reducing the immunostimulatory capability of antigen-presenting cell. Journal of molecular medicine. 2012;90(4):413–26. doi: 10.1007/s00109-011-0828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zuo D, Yu X, Guo C, Wang H, Qian J, Yi H, et al. Scavenger receptor a restrains T-cell activation and protects against concanavalin A-induced hepatic injury. Hepatology. 2013;57(1):228–38. doi: 10.1002/hep.25983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guo C, Yi H, Yu X, Hu F, Zuo D, Subjeck JR, et al. Absence of scavenger receptor A promotes dendritic cell-mediated cross-presentation of cell-associated antigen and antitumor immune response. Immunol Cell Biol. 2012;90(1):101–8. doi: 10.1038/icb.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yu X, Yi H, Guo C, Zuo D, Wang Y, Kim HL, et al. Pattern pecognition scavenger receptor CD204 attenuates toll-like receptor 4-induced NF-{kappa}B activation by directly inhibiting ubiquitination of tumor necrosis factor (TNF) receptor-associated factor 6. J Biol Chem. 2011;286(21):18795–806. doi: 10.1074/jbc.M111.224345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Andreakos E, Williams RO, Wales J, Foxwell BM, Feldmann M. Activation of NF-kappaB by the intracellular expression of NF-kappaB-inducing kinase acts as a powerful vaccine adjuvant. Proceedings of the National Academy of Sciences. 2006;103(39):14459–64. doi: 10.1073/pnas.0603493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 157.Yi H, Guo C, Yu X, Gao P, Qian J, Zuo D, et al. Targeting the immunoregulator SRA/CD204 potentiates specific dendritic cell vaccine-induced T cell response and antitumor immunity. Cancer Res. 2011;71(21):6611–20. doi: 10.1158/0008-5472.CAN-11-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guo C, Yi H, Yu X, Zuo D, Qian J, Yang G, et al. In situ vaccination with CD204 gene-silenced dendritic cell, not unmodified dendritic cell, enhances radiation therapy of prostate cancer. Mol Cancer Ther. 2012;11(11):2331–41. doi: 10.1158/1535-7163.MCT-12-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Matsutake T, Sawamura T, Srivastava PK. High efficiency CD91- and LOX-1-mediated representation of gp96-chaperoned peptides by MHC II molecules. Cancer Immun. 2010;10(7) [PMC free article] [PubMed] [Google Scholar]