Abstract
Background
Assuring adequate enteral nutritional support in critically ill patients is challenging. By describing our experience we sought to characterize the challenges, benefits and complications of an approach that stresses enteral nutrition (EN).
Study Design
We examined nutritional support received by victims of blunt trauma from eight trauma centers. We grouped subjects according to the average daily enteral caloric intake over the first 7. Group 1 received the fewest (0 kcal/kg/day) and group 5 the greatest (16 – 30 kcal/kg/day) number of calories in the first week. We focused our analyses on the patients remaining in the ICU for ≥ 8 days and compared clinical outcomes among the groups.
Results
1100 subjects were in the ICU for ≥8 days. Patients receiving the greatest number of enteral calories during the first week (group 5) had the highest incidence of VAP (49%) and the lowest incidence of bacteremia (14%). The use of parenteral nutrition was associated with bacteremia (aOR = 2.5, 95% CI = 1.8 – 3.5), VAP (aOR = 2.4, 95% CI = 1.7 – 3.3) and death (aOR = 1.9, 95% CI = 1.1 – 3.1)
Conclusions
Enteral caloric intake during the first week was related to the pattern and severity of injury and associated with important infectious outcomes. Our observations support moderating enteral intake during the first week after injury and avoiding parenteral nutrition.
BACKGROUND
Enteral support is appropriate for the majority of critically ill patients and has been linked to improved outcomes relative to starvation or parenteral nutritional support. 1-5 However, several factors frequently limit or prevent the use of gastrointestinal tract. These include prolonged or repeated NPO status due to multiple operations, the presence of gastrointestinal injuries, temporary intestinal discontinuity, or reluctance by providers to use the gastrointestinal tract in clinical states such as the open abdomen and acute pancreatitis.6 The most common consequence of an enteral-based regimen is the development of a caloric (and nitrogen) deficit and the potential for complications related to malnutrition. The use of parenteral nutrition can achieve caloric and other nutrient intake targets more readily than enteral nutrition and may result in quicker restoration of serum albumin concentrations and other nutritional indices.3,7 It is paradoxical that preventing negative nitrogen balance and avoiding caloric deficits with parenteral nutrition (PN) has generally not improved clinical outcomes in the critically ill.7,8
Few critically ill patients present a more difficult challenge to successful enteral nutritional support than the severely injured. Nevertheless, studies have generally found them to benefit from approaches that rely primarily on enteral nutrition. Our approach encourages early and safe use of enteral nutrition and includes monitoring closely for intolerance and limiting parenteral support to cases where enteral support is not succeeding.9 Guidelines similar to ours have been shown to increase the use of enteral and decrease the use of parenteral nutritional support.10 However, even when guidelines are used, inadequate enteral caloric intake is common and complications related to enteral support occur.
In this report, we describe our overall experience with nutritional support in critically ill trauma patients who were managed at trauma centers participating in the “Inflammation and Host Response to Injury” consortium. In addition to describing this experience, we also analyzed associations between nutritional support received during the first week and important clinical outcomes including death and infectious complications. Specifically, we tested whether then amount of enteral support administered during the 1st week in the intensive care unit (ICU) was associated with the development of bloodstream infections, ventilator-associated pneumonia (VAP), and death.
STUDY DESIGN
Study subjects & data collection
We analyzed data collected as part of the prospective, large-scale collaborative research program entitled, “Inflammation and the Host Response to Injury” (2U54 GM-062119-07), the details of which have been reported elsewhere.11 In brief, severely injured adults were enrolled if they had evidence of shock, as manifested by either a base deficit ≥ 6 or more or hypotension (systolic blood pressure < 90 mmHg), within 60 minutes of arrival to the emergency department and received packed red blood cell transfusion after blunt injury. Exclusion criteria included isolated severe traumatic brain injury, substantial preexisting organ dysfunction, and ongoing immunosuppression. Patient characteristics, injury characteristics and outcome data were collected prospectively from eight participating centers, de-identified, and entered into a web-based database. Institutional Review Board approval from the University of Washington was obtained to access study data. Data collection for the parent study began in September 2003. For this study, we accessed the database on November 2, 2010, and obtained data for all subjects who survived at least 48 hours.
Demographic data and measures of anatomic injury severity and physiologic compromise were obtained. These included the Abbreviated Injury Scale (AIS) Score, Injury Severity Score (ISS), APACHE II score, admission base deficit, and initial red blood cell transfusion requirement. Procedural data, although available for both abdominal and non-abdominal procedures, were limited in detail to those relating to laparotomy (nontherapeutic, splenectomy, nephrectomy, laparotomy not otherwise specified), and laparotomy with a bowel perforation (stomach, small intestine, colon, or rectum). An “open abdomen” was defined as any time the abdominal fascia was left open as part of a damage control laparotomy or where diagnosis of abdominal compartment syndrome was recorded.
The database includes all caloric intake received during each 24-hour period while the subject was in the ICU. Daily caloric intake per kilogram of body weight was calculated and used for our analyses. We were interested in understanding how enteral and parenteral caloric intake might be related to clinical outcomes. In an earlier report, we were primarily interested in how early PN influenced outcome.7 Therefore, we focused specifically on the details of the subjects’ enteral caloric support during the initial 7 days in the ICU. In order to rank subjects, we generated 5 groups based upon their enteral caloric intake over the first week in the ICU (mean kcal/kg /day days 2 through 7). The thresholds separating the groups were determined by the data and do not reflect specific clinical targets, but do provide an estimate of relative success in delivering enteral support during the initial week in the ICU. All parenteral caloric intake was also recorded daily and included dextrose and lipids (Propofol) which are not prescribed for parenteral nutrition. Subjects who received ≥750 kcal/day on 2 consecutive days were considered to have received PN as this threshold would be rarely exceeded by intravenous glucose and propofol infusions.7
Patient care protocols
Care at participating institutions was guided by standard operating procedures.9,12-19 Our approach to nutritional support is based upon the use of early enteral feeding according to an initial target of 25-30 kcal/kg/d of body weight. This could be modified according to formula estimates or metabolic cart data. Inability to achieve at least 50% of target caloric intake after 7 days prompts consideration to use parenteral nutrition. However, earlier use of parenteral nutrition was not prohibited.
Study outcomes and definition of complications
The definitions of nosocomial infections and other non-infectious complications are standardized and have been previously reported.13 The definitions for infectious and non-infectious complications are available at the Inflammation and the Host Response to Ijury website (http://www.gluegrant.org/PDFs/DefinitionsTRDBInfectComplicationsv102207.pdf) For our analyses, we were specifically interested in VAP, blood stream infections and death.
Statistical analysis
Data were managed and analyzed using STATA 11 (Stata Corp. College Station, TX) and PRISM 5.04 (GraphPad. San Diego, CA) Continuous data are presented as medians with interquartile ranges and categorical data as numbers and percentages. Comparisons among the 5 nutritional support categories were conducted using Pearson’s chi-square or ANOVA. We used logistic regression to determine the risk of complications and death associated with the nutritional support categories, adjusting for additional risk factors and important confounding factors.
RESULTS
A total of 321 subjects did not have caloric data recorded for any of their ICU stay and therefore could not be included in our analyses. They are briefly described here. Two hundred and ninety of these were intubated and 31 were not. Of the intubated subjects, 95% (290/305) spent 2 or fewer days on the ventilator and almost all (302/305) spent less than 8 days in the ICU. The case-fatality rate was 40% (128/321). Thus, these excluded subjects predominantly included 2 groups; those with devastating and non-survivable injuries who died within 6 days of injury and survivors with brief ICU stays. Only 5 subjects without recorded caloric data spent 8 or more days in the ICU.
The demographics, injury characteristics and outcomes for 1,615 subjects with caloric intake data are included in Table 1 and briefly summarized here. As previously reported, this cohort is severely injured, with a high proportion of patients with severe thoracic, lower extremity and abdominal injuries. Most followed a complicated clinical course, with prolonged ICU and hospital stays, and many developed organ dysfunction and nosocomial infections (See Table 1).
Table 1.
Demographic, Injury and Outcomes Characteristics for Entire Cohort (n = 1,615)
| Characteristic | |
|---|---|
| Demographics | |
| Age, y, median (IQR) | 42 (25-55) |
| Sex, n (%) | |
| Male | 1,062 (66) |
| Female | 553 (33) |
| Race/ethnicity, n (%) | |
| Caucasian | 1,439 (89) |
| African-American | 101 (6.3) |
| Hispanic | 196 (12) |
| Asian | 35 (2) |
| American Indian | 19 (2) |
| Injury characteristics | |
| Injury Severity Score, median (IQR) | 34 (24-42) |
| Severe brain injury, n (%) | 601 (37) |
| Severe thoracic injury, n (%) | 1084 (67) |
| Severe abdominal injury, n (%) | 725 (44) |
| Severe lower extremity injury, n (%) | 1002 (67) |
| Severe upper extremity injury, n (%) | 350 (22) |
| Severe spine injury, n (%) | 216 (13) |
| Red blood cell transfusion in 24 h, mL, median (IQR) |
1,700 (800 – 3150) |
| Initial base deficit, median (IQR) | 8.0 (6.0-11.0) |
| Outcomes | |
| Died, n (%) | 179 (11) |
| ICU length of stay, d, median (IQR) | 12 (6 - 20) |
| Hospital length of stay, d, median (IQR) | 21 (31 - 32) |
| Duration of mechanical ventilation, d, median (IQR) | 8 (4 – 15) |
Continuous data are presented as medians (25th -75th percentile) and categorical data are indicated as total for the entire cohort (%). Severe traumatic brain, thoracic, abdominal, and extremity injuries refer to the number of patients with AIS scores ≥ 3 in the indicated body region.
Trends in enteral caloric intake over the duration of ICU stay
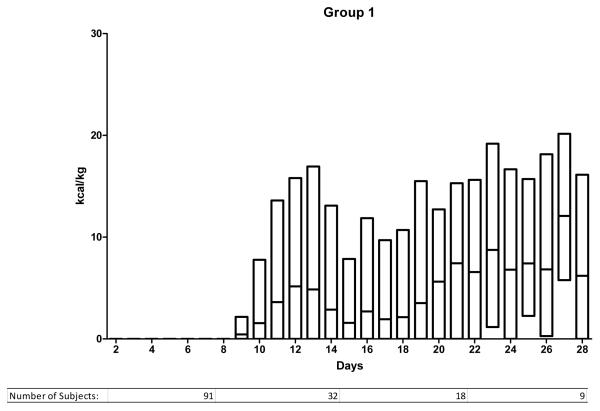
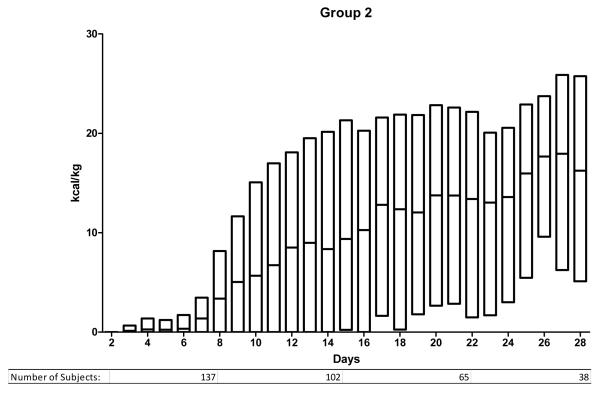
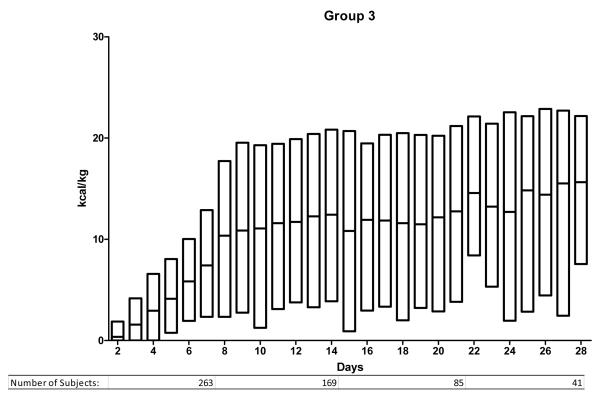
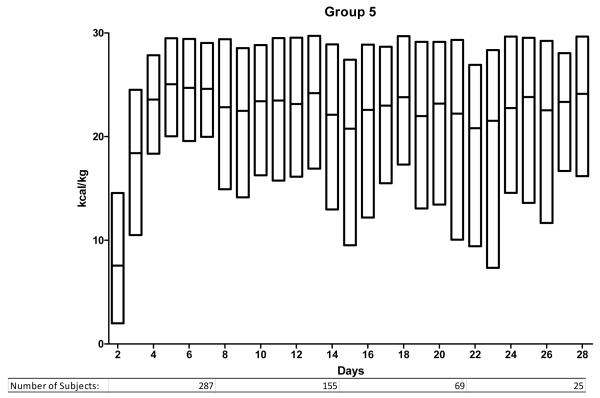
We ranked the subjects into 5 groups according to the mean daily enteral caloric intake over the first 7 days in the ICU. The caloric thresholds are shown in Table 2. Figure 1 illustrates the daily caloric intake from ICU day 2 to day 28 for the 5 groups. Group 1 (0 kcal/kg/day average during first week) includes 441 subjects, group 2 (1-2 kcal/kg/day) includes 198 subjects and the 3 remaining groups each consist of 323 subjects. Subjects in group 1 received no enteral calories during the 1st week in the ICU and had lower injury severity scores and received less transfused blood than patients in the remaining groups. They also had a shorter duration of mechanical ventilation, fewer days in the ICU and an overall shorter hospital stay than the other groups. The majority were transferred from the ICU (279) or died (60) before day 7 (total 339/441; 77%).
Table 2.
Demographic Injury Characteristics and Outcomes Based upon Enteral Tolerance during First Week for Entire Cohort (n = 1,615)
| Group 1 (n = 441) |
Group 2 (n = 198) |
Group 3 (n = 323) |
Group 4 (n = 323) |
Group 5 (n = 323) |
|
|---|---|---|---|---|---|
| Mean daily enteral caloric intake during first wk* |
0 kcal/kg/d | 1 – 2 kcal/kg/d |
3 – 8 kcal/kg/d |
9 – 15 kcal/kg/d |
16 – 30 kcal/kg/d |
| Age, y, median (IQR) |
38 (25-52) | 45 (27-58) | 43 (27-58) | 44 (28-56) | 38 (24-54) |
| Male sex, n (%) |
285 (64) | 145 (73) | 102 (68) | 204 (63) | 207 (64) |
| Injury severity score, median (IQR) |
28 (19-36) | 34 (25-41) | 34 (25-43) | 34 (27-43) | 36 (29-43) |
| Initial base deficit, median (IQR) |
9 (6-12) | 11 (8-14) | 11 (7-14) | 9.7 (7-13) | 9 (7-12) |
| Red blood cell transfusion, mL, median (IQR) |
1,400 (700- 2,800) |
2,200 (1,100-4,400) |
1,800 (1,000-3,500) |
1,750 (900- 2,800) |
1,550 (900- 2,800) |
| APACHE II Score, median (IQR) |
26 (21 – 31) | 31 (27 – 35) | 30 (26 – 34) | 30 (27 – 34) | 30 (26 – 34) |
| Case-fatality rate, n (%) |
75 (17) | 32 (16) | 24 (7) | 28 (9) | 20 (6) |
| ICU length of stay, d, median (IQR) |
5 (3-7) | 15 (7-25) | 16 (10-23) | 15 (10-23) | 14 (10-20) |
| Duration of mechanical ventilation, d, median (IQR) |
3 (2-4) | 10 (5-21) | 11 (7-18) | 11 (7-19) | 11 (7-17) |
| Hospital length of stay, d, median (IQR) |
11 (7-19) | 25 (15-39) | 24 (17-36) | 26 (18-37) | 24 (18-34) |
Continuous data are presented as medians with interquartile ranges (25th – 75th percentile) and categorical data as number in each group (%).
We ranked 5 groups calculating the mean daily caloric intake for all subjects over ICU days 2 – 7. This is described in the methods section.
Figure 1.
Enteral nutrition trends for days 2 to 28 for groups 1-5. Study subjects are divided according to nutritional support group as defined in the methods and as noted in Table 2. The y-axis indicates the number of kcal/kg delivered enterally and the x-axis indicates the ICU day. The bars indicating daily kcal/kg show the median and the upper and lower 95th percentile.
The remaining groups all received some enteral nutrition during the first 7 days. There were important differences between the groups, with trends suggesting a greater severity of injury in subjects receiving the fewest enteral calories (group 2). For example they were transfused more blood and had a higher initial base deficit than subjects in the other groups. The case-fatality rate for group 2 (16%) was similar to group 1 and higher than the other groups.
Nutritional management for subjects with ICU stays of 8 or more days
A total of 515 subjects spent ≤ 7 days in the ICU. We excluded these subjects from subsequent analyses for two reasons. First, early enteral nutritional support may be less beneficial in patients with short ICU stays. Second, these subjects were heterogeneous. Eight-eight of 515 (17%) died early as a direct result of their severe injuries. Nutritional support would not have affected their outcome. The remaining survivors were extubated and taking oral nutrition by day 4. For these reasons, we focused on the 1100 most critically ill subjects who spent greater than 1 week in the ICU.
Table 3 illustrates important characteristics for the subjects remaining in the ICU after 7 days. In conjunction with Figure 1, important information can be summarized regarding them. First, differences in enteral caloric intake persist between the 5 groups after the first week. Those in group 1 remained difficult to feed throughout their ICU stay. Their median enteral intake was < 10 kcal/kg/day. In contrast, subjects in group 5 reached daily caloric targets enterally within the 1st week and continued to receive goal enteral feeding throughout their ICU stay. Groups 2, 3 and 4 include the majority of patients remaining in the ICU beyond the 1st week and demonstrate an ongoing challenge in meeting caloric targets. In each of these 3 groups, median caloric intake remained below 20 kcal/kg/day. Comparing actual intake to the estimated target (25 kcal/kg/day) shows that most subjects do not receive goal support enterally. In group 4, only 25% of patients met this goal on any given day. The proportion was lower in groups 2 and 3. Only subjects in group 5 could be considered to have received goal enteral support. In this group, 50% received the target daily caloric intake within the first week and this proportion remained constant beyond the 1st week.
Table 3.
Demographic and Injury Characteristics for Patients in the ICU for ≥ 8 days (n = 1,100)
| Group 1 (n =109) |
Group 2 (n =144) |
Group 3 (n =274) |
Group 4 (n =282) |
Group 5 (n =291) |
|
|---|---|---|---|---|---|
| Mean daily enteral caloric intake during first wk |
0 kcal/kg/d | 1 – 2 kcal/kg/d |
3 – 8 kcal/kg/d |
9 – 15 kcal/kg/d |
16 – 30 kcal/kg/d |
| Age, y, median (IQR) |
41 (25-56) | 46 (27-58) | 46 (28-60) | 46 (30-57 | 40 (24-55) |
| Male sex, n (%) |
78 (72) | 107 (74) | 182 (66) | 177 (63) | 190 (65) |
| Initial base deficit, median (IQR) |
8 (6-11) | 10 (7-14) | 9 (6-12) | 8 (6-11) | 8 (6-11) |
| Red blood cell transfusion, mL, median (IQR) |
1,900 (1,025- 3,285) |
2,500 (1,300- 5,500) |
2,100 (1,000- 3,900) |
1,800 (900- 3,000) |
1,400 (1,000- 2,800) |
| APACHE II Score, median (IQR) |
28 (23-33) | 32 (28.5-36) | 30 (27-34) | 31 (27-34) | 30 (27-34) |
| Injury severity score, median (IQR) |
34 (25-43) | 34 (25-43) | 34 (26-43) | 34 (27-43) | 35 (29-43) |
| Severe brain injury, n (%) |
25 (23) | 56 (39) | 99 (36) | 113 (40) | 154 (53) |
| Severe thoracic injury, n (%) |
73 (67) | 95 (66) | 202 (74) | 220 (78) | 215 (74) |
| Severe abdominal injury, n (%) |
66 (61) | 78 (54) | 135 (49) | 123 (44) | 107 (37) |
| Hollow organ injury, n (%) |
4 (4) | 5 (3) | 4 (1) | 5 (2) | 1 (0.3) |
| Laparotomy, n (%) |
63 (58) | 103 (72) | 148 (54) | 119 (42) | 89 (31) |
| Open abdomen, n (%) |
31 (28) | 62(43) | 77 (28) | 39 (14) | 20 (7) |
| Parenteral nutrition, n (%) |
44 (40%) | 76 (53%) | 73 (27%) | 42 (15%) | 20 (7%) |
Continuous data are presented as medians with interquartile ranges (25th – 75th percentile) and categorical data as number in each group (percentage). Severe traumatic brain, thoracic, abdominal, and extremity injuries refer to the number of patients with AIS scores ≥ 3 in the indicated body region.
Associations between nutritional support and outcomes
Outcomes were different between the groups (Table 4). Subjects in group 1 had the shortest ICU and hospital stays and the shortest duration of mechanical ventilation, they also had the lowest risk of nosocomial infections; likely due to their relatively shorter duration of mechanical ventilation and shorter ICU stays. The duration of mechanical ventilation, ICU and hospital days were similar in groups 2 through 5. Complication rates differed among the groups. For example, bloodstream infections were most common in group 2, whereas ventilator-associated pneumonia was most common in group 5.
Table 4.
Outcomes for Patients in the ICU for ≥ 8 days (n = 1,100)
| Group 1 (n =109) |
Group 2 (n =144) |
Group 3 (n =274) |
Group 4 (n =282) |
Group 5 (n =291) |
|
|---|---|---|---|---|---|
| ICU length of stay, d, median (IQR) |
11 (8-19) | 20 (14-30) | 17 (12-24) | 17 (12-25) | 15 (11-21) |
| Duration of mechanical ventilation, d, median (IQR) |
7 (3-12) | 15 (9-25) | 13 (9-20) | 13 (9-21) | 12 (8-18) |
| Hospital length of stay, d, median (IQR) |
21 (15-34) | 31 (20-44) | 26 (20-38) | 28 (19-39) | 25 (19-34) |
| Any nosocomial infection, n (%) |
52 (48) | 94 (65) | 193 (70) | 196 (70) | 209 (72) |
| Surgical site infection, n (%) |
24 (22) | 35 (24) | 64 (23) | 52 (18) | 46 (16) |
| Ventilator- associated pneumonia, n (%) |
25 (23) | 62 (43) | 121 (44) | 129 (46) | 144 (49) |
| ARDS, n (%) | 24 (22) | 53 (37) | 85 (31) | 114 (40) | 137 (47) |
| Bloodstream infection, n (%) |
20 (18) | 46 (32) | 74 (27) | 61 (22) | 42 (14) |
| Fatality rate, n (%) |
15 (14) | 18 (13) | 16 (6) | 26 (9) | 16 (6) |
Continuous data are presented as medians with interquartile ranges (25th – 75th percentile) and categorical data as number in each group (percentage).
There were important baseline differences between the groups that might have influenced their ability to tolerate enteral nutrition and may also be associated with the outcomes of interest (Table 3). For example, group 2 subjects had a higher incidence of severe abdominal injuries; they underwent laparotomy and were treated with an open abdomen more often than subjects in the other groups. Given the high incidence of abdominal injuries, it is understandable that they received fewer enteral calories early in their ICU stay. Almost a quarter of the subjects (255/1100, 23%) received PN and was related to the amount of enteral calories received during the initial week. Group 2 had the highest (76/144, 53%) and group 5 the lowest (20/291, 7%) proportion of patients receiving supplemental PN. Group 1 was an apparent outlier; with fewer patients receiving PN support in this group than in group 2. This is likely due to the overall shorter duration of mechanical ventilation, higher case-fatality rate and earlier oral intake in the survivors. It is therefore clear that the difference in early nutritional support is, at least in part, due to important clinical differences between the groups. We performed logistic regression to adjust for these differences and to help us understand which factors, in addition to enteral nutrition, are related to VAP, bloodstream infections and mortality. The results of those analyses are shown in Tables 5, 6 & 7.
Table 5.
Regression Analyses of Risk Factors for Ventilator-Associated Pneumonia
| Factor | Adjusted odds ratio | 95% Confidence Interval |
p Value |
|---|---|---|---|
| Severe spinal injury | 1.6 | 1.2 – 2.3 | 0.007 |
| Severe lower extremity injury |
0.61 | 0.47 – 0.79 | < 0.001 |
| Parenteral nutrition | 2.4 | 1.7 – 3.3 | < 0.001 |
| Female sex | 0.48 | 0.37 – 0.63 | < 0.001 |
| Enteral nutrition group | < 0.001 | ||
| Group 2 | 2.0 | 1.7 – 3.3 | < 0.001 |
| Group 3 | 2.8 | 1.7 – 4.7 | < 0.001 |
| Group 4 | 3.6 | 2.2 – 6.1 | < 0.001 |
| Group 5 | 4.3 | 2.5 – 7.3 | < 0.001 |
The adjusted odds ratios, confidence intervals and p-values are from forward stepwise logistic regression. The variables included in the initial regression model are summarized in the text and are common to the models used for each of the outcomes evaluated in Tables 5, 6 & 7. The enteral groups are detailed in the text and defined in Table 2. The adjusted odds ratios are all relative to the referent category (group 1).
Table 6.
Regression Analyses of Risk Factors for Bloodstream Infection
| Factor | Adjusted odds ratio | 95% Confidence Interval |
p Value |
|---|---|---|---|
| Parenteral nutrition | 2.5 | 1.8 – 3.5 | < 0.001 |
| Volume of blood transfused in first 24 h |
1.2 | 1.0 – 1.3 | 0.035 |
| Enteral nutrition group | 0.031 | ||
| Group 2 | 1.9 | 1.0 – 3.5 | 0.05 |
| Group 3 | 1.9 | 1.1 – 3.5 | 0.02 |
| Group 4 | 1.6 | 0.9 – 3.0 | 0.09 |
| Group 5 | 1.1 | 0.6 – 2.0 | 0.77 |
The adjusted odds ratios, confidence intervals and p-values are from forward stepwise logistic regression. The variables included in the initial regression model are summarized in the text and are common to the models used for each of the outcomes evaluated in Tables 5, 6 & 7. The enteral groups are detailed in the text and defined in Table 2. The adjusted odds ratios are all relative to the referent category (group 1).
Table 7.
Regression Analyses of Risk Factors for Mortality
| Factor | Adjusted odds ratio | 95% Confidence Interval |
p Value |
|---|---|---|---|
| Age group | 1.5 | 1.2 – 1.8 | 0.001 |
| Volume of blood transfused in first 24 h |
1.4 | 1.1 – 1.7 | 0.005 |
| Parenteral nutrition | 1.9 | 1.1 – 3.1 | 0.014 |
| Enteral nutrition group | 0.11 | ||
| Group 2 | 0.7 | 0.3 – 1.6 | 0.41 |
| Group 3 | 0.4 | 0.2 – 0.8 | 0.015 |
| Group 4 | 0.7 | 0.4 – 1.5 | 0.39 |
| Group 5 | 0.5 | 0.2 – 1.1 | 0.08 |
The adjusted odds ratios, confidence intervals and p-values are from forward stepwise logistic regression. The variables included in the initial regression model are summarized in the text and are common to the models used for each of the outcomes evaluated in Tables 5, 6 & 7. The enteral groups are detailed in the text and defined in Table 2. The adjusted odds ratios are all relative to the referent category (group 1).
Overall, VAP (Table 5) was common, occurring in 516 (47%). Compared to group 1, subjects in all other groups had a higher risk for developing VAP, the highest was for subjects in group 5 (aOR = 4.3, 95% CI = 2.5 – 7.3). Severe spinal injury and parenteral nutrition were also associated with risk for VAP. Female sex and severe lower extremity injuries were associated with a lower risk of VAP. Parenteral nutrition use was associated with the development of VAP [adjusted odds ratio (aOR) = 2.4, 95% CI = 1.7 – 3.3]. A total of 243 subjects (23%) developed a bloodstream infection. Subjects receiving some, but relatively few enteral calories (groups 2 and 3) had a 1.9-fold increased risk of a bloodstream infection relative to patients in group 1, even after adjustment for PN use (Table 6). Mortality was associated with age, volume of packed red blood cells transfused in the first 24 hours and the use of parenteral nutrition. There was no association between the amount of enteral nutrition received during the first week and mortality.
All of the analyses above report differences relative to group 1. Subjects in group 1 may differ from those in the other groups in ways that may confound or bias the analyses. Therefore, the results that may be misleading and related to baseline differences rather than to differences in nutrition therapy. We thought it would be important to understand differences in subjects who received at least some enteral support during the first week, and repeated the analyses with group 1 excluded. The following results are from analyses limited to the 991 subjects in groups 2 through 5. Relative to group 2, the remaining groups each had a significant increased risk for VAP. Group 5 has the highest associated risk (aOR = 1.9, 95% CI= 1.3 – 3.1). As in the previous analysis, parenteral nutrition and spinal cord injury were associated with a higher risk for VAP. Only parenteral nutrition remained associated (aOR = 2.3, 95% CI = 1.6 – 3.2) with bloodstream infections. Lastly, the volume of blood transfused (aOR = 1.3, 95% CI = 1.1 – 1.7) and age group (aOR = 1.5, 95% CI = 1.2 – 1.9) remained associated with mortality. In summary, these analyses, limited to subjects that received some enteral support during the first week, confirmed that PN was an important risk factor for bloodstream infections and that the volume of enteral support during the first week was associated with VAP.
DISCUSSION
This study of nutritional support in a large group of severely injured blunt trauma patients offers some important observations and highlights the challenges faced in their management. We observed that a large fraction of severely injured trauma patients do not reach caloric goals enterally and this persists well beyond the first few days of their ICU stay. The proportion of subjects receiving at least 50% of target daily caloric intake did gradually increase over the first week, but only 441 (42%) of those remaining in the ICU beyond the first week had reached this threshold by the 7th post injury day. Resuscitation, diagnostic imaging and operative procedures all take priority over nutritional support and clearly limit caloric intake during the first few days after severe trauma but our observations indicate that these limitations persist and likely lead to caloric deficits. By the 14th day in the ICU, the fraction of subjects receiving >50% of estimated caloric needs had increased somewhat to 344/629 (55%). Despite our guidelines and despite current recommendations by multiple critical care organizations, we observed that hypocaloric enteral feeding is a standard de facto practice in severely ill trauma patients during the first post-injury week, which was occurring across all involved institutions, with some variability. The nutritional support and other standard operating procedures were designed to minimize variations in care across the participating institutions. Compliance was recorded for each institution and reported at quarterly meetings of the investigators. Compliance with the nutritional support guidelines was assessed on ICU days 5 and 7. There was some variation between the sites in the provision of enteral nutrition. For example, as documented in the most recent compliance report, the mean total daily calories on ICU day 5 ranged from a low of 980 kcal/day to 1680 kcal/day. This was consistent with the observations in this study in which the various institutions were represented in different proportions across the 5 nutritional support groups. However, when site/institution was included as a variable in the regression models, there was no association with the infectious outcomes or with mortality. This suggests that there were some differences in practice between institutions, but these differences were small enough to not influence outcomes. Nevertheless, small differences in practice between institutions might warrant more detailed study to help understand why they were present and if they could be further reduced.
Our previously published guidelines include measures to identify evidence of feeding intolerance (abdominal distention, elevated gastric residual volumes, etc.) meant to reduce the risk of complications, such as aspiration.9 However, aspiration can be subclinical in mechanically ventilated patients with endotracheal tubes and suppressed cough reflexes. Endotracheal tube cuffs do not provide complete protection from aspiration; nasogastric tubes impair normal esophageal motility, inhibit lower esophageal sphincter function and together may permit micro-aspiration. We observed that those who received the highest amount of enteral calories during the first week also had the highest incidence of VAP. This risk remained elevated after adjustment for other risk factors, and after excluding the lowest risk (group 1) subjects. Our observation raises the possibility that aggressive early enteral nutrition contributes to aspiration and pneumonia. Given that early ileus is a recognized complication after abdominal surgery and that supine immobilization is a common logistical limitation after blunt trauma, our observations document that these constitute major VAP risks in our patient cohort. We should not over-interpret this observation or its implications. Despite having a higher incidence of ventilator associated pneumonia, subjects in group 5 did not spend a longer time on the ventilator, in the ICU or in the hospital, nor did they have a higher case-fatality rate than subjects in the other groups. The EDEN Randomized Trial attempted to determine whether initial trophic feeding influences outcomes in patients with ARDS.20 In that study, subjects were randomized to either trophic feeding (~400 kcal/day) to full feeding (~1300 kcal/day). There were no differences in important outcomes in that study. Despite the subjects being substantially different, our observations are consistent with the results of that trial. In our study, groups 3, 4 and 5 most closely approximate the description of subjects in the EDEN trail. We observed no differences in ICU or hospital length of stay or in mortality between these 3 groups. Bloodstream infections were more common in group 3, perhaps due to the greater use or parenteral nutrition in that group relative to the others.
Supplementation with parenteral nutrition when prescribed caloric goals are not reached has been recommended in order to avoid caloric and nutrient deficits. We previously reported that such an approach, despite improving nutritional markers, does not improve clinical outcomes.7 Supporting our previous observations, the results of a large clinical trial (referred to as the EPaNIC trial) suggest that early supplemental parenteral nutrition is harmful. Based upon their results, the authors of that study concluded that early (day 3) initiation of parenteral nutrition in patients receiving insufficient enteral nutrition is inferior to waiting until day 7 to initiate parenteral nutrition.8 More recently, another reported clinical trial also failed to identify a clear benefit to the early administration of PN in critically ill adults.21,22 However, unlike the EPaNIC trial, this study did not find PN to be harmful. Taken together, our observations, and previous published literature indicate that early supplemental parenteral support does not improve clinical outcomes and is associated with increased mortality.
Limitations of our study include that it is observational and that there were clear differences between the groups that could account for, at least in part, the outcome differences we observed. For example, subjects who underwent laparotomy and those who were managed with an open abdomen received less enteral support during the first week. Although, patients with abdominal injuries were still expected to be managed according to the nutritional support algorithm, we cannot determine whether they did not tolerate advances in enteral support or whether clinicians simply chose to withhold or advance support more slowly. It is likely that both of these factors contributed to subjects with abdominal injuries receiving less enteral support during the first week. Despite this and other baseline differences possibly contributing to patients receiving less enteral support during the first week, it is unlikely that these are entirely responsible for the differences in outcomes that we observed. Also, a small number of patients received parenteral nutrition during the first week. Most of these subjects were in group 1 (37%) or group 2 (45%). While the decision to use parenteral nutrition earlier than day 7 deviates from our guidelines, it is consistent with the literature that existed at the time these subjects were enrolled. The database does not include information regarding the indications for PN in individual cases and we cannot determine why PN was initiated earlier than recommended. While unmeasured differences, associated with PN use may have contributed to the increased risk for bloodstream infections, there is considerable evidence that implicates PN directly in this complication. Lastly, we observed an association between the number of enteral calories received during the first week and VAP; implicating micro-aspiration exacerbated by aggressive early feeding practices. It is important to recognize that other factors may also be implicated. Subjects receiving the highest amount of calories in the first week (group 5) had a higher incidence of severe thoracic injuries, and despite controlling for this variable in our analysis, it is likely that part of the increased risk for VAP is related to chest trauma.
The basis for the benefits of enteral support in critically ill patients may not be solely due to the provision of calories, nitrogen or other macronutrients. Many benefits may be related to the use of the enteral route, including improvement of splanchnic blood flow, better glucose tolerance and improved nutrient utilization. This has led some to postulate that hypocaloric feeding is sufficient. The data in this regard are not clear and there is evidence of improved clinical outcomes associated with increased delivery of enteral calories.23 We do not advocate deliberate attempts to provide hypocaloric feeding but also recognize that the addition of parenteral support in order to reach a total caloric and macronutrient target is not appropriate for the majority of critically ill trauma patients.
We conclude that the majority of severely injured trauma patient can be supported enterally during their ICU stay, but that actual caloric intake is often less than recommended. The high risk of VAP in the subjects receiving the most calories enterally suggests that feeding practices during the first week may contribute to aspiration and pneumonia. However, this does not prolong the duration of care or increase mortality. Finally, we observed parenteral nutrition to be associated with an increased risk of blood stream infections, a higher risk for VAP and a greater risk of death.
Acknowledgments
The Inflammation and the Host Response to Injury “Glue Grant” program is supported by the National Institute of General Medical Sciences. This article was prepared using a dataset obtained from the Glue Grant program and does not necessarily reflect the opinions or views of the Inflammation and the Host Response to Injury Investigators or the National Institute of General Medical Sciences. This study is supported by the Inflammation and the Host Response to Injury Large-Scale Collaborative Project Award 2-U54-GM062119 from the National Institute of General Medical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
References
- 1.Gramlich L, Kichian K, Pinilla J, et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition. 2004;20:843–848. doi: 10.1016/j.nut.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992;216:172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore FA, Moore EE, Jones TN, et al. TEN versus TPN following major abdominal trauma--reduced septic morbidity. J Trauma. 1989;29:916–922. doi: 10.1097/00005373-198907000-00003. discussion 922-913. [DOI] [PubMed] [Google Scholar]
- 4.Peterson VM, Moore EE, Jones TN, et al. Total enteral nutrition versus total parenteral nutrition after major torso injury: attenuation of hepatic protein reprioritization. Surgery. 1988;104:199–207. [PubMed] [Google Scholar]
- 5.Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr. 2001;74:534–542. doi: 10.1093/ajcn/74.4.534. [DOI] [PubMed] [Google Scholar]
- 6.Dissanaike S, Pham T, Shalhub S, et al. Effect of immediate enteral feeding on trauma patients with an open abdomen: protection from nosocomial infections. J Am Coll Surg. 2008;207:690–697. doi: 10.1016/j.jamcollsurg.2008.06.332. [DOI] [PubMed] [Google Scholar]
- 7.Sena MJ, Utter GH, Cuschieri J, et al. Early supplemental parenteral nutrition is associated with increased infectious complications in critically ill trauma patients. J Am Coll Surg. 2008;207:459–467. doi: 10.1016/j.jamcollsurg.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 9.O’Keefe GE, Shelton M, Cuschieri J, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care VIII--Nutritional support of the trauma patient. J Trauma. 2008;65:1520–1528. doi: 10.1097/TA.0b013e3181904b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin CM, Doig GS, Heyland DK, et al. Multicentre, cluster-randomized clinical trial of algorithms for critical-care enteral and parenteral therapy (ACCEPT) CMAJ. 2004;170:197–204. [PMC free article] [PubMed] [Google Scholar]
- 11.Maier RV, Bankey P, McKinley B, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care. J Trauma. 2005;59:762–763. [PubMed] [Google Scholar]
- 12.Cuschieri J, Freeman B, O’Keefe G, et al. Inflammation and the host response to injury a large-scale collaborative project: patient-oriented research core standard operating procedure for clinical care X. Guidelines for venous thromboembolism prophylaxis in the trauma patient. J Trauma. 2008;65:944–950. doi: 10.1097/TA.0b013e3181826df7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans HL, Cuschieri J, Moore EE, et al. Inflammation and the host response to injury, a Large-Scale Collaborative Project: patient-oriented research core standard operating procedures for clinical care IX. Definitions for complications of clinical care of critically injured patients. J Trauma. 2009;67:384–388. doi: 10.1097/TA.0b013e3181ad66a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harbrecht BG, Minei JP, Shapiro MB, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care: VI. Blood glucose control in the critically ill trauma patient. J Trauma. 2007;63:703–708. doi: 10.1097/TA.0b013e31811eadea. [DOI] [PubMed] [Google Scholar]
- 15.Minei JP, Nathens AB, West M, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: patient-oriented research core--standard operating procedures for clinical care. II. Guidelines for prevention, diagnosis and treatment of ventilator-associated pneumonia (VAP) in the trauma patient. J Trauma. 2006;60:1106–1113. doi: 10.1097/01.ta.0000220424.34835.f1. discussion 1113. [DOI] [PubMed] [Google Scholar]
- 16.Moore FA, McKinley BA, Moore EE, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care. III. Guidelines for shock resuscitation. J Trauma. 2006;61:82–89. doi: 10.1097/01.ta.0000225933.08478.65. [DOI] [PubMed] [Google Scholar]
- 17.Nathens AB, Johnson JL, Minei JP, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: Patient-Oriented Research Core--standard operating procedures for clinical care. I. Guidelines for mechanical ventilation of the trauma patient. J Trauma. 2005;59:764–769. [PubMed] [Google Scholar]
- 18.West MA, Moore EE, Shapiro MB, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care VII--Guidelines for antibiotic administration in severely injured patients. J Trauma. 2008;65:1511–1519. doi: 10.1097/TA.0b013e318184ee35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West MA, Shapiro MB, Nathens AB, et al. Inflammation and the host response to injury, a large-scale collaborative project: Patient-oriented research core-standard operating procedures for clinical care. IV. Guidelines for transfusion in the trauma patient. J Trauma. 2006;61:436–439. doi: 10.1097/01.ta.0000232517.83039.c4. [DOI] [PubMed] [Google Scholar]
- 20.Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doig GS. Parenteral versus enteral nutrition in the critically ill patient: additional sensitivity analysis supports benefit of early parenteral compared to delayed enteral nutrition. Intensive Care Med. 2013;39:981–982. doi: 10.1007/s00134-013-2856-5. [DOI] [PubMed] [Google Scholar]
- 22.Doig GS, Simpson F, Sweetman EA, et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA. 2013;309:2130–2138. doi: 10.1001/jama.2013.5124. [DOI] [PubMed] [Google Scholar]
- 23.Alberda C, Gramlich L, Jones N, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. 2009;35:1728–1737. doi: 10.1007/s00134-009-1567-4. [DOI] [PubMed] [Google Scholar]