Abstract
Previous studies have indicated increased plasma levels of inducible nitric oxide synthase in lung. This study further examines the pulmonary expression of nitric oxide synthase (NOS) isoforms in an ovine model of acute lung injury induced by smoke inhalation and burn injury (S+B injury). Female range bred sheep (4 per group) were sacrificed at 4, 8, 12, 24, and 48 hours after injury and immunohistochemistry was performed in tissues for various NOS isoforms. The study indicates that in uninjured sheep lung, endothelial (eNOS) is constitutively expressed in the endothelial cells associated with the airways and parenchyma, and in macrophages. Similarly, neuronal (nNOS) is constitutively present in the mucous cells of the epithelium and in neurons of airway ganglia. In uninjured lung, inducible (iNOS) was present in bronchial secretory cells and macrophages. In tissue after S+B injury, new expression of iNOS was evident in bronchial ciliated cells, basal cells, and mucus gland cells. In the parenchyma, a slight increase in iNOS immunostaining was seen in type I cells at 12 and 24 hours after injury only. Virtually no change in eNOS or nNOS was seen after injury.
Keywords: acute lung injury, airway, immunohistochemistry, lung, mucous glands, nitric oxide, nitric oxide isoforms, sheep
Inhalation injury increases the morbidity and mortality of burn injury patients [1]. Previous studies have shown that the combination of burn and inhalation of cooled cotton smoke (S+B injury) in sheep effectively models the pathophysiology of these injuries in humans [2] and is neutrophil dependent [3]. However, recent studies have demonstrated that nitric oxide (NO), derived from nitric oxide synthases (NOS), contributes to the development of acute lung injury (ALI) in sheep after S+B injury [4, 5].
Numerous studies have examined the mechanisms by which NO may be involved in the pathophysiology of disease processes. NO has been shown to react with superoxide to yield the free radical peroxynitrite (ONOO−) [6–8]. Peroxynitrite has cytotoxic potential through reactions with protein and nonprotein sulfyhdryls, oxidation of lipids, and nitrosylation of tyro-sine [9, 10]. However, studies have also shown that NO may promote the chemotactic potential of neutrophils [11–14] and modulate the production of proinflammatory agents [13].
To more fully understand the dynamics by which NOS inhibitors can modify the responses of the lung to injury, and to understand the mechanisms by which NO contributes to ALI, requires identification of the cells that express these enzymes and the dynamics of their expression. The purpose of this study is to use immunohistochemical techniques to identify the nitric oxide synthase (NOS) isoforms, endothelial (eNOS), neuronal (nNOS), and inducible (iNOS), that are expressed in uninjured lung and to examine the temporal changes in expression of these enzymes in the respiratory system following burn and smoke inhalation injury in sheep.
MATERIALS AND METHODS
Animal Care and Use
This study was approved by the Animal Care and Use Committee of the University of Texas Medical Branch and conducted in compliance with the guidelines for the care and use of laboratory animals of the National Institutes of Health and the American Physiological Society.
Animals used in this study were female, range bred, sheep weighing approximately 40 kg. Preparation for injury and chronic study was done as previously described [5]. Briefly, for surgical preparation, the animals received endotracheal intubation and ventilation while under halothane anesthesia, followed by cannulation of the right femoral artery and vein. Additionally, a thermodilution catheter was introduced through the right common jugular vein into the pulmonary artery. A Silastic catheter was also positioned in the left atrium to directly measure left atrial pressure. Following surgical preparation, the animals were allowed to recover for 5 to 7 days and given free access to food and water. Prior to injury, the animals were deeply anesthetized with 3% halothane.
Smoke inhalation and Burn Injury
Previous studies have described in detail the protocols for smoke inhalation and burn injury [2, 15]. Briefly, smoke inhalation injury was induced by placing 40 g of ignited cotton toweling in a modified bee smoker attached to a tracheostomy tube containing a thermistor. The temperature of the smoke was continuously monitored to ensure that it did not exceed 40°C. Four series of 12 breaths (total 48 breaths) were delivered. Carboxyhemoglobin concentration in the arterial blood was monitored (CO-Oximeter 282; Instrumentation Laboratory, Lexington, MA) after each 12 breaths to ensure that each animal had received an equivalent dose.
Cutaneous burn injury, 40%, 3rd degree, was inflicted with a Bunsen burner, with each flank of the animal receiving a 20% total body surface area burn. The injury was a full-thickness burn, including both the epidermis and dermis, such that the nerve endings were destroyed, and the sheep did not show pain or distress after the procedure. Following injury, animals were given free access to food. Water intake was restricted and the animals were resuscitated with Ringer’s lactate solution at 4 mL/kg/24 h [16]. During the study period, the animals were monitored in a critical care facility. A total of 18 sheep were used in this study. Three sheep with surgical preparation and no injury or ventilation (sham animals) were sacrificed to determine expression of NOS isoforms in uninjured sheep lung. To examine the cellular and temporal changes in NOS isoform expression, 3 sheep per group were sacrificed at 4, 8, 12, 24, and 48 hours after S+B injury. Sacrifice of the animals was accomplished with an overdose of ketamine followed by injection of saturated potassium chloride solution.
Tissue Sampling and Scoring
Following verification of death, the trachea and lungs were removed and tissue for histology was collected in a systematic way. A cross-section approximately 1 cm thick was taken through the lower lobe of the right lung, at right angles to the main bronchus, midway from the hilum to the distal tip, injected with 10% formalin, and immersed in fixative for 3 to 5 days. Following fixation, the tissue slice was sampled into 4 blocks for light microscopic analysis. A technician unaware of the treatment group accomplished sampling of the lung slice for histological processing. The major bronchus was always included in one block, with other blocks sampled to assess more distal parenchyma. All tissues were placed into processing cassettes to maintain the orientation of the histological sections parallel to the original midline cross-section of lung tissue. Immunohistochemical staining for NOS isoforms was conducted on tissue samples containing the cross-section of the main bronchus and surrounding parenchyma.
Immunohistochemistry
For immunohistochemical staining, sheep lung tissue was sectioned at 4 microns from 3 animals at each time point. Slides were heated overnight at 60°C, deparaffinized, and treated with HIER (heat-induced epitope retrieval) for 20 minutes in a steamer. Endogenous peroxidase was blocked using 0.6% hydrogen peroxide in methanol for 5 minutes. Nonspecific binding was blocked with normal serum for 30 minutes and incubated with the appropriate antibody overnight at 4°C. Antigen detection was performed with the Vectastain elite peroxidase kit with diaminobenzidine (DAB) as the chromogen (Vector Laboratories, Burlingame, CA). A polyclonal antibody to nNOS (Upstate Biotechnology, Lake Placid, NY) was used at a concentration of 0.25 μg/mL. For eNOS immunostaining, a monoclonal antibody (Transduction Laboratories, San Diego, CA) was used at a concentration of 0.125 μg/mL. Immunolocalization of iNOS was accomplished with a polyclonal antibody (Affinity Bioreagents, Golden, CO) at a concentration of 0.5 μg/mL. Following immunostaining procedures, slides were counter-stained with hematoxylin. Appropriate controls were run with mouse or rabbit immunoglobulin G (IgG) at the same concentrations as the primary antibodies.
Following completion of immunohistochemical procedures, 3 investigators independently examined the slides, identifying cell types of the bronchi and parenchyma that were immunostained for each specific isoform, and assigning a score of relative intensity. A score of 0 represented the absence of any immunolocalization, with 1= light, 2 = moderate, 3 = strong, and 4= intense staining. Following analysis, all scores were compared to confirm the identification and intensity of cellular staining for each isoform. The 3 independent scores were then averaged and used to assess changes in temporal expression of each isoform.
Statistical Analysis
Statistical analysis of scoring results was done by the unpaired Student’s t test using GraphPad PRISM software (San Diego, CA) and statistical significance was accepted when P < .05.
RESULTS
Comparison of scoring from 3 independent examinations of the stained slides showed similar identification of cells staining positive for each isoform, and similar trends in changes that were evident between isoforms and between uninjured and injured tissues. All immunohistochemical control slides, using the appropriate IgG concentrations, showed virtual absence of any staining.
Results showed that eNOS and nNOS isoforms were constitutively expressed in uninjured tissue. Light to moderate immunostaining for eNOS was localized in ciliated, secretory, and basal cells of the bronchial epithelium, whereas endothelial cells of the submucosa had much more intense staining (Table 1, Figure 1). Light staining of the bronchiolar epithelium and moderate staining of the endothelium were also seen using the antibody for eNOS. Within the parenchyma of the uninjured tissue, both type I and II pneumocytes, interstitial macrophages, and capillary endothelial cells exhibited light to moderate immunostaining for eNOS (Table 2, Figure 2). Examination of tissue following S+B injury detected no significant difference in staining intensity among the cells that stained positive in the uninjured tissue, nor was any new cellular localization detected following injury.
TABLE 1.
Relative Intensity and Cellular Sites of eNOS and nNOS Expression in Bronchial Tissue of Uninjured Sheep
| NOS isoform | Bronchial epithelium
|
Submucosal tissue
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CC | SC | BC | Endo | MG | MØ | FB | IF | SM | |
| eNOS | + | ++ | ++ | + | + | + | – | – | + |
| nNOS | – | ++ | – | + | + | + | – | – | + |
Intensity of immunostaining (– = absent, + = light, ++ = moderate, + + + = strong) in CC = ciliated cells, SC = secretory cells, BC = basal cells, Endo = endothelial cells, MG = mucous gland acinar cells, MØ = macrophages, FB = fibroblasts, IF = inflammatory cells, SM = smooth muscle cells. Identical results were obtained in tissues from injured sheep.
FIGURE 1.
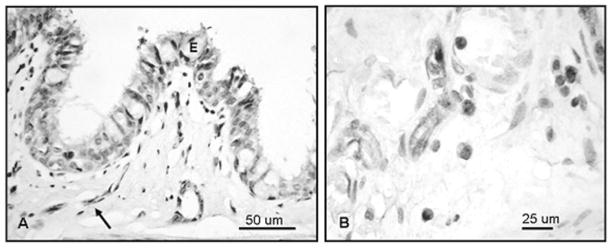
Immunostaining for eNOS in main bronchial tissue of an uninjured sheep lung. (A) Staining is present in ciliated, secretory, and basal cells of the lining epithelium (E) and endothelial cells (arrow). (B) Cells with morphology of macrophages show cytoplasmic staining as intense as that of endothelial cells.
TABLE 2.
Relative Intensity and Cellular Sites of eNOS and nNOS Expression in Bronchioles and Parenchyma of Uninjured Sheep
| NOS isoform | Broncheoles
|
Parenchyma
|
||||||
|---|---|---|---|---|---|---|---|---|
| CC | SC | BC | T1 | T2 | IMØ | AM | CEndo | |
| eNOS | + | ++ | ++ | + | + | + | – | ++ |
| nNOS | – | – | – | + | – | + | – | – |
Note. Intensity of immunostaining (– = absent, + = light, ++ = moderate, + + + = strong) in Epith = epithelium, Endo = endothelial cells, T1 = type I cells, T2 = type II cells, IMØ = interstitial macrophages, AMØ = alveolar macrophages, and CEndo = septal capillary endothelial cells. Similar results were obtained in injured sheep lung for these constitutive NOS enzymes.
FIGURE 2.
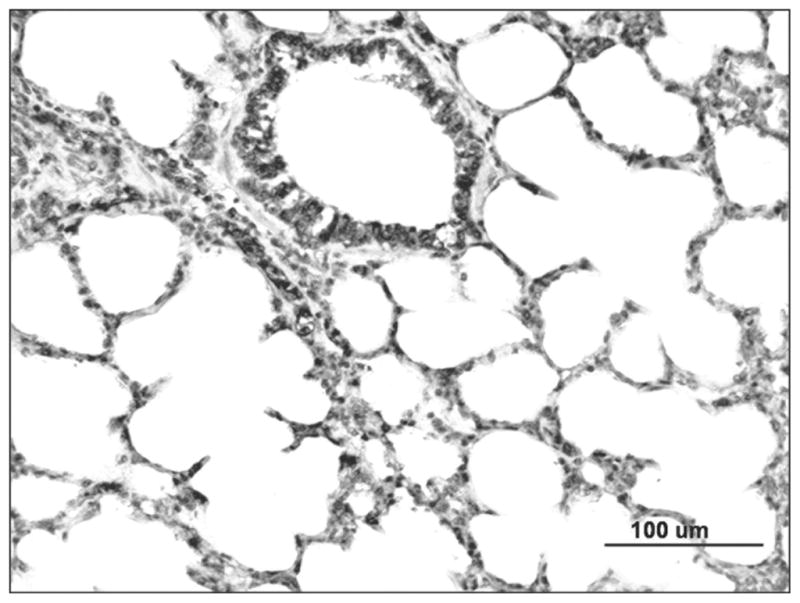
Localization of eNOS staining in the parenchyma of uninjured sheep lung shows positive staining in airway epithelial and endothelial cells, including type I and II epithelial cells and interstitial macrophages.
In tissue from uninjured sheep, nNOS immunostaining was localized in secretory cells of the bronchial lining epithelium and submucosal glands. Light staining was also evident in bronchial smooth muscle tissue and macrophages. Localization of nNOS immunostaining to the above cell types in uninjured sheep bronchi is depicted in Figure 3A. Intense nNOS immunostaining was also seen in a few neurons within intrinsic airway parasympathetic ganglia, as shown in Figure 3B. The ganglia containing nNOS-staining neurons tended to be located in the angles between the bronchial cartilage and the pulmonary artery. Immunostaining was absent in the epithelium of the smaller bronchioles and in the parenchyma, except for light immunostaining in alveolar and interstitial macrophages. Examination of tissue from injured sheep showed no significant change in the types of cells staining positive for the nNOS isoform, or in the intensity of staining. Among the 3 independent scores, a trend of decreased immunostaining for nNOS was detected in the bronchial secretory cells, perhaps because secretory cells express their contents and detach after S+B injury. No change in the intensity of nNOS immunostaining in the other cell types was evident after injury. The patterns of cellular expression and relative intensity of staining for eNOS and nNOS in uninjured and injured tissue are depicted in Tables 1 and 2.
FIGURE 3.
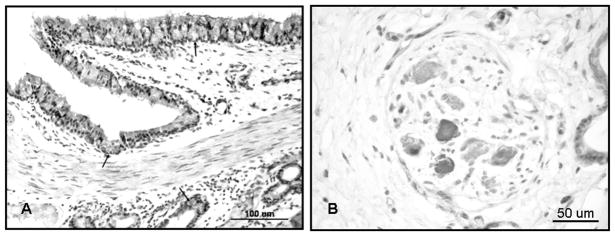
Immunolocalization of nNOS in bronchial tissue of an uninjured sheep (A). Staining was prominent in secretory cells of the lining epithelium and submucosal glands (arrows). Light staining was also present in endothelial cells, smooth muscle tissue, and macrophages (B).
Examination of uninjured tissue immunostained for iNOS showed moderate expression of this isoform in secretory cells of the bronchial epithelium and submucosal macrophages, as well as in alveolar and interstitial macrophages of the parenchyma. Light immunostaining for iNOS was also detected in bronchial ciliated cells and in submucosal glands (Figure 4A), and in the bronchiolar epithelium (Figure 4B).
FIGURE 4.
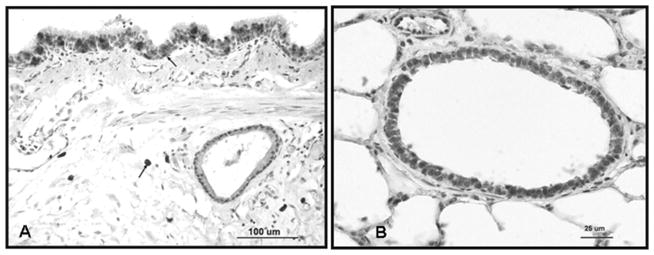
Immunolocalization of iNOS in uninjured sheep lung (A). Micrograph showing expression in secretory cells of the bronchial epithelium and submucosal macrophages (arrows). (B) Micrograph shows iNOS expression in alveolar and interstitial macrophages of the parenchyma.
In tissue after S+B injury, there was a striking increase in the intensity of iNOS immunostaining in the bronchial epithelium, including all cell types, along with intense expression within submucosal gland secretory cells, beginning with the earliest time interval studied, 4 hours after injury (Figure 5A and B). The new expression of iNOS in basal cells was retained through the 48-hour study period, though columnar epithelial cells in the main bronchus were progressively detached after injury. More intense iNOS immunostaining was also evident in the bronchial smooth muscle tissue and in the bronchiolar epithelium that was retained through the 48-hour study period. These changes were relatively uniform throughout the lung. Tables 3 and 4 contain the relative intensity scores for iNOS immunoreactivity, showing new and increased expression of this isoform in the main bronchus, bronchial mucous glands, and bronchioles, respectively. Within the parenchyma, only a mild increase in iNOS immunoreactivity was evident in type I cells at 12 and 24 hours after injury. In Table 3, statistical analysis of the staining intensity scores for basal cells of the bronchial epithelium and mucous glands showed a significant increase at all time points after injury compared to uninjured tissue. These data are represented graphically in Figure 6A and B.
FIGURE 5.
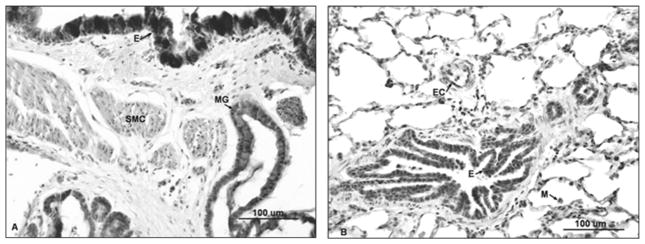
Intense staining for iNOS in sheep lung 4 hours after S+B injury. (A) Micrograph depicting increased staining of the entire bronchial epithelium (E), mucous glands (MG), and smooth muscle cells. (B) After injury, increased iNOS expression is also present in bronchiolar epithelium (E), endothelial cells (EC), and alveolar macrophages (M) in the parenchyma.
TABLE 3.
Relative Intensity and Cellular Sites of iNOS Expression in the Main Bronchus in Uninjured Sheep and in Sheep after S+B Injury
| Bronchial epithelium
|
Submucosal tissue
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (hour) | CC | SC | BC | Endo | MG | MØ | FB | IC | SM |
| 0 | + | + + + | – | + | + | + + + | – | – | – |
| 4 | + + + | + + + | ++ | ++ | + + + | + + + | ++ | + | ++ |
| 8 | ++ | + + + | + + + | ++ | + + + | + + + | ++ | + | + |
| 12 | + + + | + + + | + + + | ++ | + + + | + + + | ++ | ++ | ++ |
| 24 | * | * | + + + | ++ | + + + | + + + | + | ++ | + |
| 48 | * | * | + + + | ++ | + + + | + + + | ++ | ++ | + |
Note. Intensity of immunostaining (– = absent, + = light, ++ = moderate, + + + = strong) in CC = ciliated cells, SC = secretory cells, BC = basal cells, Endo = endothelial cells, MG = mucous gland acinar cells, MØ = macrophages, FB= fibroblasts, IF = inflammatory cells, and SM = smooth muscle cells.
Cells displaced from the epithelium were not scored.
Note Figure 6.
TABLE 4.
Relative Intensity and Cellular Sites of iNOS Expression in Bronchioles and Parenchyma of Uninjured Sheep and in Sheep after S+B Injury
| time (hour) | Bronchioles
|
Parenchyma
|
|||||
|---|---|---|---|---|---|---|---|
| Epith | Endo | T1 | T2 | IMØ | AMØ | CEndo | |
| 0 | + | + | + | ++ | ++ | ++ | – |
| 4 | ++ | ++ | + | ++ | ++ | ++ | – |
| 8 | ++ | ++ | + | ++ | ++ | ++ | – |
| 12 | + + + | ++ | ++ | ++ | ++ | ++ | – |
| 24 | + + + | + + + | ++ | ++ | ++ | ++ | – |
| 48 | ++ | ++ | + | ++ | ++ | ++ | – |
Note. Intensity of immunostaining (– = absence, + = light, ++ = moderate, + + + = strong) in Epith = epithelium, Endo = endothelial cells, T1 = type I cells, T2 = type II cells, IMØ = interstitial macrophages, AMØ = alveolar macrophages, and CEndo = septal capillary endothelial cells.
FIGURE 6.
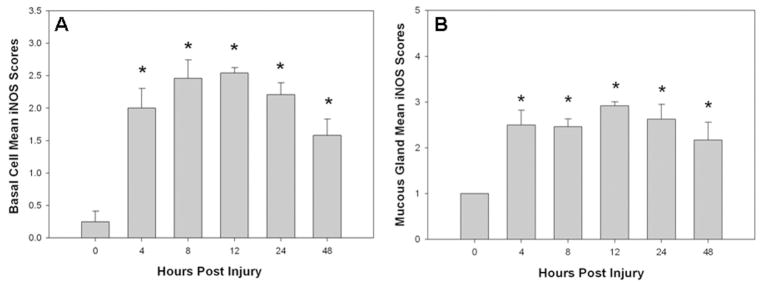
(A) Graph showing that the mean of the semiquantitative scores for iNOS expression in basal cells is significantly increased and maintained at 4 though 48 hours after injury. (B) Graph showing that the mean of the semiquantitative scores for iNOS expression in mucous glands was also significantly increased at all time points after injury. *Significantly different from uninjured, P < .05.
DISCUSSION
This study has demonstrated that all 3 isoforms of NOS are expressed in the lung of uninjured sheep. Following injury, the most pronounced change in expression of these enzymes was in the iNOS isoform. The expression of the eNOS and nNOS isoforms remained relatively unchanged, consistent with the roles of these isoforms as constitutive types of nitric oxide synthase.
Endothelial nitric oxide synthase (eNOS) was localized in ciliated cells of the lining epithelium, endothelial cells, macrophages, alveolar type I and II pneumocytes, and in the lining epithelial cells of the bronchial submucosal glands. These results are in agreement with previous studies demonstrating vascular endothelial and large airway expression of eNOS in sheep lung [17–19]. Expression of eNOS has also been described in macrophages, consistent with the faint expression described here [20, 21]. Expression of eNOS has also been observed in ciliated, Clara, and type II cells of rat airway epithelial cells [22, 23]. In this study, clearly, goblet cells showed no eNOS immunoreactivity; however, other secretory or nonciliated cells stained positive for eNOS. A study by Shaul and colleagues in a nonciliated human bronchial epithelial cell line demonstrated that a calcium-dependent NOS isoform was present, and confirmed this with immunohistochemistry [24]. Further studies are needed to identify the nonciliated airway epithelial cells in sheep or humans that contain eNOS. Following injury, no new expression of eNOS immunostaining was detected, nor was any change in staining intensity detected in any cell type, as compared to uninjured tissue.
Examination of nNOS immunostaining showed that in uninjured animals, the bronchial secretory cells of the lining and glandular epithelium, interstitial macrophages, and smooth muscle tissue were lightly stained. Neuronal cell bodies were also positive for nNOS in ganglia around bronchi. In the smaller airways and parenchyma, staining for nNOS was absent, except for light localization in the type I cells of the alveolar epithelium and faint staining of macrophages. nNOS is not normally observed in tissue macrophages, so it may be that the light staining for nNOS that we observed in bronchial submucosal, interstitial, and alveolar macrophages, as well as faint staining of airway smooth muscle, may have been due to nonspecific binding of primary antibody. In review of the literature, previous studies localizing pulmonary nNOS expression have shown localization in neuronal cell bodies and fibers in frogs [25] and mice [26]. In developing and adult sheep lung, Sherman and colleagues showed similar expression of nNOS as observed in this study, except that the airway expression extended into the small bronchiolar airways and was more extensive in the alveolar epithelium [19]. Possibly the greater intensity of staining and increased range of cellular expression in their study may have been due to use of a higher concentration of primary antibody.
Results of iNOS staining in this study showed that in uninjured sheep, secretory cells of the lining epithelium and macrophages in the bronchial submucosa stained strongly for iNOS. Light staining for iNOS was seen in the submucosal glands and vascular endothelium. Light staining was also localized in bronchiolar epithelium. In the parenchyma, strong staining for iNOS was detected in type II pneumocytes and in interstitial and alveolar macrophages, whereas light staining was localized in type I alveolar lining cells. Following injury, at all time intervals, more intense staining was seen in all cells of the bronchial epithelium and submucosal glands, endothelium, and smooth muscle tissue. Independent intensity scores also showed increases in staining in bronchiolar epithelium and type I alveolar epithelial cells after injury. The results of iNOS expression observed in uninjured sheep are similar to the results shown by Sherman and colleagues who reported the expression of iNOS in the epithelium of both large and small airways. However, in their study, iNOS was not detected in the alveolar epithelium or endothelium [19]. In our study, light staining for iNOS was seen in submucosal endothelium, and expression at that site was increased after injury. No expression was observed in capillary endothelial cells of the parenchyma of either uninjured or injured sheep.
The pathophysiology of smoke inhalation injury includes increased bronchial blood flow, increased lung lymph flow, and pulmonary edema [27]. Studies have demonstrated attenuation of the pathophysiological responses to S+B injury after interventions that prevented the rise in bronchial blood flow by bronchial artery ligation and ethanol injection [28, 29]. Recent studies from our laboratory have also demonstrated a 2- to 3-fold increase in NO metabolites in sheep after smoke inhalation injury [4, 5, 30]. The mechanisms by which inhalation of toxic smoke induced production of nitric oxide and increased expression of iNOS are not yet clear, but are subjects of active investigation. Previous studies indicated that iNOS produced in the airway circulation plays a major role on the significant increase in airway blood flow, which may contribute to the spread of injury from injured airway to the lung parenchyma [31, 32]. Sensory neural reflex mechanisms, nuclear oxidative signaling and activation of nuclear factor (NF)-κB are also suspected.
In recent studies, we have used specific inhibitors of the iNOS isoform in sheep after S+B injury and observed significantly reduced bronchial blood flow, pulmonary vascular resistance, and pulmonary edema. The use of these iNOS inhibitors attenuated the development of ALI in this injury model [4, 5]. An additional study has demonstrated that nNOS inhibition attenuates ALI in sheep with S+B injury. Enkhbaatar and colleagues have also confirmed the contribution of nNOS to ALI in a model of smoke inhalation injury and lung sepsis [33]. The results of this study demonstrating the acute, progressive increase in iNOS isoform expression support the concept that the contribution of NO to ALI may be important in this injury model. However, mechanisms besides modulation of blood flow and tissue injury may exist, through which NO may contribute to the pathophysiology of ALI in this injury model.
One important feature of this injury model is the formation of bronchial obstructive “casts” that are composed of fibrin, neutrophils, and mucus. Life-threatening cast formation is also observed in burn patients with severe inhalation injury [34]. Two studies have shown an association between reduced cast formation and improvement of pulmonary function in an ovine model of inhalation injury [35, 36]. Further, the work of Hubbard and colleagues, also using an ovine model of inhalation injury, demonstrated extensive obstruction of airways, to which these authors attributed all the animal deaths in their study [37]. Recent studies have measured the degree of airway obstruction in sheep after S+B injury and demonstrated that approximately 10% of bronchi are extensively obstructed after injury and that the obstructive material appears to migrate into distal airways and parenchyma over time [38]. The major components of the obstructive material identified in that study were mucus derived from submucosal gland secretion and neutrophils from the intense inflammatory reaction following injury to the upper airways. Studies have shown that NO is a mediator in both glandular secretion and in the acute inflammatory reaction.
Hypersecretion of mucus is common in airway diseases. Studies have shown that endogenous NO modulates submucosal gland secretion. Studies using isolated feline and human bronchial submucosal glands have demonstrated that the NO generating compound isosorbide dinitrate can promote secretion, and that inhibition of NOS isoforms reduced methacholine and bradykinin-induced secretion [39]. In this study, we found light staining of all 3 isoforms of NOS in mucous glands, and increased expression of iNOS that was evident in sheep 4 hours after injury and continued to 48 hours. Together, these studies suggest a functional role for NO in bronchial gland secretion in sheep after S+B injury and, may help explain the reduced airway obstruction that has been seen in sheep treated with specific inhibitors of iNOS [4, 40].
Recent studies have also implicated a role for NO in recruitment of acute inflammatory cells. A study by Tassiopoulos and colleagues in a rat model of acute lung injury induced by aortic occlusion demonstrated that inhibition of iNOS decreased pulmonary sequestration of neutrophils and neutrophil chemotaxis, thus reducing lung injury [11]. In an in vitro study, chemotaxis of human neutrophils to formyl-met-leu-phe (fMLP) was shown to be inhibited by pretreatment of the cells with monomethyl-l-arginine, a nonspecific inhibitor of NOS [12]. Together, these studies suggest that induction of NOS and NO generation in the lungs of sheep after injury may promote or enhance the inflammatory reaction induced by smoke inhalation injury.
The relationship of NO to mucous secretion and neutrophil activation and recruitment, though discussed as separate processes, are integrally related processes. Studies have shown that neutrophil elastase is able to promote submucosal gland mucous secretion [41] and that glands can produce proinflammatory mediators [42–44]. Studies in our laboratory have also shown that smoke inhalation injury promotes bronchial lining and glandular expression of endothelin-1, a neutrophil chemokinetic agent capable of stimulating inflammatory reactions [45]. These studies suggest a role of gland cells in the acute inflammatory reaction that follows inhalation injury. Clearly, further studies are needed to define the contributions and interactions of the inflammatory reaction in the upper airways and sub-mucosal gland secretion.
In summary, the present study has identified the cell types that express the constitutive endothelial and neuronal isoforms of nitric oxide synthase (eNOS and nNOS) and the inducible form (iNOS), and the changes in protein expression of these isoforms that occur after combined burn and smoke inhalation injury in this ovine model. All 3 isoforms are present in multiple cell types in both uninjured and injured sheep. As might be expected, the 2 constitutive, calcium-dependent isoforms, eNOS and nNOS, were not found to have significant differences in their intensity or sites of expression after injury. The inducible form (iNOS), however, showed a striking increase in apparent protein expression in certain cell types, particularly in the basal cells of the bronchial epithelium and mucous glands, beginning at 4 hours after injury and persisting for at least 48 hours. This increase in expression of iNOS is consistent with recent results showing a beneficial effect of inhibition of nitric oxide synthase using agents selective for iNOS, particularly during the second 24 hours after S+B injury. Although activation of pre-existing eNOS and nNOS may well have a role in this model of acute lung injury, new expression of enzyme nitric oxide synthase appears to be limited to the inducible isoform, iNOS.
Acknowledgments
This study was supported by grants 8469, 8450, and 8460 from the Shriners of North America and grants GM60688 and GM066312 from the National Institutes of Health.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Contributor Information
Robert A. Cox, Department of Pathology, University of Texas Medical Branch and Shriners Hospitals for Children, Galveston, Texas, USA
Sam Jacob, Department of Pathology, University of Texas Medical Branch and Shriners Hospitals for Children, Galveston, Texas, USA.
Gloria Oliveras, Department of Pathology, University of Texas Medical Branch and Shriners Hospitals for Children, Galveston, Texas, USA.
Kazunori Murakami, Department of Anesthesiology, University of Texas Medical Branch, Galveston, Texas, USA.
Perenlei Enkhbaatar, Department of Anesthesiology, University of Texas Medical Branch, Galveston, Texas, USA.
Lillian Traber, Department of Anesthesiology, University of Texas Medical Branch, Galveston, Texas, USA.
Frank C. Schmalstieg, Department of Pediatrics, University of Texas Medical Branch, Galveston, Texas, USA
David N. Herndon, Department of Surgery, University of Texas Medical Branch and Shriners Hospitals for Children, Galveston, Texas, USA
Daniel L. Traber, Department of Anesthesiology, University of Texas Medical Branch, Galveston, Texas, USA
Hal K. Hawkins, Department of Pathology, University of Texas Medical Branch and Shriners Hospitals for Children, Galveston, Texas, USA
References
- 1.Shirani KZ, Pruitt BA, Mason AD., Jr The influence of and pneumonia on burn mortality. Ann Surg. 1987;205:82–87. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herndon DN, Traber DL, Niehaus GD, Linares HA, Traber LD. The pathophysiology of smoke inhalation injury in sheep model. J Trauma. 1984;24:1044–1051. doi: 10.1097/00005373-198412000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Basadre JO, Sugi K, Traber DL, Traber LD. The effect of leukocyte depletion on smoke inhalation injury in sheep. Surgery. 1988;104:208–215. [PubMed] [Google Scholar]
- 4.Enkhbaatar P, Murakami K, Shimoda K, Mizutani A, Traber L, Phillips GB, Parkinson JF, Cox R, Hawkins H, Herndon D, Traber D. The inducible nitric oxide synthase inhibitor BBS-2 prevents acute lung injury in sheep after burn and smoke inhalation injury. Am J Respir Crit Care Med. 2003;167:1021–1026. doi: 10.1164/rccm.200209-1031PP. [DOI] [PubMed] [Google Scholar]
- 5.Soejima K, McGuire R, Snyder N, Uchida T, Szabo C, Salzman A, Traber LD, Traber DL. The effect of inducible nitric oxide synthase (iNOS) inhibition on smoke inhalation injury in sheep. Shock. 2000;13:261–266. doi: 10.1097/00024382-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 8.Schiffrin EL, Touyz RM. Vascular biology of endothelin. J Cardiovasc Pharmacol. 1998;32(Suppl 3):S2–S13. [PubMed] [Google Scholar]
- 9.Stroes ES, van Faassen EE, van Londen GJ, Rabelink TJ. Oxygen radical stress in vascular disease: the role of endothelial nitric oxide synthase. J Cardiovasc Pharmacol. 1998;32(Suppl 3):S14–S21. [PubMed] [Google Scholar]
- 10.Royall JA, Kooy NW, Beckman JS. Nitric oxide-related oxidants in acute lung injury. New Horiz. 1995;3:113–122. [PubMed] [Google Scholar]
- 11.Tassiopoulos AK, Hakim TS, Finck CM, Pedoto A, Hodell MG, Landas SK, McGraw DJ. Neutrophil sequestration in the lung following acute aortic occlusion starts during ischaemia and can be attenuated by tumour necrosis factor and nitric oxide blockade. Eur J Vasc Endovasc Surg. 1998;16:36–42. doi: 10.1016/s1078-5884(98)80089-0. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan SS, Billiar T, Curran RD Zdziarski UE, Simmons RL, Basford RE. Inhibition of chemotaxis NG-monomethyl-l-arginine: a role for cyclic GMP. Blood. 1989;74:1885–1887. [PubMed] [Google Scholar]
- 13.de Mello SB, Novaes GS, Laurindo IM, Muscara MN, Maciel FM, Cossermelli W. Nitric oxide synthase inhibitor influences prostaglandin and interleukin-1 production in experimental arthritic joints. Inflamm Res. 1997;46:72–77. doi: 10.1007/s000110050086. [DOI] [PubMed] [Google Scholar]
- 14.Moilanen E, Vuorinen P, Kankaanranta H, Metsa-Ketela T, Vapaatalo H. Inhibition by nitric oxide-donors of human polymorphonuclear leucocyte functions. Br J Pharmacol. 1993;109:852–858. doi: 10.1111/j.1476-5381.1993.tb13653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herndon DN, Trabe LD, Linares H, Flynn JD, Niehaus G, Kramer G, Trabe DL. Etiology of the pulmonary pathophysiology associated with inhalation injury. Resuscitation. 1986;14:43–59. doi: 10.1016/0300-9572(86)90006-7. [DOI] [PubMed] [Google Scholar]
- 16.Baxter CR, Shires T. Physiological response to crystalloid resuscitation of severe burns. Ann N Y Acad Sci. 1968;150:874–894. doi: 10.1111/j.1749-6632.1968.tb14738.x. [DOI] [PubMed] [Google Scholar]
- 17.Tzao C, Nickerson PA, Russell JA, Noble BK, Steinhorn RH. Paracrine role of soluble guanylate cyclase and type III nitric oxide synthase in ovine fetal pulmonary circulation: a double labeling immunohistochemical study. Histochem Cell Biol. 2003;119:125–130. doi: 10.1007/s00418-002-0494-z. [DOI] [PubMed] [Google Scholar]
- 18.Parker TA, Le Cras TD, Kinsella JP, Abman SH. Developmental changes in endothelial nitric oxide synthase expression and activity in ovine fetal lung. Am J Physiol Lung Cell Mol Physiol. 2000;278:L202–L208. doi: 10.1152/ajplung.2000.278.1.L202. [DOI] [PubMed] [Google Scholar]
- 19.Sherman TS, Chen Z, Yuhanna IS, Lau KS, Margraf LR, Shaul PW. Nitric oxide synthase isoform expression in the developing lung epithelium. Am J Physiol. 1999;276:L383–L390. doi: 10.1152/ajplung.1999.276.2.L383. [DOI] [PubMed] [Google Scholar]
- 20.Molinuevo MS, Etcheverry SB, Cortizo AM. Macrophage activation by a vanadyl-aspirin complex is dependent on L-type calcium channel and the generation of nitric oxide. Toxicology. 2005;210:205–212. doi: 10.1016/j.tox.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Choi HS, Rai PR, Chu HW, Cool C, Chan ED. Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am J Respir Crit Care Med. 2002;166:178–186. doi: 10.1164/rccm.2201023. [DOI] [PubMed] [Google Scholar]
- 22.Zhan X, Li D, Johns RA. Immunohistochemical evidence for the NO cGMP signaling pathway in respiratory ciliated epithelia of rat. J Histochem Cytochem. 1999;47:1369–1374. doi: 10.1177/002215549904701103. [DOI] [PubMed] [Google Scholar]
- 23.Zhan X, Li D, Johns RA. Expression of endothelial nitric oxide synthase in ciliated epithelia of rats. J Histochem Cytochem. 2003;51:81–87. doi: 10.1177/002215540305100110. [DOI] [PubMed] [Google Scholar]
- 24.Shaul PW, North AJ, Wu LC, Wells LB, Brannon TS, Lau KS, Michel T, Margraf LR, Star RA. Endothelial nitric oxide synthase is expressed in cultured human bronchiolar epithelium. J Clin Invest. 1994;94:2231–2236. doi: 10.1172/JCI117585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bodegas ME, Villaro AC, Montuenga LM, Moncada S, Riveros-Moreno V, Sesma P. Neuronal nitric oxide synthase immunoreactivity in the respiratory tract of the frog, Rana temporaria. Histochem J. 1995;27:812–818. [PubMed] [Google Scholar]
- 26.Guembe L, Villaro AC. Histochemical demonstration of neuronal nitric oxide synthase during development of mouse respiratory tract. Am J Respir Cell Mol Biol. 1999;20:342–351. doi: 10.1165/ajrcmb.20.2.3319. [DOI] [PubMed] [Google Scholar]
- 27.Stothert JC, Jr, Ashley KD, Kramer GC, Herndon DN, Traber LD, Deubel-Ashley K, Traber DL. Intrapulmonary distribution of bronchial blood flow after moderate smoke inhalation. J Appl Physiol. 1990;69:1734–1739. doi: 10.1152/jappl.1990.69.5.1734. [DOI] [PubMed] [Google Scholar]
- 28.Sakurai H, Johnigan R, Kikuchi Y, Harada M, Traber LD, Traber DL. Effect of reduced bronchial circulation on lung fluid flux after smoke inhalation in sheep. J Appl Physiol. 1998;84:980–986. doi: 10.1152/jappl.1998.84.3.980. [DOI] [PubMed] [Google Scholar]
- 29.Efimova O, Volokhov AB, Iliaifar S, Hales CA. Ligation of the bronchial artery in sheep attenuates early pulmonary changes following exposure to smoke. J Appl Physiol. 2000;88:888–893. doi: 10.1152/jappl.2000.88.3.888. [DOI] [PubMed] [Google Scholar]
- 30.Soejima K, Traber LD, Schmalstieg FC, Hawkins H, Jodoin JM, Szabo C, Szabo E, Varig L, Salzman A, Traber DL. Role of nitric oxide in vascular permeability after combined burns and smoke inhalation injury. Am J Respir Crit Care Med. 2001;163:745–752. doi: 10.1164/ajrccm.163.3.9912052. [DOI] [PubMed] [Google Scholar]
- 31.Soejima K, McGuire N, Snyder N, Uchida TC, Szabó A, Salzman A, Traber LD, Traber DL. The ‘effect of inducible nitric oxide synthase (iNOS) inhibition on smoke inhalation injury. Shock. 2000;13:261–266. doi: 10.1097/00024382-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Mizutani A, Enkhbaatar P, Esechie A, Traber LD, Cox RA, Hawkins HK, Deyo DJ, Murakami K, Takayuki N, Traber DL. Pulmonary changes in a mouse model of combined burn and smoke inhalation-induced injury. Appl Physiol. 2008;105:678–684. doi: 10.1152/japplphysiol.00232.2007. [DOI] [PubMed] [Google Scholar]
- 33.Enkhbaatar P, Murakami K, Shimoda K, Mizutani A, McGuire R, Schmalstieg F, Cox R, Hawkins H, Jodoin J, Lee S, Traber DL, Herndon DN, Traber LD. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole attenuates acute lung injury in an ovine model. Am J Physiol Regul Integr Comp Physiol. 2003;285:R366–R372. doi: 10.1152/ajpregu.00148.2003. [DOI] [PubMed] [Google Scholar]
- 34.Pietak SP, Delahaye DJ. Airway obstruction following smoke inhalation. Can Med Assoc J. 1976;115:329–331. [PMC free article] [PubMed] [Google Scholar]
- 35.Cox CS, Jr, Zwischenberger JB, Traber DL, Traber LD, Haque AK, Herndon DN. Heparin improves oxygenation and minimizes barotrauma after severe smoke inhalation in an ovine model. Surg Gynecol Obstet. 1993;176:339–349. [PubMed] [Google Scholar]
- 36.Sasaki T, Shimura S, Sasaki H, Takishima T. Effect of epithelium on mucus secretion from feline tracheal submucosal glands. J Appl Physiol. 1989;66:764–770. doi: 10.1152/jappl.1989.66.2.764. [DOI] [PubMed] [Google Scholar]
- 37.Hubbard GB, Langlinais PC, Shimazu T, Okerberg CV, Mason AD, Pruitt BA. The morphology of smoke inhalation injury in sheep. J Trauma. 1991;31:1477–1486. doi: 10.1097/00005373-199111000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Cox RA, Burke AS, Soejima K, Murakami K, Katahira J, Traber LD, Herndon DN, Schmalstieg FC, Trabe DL, Hawkins HK. Airway obstruction in sheep with burn and smoke inhalation injuries. Am J Respir Cell Mol Biol. 2003;29:295–302. doi: 10.1165/rcmb.4860. [DOI] [PubMed] [Google Scholar]
- 39.Nagaki M, Shimura MN, Irokawa T, Sasaki T, Shirato K. Nitric oxide regulation of glycoconjugate secretion from feline and human airways in vitro. Respir Physiol. 1995;102:89–95. doi: 10.1016/0034-5687(95)00042-c. [DOI] [PubMed] [Google Scholar]
- 40.Cox RA, Soejima K, Katahira J, Murakami K, Burke AS, Traber LD, Zwischenberger JB, Schmalstieg FC, Traber DL, Hawkins HK. Inhibition of inducible nitric oxide synthase decreases airway obstruction in sheep following smoke inhalation injury. Presented at the Twenty-Forth Annual Conference on Shock; Marco Island, FL. 2001. p. Abstract 225. [Google Scholar]
- 41.Dwyer TM, Farley JM. Human neutrophil elastase releases two pools of mucinlike glycoconjugate from tracheal submucosal gland cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L675–L682. doi: 10.1152/ajplung.2000.278.4.L675. [DOI] [PubMed] [Google Scholar]
- 42.Tsicopoulos A, Janin A, Akoum H, Lamblin C, Vorng H, Hamid Q, Tonnel AB, Wallaert B. Cytokine profile in minor salivary glands from patients with bronchial asthma. J Allergy Clin Immunol. 2000;106:687–696. doi: 10.1067/mai.2000.109826. [DOI] [PubMed] [Google Scholar]
- 43.Cox RA, Burke AS, Oliveras G, Zwishenberger JB, Jeschke MG, Schmalstieg FC, Herndon DN, Traber D, Hawkins HK. Acute bronchial obstruction in sheep: histopathology and gland cytokine expression. Exp Lung Res. 2005;31:819–837. doi: 10.1080/01902140600574967. [DOI] [PubMed] [Google Scholar]
- 44.Cox RA, Burke AS, Traber DL, Herndon DN, Hawkins HK. Production of pro-inflammatory polypeptides by airway mucous glands and its potential significance. Pulm Pharmacol Ther. 2007;20:172–177. doi: 10.1016/j.pupt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Cox RA, Soejima K, Burke AS, Traber LD, Herndon DN, Schmalstieg FC, Traber DL, Hawkins HK. Enhanced pulmonary expression of endothelin-1 in an ovine model of smoke inhalation injury. J Burn Care Rehabil. 2001;22:375–383. doi: 10.1097/00004630-200111000-00005. [DOI] [PubMed] [Google Scholar]


