Aromatic nitriles have application in a variety of fields as both synthetic intermediates and final targets.[1] For example, the antineoplastic Letrozole, antidepressant Citalopram, and anti-HIV drug Etravirine all possess an aryl nitrile moiety. Traditionally, benzonitriles are synthesized via diazotization of anilines followed by a Sandmeyer reaction with superstoichiometric amounts of copper (I) cyanide.[2] Another common route to benzonitriles is the Rosenmund-von Braun reaction, which typically entails heating superstoichiometric amounts of copper (I) cyanide with an aryl iodide at elevated temperature.[3] Recent advances have allowed for the use of catalytic quantities of copper, however these protocols still have limitations.[4]
The palladium-catalyzed coupling of cyanide with aryl (pseudo)halides proceeds under milder conditions and displays increased functional group tolerance. The first Pd–catalyzed cyanation method was reported by Takagi forty years ago.[5] Despite great advances,[1, 6] cross-coupling procedures to form ArCN have obtained a reputation as being highly irreproducible.[7] Mechanistic studies by Grushin have shown this is due, in part, to catalyst deactivation by cyanide, which is able to poison all of the intermediates in the catalytic cycle.[8] Common methods to avoid catalyst poisoning include the addition of reducing agents[9] or exploiting the low solubility of NaCN, KCN, and Zn(CN)2 in organic solvents. Grushin's NaCN method,[7] while of high industrial relevance, requires the use of rigorously anhydrous conditions (glovebox setup), which renders this chemistry inconvenient or inaccessible to many synthetic chemists. Further, MCN salts (M = Na or K) are often milled prior to their use in order to guarantee solubility and reproducibility in their application. Milling is problematic considering the high toxicity in conjunction with the possibility of aerosolizing fine cyanide salts. Zinc cyanide finds the widest use in Pd-catalyzed cyanations of functionalized substrates.[10] At approximately 10% the toxicity of its sodium or potassium congeners, Zn(CN)2 is less hazardous, but still poses a significant risk.
To address safety concerns, several Pd-catalyzed methods using alternative cyanide sources have been described.[11] Beller[12] and Weissman[13] discovered that K4[Fe(CN)6], a non-toxic food additive, could serve as a cyanide source for Pd-catalyzed coupling reactions. Aqueous systems using K4[Fe(CN)6]•3H2O with a phase transfer catalyst have been reported, but these still require temperatures greater than 140 °C.[14] Significant advances were reported by Huang[15] and Kwong[16] who employed a 1:1 organic:aqueous solvent mixture to enable cyanide transfer from K4[Fe(CN)6]•3H2O under milder conditions. Still, the scope is narrow, with examples of five-membered heterocycles being rare. Thus, while progress has been made towards a practical benzonitrile synthesis using non-toxic cyanide sources, a general, efficient method for the cyanation of (hetero)aryl halides is still needed. Herein, we disclose a Pd-catalyzed cyanation system that: 1) is applicable to aryl chlorides at low to modest catalyst loadings; 2) works well with a wide range of heterocyclic halides, including in many instances five-membered heterocycles bearing free N–H groups; and 3) is complete in one hour at ≤ 100 °C.
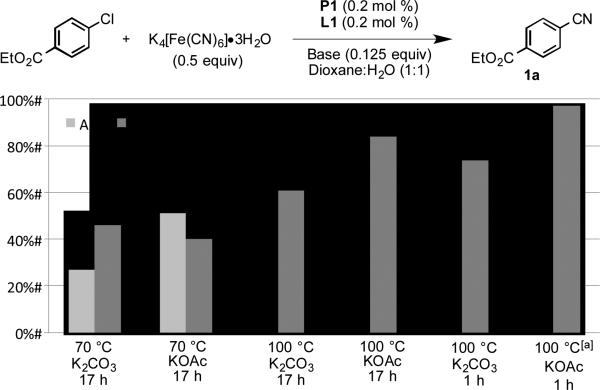
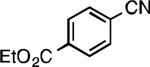
Our initial experiments focused on identifying conditions to prepare benzonitrile 1a from the corresponding aryl chloride. Using our third generation palladacycle precatalysts (P1-P3),[17] a preliminary survey of ligands revealed that use of a catalyst based on XPhos (L1) provided superior yields of benzonitrile 1a, outperforming tBuXPhos (L2) and tBuBrettPhos (L3), as well as other phosphines commonly used for aryl cyanation reactions such as tri-tert-butylphosphine [(P(t-Bu)3] and 1,1’-bis(diphenylphosphino)ferrocene (dppf) (see supporting information for details). Next, we examined the effect of base, temperature, and reaction time on overall yield, utilizing 0.2 mol % P1 and L1 in a 1:1 mixture of dioxane and water (Figure 1). Although carbonate bases are most often used to promote cyanide dissociation from K4[Fe(CN)6]•3H2O, we observed significant substrate and/or product decomposition in experiments employing K2CO3. However, we obtained excellent results using a weaker base, KOAc, and by conducting the reactions at 100 °C for 1h.[18,19]
Figure 1.
Optimization of base, temperature, and time. Reaction conditions: ethyl 4-chlorobenzoate (1 mmol), K4[Fe(CN)6]•3H2O (0.5 equiv), P1 (0.2 mol %), L1 (0.2 mol %), base (0.125 equiv), dioxane (2.5 mL), H2O (2.5 mL). Yields determined by GC analysis of the crude reaction mixture [a] Isolated yield, average of 2 independent runs.
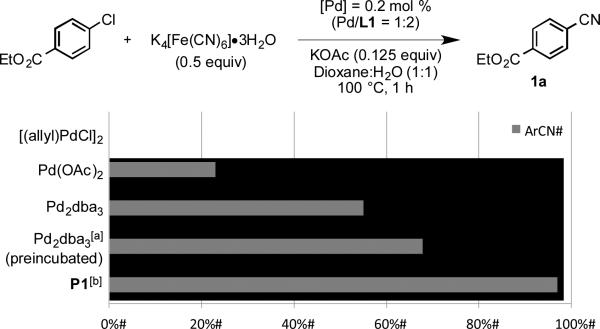
As shown in Figure 2, palladacycle precatalyst P1 proved to be the most effective palladium precursor when compared to commonly used Pd complexes. The commonly used Pd sources Pd(OAc)2 and [(allyl)PdCl]2 require reduction and ligand complexation to form the active catalyst, and poisoning by cyanide likely occurs when activation is performed under the reaction conditions.[20] Another palladium source, Pd2dba3, has been shown to engender higher catalytic activity in C–N coupling processes when preincubated with phosphine ligand at 120 °C.[21] However, implementing this protocol delivered only a moderate improvement in yield over that obtained from the direct use of Pd2dba3 without preincubation.[22,23] In contrast, use of P1 afforded 1a in excellent yield (97%) and was operationally simple as the active catalyst is generated efficiently in situ upon exposure to base. Other precatalysts have been applied to Pd-catalyzed cyanations,[24] but this system represents the only example capable of converting aryl chlorides to benzonitriles at low catalyst loading with a non-toxic cyanide source.
Figure 2.
Comparison of palladium sources. Reaction conditions: ethyl 4-chlorobenzoate (1 mmol), K4[Fe(CN)6]•3H2O (0.5 equiv), [Pd] (0.2 mol %), L1 (Pd/L 1:2), KOAc (0.125 equiv), dioxane (2.5 mL), H2O (2.5 mL). Yields determined by GC analysis of the crude reaction mixture [a] Pd2dba3 and ligand was preincubated in dioxane at 120 °C for 3 min before addition to other reagents. [b] Isolated yield, average of 2 independent runs.
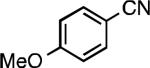
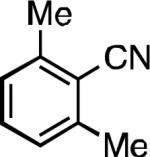
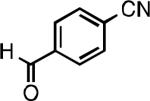
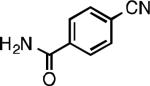
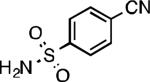
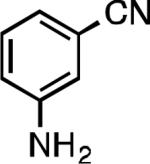
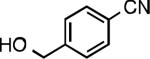
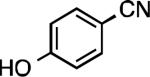
We next explored the substrate scope of our protocol (Table 1). Electron-rich (1b), electron-poor (1a, 1d), and di-ortho-substituted (1c) aryl chlorides can efficiently be converted to benzonitriles using a catalyst based on L1. Interestingly, more electron-deficient aryl chlorides (such as 1e) required the use of the di-tertbutylphosphino analog L2. Recently, Hartwig showed that reductive elimination to form ArCN is slower for electron-deficient aryl groups.[25] That a catalyst based on the larger ligand L2 allows for more facile reductive elimination for electron-deficient substrates is in accord with this view. This method exhibits high tolerance for substrates bearing free N–H or O–H groups, such as primary amides (1g), sulfonamides (1h), anilines (1j), and benzylic alcohols (1j), all of which could be converted to the corresponding benzonitrile using less than 1 mol % Pd. In general, aryl chlorides with coordinating functional groups afforded higher yields when L2 was employed. Coupling an aryl chloride containing a phenol (1k) proved more challenging, yet could be accomplished in 85% yield using of 3 mol % P2. In contrast, cyanation of the less acidic benzylic alcohol (1j) proceeded using only 0.5 mol % P2. Additionally, reactive substituents such as aldehydes (1f) are tolerated as well. Treatment of aldehydes with cyanide and base typically results in benzoin condensation, which occurred under standard conditions at 100 °C. At 70 °C, no benzoin condensation was observed and the benzonitrile product could be isolated in high yield, albeit using slightly higher catalyst loadings.
Table 1.
| |||
|---|---|---|---|
|
|
|

|
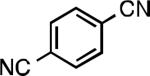
| L1 97% (0.2% P1) | L1 95% (0.4% P1) | L1 90% (0.6% P1) | L1 97% (0.3% P1)[c] |
|
|
|
|
| L2 94% (0.8% P2) | L2 84% (1.8% P2)[d] | L2 90% (0.8% P2) | L2 92% (0.7% P2) |
|
|
|
|
| L2 91% (0.8% P2) | L2 92% (0.5% P2) | L2 85% (3% P2) | |
Reaction conditions: aryl chloride (1 mmol), K4[Fe(CN)6]•3H2O (0.5 equiv), precatalyst (× mol %), ligand (× mol %) KOAc (0.125 equiv), dioxane (2.5 mL), H2O (2.5 mL).
Isolated yields, average of 2 independent runs.
10 mmol scale: 96% yield.
70 °C, 12 h.
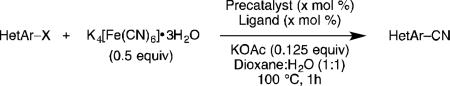
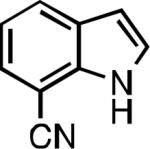
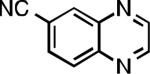
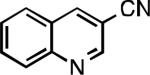
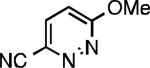
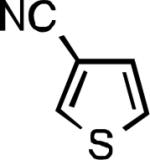
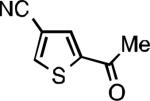
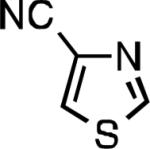
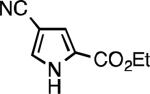
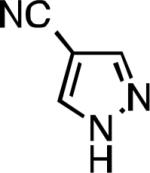
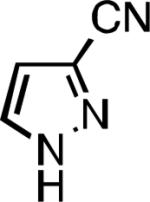
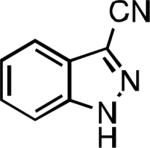
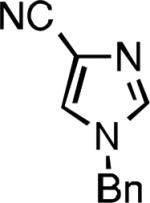
Five-membered heterocycles are extremely important in the fields of pharmaceutical, agrochemical, and materials science, however general procedures for their cyanation by cross-coupling are lacking. We have found that our method accommodates a wide range of heteroaryl halides (Table 2). For example, unprotected indole 2a, bearing an ortho N–H group, yields 95% of product using only 0.7 mol % P1. Sulfur-containing heterocycles, such as thiophenes (2e, 2f) and thiazole (2g), also afforded product, although use of higher catalyst loading was required. Further, pyrroles (2h), pyrazoles (2i, 2j), and indazoles (2k) are all well handled. The latter represent, to the best of our knowledge, the first Pd-catalyzed cyanation of 4-bromopyrazole (2i),[26] 3-bromopyrazole (2j), and 3-chloroindazole (2k).[27]
Table 2.
| |||
|---|---|---|---|
|
|
|
|
| L1 95% (0.7% P1) | L2 97% (1% P2) | L2 93% (2% P2) | L2 64% (4% P2)[c] |
|
|
|
|
| L2 66% (1.6% P2)[d] | L2 91% (4% P2) | L2 91% (4% P2) | L2 92% (1.4% P2) |
|
|
|
|
| L2 93% (1% P2) | L3 64% (5% P3)[e] | L2 94% (4% P2) | L3 99% (1.5% P3) |
Reaction conditions: aryl chloride (1 mmol), K4[Fe(CN)6]•3H2O (0.5 equiv), precatalyst (× mol %), ligand (× mol %) KOAc (0.125 equiv), dioxane (2.5 mL), H2O (2.5 mL).
Isolated yields, average of 2 independent runs.
0.5 equiv KOAc.
Low yield due to product volatility.
Isolated as HCl salt, 95:5 mixture of HetArCN:HetArBr.
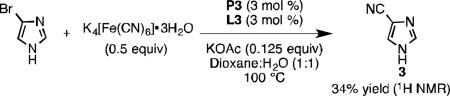
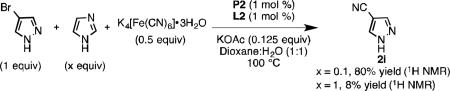
Unprotected imidazoles are difficult substrates for many Pd-catalyzed cross-coupling reactions due to their propensity to bind to transition metal centers.[21b] For example, 4-cyanoimidazole (3) was obtained in 34% yield when the corresponding heteroaryl bromide
 |
(1) |
 |
(2) |
 |
(3) |
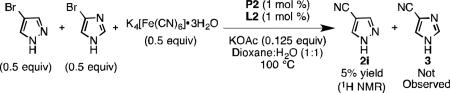
was subjected to the conditions depicted in Eq 1. Increasing the catalyst loading did not aid in driving the reaction to full conversion or increasing the yield in an appreciable manner. A competition experiment showed that in the presence of 4-bromoimidazole, cyanation of 4-bromopyrazole yielded only a small amount of product 2i (Eq 2). Imidazole appears to have an inhibitory effect on catalysis, but only when present in stoichiometric quantities (Eq 3). We hypothesized N-protection of 4-bromoimidazole would prevent inhibition. Indeed, subjecting 4-bromo-N-benzylimidazole[28] to our protocol yielded 2l in 99% yield at 1.5 mol % Pd loading.
 |
(4) |
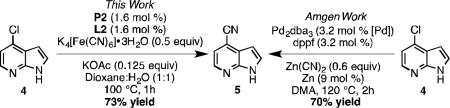
Application of this methodology to the preparation of a pharmaceutical intermediate was also investigated. For the preparation of 2-[(1H-pyrrolo[2,3-b]pyridine-4-yl)methylamino]-5-fluoronicotinic acid, 4-cyano-7-azaindole (5) was used as an intermediate.[29] The reported synthesis utilized Zn(CN)2 with 3.2 mol % (dppf)Pd (Eq 4). Using our procedure, 5 was obtained in similar yield using half the catalyst, in half the time, at lower temperature, and using K4[Fe(CN)6]•3H2O in lieu of Zn(CN)2.[30]
Finally, the transmetallation of cyanide with oxidative addition complex 6 was examined (Table 3). Grushin has reported that (PPh3)2Pd(Ph)(I) reacts with [Bu4N]+[CN]− to afford PhCN;[8a] similarly, we observed rapid transmetallation of KCN with 6 and reductive elimination to form 7 at room temperature. Allowing 6 to react with K4[Fe(CN)6]•3H2O at room temperature for 30 minutes afforded no Pd–CN complex or 7 (via 19F NMR), suggesting that dissociation of cyanide from the iron center requires higher temperatures. In accord with this, when the reaction was conducted at 100 °C, cyanide transfer from K4[Fe(CN)6]•3H2O and reductive elimination to form 7 proceeded at a rate and efficiency comparable to the use of KCN at room temperature. Future efforts will focus on promoting more facile transfer of cyanide from K4[Fe(CN)6]•3H2O to enable room temperature cyanation.
Table 3.
Transmetallation studies

| |||||
|---|---|---|---|---|---|
| Entry | Cyanide source | Base | Temp | Time (min) | Yield (7)[a] |
| 1[b] | KCN | KOAc | rt | 5 | 56% |
| 2[b] | KCN | KOH | rt | 5 | 69% |
| 3[b] | [K4Fe(CN)6]•3H2O | KOAc | rt | 30 | 0% |
| 4[c] | [K4Fe(CN)6]•3H2O | KOAc | 100 °C | 5 | 66% |
19F NMR yield.
Solvent = THF.
Solvent = dioxane.
In conclusion, we have disclosed a general method for the cyanation of (hetero)aryl halides. The use of a non-toxic cyanide source in conjunction with wide functional group tolerance and fast reaction times make this method particularly convenient to synthetic chemists.
Experimental Section
General Procedure for the Pd-catalyzed cyanation of (hetero)aryl chlorides and bromides
To a screw-top test tube equipped with a magnetic stir bar was added precatalyst, ligand, K4[Fe(CN)6]•3H2O (211 mg, 0.5 equiv), and (if solid) (hetero)aryl halide (1 mmol). After sealing with a Teflon-lined screw-cap septum, the vessel was evacuated and backfilled with nitrogen (this process was repeated for a total of three cycles). (Hetero)aryl halide (if liquid) (1 mmol), dioxane (2.5 mL), and 0.05 M KOAc in degassed water (2.5 mL, see supporting information for degassing procedure) were then added to the reaction tube via syringe. The test tube was placed in an oil bath preheated to 100 °C and stirred for 1 h. Upon initial stirring, a clear, yellow solution was observed. During the course of the reaction, a yellow or green precipitate formed on the walls of the reaction vessel. After 1 h of stirring at 100 °C, the reaction mixture was then cooled to room temperature. The contents of the test tube were transferred to a separatory funnel using EtOAc (15 mL) and brine (15 mL), and the organic layer was separated from the aqueous layer. If the reaction was successful, during the extraction process the color of the aqueous layer turns dark blue. This is a colloidal suspension of insoluble fine particles. Isolation and PXRD analysis revealed this solid to be Prussian Blue. The aqueous layer was further extracted with EtOAc (total 2 × 15 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated in vacuo. The resulting mixture was adsorbed onto silica gel, dried in vacuo, and purified via column chromatography to yield the product. Notes: In order for this procedure to be successful for the cyanation of (hetero)aryl halides, efficient stirring is absolutely essential. All reactions in Figures 1-2 and Tables 1-2 were conducted on a 1 mmol scale, except for entry 1d which was also carried out using 10 mmol of aryl chloride. All 1 mmol scale reactions were performed using a stir plate set to 900 rpm. Deviations in reaction vessel size, stir bar size, and reaction scale may require optimization of stirring efficiency to guarantee best results. Additionally, deviation from a 1:1 organic:water solvent mixture can result in incomplete conversions. It is important to make sure no solvent can escape the vessel or be absorbed by the septum to ensure optimal yields. See Supporting Information for more details.
Supplementary Material
Scheme 1.
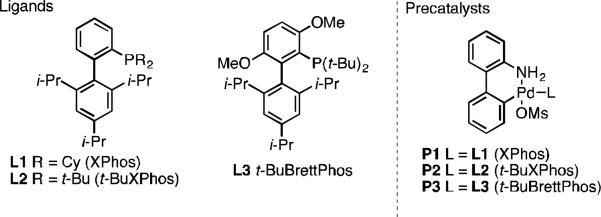
Ligands and precatalysts used in this study
Footnotes
Research reported in this publication was supported by the National Institutes of Health under award number GM46059. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Heath. We thank Dr. Robb DeBergh and Dr. Nathan Jui for help with preparation of this manuscript. MIT has patents on some of the ligands and precatalysts used in this work from which S.L.B. receives royalty payments.
Supporting information for this article is available on the WWW under http://www.angewandte.org
References
- 1.Anbarasan P, Schareina T, Beller M. Chem. Soc. Rev. 2011;40:5049–5067. doi: 10.1039/c1cs15004a. [DOI] [PubMed] [Google Scholar]
- 2.a Sandmeyer T. Ber. Dtsch. Chem. Ges. 1884;17:2650–2653. [Google Scholar]; b Hodgson HH. Chem. Rev. 1947;40:251–277. doi: 10.1021/cr60126a003. [DOI] [PubMed] [Google Scholar]
- 3.a Rosenmund KW, Struck E. Ber. Dtsch. Chem. Ges. 1919;52:1749–1756. [Google Scholar]; b von Braun J, Manz G. Liebigs Ann. Chem. 1931;488:111–126. [Google Scholar]; c Ellis GP, Romney-Alexander TM. Chem. Rev. 1987;87:779–794. [Google Scholar]
- 4.a Monnier F, Taillefer M. Angew. Chem. Int. Ed. 2009;48:6954–6971. doi: 10.1002/anie.200804497. [DOI] [PubMed] [Google Scholar]; b Zanon J, Klapars A, Buchwald SL. J. Am. Chem. Soc. 2003;125:2890–2891. doi: 10.1021/ja0299708. [DOI] [PubMed] [Google Scholar]
- 5.Takagi K, Okamoto T, Sakakiba Y, Oka S. Chem. Lett. 1973:471–474. [Google Scholar]
- 6.Jones LH, Summerhill NW, Swain NA, Mills JE. Med. Chem. Comm. 2010;1:309–318. [Google Scholar]
- 7.Ushkov AV, Grushin VV. J. Am. Chem. Soc. 2011;133:10999–11005. doi: 10.1021/ja2042035. [DOI] [PubMed] [Google Scholar]
- 8.a Dobbs KD, Marshall WJ, Grushin VV. J. Am. Chem. Soc. 2007;129:30–31. doi: 10.1021/ja066931d. [DOI] [PubMed] [Google Scholar]; b Erhardt S, Grushin VV, Kilpatrick AH, Macgregor SA, Marshall WJ, Roe DC. J. Am. Chem. Soc. 2008;130:4828–4845. doi: 10.1021/ja078298h. [DOI] [PubMed] [Google Scholar]
- 9.Jin FQ, Confalone PN. Tetrahedron Lett. 2000;41:3271–3273. [Google Scholar]
- 10.Magano J, Dunetz JR. Chem. Rev. 2011;111:2177–2250. doi: 10.1021/cr100346g. [DOI] [PubMed] [Google Scholar]
- 11.a Luo F-H, Chu C-I, Cheng C-H. Organometallics. 1998;17:1025–1030. [Google Scholar]; b Sawant DN, Wagh YS, Tambade PJ, Bhatte KD, Bhanage BM. Adv. Synth. Catal. 2011;353:781–787. [Google Scholar]; c Zheng S, Yu C, Shen Z. Org. Lett. 2012;14:3644–3647. doi: 10.1021/ol3014914. [DOI] [PubMed] [Google Scholar]; d Zhang G-Y, Yu J-T, Hu M-L, Cheng J. J. Org. Chem. 2013;78:2710–2714. doi: 10.1021/jo3025829. [DOI] [PubMed] [Google Scholar]
- 12.a Schareina T, Zapf A, Beller M. Chem. Commun. 2004:1388–1389. doi: 10.1039/b400562g. [DOI] [PubMed] [Google Scholar]; b Schareina T, Zapf A, Beller M. J. Organomet. Chem. 2004;689:4576–4583. [Google Scholar]; c Schareina T, Zapf A, Maegerlein W, Mueller N, Beller M. Tetrahedron Lett. 2007;48:1087–1090. [Google Scholar]; d Schareina T, Jackstell R, Schulz T, Zapf A, Cotte A, Gotta M, Beller M. Adv. Synth. Catal. 2009;351:643–648. [Google Scholar]
- 13.Weissman SA, Zewge D, Chen C. J. Org. Chem. 2005;70:1508–1510. doi: 10.1021/jo0481250. [DOI] [PubMed] [Google Scholar]
- 14.a Chen G, Weng J, Zheng Z, Zhu X, Cai Y, Cai J, Wan Y. Eur. J. Org. Chem. 2008:3524–3528. [Google Scholar]; b Velmathi S, Leadbeater NE. Tetrahedron Lett. 2008;49:4693–4694. [Google Scholar]
- 15.Zhang J, Chen X, Hu T, Zhang Y, Xu K, Yu Y, Huang J. Catal. Lett. 2010;139:56–60. [Google Scholar]
- 16.a Yeung PY, So CM, Lau CP, Kwong FY. Angew. Chem. Int. Ed. 2010;49:8918–8922. doi: 10.1002/anie.201005121. [DOI] [PubMed] [Google Scholar]; b Yeung PY, So CM, Lau CP, Kwong FY. Org. Lett. 2011;13:648–651. doi: 10.1021/ol1028892. [DOI] [PubMed] [Google Scholar]; c Yeung PY, Tsang CP, Kwong FY. Tetrahedron Lett. 2011;52:7038–7041. [Google Scholar]
- 17.a Bruno NC, Tudge MT, Buchwald SL. Chem. Sci. 2013;4:916–920. doi: 10.1039/C2SC20903A. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Bruno NC, Buchwald SL. Org. Lett. 2013;15:2876–2879. doi: 10.1021/ol401208t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reactions performed with base loading below 0.125 equiv resulted in incomplete conversions. Use of excess base (>0.5 equiv) is not recommended as it may lead to an increased rate of cyanide dissociation from the iron center and catalyst poisoning.
- 19.Other bases examined in initial experiments: Na2CO3 and K3PO4.
- 20.To circumvent this issue, Kwong precomplexed ligand and Pd(OAc)2 in freshly distilled dichloromethane and Et3N with the aid of a heat gun, see ref. [16].
- 21.a Ueda S, Su M, Buchwald SL. Angew. Chem. Int. Ed. 2011;50:8944–8947. doi: 10.1002/anie.201103882. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ueda S, Su M, Buchwald SL. J. Am. Chem. Soc. 2012;134:700–706. doi: 10.1021/ja2102373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dibenzylideneacetone (dba) is known to compete with phosphine ligation, see: Amatore C, Broeker G, Jutand A, Khalil F. J. Am. Chem. Soc. 1997;119:5176–5185.
- 23.The quality of commercially available Pd2dba3 is highly variable, see: Zalesskiy SS, Ananikov VP. Organometallics. 2012;31:2302–2309.
- 24.a Okano T, Iwahara M. J. Kiji, Synlett. 1998:243–244. [Google Scholar]; b Okano T, Kiji J, Toyooka Y. Chem. Lett. 1998:425–426. [Google Scholar]; c Grossman O, Gelman D. Org. Lett. 2006;8:1189–1191. doi: 10.1021/ol0601038. [DOI] [PubMed] [Google Scholar]; d Cheng Y.-n., Duan Z, Li T, Wu Y. Synlett. 2007:543–546. [Google Scholar]; e Hajipour AR, Karami K, Pirisedigh A. Appl. Organomet. Chem. 2010;24:454–457. [Google Scholar]; f Hajipour AR, Karami K, Tavakoli G, Pirisedigh A. J. Organomet. Chem. 2011;696:819–824. [Google Scholar]
- 25.Klinkenberg JL, Hartwig JF. J. Am. Chem. Soc. 2012;134:5758–5761. doi: 10.1021/ja300827t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.For Pd-catalyzed cyanation of 4-iodopyrazoles, see: Fraley ME, Peckham JP, Arrington KL, Hoffman WF, Hartman GD. Merck & Co., Inc.; 2003. WO-03011837.Aventis Pharma GMBH; 2004. EP-1433788.Sakagami M, Hashizume H, Tanaka S, Okuno T, Yari H, Tonogaki K, Kouyama K. Shionogi & Co., Ltd.; 2009. WO-2009131096.
- 27.For Pd-catalyzed cyanation of 3-bromoindazoles, see: Cottyn B, Vichard D, Terrier F, Nioche P, Raman CS. Synlett. 2007:1203–1206.Ahrendt KA, Buchmelter AJ, DeMesse J, Grina J, Hansen JD, Laird ER, Lunghoffer P, Moreno D, Newhouse B, Ren L, Seo J, Tian H, Wenglowsky SM, Feng B, Guzner J, Malesky K, Mathieu S, Rudolph J, Wen Z, Young WB. Array Biopharma, Inc.; Genentech, Inc.; 2009. WO-2009111279.Degnan AP, Tora GO, Denhart DJ, Vrudhula VM, Macor JE, Bronson JJ. Bristol-Myers Squibb Company; 2009. WO-2009009411.
- 28.Cyanation of 4-bromo-N-tritylimidazole yielded little product due to low solubility under the reaction conditons.
- 29.Wang X, Zhi B, Baum J, Chen Y, Crockett R, Huang L, Eisenberg S, Ng J, Larsen R, Martinelli M, Reider P. J. Org. Chem. 2006;71:4021–4023. doi: 10.1021/jo0602571. [DOI] [PubMed] [Google Scholar]
- 30.Product was isolated as 95:5 mixture of 5:4.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.