Abstract
In the past decade, evidence has emerged that there is a variety of bidirectional cell-cell and/or cell-extracellular matrix interactions within the neurovascular unit (NVU), which is composed of neuronal, glial, and vascular cells along with extracellular matrix. Many central nervous system (CNS) diseases, which lead to NVU dysfunction, have common features such as glial activation/transformation and vascular/blood-brain-barrier alteration. These phenomena show dual opposite roles, harmful at acute phase and beneficial at chronic phase. This diverse heterogeneity may induce biphasic clinical courses, i.e. degenerative and regenerative processes in the context of a dynamically coordinated cell-cell/cell-matrix interactions in the NVU. A deeper understanding of the seemingly contradictory actions in cellular levels is essential for NVU protection or regeneration to suppress the deleterious inflammatory reactions and promote adaptive remodeling after CNS injury. This mini-review will present an overview of recent progress in the biphasic roles of the NVU and discuss the clinical relevance of NVU responses associated with CNS diseases, such as stroke and other chronic neurodegenerative disease.
Keywords: Neurovascular Unit, Stroke, CNS injury, Neuroprotection, Remodeling, Astrocyte, Cerebral Endothelial Cell, Microglia
1. Introduction
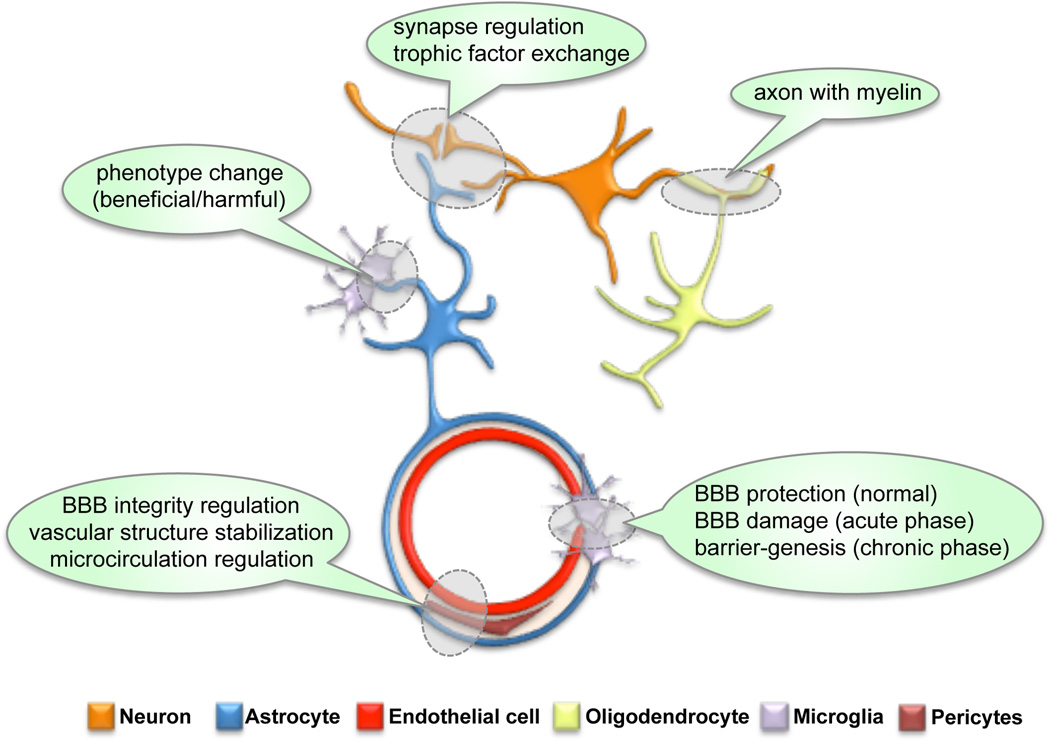
The neurovascular unit (NVU) is composed of neurons, glial cells (astrocytes, microglia, oligodendrocytes), vascular cells (endothelial cells, smooth muscle cells, and pericytes), and extracellular matrix (ECM)1–7 (Figure1). The dynamic interactions among each component within the NVU lead to multifactorial clinical outcomes of central nervous system (CNS) diseases. Over the past decade, since the 2002 Report of the Stroke Progress Review Group, more attention has been paid to this concept of NVU in the field of stroke, because neuron-centered approaches have not achieved successful therapeutic translation to stroke patients. Recently, besides stroke, the multifaceted events in the NVU has been shown to be closely related with the pathogenesis of other CNS diseases including Alzheimer’s disease, vascular dementia, Parkinson’s disease, amyotrophic lateral sclerosis, and multiple sclerosis8.
Figure 1.
Schematic of the Neurovascular Unit. Neuron, astrocyte, cerebral endothelium, oligodendrocyte, microglia, and pericyte compose the neurovascular unit (NVU), and cell-cell interactions between the NVU components may maintain the brain homeostasis. BBB: blood-brain barrier
Recent research advances have shown that NVU components have diverse heterogeneity in their morphology, developmental origin, gene expression profile, physiological properties, function, and response to injury and disease9–14. These diversities may endow the NVU with its dual roles of deleterious and protective properties at different phases after injury. For instance, NVU injury by ischemia and reperfusion after stroke triggers delayed restorative processes, such as neuronal and microvascular sprouting, synaptogenesis, and glial activation/transformation to create a beneficial environment for neuronal growth and plasticity15–19. Innate and adaptive immune systems also show fine-tuned balance reactions in both inflammatory (deleterious) and anti-inflammatory (beneficial) responses after stroke20. Similar to conditions after stroke, many other CNS diseases have been reported to accompany the endogenous repair and remodeling mechanisms in delayed phase. However, when prolonged inflammation and injury outweighs endogenous regenerative reactions, cumulative brain damage leads to poor clinical outcome15, 21. Hence, the dynamic and coordinated mechanisms of cellular function within the NVU under pathologic conditions needs to be elucidated for us to protect or restore the brain homeostasis after injury or disease.
In this mini-review, we will survey recent progress in understanding the roles of NVU components after brain injury, with a focus on their heterogeneous and dual nature properties. Then we will summarize how this complexity affects clinical outcomes of CNS diseases, such as stroke and other neurodegenerative diseases.
2. Components of the neurovascular unit
Over the past several decades, studies of CNS pathologies have focused on intra-neuronal mechanisms. However, as the concept of the NVU emphasizes, it is not easy to achieve neuroprotection without considering other brain cell types. The trophic coupling between neurons and other NVU cells plays important roles under both normal and pathologic conditions. Moreover, these non-neuronal NVU components may also affect cellular function of neighboring non-neuronal brain cells. Here, we will introduce key mechanisms of those non-neuronal NVU components, focusing on their cell-cell/cell-matrix interactions.
2.1 Astrocytes
The astrocyte is particularly important in the NVU. They constitute nearly half of brain cells, and outnumber neurons in the human brain. There is no CNS disease that does not substantially involve astrocytes13, 22. A single astrocyte extends thousands of fine membranous processes that ensheath synapses/micro-vessels and help fill the neuropil. In addition to the traditionally recognized role of regulating extracellular ionic balance, emerging research has now revealed a wide variety of astrocyte functions on NVU regulation.
First, astrocytes play a pivotal role on the formation, function, and elimination of synapses, which regulate neuronal activities13. Previous in vitro study showed that retinal ganglion cells co-cultured with astrocytes form functional synapses whose activity increases by nearby 100-fold23. Astrocyte-secreted thrombospondins (TSP)24, cholesterol25, and glypican 4 and 626 have been shown to powerfully promote synaptic formation, presynaptic function, and postsynaptic function, respectively. Also, astrocytes possess many receptors as neurons do. Neurotransmitters from neurons activate calcium-based signaling cascades in astrocytes to release various active substances such as ATP. These astrocyte-derived substances (i.e. gliotransmitters) act back on neurons to either inhibit or enhance neuronal activities.
Second, astrocytes control vascular tone and cerebral blood flow through their numerous fine processes, which form close associations with both blood vessels and synapse27, 28. In response to enhanced neuronal activity, astrocytes signal to blood vessels directly (through gap junctions) or indirectly (releasing soluble factors) about the need for regional increase in blood flow, resulting in enhanced delivery of oxygen and glucose to the active brain region.
Third, astrocytes regulate blood brain barrier (BBB). Scar-forming reactive astrocytes play a critical role in sealing the BBB injury29. Conversely, in a mouse model of multiple sclerosis (MS), recent cell-specific loss-of-function study has shown that reactive astrocyte-derived vascular endothelial growth factor (VEGF)-A causes BBB breakdown accompanied with lymphocyte infiltration, tissue damage, and clinical deficit30.
Forth, astrocytes are highly secretory cells and communicate with other cells by releasing various molecules including soluble trophic factors. It is well known that astrocytes nourish neurons31. They have been also shown to influence either positively or negatively oligodendrocyte lineage cells through releasing multiple trophic factors32, 33. Moreover, interaction between astrocytes and endothelial progenitor cells (EPCs) may mediate neurovascular remodeling after stroke34.
Fifth, astrocytes are interconnected with neighboring cells through gap junction channels that are regulated by extra- and intracellular signals and allow exchange of information35. Astrocyte-endothelial and astrocyte-neuronal gap junctions are mediated by connexin-43 (CX43) and CX30 hemichannels that allow cell-cell transfer of nutrients, metabolite, secondary messengers and ions36.
Notably, reactive astrocytes after brain injury can be beneficial under some conditions, while they are traditionally thought as detrimental. Reactive astrocytes are harmful in that they produce several proinflammatory cytokines and astroglial scar can inhibit axon regeneration. Inhibitory substrates from reactive gliosis interfere with neuroplasticity37, and preventing the accumulation of the inhibitory signals promotes recovery in animal models of stroke or trauma38. However, they can be also helpful for neurons through upregulating synaptogenesis-inducing genes24 or secreting trophic factors39. A conditional knockdown of reactive astrocytes showed larger lesions with more inflammation responses in mice after brain trauma40. Moreover, reactive astrocytes may release tissue-type plasminogen activator to enhance neuronal dendrite formation41. Of course, it remains mostly unknown why reactive astrocytes show the dual roles after injury. But, astrocytes may be a far more heterogenous group of cells than previously thought. Astrocytes are broadly divided into two main classes in their morphology, antigenic phonotype, and location. Protoplasmic astrocytes in gray matter express low levels of glial fibrillary acidic protein (GFAP) and their processes ensheath synapses and blood vessels11, 13, 42. On the other hand, fibrous astrocytes in white matter express high levels of GFAP and contact nodes of Ranvier and blood vessels11, 13, 42. Thus far, the most widely and homogeneously expressed astrocyte specific protein is Aldh1L142. Recent translational profiling approach has revealed a surprising amount of regional astrocyte heterogeneity. Translated mRNAs in cortical astrocytes, cerebellar astrocytes, and cerebellar Bergman glia are substantially heterogenous at the different brain regions43. Physiological studies have revealed that astrocytes are highly diverse in their electrophysiological properties, calcium dynamics, and gap junction coupling11. Interestingly, different groups of astrocytes exhibit distinct features of spontaneous Ca2+ activity. Cortical layer astrocytes show frequent asynchronous Ca2+ activity, while layer 2/3 astrocytes showed infrequent synchronous Ca2+ activity44. After brain injury, gene profiles of reactive astrocytes are radically altered at different time points. The profile changes occur at different brain regions and even within the same brain regions11. However, whether each single astrocyte can transform its phenotype and whether there exists phenotypically different cell populations in specific brain regions remain to be determined. Furthermore, the altered gene expression of reactive astrocytes is specific to a given injury type, as was shown by the transcriptomic study using mouse models of middle cerebral artery occlusion (MCAO) and systemic LPS injection12. Hence, this highly heterogeneity of astrocytes might contribute to the dual opposite roles of the NVU after brain injury. How disease processes affect the phenotype of astrocytes, including transcriptional, posttranscriptional, or epigenetic regulation as well as autocrine or paracrine mechanisms, needs to be clarified in future studies.
2.2 Cerebral Endothelium
Vascular cells are major cellular constituent of the brain. The importance of the circulatory system to the human brains is highlighted by the fact that, while the brain comprises ~2% of total body mass, it receives up to 20% of cardiac output and is responsible for ~20% and ~25% of the body’s oxygen and glucose consumption, respectively45. The cerebrovascular tree originates from large, interconnected arteries forming the circle of Willis at the base of the brain. These arteries sequentially divide and once inside the brain parenchyma, give rise to pial arteries, penetrating intracerebral arteries, arterioles and a vast capillary network. The cerebrovascular system was traditionally thought as a passive conduit for blood stream, however, recent research has proposed that the system plays a more active role in maintaining the CNS homeostasis with neighboring cells in the NVU.
First, cerebral endothelial cells with astrocytes, and pericytes form BBB. Astrocytic-endfeets ensheath both endothelial cells, and pericytes, while a basal lamina surrounding these cells is shared with astrocytes. The BBB constitutes anatomical, physiochemical, and biochemical barrier that controls the exchange of materials between blood, brain and cerebrospinal fluid. Vascular integrity, specifically the permeability of the BBB, is an important mediator of brain damage. BBB breakdown due to endothelial dysfunction is frequently associated with a myriad of neurological pathologies, including chronic CNS diseases4, 46, 47. Therefore, cerebral endothelial function has attracted growing attention as a novel and important target for intervention in the setting of brain injury.
Second, cerebral endothelial cells nourish neighboring neurons. They guide developing axons48, protect neurons against stress49, 50, and provide a niche for supporting neural stem cells51. In this so-called neurovascular niche, cell-cell signaling between cerebral endothelial cells and neuronal precursor cells help mediate and sustain pockets of ongoing neurogenesis and angiogenesis in adult brain51, 52.
Third, cerebral endothelial cells are also supportive for oligodendrocyte lineage cells. Cerebral endothelial cells and oligodendrocyte precursor cells (OPCs) may provide an oligovascular niche, wherein endothelial-derived growth factors promote the proliferation of OPCs53. Notably, those trophic support from endothelial cells might be attenuated under pathologic conditions54. Moreover, recent in vitro studies have shown that VEGF-A secreted from cerebral endothelial cells can promote the migration but not the proliferation of OPCs55, 56. As seen here, cerebral endothelial cells are rich source of soluble factors. Because the endothelium in blood vessels surveys the entire brain, cerebral endothelial cells might play central roles in cell-cell signaling in the NVU.
2.3 Oligodendrocytes
As noted, the NVU is now relatively well-accepted as a conceptual model to understand phenomena in acute injury and chronic recovery. Although, the NVU has been mostly utilized to discuss phenomena in gray matter, cell-cell interactions could be also very important for white matter. Oligodendrocytes are one of the major cell types in the white matter, and within the CNS, they produce a lipid-rich membrane called myelin to enwrap axons for efficient conduction of electrical impulses. Developmentally, myelin-forming oligodendrocytes are generated by subventricular cells that give rise to committed OPCs57. Although most myelination occur early in the life, myelination continues at least into late adolescence and, in some regions of the CNS, may increase throughout much of adult life58. Interestingly, myelin in the adult CNS may have some plasticity in response to changes in neural activity. Successful learning of juggling is associated with an increase in fractional anisotropy in the white matter underlying the intraparietal sulcus, suggesting an increase in myelination even in adult brain59. Animal models have also shown detectable increases in myelination in the adulthood under environmental enrichment60. Notably, adult OPCs are abundant in both grey and white matter areas, comprising 5–8% of all the cells in adult brain61. Here we introduce key mechanisms of mature oligodendrocytes and their precursors on the cell-cell interactions with other types of brain cells.
Oligodendrocyte-neuron interactions have been extensively examined because matured oligodendrocytes myelinate axons. It is well known that oligodendrocytes can signal to neurons via myelin-axon interactions62, 63. Mouse models of oligodendrocyte injury, such as proteolipid protein (plp1)-null mice64 and Cnp mutant mice65, have demonstrated axon loss without considerable demyelination, suggesting that oligodendrocytes support axon survival through a myelin-independent mechanism66. Recently it has been demonstrated that oligodendrocytes metabolically support neuronal axons. Oligodendrocytes may serve as a principal metabolic supplier of lactate, which is integral for axonal energy support, through monocarboxylate transporter 1 (MCT1)67. In addition, oligodendrocyte-derived trophic factors, such as IGF1 and GDNF, promote neuron survival and axon outgrowth in vitro68. On the other hand, axonal surface ligands, axon-secreted molecules, and axonal activities control highly-regulated processes for oligodendrocyte differentiation/maturation63. One of the axonally expressed ligands Jagged signals OPCs via Notch pathway to inhibit their differentiation69. In addition, other axonal ligands PSA-NCAM70 or LINGO-171 are also known as inhibitory molecules for myelination. By contrast, promyelination signals such as laminin and neuregulin can activate certain intracellular signaling pathways in oligodendrocyte lineage cells63. For instance, Wnt signaling may exert complex roles in myelination. The signal acts in conjunction with Tcf4 to promote the initial stage of oligodendrocyte differentiation, but prevent subsequent differentiation steps unless down-regulated63. Furthermore, there is evidence that myelination is at least in part driven by the level of electrical activity in the axons themselves. OPCs can generate postsynaptic potentials in response to synaptic input such as glutamate stimulation. This response is rapidly lost as the cells differentiate into mature oligodendrocytes72. Finally, SVZ-derived OPCs receive synaptic input in the white matter in a mouse model of remyelination, suggesting that like developmental myelination, remyelination may be in part mediated by neuronal activity72.
As shown previously, endothelial-oligodendrocyte interactions in the so-called oligovascular niche may contribute to ongoing angiogenesis and oligodendrogenesis in adult white matter, particularly after brain injury. During chronic phase after white matter injury, MMP-9 from oligodendrocytes may promote vascular remodeling73. Also in demyelinating diseases such as MS, leukodystrophy, and vascular dementia, OPCs attempt to remyelinate in areas of myelin damage62. While there is no evidence that cerebral endothelial cells can promote oligodendrogenesis during chronic phase in those diseases, cerebral endothelium show some potentials to enhance OPC proliferation and migration in cell culture studies53. Hence, it seems likely that strategies targeting the oligovascular signaling may help promote remyelination along with vascular remodeling in white matter-related diseases.
2.4 Microglia
Microglial cells are the resident immune cells of the CNS, constituting about 10% of CNS glia. In contrast to neuroglia (astrocytes, ependymal cells, and oligodendrocytes), microglial cells are mesodermal in origin and serve as the CNS’s innate immune apparatus (i.e. perform immunological functions). They constantly monitor the CNS environment, and act as first responders to CNS damage. Unlike other glial cells such as astrocytes or oligodendrocytes, microglia are not electronically coupled with syncytial network. Rather, microglia are more individualistic and keep their own surveillance territory74. Hence, this cell type may contribute to the NVU function in a unique way. Indeed, microglial processes and arborizations are highly mobile and continually rebuilt, with de novo formation and withdrawal of processes as well as motile filopodium-like protrusions75. The random scanning by their processes rapidly changes to a targeted movement toward the site of injury when microlesions are induced76. Those processes may involve assistance from neighboring astrocytes releasing purinoreceptor ligands77. Furthermore, microglial cells can react rapidly to even tiny ruptures in blood vessels76. Notably, circulating immune cells including microglia can open the BBB under certain conditions, such as toxins, pathogens and some drugs in the blood stream78.
One more important feature of microglia in the NVU would be their phenotypic heterogeneity74, 75. When microglia are challenged by bacterial invasion, phagocytosis occurs together with the release of inflammatory mediators (M1-like phenotype). By contrast, when removing apoptotic cells or myelin debris, microglia release anti-inflammatory factors (M2-like phenotype). Although parenchymal microglia are distinct from other macrophage-like populations such as perivascular or meningeal locations, microglia may contain region-specific phenotype74. The structural organization (white or grey matter), proximity to the vasculature, BBB features and biochemical micromilieu could impose specific phenotypic adjustments79. For instance, hippocampal microglia express higher levels of mRNA for TNFα, CD4, and FcγRII compared with those from the diencephalon, tegmentum, cerebellum, and cerebral cortex80. Neurotrophin-3 expression is selectively found in microglia from the cerebral cortex, globus pallid us and medulla, but not in those from other CNS tissue74.
Those phenotypic heterogeneities may be related to complex microglial roles after brain injury. Following insults, if microglial cells do not efficiently limit damage and keep homeostasis, they transform from the surveillance mode to activated phenotype, by expanding their ramified morphology and assuming an ameboid (macrophagelike) structure that enables them to migrate75. Chemotactic reorientations and other nontranscriptional adjustments can occur in seconds to minutes, and even massive induction of complex gene sets is achieved within a few hours, and can be long-lasting. Reactive microglia upregulate cytokine and surface receptor expression, and increase phagocytosis. Notably, as mention in the astrocyte section above, microglia also have seemingly contradictory cyto-protective and cyto-toxic properties under pathological conditions. Reactive microglia secrete proinflammatory molecules, such as tumor necrosis factor (TNF) α, interferon (IFN) γ, and interleukin (IL) β. On the other hand, they also release trophic and anti-inflammatory factors, such as IGF1, IL4, and IL1075, 81–83. In injured brains, accumulated dead cells and tissue debris inhibit brain repairing, and microglia (and infiltrating macrophages) constitute the predominant phagocytes to clean up them to promote the brain remodeling.20, 84 Furthermore, recent studies have shown that microglia may also affect neuronal and synaptic functions75. Interestingly, inflammation-associated-microglia can attenuate neurogenesis, while microglia activated by certain T cell cytokines can promote neurogenesis85. Overall, there may be analogies within the concept of M1 versus M2 macrophages, wherein classically acknowledged macrophases are detrimental while under some conditions, they may promote tissue repair in the diseased brain86.
2.5 Pericytes
Pericytes are located surrounding the endothelial cell layers of the capillary network in the brain. They are a very important component of the NVU in serving as integrators, coordinators and effectors of neurovascular functions, including regulation of BBB integrity47, 87, 88, regulation of CBF47, 89, clearance and phagocytosis of cellular debris or byproducts45, 90, and a source of pluripotent stem cells.90 The pericyte density and the proportion of the pericyte coverage to endothelial cells vary among different organs and vascular beds. The pericyte coverage in CNS is higher than in other organs, with 1:1–3:1 ratio between endothelial cells and pericytes, and an approximately 30% coverage of the endothelial abluminal surface.90–93 Within the NVU, pericyte is closely associated with cerebral endothelial cells to maintain normal NVU functions. Here we focus on the key mechanisms of pericytes on vascular systems.
First, pericytes project elongated processes that ensheash the capillary wall. In areas lacking a basement membrane, interdigitations of pericyte and endothelial cell membrane make direct peg-and-socket contacts containing cell-cell junction proteins, including N-cadherin and connexin which form adherence and gap junction, respectively94. The crosstalk and functional coupling between pericytes and endothelial cells is the result of several transduction cascades, including PDGF-B, transforming growth factor-β (TGF-β), Notch, sphingosine-1 phosphate and angiopoietin signaling95. For instance, pericyte-derived TGF-β binds to TGFβR2 in endothelium to activate ALK1-Smad1/5/8 pathway and ALK5-Smad2/3/4. The former pathway favors proliferation and migration of pericyte, while the latter pathway inhibits pericyte proliferation94.
Second, pericytes have several angiogenic actions after initial embryonic CNS vascular establishment. For instance, pericytes express several MMPs and urokinase plasminogen activator receptor capable of enhancing extracellular matrix degradation early in angiogenesis, thereby removing mechanical restraints to endothelial cell migration and facilitating the release of more matrix-sequestered angiogenic factors96, 97. Furthermore, pericytes directly contribute to the synthesis of essential extracellular matrix proteins, including laminin, nidogen and fibronection94. They also secrete tissue inhibitor of metalloproteinase 3 (TIMP3), a potent inhibitor of several MMPs, which inhibits degradation of basement membrane proteins during the vessel stabilization phase94. Pericytes-derived angiogenic factors such as VEGF-A have also been suggested to stimulate endothelial survival, proliferation, and sprout formation98. Thus pericytes have multiple and sometimes opposing roles in angiogenesis or vascular stabilization.
Third, pericytes play essential roles in maintaining BBB. Recent work using mice with deficient PDGFRβ signaling and resulting deficits in embryonic pericyte recruitment have demonstrated that pericyte loss increases the BBB permeability mediated by endothelial transcytosis88. BBB forms early in embryogenesis, during a time period that coincides with initial pericyte recruitment and precedes astrocyte generation88. The role of pericytes in maintaining BBB integrity has been demonstrated not only at the perinatal period but also in the adult and aging brain47, 87. Pericyte-deficient mice with deficient PDGFRβ signaling demonstrate both BBB breakdown and reductions in brain microcirculation, which lead to neuroinflammation and neurodegeneration in the adult and aging brain47. A loss of brain pericytes and resulting BBB breakdown have been shown to impair neurovascular function through leakage and deposition of several vasculotoxic and/or neurotoxic blood-derived macromolecules, such as fibrin, thrombin, plasmin, and hemoglobin-derived hemosiderin, which causes accumulation of iron and reactive oxygen species47, 94.
3. CNS diseases and Neurovascular Unit
As seen above, glial and vascular cells in the NVU work together to maintain brain function. The diverse phenotypic plasticity of those cells may exert dual actions – detrimental vs. beneficial – under diseased conditions. Here we introduce key mechanisms for the biphasic properties of NVU responses in representative CNS diseases.
3.1 Stroke
Stoke is the second most common cause of death worldwide and a major cause of acquired disability in adults99. In the past several decades, remarkable progress in understanding stroke pathophysiology has been made. However, most translational efforts into effective therapies have failed, except for the thrombolytic or intravascular clot removal therapy that only benefits a small proportion of patients1, 3, 15, 17, 18, 20, 100. In regard to the NVU responses, stroke is the most well examined CNS disease because stroke pathophysiology shows relatively significant biphasic phenomena (Figure 2). Under acute phase after stroke onset, ischemic injury results in an abrupt deprivation of nutrient supplies that quickly leads to irreversible damage in the core of affected area. On the other hand, during chronic phase, remodeling signaling may occur in the partly preserved peri-infarct area (so-called penumbra). While precise mechanisms of the biphasic properties after stroke onset are still mostly elucidated, some key molecules are being identified to modulate the NVU responses.
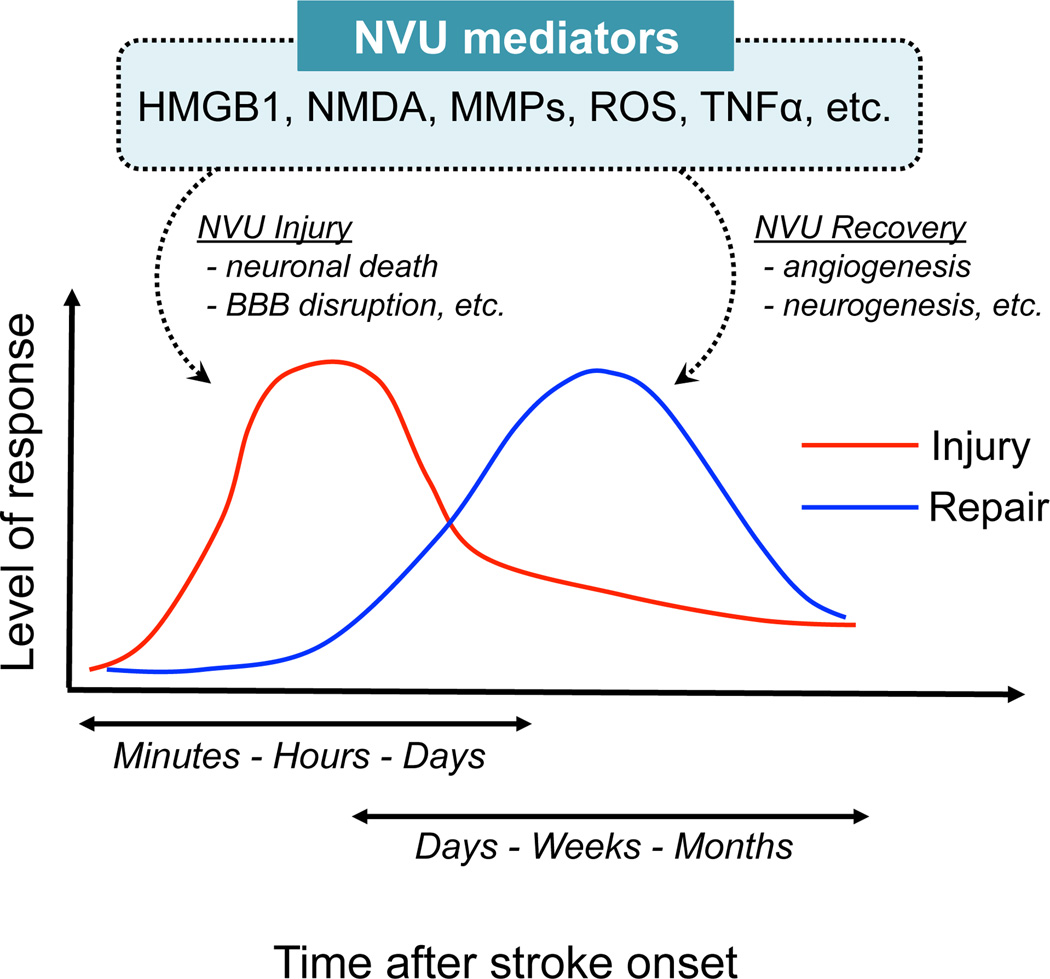
Figure 2.
Schematic to summarize the biphasic responses after stroke onset. In the acute phase, deleterious responses occur. On the other hand, remodeling signaling may emerge at the later time point. Interestingly, NVU mediators such as HMGB-1 would work in both phases, with opposite actions. NVU: neurovascular unit, BBB: blood-brain barrier
After stroke onset, ischemia-reperfusion injury induces a robust inflammatory response, which would be necessary for tissue repair and regeneration in the later time points15, 17, 18. Within minutes to hours after the onset of cerebral ischemia, a cascade of inflammatory events is initiated through activation of resident cells and recruitment of circulating leucocytes. Primary signals that trigger the upregulation of inflammatory mediators are endogenous molecules (i.e. damage-associated molecular patterns; DAMPs). Most DAMPs including HMGB-1, hyaluronan, or heat shock proteins are released from dying and dead cells101, and sensed by pattern recognition receptors, including Toll-like receptors (TLRs) and scavenger receptors, which are widely expressed on microglia, privascular macrophages, and brain endothelial cells. Once the DAMP-receptor signaling is activated, inflammatory mediators such as cytokines (IL-1β, TNF-α, and IL-6 etc.), chemokines, nitrix oxide, and reactive oxygen species are released from the NVU component cells to exacerbate cell death and lead to BBB breakdown20, 100. Also, the cytokines and chemokines induce upregulation of adhesion molecules including P-selectin, E-selectin, and ICAM1, on the vascular endothelium20, 100. These molecules then attract circulating leucocytes in the brain parenchyma. Notably, the inflammatory cytokine TNF-α released by the DAMP family may show both noxious and beneficial actions under stroke conditions20, 100. The dual roles of TNF-α might depend on its cellular target or corresponding selective signals recruited after binding to TNF-α receptors102. Among the DAMP family, HMGB-1 has now attracted the attention as a biphasic mediator. HMGB-1 shows an acute inflammatory noxious effect at the early stage, followed by a promotion of delayed neurovascular remodeling, such as neurogenesis and angiogenesis102.
Similar to HMGB1, some other molecules can also play dual roles, i.e., harmful and beneficial at different phases after stroke (Figure 2). N-methyl-D-aspartic acid (NMDA) signaling, which induces excitotoxicity if overactivated at acute neurodestructive phase, is necessary for delayed neuronal plasticity103. Matrix metalloproteinases (MMPs) are traditionally thought as a biphasic mediator for neurovascular signaling. At early phase of stroke, MMPs cause multiple NVU dysfunctions, such as anoikis-like neuronal death and BBB leakage104. On the other hand, during stroke recovery, the same proteinases contribute to beneficial neurovascular remodeling including angiogenesis and neurogenesis105–109. Reactive oxygen species (ROS) would be another example. ROS is well known to cause multiple harmful events in the multiple cells after stroke. But low levels of ROS may be essential for cellular homeostasis. Recently, ROS is shown to mediate the VEGF-induced OPC migration, which is an essential step for oligodendrogenesis56. Importantly, all these molecules may work as critical mediators for bidirectional cell-cell interactions in the NVU.
Although the biphasic properties (i.e. detrimental at acute phase and beneficial at delayed phase) after stroke onset are now relatively well-accepted, chronic neurodegenerative process might also exist, leading to the progression of the disease. About 30% of stroke survivors have dementia, often associated with brain atrophy110. Pathological studies have shown that an inflammatory infiltrate, such as mononuclear cells, perivascular cuffing, macrophages, T cells, and dendritic cells, persists for years after the stroke113, 114. These findings may suggest that a long-lasting immune response is related to neurodegenerative processes in chronic phase after stroke20, 111, 112. Therefore, future studies might need to carefully examine the mechanisms of harmful responses in the chronic phase.
Cell-cell interactions in the NVU may stimulate the endogenous repairing systems during the delayed phase after stroke onset. Neurogenesis, axonal sprouting, and angiogenesis have been reported to take place after stroke in humans as well as animals. In rodent stoke models, cerebral ischemia increases neurogenesis in the ipsilateral SVZ, along with neuroblast migration from the SVZ site to lesion area113. After focal ischemia, neuronal progenitor cells show dynamic changes in the G1 phase of the actively dividing cell cycle at 2 to 4 days, resulting in subsequent neuronal differentiation114. In addition, between 2 and 28 days after stroke, endothelial sprouting is initiated at the border of the infarct area, and new vessels develop in the ischemic boundary17. At the early stages, angiogenic vessels are permeable, which are associated with the increase of VEGF while angiopoietin1/Tie2 decrease. However, new vessels become less penetrable as they mature, as both VEGF and angiopoietin1/Tie2 increase115. Interestingly, those stoke-induced angiogenesis and neurogenesis are closely linked together in the so-called neurovascular niche17, 51. After stroke, neuroblasts born in the SVZ migrate to the ischemic boundary, where angiogenesis takes place116, 117. During their migration, these neuroblasts are associated with cerebral vessels118. On the contrary, some angiogenic factors can regulate neuroregeneration, and have direct neuroprotective properties119–121. Also, as noted previously, cerebral endothelial cells guide developing axons, provide trophic support to neurons and their progenitor cells51, 52. Taken together, cell-cell trophic coupling in the NVU is an important mechanism for the biphasic responses in stroke pathophysiology, and therefore, deeper understanding of the NVU mechanisms may lead to novel approaches for this devastating disease.
3.2 Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative disorder associated with accumulation of amyloid β-peptide (Aβ) in brain and blood vessels, as well as the formation of neurofibrillary tangles122. The pathogenesis of AD is also closely associated with NVU dysfunction7, 46, 123. Pathophysiological levels of Aβ accelerate neuronal122 and neurovascular124, 125 dysfunctions, which lead to the development of cerebral β-amyloidosis46. Changes in the brain vasculature have been shown to contribute to the onset and progression of the pathological processes, such as BBB breakdown45, 126, microvascular reduction46, or impaired vascular reactivity. Vascular dysfunction impairs the clearance of brain Aβ46, and may also increase the influx of peripheral Aβ into the brain46. Furthremore, reduction of blood perfusion enhances the expression and processing of Aβ-precursor protein (APP)124, which induces Aβ accumulation in the brain. Recently, astrocyte-secreted APOE4 has been shown to mediate BBB breakdown (e.g. degradation of basement membrane and tight junction) through pericytes under the pathologic conditions of AD. Astrocytic-APOE4 binds to the LRP1 receptor in pericytes, and activates the proinflammatory cyclophilin A-nuclear factor-κ B-MMP-9 pathway. Subsequently, the pericytic MMP-9 causes neurovascular defects along with neuronal and synaptic degeneration127.
As seen in other CNS diseases, glial (e.g. astrocyte and microglia) activation is also common features of AD. One of the earliest neurovascular changes in AD brains is the accumulation of reactive astrocytes at sites of Aβ deposition.128 Normal astrocytes can remove and degrade Aβ without additional stimuli such as opsonins or cytokines129, while reactive astrocytes surrounding Aβ deposits in the AD brain seem incapable of removing Aβ130. Hence, this dysregulation of Aβ clearance by astrocytes may precede the Aβ accumulation in AD. As for the microglial activation in AD brains, one important feature is that activated microglia show both beneficial (i.e. phagocytosis and clearance) and detrimental (i.e. inflammation) responses46. In a mouse model of AD (PS1/APP-transgenic), recruited blood-derived cells transform into microglia and the phenotype change of microglia may occur as AD-like pathology progresses131. Depletion of microglia results in increased plaque load, indicating that the newly recruited populations have phagocytic properties132. In terms of the importance of microglia for plaque removal, there is another study that has demonstrated intraventricular transplantation of exogenous microglia can migrate into the parenchyma and increase the clearance of amyloid plaques133. Interestingly, microglia under AD pathology may also exhibit functional changes with increasing aging. Microglia from aged PS1/APP-transgenic mice showed less expression levels of Aβ-binding scavenger receptors (SRA, CD36, and RAGE) and Aβ-degrading enzymes (insulysin, neprilysin, and MMP-9) compared to young transgenic mice134. Furthermore, proinflammatory cytokines (e.g. IL-1β and TNFα) were increased in the aged microglia, which might act in an autocrine manner to decrease the Aβ clearance capacity.134
Those heterogeneous function of activated glial cells on Aβ clearance would contribute to complex NVU responses during the chronic phase of this disease. An increasing number of studies have reported reduced vascular density and impaired neurogenesis in AD brains135. On the other hand, some enhancing angiogenic responses may occur at the later time points136. Probably, the capacity of endogenous restoration and plasticity in this disease depends on the amount of amyloid burden, the property of Aβ, the stage of disease, or surrounding environment. For example, while physiological levels of Aβ stimulate angiogenesis through a synergic effect with FGF-2 in endothelial cells137 and regulate neuronal/synaptic activities138, pathological levels of Aβ would inhibit angiogenesis139. Furthermore, a decreased circulating pool of angiogenic cells may contribute to the impaired angiogenic responses in AD brains.140 However, in APP23-transgenic mice, the surrounding vascular array around the vascular holes at amyloid plaques appears denser and shows typical features for angiogenesis, suggesting that angiogenic processes could be triggered to compensate for the absence of blood vessels.141 Ultimately, as the pathology progresses, the endogenous responses may become suboptimal and can not compensate for all the abnormal neurovascular changes, presumably leading to neurodegenerative outcomes.142
3.3 Parkinson’s disease
Parkinson’s disease (PD) is the second most common, progressive neurodegenerative disorder characterized by numerous motor and non-motor symptoms143. The motor symptoms of PD are mainly related to the reduction of striatal dopamine secondary to loss of dopaminergic neurons in the substantia nigra. The previous studies have focused on the molecular pathways underlying neuronal damages in PD, and revealed that neuronal cell death are largely caused by α-synuclein aggregation, proteosomal and lysosomal system dysfunction, reduced mitochondrial activity, and oxidative stress143. However, little is known about what drives PD to unstoppable progression, and still no effective, mechanism-based treatment is available to retard the PD progression. Therefore, recent research has gradually taken places on the mechanisms by which initial neuronal damages are transformed into chronic progressive neurodegeneration. Thus far, several key mechanisms are elucidated as to the PD progression, and some NVU responses may contribute to the pathogenesis.
First, chronic inflammation exacerbates the pathology in PD144. Reactive astrocytes and microglia are abundant in the substantia nigra of PD cases in human brains145. In cellular and animal models of PD, their toxicities toward dopaminergic neurons have been well demonstrated144. Whether inflammation is an initiating factor of PD in humans is still unclear, however, intracranial infusion of bacterial lipopolysaccaharide, a ligand for TLR-4 and a potent activator of microglia, is sufficient to induce the loss of tyrosine hydroxylase+ neurons in rodents146. Under the pathophysiologic conditions, astrocytes can act as amplifiers of microglia-derived toxic mediators147. But again, as in the other CNS diseases, they have been shown to secrete a number of neurotophic factors such as GDNF and BDNF for injured dopaminergic neurons148.
Second, extracellular α-synuclein is associated with the disease progression. Recent studies have suggested that α-synuclein is actively secreted or released by dying neurons to the extracellular space. Then, α-synuclein can be transferred between neurons (cell-cell propagation), exerting the toxic actions on the recipient neurons. Interestingly, extracellular α-synuclein can be also transferred to glial cells, and these α-synuclein-activated cells would exacerbate the PD pathology149.
Self-repairing systems may also work during the chronic phase in PD brains. Sprouting of dopaminergic neuron terminals and angiogenesis have been observed in both PD patients and animal models150. Doparminergic neurons that survived injury sprout to maintain doparminergic neuron terminal density as a compensatory response150. However, newly formed dopaminergic neuron terminals have altered structure and function. This maladaptive remodeling induces a diminished capacity for dopamine re-uptake, which leads to dysregulated striatal dopamine release151. As for the vascular remodeling, post-mortem investigations on PD brains of humans and animal models have provided evidence of angiogenesis in the substantia nigra152. However, the angiogenic activity accompanies with BBB dysfunction in PD patients who had history of L-dopa-induced dyskinesia153. What mechanisms regulate the balance between maladaptive and adaptive neurogenesis/angiogenesis should be carefully dissected in future studies.
3.4 Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a fatal disease characterized by progressive motor neuron degeneration in the brain and spinal cord, and leads to muscle atrophy, paralysis and death typically within three to five years from diagnosis154, 155. As is the case of other CNS diseases seen above, microglial and astrocytic activations may modulate complex pathological processes.
Microglial cells become activated before clinical disease outcome, and the numbers of activated microglia and astrocytes increase further until the end stage of disease in a mouse model of ALS156. The transgenic mice expressing a mutant form of human SOD1 have been well used to examine the pathophysiology of ALS. Interestingly, selective expression of mutant SOD1 in motor neurons, astrocytes, or microglia does not result in motor-neuron degeneration in neither cases157–159, suggesting that not only neurons but also glial cells play an important part in motor-neuron degeneration155. Microglial cells may degenerate motor-neurons through T-cell infiltration. Indeed, clinical studies in post-mortem tissue of patients with ALS confirmed the increased infiltration of T cells160. T-cells in the CNS are rarely detected at the early disease stage, but readily infiltrate the spinal cord as disease progresses. Notably, some types of T cells can enhance neuroinflammatory state of microglia, while CD4+ T cells can be protective by enhancing the M2 character of microglia over their M1 character in ALS model mice155, 161. But, cytotoxic T cells may predominate at the end stage of disease155, 161.
Besides microglia and astrocytes, oligodendrocytes may also mediate ALS pathogenesis. ALS model mice exhibited enhanced proliferation and differentiation of OPCs in regions of neurodegeneration162, presumably in response to oligodendrocyte injury67. Additionally, cytoplasmic inclusions are found in human ALS oligodendrocytes163. MCT1, which is predominantly localized to oligodendrocytes and may metabolically support axons, is reduced in affected brain regions in ALS patients as well as the ventral horn of the spinal cord of mutant SOD1 transgenic mice67. These findings suggest that dysfunction of oligodendrocytes may be related to neurotoxicity in ALS.
Thus far, a wide range of factors such as chemokine motif ligand 2 (CCL2) and colony stimulating factor 1 (CSF1), has been suggested to mediate the dual roles of glial cells on motor neuron164. Under pathological conditions of ALS, surrounding environment or timing of activation may switch the phenotype of glial cells from neurotoxic to neuroprotective155. These glial heterogeneities would be also important in the pathogenic mechanisms of vascular functions. Clinical and experimental studies have shown that under the diseased conditions, all the BBB, blood-spinal cord barrier (BSCB), and blood-cerebrospinal fluid barrier (BCSFB), are impaired. Structural and functional BBB/BSCB impairments were shown in animal models of ALS in the early stage, worsening with disease progression154, 165. Recently, PDGFRβ-deficient mice showed gross pericyte reductions in the spinal cord, which may cause the BSCB disruption with accumulation of blood-derived toxic proteins in motor neurons and induce spinal neurodegenerative changes.166 Evidence of BBB/BSCB damage has also been observed in post-mortem tissue from ALS patients167. In addition, reduced capillary blood flow in spinal cords of ALS mice165 and brains of ALS patients168 was noted.
3.5 Multiple sclerosis
Multiple sclerosis (MS) is characterized by inflammation, demyelination, axonal and neurodegeration in the CNS with different degrees of autoimmune involvement169, 170. The typical disease course after the first attack consists of remissions and relapses with slowly progressing disability.170 Many components of the innate and adaptive immune systems contribute to different aspects of the disease process. Entry of autoreactive T cells (adaptive immune effectors) from the peripheral blood through the BBB into the CNS initiates an abnormal immunopathogenic cascade. Subsequently, innate immune effectors, including resident CNS microglia, macrophages, dendritic and natural killer cells, and astrocytes are activated. They produce proinflammatory cytokines and toxic molecules such as TNF-α, IFN-γ, glutamate, ROS, and RNS. Furthermore, they recruit other immune cells, including other T cells, B cells and mast cells from the peripheral blood.169 Disease progression from the initial focal inflammatory phase to chronic neurodegenerative phase may relate an immune balance shift characterized by abnormal activation of innate immune system. This may in part explain why currently approved MS therapies that target the adaptive immune system can prevent the appearance of new lesions but show little effect in progressive stages.171
Although remyelination is often inadequate in MS patients62, 172, endogenous microglia and infiltrating macrophages would work for promoting the oligodendrocyte remodeling (i.e. oligodendrogenesis)84. The myelin debris, which contains proteins that inhibit OPC differentiation, is generated during demyelination. But microglia and macrophages try to remove the myelin debris. Also, they can produce soluble mediators, which attract the phagocytic and repair-promoting effectors and precursor cells. Lack of TNF-α leads to a significant delay in remyelination with a reduction of proliferation and maturation of OPCs in mouse MS models. Analysis with mice lacking TNFR1 or TNFR2 has demonstrated that TNF-α signaling through TNFR2 promotes the accumulation of proliferating OPCs.173 Furthermore, recent transcriptomic analysis in a mouse model of MS has demonstrated that under the diseased conditions, microglia can exhibit the phenotype of supporting remyelination. Those microglia produce a rich repertoire of cytokines and chemokines, which activate and recruit endogenous OPCs to the lesion site for repairing the damaged myelin sheathes.10
As noted, axonal damage occurs early in the disease and increases over time, becoming the main contributor to permanent disability. Post-mortem studies have revealed that remyelination failure in chronic MS is associated with reduced recruitment and/or disturbed maturation of OPCs172. Many of the positive regulators for remyelination are provided in the context of acute inflammation at the early stages of lesion formation, but diminished in the chronic inflammatory environment62, 172, 174. On the other hand, early stage inhibitors of OPC differetiation, such as Semaphorin 3A and Notch-jagged1 signaling, appear during phases of ongoing inflammation and may persist for extended periods. Appropriate interactions of axons with oligodendrocyte processes are necessary to form myelin, and therefore, effective therapeutic time window to promote remyelination following demyelination would be pivotal for functional recovery treatment172. Notably, however, recent study using multiphoton in vivo imaging techniques has demonstrated that axonal injury may also occur independently from demyelination. The focal axonal degeneration was induced by macrophage-derived ROS and RNS in a mouse model of MS.175 Thus, the therapeutic strategies of MS should be also targeted for protection and restoration of axons, according to the stage of axonal degeneration independent of demyelination.
4. Conclusions
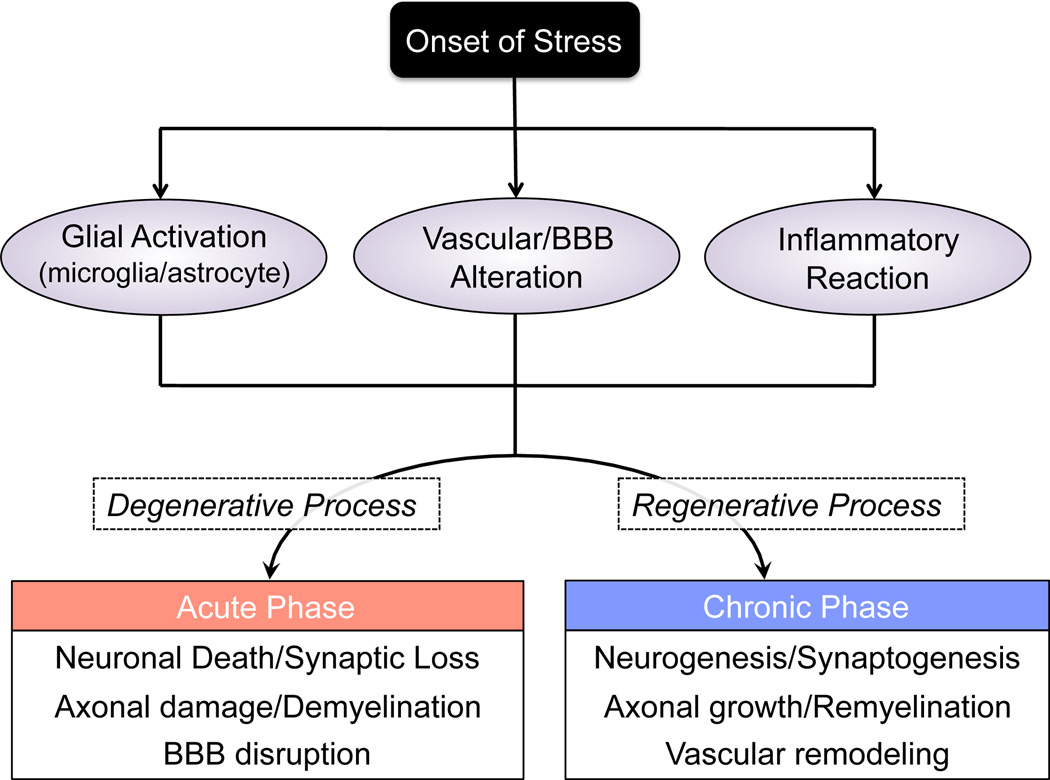
The concept of NVU has provided a novel framework for both basic and clinical research in CNS disease field. A purely neuro-centric focus has been shifted into a more integrated view, wherein dynamic interactions between all cell types contribute to function and dysfunction in the brain. As we have discussed in this mini-review, many CNS diseases have common features, such as glial activation/transformation, vascular/BBB alteration, and inflammatory reactions. These responses may play dual opposite roles (i.e. harmful and beneficial) with diverse heterogeneity, leading to biphasic clinical course (Figure 3). Multiple NVU mediators induce neurovascular dysfunction in the acute phase of CNS diseases. In contrast, the same mediators in turn may underlie neurovascular repair processes in the chronic phase. The notion that a damaged brain is surprisingly plastic will surely present many parallels with many other CNS diseases. A deeper understanding of cellular mechanisms of the transition zones between injury and repair will give us new directions for more effective treatments.
Figure 3.
Schematic to summarize the pathologic processes in CNS diseases. In most cases of CNS diseases, glial activation, vascular alteration, and inflammatory reaction are observed after the onset of stress. These responses lead to brain injury at the acute phase, but may contribute to neurovascular remodeling in the chronic phase. BBB: blood-brain barrier
Acknowledgements
Supported in part by Research Abroad from the Japan Society for the Promotion of Science (T.M.), the Deane Foundation (E.H.L and K.A.), American Heart Association (E.H.L and K.A.), and National Institutes of Health (E.H.L and K.A.). The authors thank Drs. Ji Hae Seo and Nobukazu Miyamoto for many helpful discussions.
References
- 1.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, van Leyen K, et al. Cell-cell signaling in the neurovascular unit. Neurochem Res. 2007;32:2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- 3.del Zoppo GJ. Stroke and neurovascular protection. N Engl J Med. 2006;354:553–555. doi: 10.1056/NEJMp058312. [DOI] [PubMed] [Google Scholar]
- 4.Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med. 2010;16:1370–1371. doi: 10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]
- 5.Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: Molecular mechanisms and therapeutic implications. Neuron. 2011;71:406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 7.Iadecola C. Neurovascular regulation in the normal brain and in alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 8.Arai K, Lok J, Guo S, Hayakawa K, Xing C, Lo EH. Cellular mechanisms of neurovascular damage and repair after stroke. J Child Neurol. 2011;26:1193–1198. doi: 10.1177/0883073811408610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urich E, Lazic SE, Molnos J, Wells I, Freskgard PO. Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood-brain barrier models. PLoS One. 2012;7:e38149. doi: 10.1371/journal.pone.0038149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olah M, Amor S, Brouwer N, Vinet J, Eggen B, Biber K, et al. Identification of a microglia phenotype supportive of remyelination. Glia. 2012;60:306–321. doi: 10.1002/glia.21266. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Barres BA. Astrocyte heterogeneity: An underappreciated topic in neurobiology. Curr Opin Neurobiol. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barres BA. The mystery and magic of glia: A perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Allen NJ, Barres BA. Neuroscience: Glia - more than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- 15.Lo EH. Degeneration and repair in central nervous system disease. Nat Med. 2010;16:1205–1209. doi: 10.1038/nm.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermann DM, Chopp M. Promoting brain remodelling and plasticity for stroke recovery: Therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012;11:369–380. doi: 10.1016/S1474-4422(12)70039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: Underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo EH. A new penumbra: Transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 19.Arai K, Jin G, Navaratna D, Lo EH. Brain angiogenesis in developmental and pathological processes: Neurovascular injury and angiogenic recovery after stroke. FEBS J. 2009;276:4644–4652. doi: 10.1111/j.1742-4658.2009.07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iadecola C, Anrather J. The immunology of stroke: From mechanisms to translation. Nature medicine. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing C, Hayakawa K, Lok J, Arai K, Lo EH. Injury and repair in the neurovascular unit. Neurological research. 2012;34:325–330. doi: 10.1179/1743132812Y.0000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- 24.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, et al. Thrombospondins are astrocyte-secreted proteins that promote cns synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, et al. Cns synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 26.Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via glua1 ampa receptors. Nature. 2012;486:410–414. doi: 10.1038/nature11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 28.Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 30.Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, et al. Astrocyte-derived vegf-a drives blood-brain barrier disruption in cns inflammatory disease. J Clin Invest. 2012;122:2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricci G, Volpi L, Pasquali L, Petrozzi L, Siciliano G. Astrocyte-neuron interactions in neurological disorders. J Biol Phys. 2009;35:317–336. doi: 10.1007/s10867-009-9157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arai K, Lo EH. Astrocytes protect oligodendrocyte precursor cells via mek/erk and pi3k/akt signaling. J Neurosci Res. 2010;88:758–763. doi: 10.1002/jnr.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore CS, Abdullah SL, Brown A, Arulpragasam A, Crocker SJ. How factors secreted from astrocytes impact myelin repair. Journal of neuroscience research. 2011;89:13–21. doi: 10.1002/jnr.22482. [DOI] [PubMed] [Google Scholar]
- 34.Hayakawa K, Pham LD, Katusic ZS, Arai K, Lo EH. Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc Natl Acad Sci U S A. 2012;109:7505–7510. doi: 10.1073/pnas.1121146109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: A step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- 36.Chew SS, Johnson CS, Green CR, Danesh-Meyer HV. Role of connexin43 in central nervous system injury. Exp Neurol. 2010;225:250–261. doi: 10.1016/j.expneurol.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Fitch MT, Silver J. Cns injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Experimental neurology. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silver J, Miller JH. Regeneration beyond the glial scar. Nature reviews. Neuroscience. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 39.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 40.Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain: a journal of neurology. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- 41.Xin H, Li Y, Shen LH, Liu X, Wang X, Zhang J, et al. Increasing tpa activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in the mouse. PloS one. 2010;5:e9027. doi: 10.1371/journal.pone.0009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, et al. Application of a translational profiling approach for the comparative analysis of cns cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takata N, Hirase H. Cortical layer 1 and layer 2/3 astrocytes exhibit distinct calcium dynamics in vivo. PLoS One. 2008;3:e2525. doi: 10.1371/journal.pone.0002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Zlokovic BV. Neurovascular pathways to neurodegeneration in alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makita T, Sucov HM, Gariepy CE, Yanagisawa M, Ginty DD. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature. 2008;452:759–763. doi: 10.1038/nature06859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dugas JC, Mandemakers W, Rogers M, Ibrahim A, Daneman R, Barres BA. A novel purification method for cns projection neurons leads to the identification of brain vascular cells as a source of trophic support for corticospinal motor neurons. J Neurosci. 2008;28:8294–8305. doi: 10.1523/JNEUROSCI.2010-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, et al. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc Natl Acad Sci U S A. 2008;105:7582–7587. doi: 10.1073/pnas.0801105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, et al. Adult svz lineage cells home to and leave the vascular niche via differential responses to sdf1/cxcr4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arai K, Lo EH. Oligovascular signaling in white matter stroke. Biol Pharm Bull. 2009;32:1639–1644. doi: 10.1248/bpb.32.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arai K, Lo EH. An oligovascular niche: Cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayakawa K, Seo JH, Pham LD, Miyamoto N, Som AT, Guo S, et al. Cerebral endothelial derived vascular endothelial growth factor promotes the migration but not the proliferation of oligodendrocyte precursor cells in vitro. Neurosci Lett. 2012 doi: 10.1016/j.neulet.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayakawa K, Pham LD, Som AT, Lee BJ, Guo S, Lo EH, et al. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J Neurosci. 2011;31:10666–10670. doi: 10.1523/JNEUROSCI.1944-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, et al. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 59.Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Juraska JM, Kopcik JR. Sex and environmental influences on the size and ultrastructure of the rat corpus callosum. Brain Res. 1988;450:1–8. doi: 10.1016/0006-8993(88)91538-7. [DOI] [PubMed] [Google Scholar]
- 61.Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- 62.Franklin RJ, Ffrench-Constant C. Remyelination in the cns: From biology to therapy. Nature reviews. Neuroscience. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 63.Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- 64.Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- 65.Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, et al. Disruption of cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 66.Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilkins A, Majed H, Layfield R, Compston A, Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: A novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci. 2003;23:4967–4974. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- 70.Charles P, Hernandez MP, Stankoff B, Aigrot MS, Colin C, Rougon G, et al. Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc Natl Acad Sci U S A. 2000;97:7585–7590. doi: 10.1073/pnas.100076197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, et al. Lingo-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- 72.Etxeberria A, Mangin JM, Aguirre A, Gallo V. Adult-born svz progenitors receive transient synapses during remyelination in corpus callosum. Nat Neurosci. 2010;13:287–289. doi: 10.1038/nn.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pham LD, Hayakawa K, Seo JH, Nguyen MN, Som AT, Lee BJ, et al. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia. 2012;60:875–881. doi: 10.1002/glia.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Graeber MB. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- 75.Hanisch UK, Kettenmann H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 76.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 77.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. Atp mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 78.Hughes V. Microglia: The constant gardeners. Nature. 2012;485:570–572. doi: 10.1038/485570a. [DOI] [PubMed] [Google Scholar]
- 79.Binstadt BA, Patel PR, Alencar H, Nigrovic PA, Lee DM, Mahmood U, et al. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nat Immunol. 2006;7:284–292. doi: 10.1038/ni1306. [DOI] [PubMed] [Google Scholar]
- 80.Ren L, Lubrich B, Biber K, Gebicke-Haerter PJ. Differential expression of inflammatory mediators in rat microglia cultured from different brain regions. Brain Res Mol Brain Res. 1999;65:198–205. doi: 10.1016/s0169-328x(99)00016-9. [DOI] [PubMed] [Google Scholar]
- 81.Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, et al. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Narantuya D, Nagai A, Sheikh AM, Masuda J, Kobayashi S, Yamaguchi S, et al. Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement. PLoS One. 2010;5:e11746. doi: 10.1371/journal.pone.0011746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol. 2009;210:3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Napoli I, Neumann H. Protective effects of microglia in multiple sclerosis. Experimental neurology. 2010;225:24–28. doi: 10.1016/j.expneurol.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 85.Olah M, Ping G, De Haas AH, Brouwer N, Meerlo P, Van Der Zee EA, et al. Enhanced hippocampal neurogenesis in the absence of microglia t cell interaction and microglia activation in the murine running wheel model. Glia. 2009;57:1046–1061. doi: 10.1002/glia.20828. [DOI] [PubMed] [Google Scholar]
- 86.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews. Immunology. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 88.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of cns capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sa-Pereira I, Brites D, Brito MA. Neurovascular unit: A focus on pericytes. Molecular neurobiology. 2012;45:327–347. doi: 10.1007/s12035-012-8244-2. [DOI] [PubMed] [Google Scholar]
- 91.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3d reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 92.Sims DE. The pericyte--a review. Tissue & cell. 1986;18:153–174. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- 93.Armulik A, Genove G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Developmental cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 94.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 96.Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Virgintino D, Girolamo F, Errede M, Capobianco C, Robertson D, Stallcup WB, et al. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis. 2007;10:35–45. doi: 10.1007/s10456-006-9061-x. [DOI] [PubMed] [Google Scholar]
- 98.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D'Amore PA. Pericyte production of cell-associated vegf is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 99.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 100.Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, et al. Stroke and the immune system: From pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10:471–480. doi: 10.1016/S1474-4422(11)70066-7. [DOI] [PubMed] [Google Scholar]
- 101.Chen GY, Nunez G. Sterile inflammation: Sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hayakawa K, Qiu J, Lo EH. Biphasic actions of hmgb1 signaling in inflammation and recovery after stroke. Ann. NY Acad. Sci. 2010;1207:50–57. doi: 10.1111/j.1749-6632.2010.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, et al. Apolipoprotein e controls cerebrovascular integrity via cyclophilin a. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol. 2008;8:82–89. doi: 10.1016/j.coph.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 105.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 106.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Some remarks on the growth-rate and angiogenesis of microvessels in ischemic stroke. Morphometric and immunocytochemical studies. Patol Pol. 1993;44:203–209. [PubMed] [Google Scholar]
- 107.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 108.Szpak GM, Lechowicz W, Lewandowska E, Bertrand E, Wierzba-Bobrowicz T, Dymecki J. Border zone neovascularization in cerebral ischemic infarct. Folia Neuropathol. 1999;37:264–268. [PubMed] [Google Scholar]
- 109.Greenberg DA. Neurogenesis and stroke. CNS Neurol Disord Drug Targets. 2007;6:321–325. doi: 10.2174/187152707783220901. [DOI] [PubMed] [Google Scholar]
- 110.Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet neurology. 2005;4:752–759. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- 111.Mena H, Cadavid D, Rushing EJ. Human cerebral infarct: A proposed histopathologic classification based on 137 cases. Acta neuropathologica. 2004;108:524–530. doi: 10.1007/s00401-004-0918-z. [DOI] [PubMed] [Google Scholar]
- 112.Yilmaz A, Fuchs T, Dietel B, Altendorf R, Cicha I, Stumpf C, et al. Transient decrease in circulating dendritic cell precursors after acute stroke: Potential recruitment into the brain. Clinical science. 2010;118:147–157. doi: 10.1042/CS20090154. [DOI] [PubMed] [Google Scholar]
- 113.Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 114.Zhang RL, Zhang ZG, Lu M, Wang Y, Yang JJ, Chopp M. Reduction of the cell cycle length by decreasing g1 phase and cell cycle reentry expand neuronal progenitor cells in the subventricular zone of adult rat after stroke. J Cereb Blood Flow Metab. 2006;26:857–863. doi: 10.1038/sj.jcbfm.9600237. [DOI] [PubMed] [Google Scholar]
- 115.Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, et al. Correlation of vegf and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 116.Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and mri indices of functional recovery from stroke. Stroke. 2007;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- 117.Chopp M, Li Y, Zhang J. Plasticity and remodeling of brain. J Neurol Sci. 2008;265:97–101. doi: 10.1016/j.jns.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 118.Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 119.Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35:2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- 120.Hansen TM, Moss AJ, Brindle NP. Vascular endothelial growth factor and angiopoietins in neurovascular regeneration and protection following stroke. Curr Neurovasc Res. 2008;5:236–245. doi: 10.2174/156720208786413433. [DOI] [PubMed] [Google Scholar]
- 121.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. Vegf enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 123.Kalaria RN, Akinyemi R, Ihara M. Does vascular pathology contribute to alzheimer changes? J Neurol Sci. 2012 doi: 10.1016/j.jns.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 124.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and alzheimer's disease. Brain Pathol. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bell RD, Deane R, Chow N, Long X, Sagare A, Singh I, et al. Srf and myocardin regulate lrp-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zlokovic BV. Neurovascular mechanisms of alzheimer's neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 127.Ikonomidou C, Turski L. Why did nmda receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 128.Miao J, Xu F, Davis J, Otte-Holler I, Verbeek MM, Van Nostrand WE. Cerebral microvascular amyloid beta protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid beta precursor protein. Am J Pathol. 2005;167:505–515. doi: 10.1016/s0002-9440(10)62993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, et al. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 130.Allaman I, Gavillet M, Belanger M, Laroche T, Viertl D, Lashuel HA, et al. Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: Impact on neuronal viability. J Neurosci. 2010;30:3326–3338. doi: 10.1523/JNEUROSCI.5098-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Naert G, Rivest S. Hematopoietic cc-chemokine receptor 2 (ccr2) competent cells are protective for the cognitive impairments and amyloid pathology in a transgenic mouse model of alzheimer's disease. Mol Med. 2012;18:297–313. doi: 10.2119/molmed.2011.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rogers J, Strohmeyer R, Kovelowski CJ, Li R. Microglia and inflammatory mechanisms in the clearance of amyloid beta peptide. Glia. 2002;40:260–269. doi: 10.1002/glia.10153. [DOI] [PubMed] [Google Scholar]
- 133.Takata K, Kitamura Y, Yanagisawa D, Morikawa S, Morita M, Inubushi T, et al. Microglial transplantation increases amyloid-beta clearance in alzheimer model rats. FEBS Lett. 2007;581:475–478. doi: 10.1016/j.febslet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 134.Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging alzheimer's disease mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of alzheimer's disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 136.Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in alzheimer's disease. Mol Neurodegener. 2011;6:85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cecchini S, Girouard S, Huels MA, Sanche L, Hunting DJ. Single-strand-specific radiosensitization of DNA by bromodeoxyuridine. Radiation research. 2004;162:604–615. doi: 10.1667/rr3267. [DOI] [PubMed] [Google Scholar]
- 138.Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nature neuroscience. 2009;12:1567–1576. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- 139.Hayashi S, Sato N, Yamamoto A, Ikegame Y, Nakashima S, Ogihara T, et al. Alzheimer disease-associated peptide, amyloid beta40, inhibits vascular regeneration with induction of endothelial autophagy. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1909–1915. doi: 10.1161/ATVBAHA.109.188516. [DOI] [PubMed] [Google Scholar]
- 140.Lee ST, Chu K, Jung KH, Park HK, Kim DH, Bahn JJ, et al. Reduced circulating angiogenic cells in alzheimer disease. Neurology. 2009;72:1858–1863. doi: 10.1212/WNL.0b013e3181a711f4. [DOI] [PubMed] [Google Scholar]
- 141.Meyer EP, Ulmann-Schuler A, Staufenbiel M, Krucker T. Altered morphology and 3d architecture of brain vasculature in a mouse model for alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3587–3592. doi: 10.1073/pnas.0709788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pimentel-Coelho PM, Rivest S. The early contribution of cerebrovascular factors to the pathogenesis of alzheimer's disease. The European journal of neuroscience. 2012;35:1917–1937. doi: 10.1111/j.1460-9568.2012.08126.x. [DOI] [PubMed] [Google Scholar]
- 143.Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, et al. Missing pieces in the parkinson's disease puzzle. Nat Med. 2010;16:653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- 144.McGeer PL, McGeer EG. Glial reactions in parkinson's disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 145.Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: Relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Meredith GE, Sonsalla PK, Chesselet MF. Animal models of parkinson's disease progression. Acta Neuropathol. 2008;115:385–398. doi: 10.1007/s00401-008-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saavedra A, Baltazar G, Santos P, Carvalho CM, Duarte EP. Selective injury to dopaminergic neurons up-regulates gdnf in substantia nigra postnatal cell cultures: Role of neuron-glia crosstalk. Neurobiol Dis. 2006;23:533–542. doi: 10.1016/j.nbd.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 149.Marques O, Outeiro TF. Alpha-synuclein: From secretion to dysfunction and death. Cell Death Dis. 2012;3:e350. doi: 10.1038/cddis.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 151.Lee J, Zhu WM, Stanic D, Finkelstein DI, Horne MH, Henderson J, et al. Sprouting of dopamine terminals and altered dopamine release and uptake in parkinsonian dyskinaesia. Brain. 2008;131:1574–1587. doi: 10.1093/brain/awn085. [DOI] [PubMed] [Google Scholar]
- 152.Wada K, Arai H, Takanashi M, Fukae J, Oizumi H, Yasuda T, et al. Expression levels of vascular endothelial growth factor and its receptors in parkinson's disease. Neuroreport. 2006;17:705–709. doi: 10.1097/01.wnr.0000215769.71657.65. [DOI] [PubMed] [Google Scholar]
- 153.Ohlin KE, Francardo V, Lindgren HS, Sillivan SE, O'Sullivan SS, Luksik AS, et al. Vascular endothelial growth factor is upregulated by l-dopa in the parkinsonian brain: Implications for the development of dyskinesia. Brain. 2011;134:2339–2357. doi: 10.1093/brain/awr165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Garbuzova-Davis S, Rodrigues MC, Hernandez-Ontiveros DG, Louis MK, Willing AE, Borlongan CV, et al. Amyotrophic lateral sclerosis: A neurovascular disease. Brain Res. 2011;1398:113–125. doi: 10.1016/j.brainres.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 155.Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: Role of glial activation in motor neuron disease. Lancet Neurol. 2011;10:253–263. doi: 10.1016/S1474-4422(11)70015-1. [DOI] [PubMed] [Google Scholar]
- 156.Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial als correlates with disease progression. Neurology. 2001;57:1282–1289. doi: 10.1212/wnl.57.7.1282. [DOI] [PubMed] [Google Scholar]
- 157.Lino MM, Schneider C, Caroni P. Accumulation of sod1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J Neurosci. 2002;22:4825–4832. doi: 10.1523/JNEUROSCI.22-12-04825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, et al. Wild-type microglia extend survival in pu. 1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gong YH, Parsadanian AS, Andreeva A, Snider WD, Elliott JL. Restricted expression of g86r cu/zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J Neurosci. 2000;20:660–665. doi: 10.1523/JNEUROSCI.20-02-00660.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Engelhardt JI, Tajti J, Appel SH. Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch Neurol. 1993;50:30–36. doi: 10.1001/archneur.1993.00540010026013. [DOI] [PubMed] [Google Scholar]
- 161.Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. Cd4+ t cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited als. Proc Natl Acad Sci U S A. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. Ng2+ cns glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Seilhean D, Cazeneuve C, Thuries V, Russaouen O, Millecamps S, Salachas F, et al. Accumulation of tdp-43 and alpha-actin in an amyotrophic lateral sclerosis patient with the k17i ang mutation. Acta Neuropathol. 2009;118:561–573. doi: 10.1007/s00401-009-0545-9. [DOI] [PubMed] [Google Scholar]
- 164.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O'Banion MK, et al. Als-causing sod1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Winkler EA, Sengillo JD, Bell RD, Wang J, Zlokovic BV. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:1841–1852. doi: 10.1038/jcbfm.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Henkel JS, Beers DR, Wen S, Bowser R, Appel SH. Decreased mrna expression of tight junction proteins in lumbar spinal cords of patients with als. Neurology. 2009;72:1614–1616. doi: 10.1212/WNL.0b013e3181a41228. [DOI] [PubMed] [Google Scholar]
- 168.Rule RR, Schuff N, Miller RG, Weiner MW. Gray matter perfusion correlates with disease severity in als. Neurology. 2010;74:821–827. doi: 10.1212/WNL.0b013e3181d3e2dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sospedra M, Martin R. Immunology of multiple sclerosis. Annual review of immunology. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 170.Fugger L, Friese MA, Bell JI. From genes to function: The next challenge to understanding multiple sclerosis. Nature reviews. Immunology. 2009;9:408–417. doi: 10.1038/nri2554. [DOI] [PubMed] [Google Scholar]
- 171.Lopez-Diego RS, Weiner HL. Novel therapeutic strategies for multiple sclerosis--a multifaceted adversary. Nature reviews. Drug discovery. 2008;7:909–925. doi: 10.1038/nrd2358. [DOI] [PubMed] [Google Scholar]
- 172.Kotter MR, Stadelmann C, Hartung HP. Enhancing remyelination in disease--can we wrap it up? Brain: a journal of neurology. 2011;134:1882–1900. doi: 10.1093/brain/awr014. [DOI] [PubMed] [Google Scholar]
- 173.Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. Tnf alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nature neuroscience. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 174.Foote AK, Blakemore WF. Inflammation stimulates remyelination in areas of chronic demyelination. Brain: a journal of neurology. 2005;128:528–539. doi: 10.1093/brain/awh417. [DOI] [PubMed] [Google Scholar]
- 175.Nikic I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM, et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nature medicine. 2011;17:495–499. doi: 10.1038/nm.2324. [DOI] [PubMed] [Google Scholar]