Abstract
Objective
Acute lung injury secondary to smoke inhalation is a major source of morbidity and mortality in burn patients. We tested the hypothesis that nebulized epinephrine would ameliorate pulmonary dysfunction secondary to acute lung injury by reducing airway hyperemia and edema formation and mediating bronchodilatation in an established, large animal model of inhalation injury.
Design
Prospective, controlled, randomized trial.
Setting
University research laboratory.
Subjects
Twenty-four chronically instrumented, adult, female sheep.
Interventions
Following baseline measurements, the animals were allocated to a sham-injured group (n = 5), an injured and saline-treated group (n = 6), or an injured group treated with 4 mg of nebulized epinephrine every 4 hrs (n = 6). Inhalation injury was induced by 48 breaths of cotton smoke. The dose of epinephrine was derived from dose finding experiments (n = 7 sheep).
Measurements and Main Results
The injury induced significant increases in airway blood flows, bronchial wet/dry weight ratio, airway obstruction scores, ventilatory pressures, and lung malondialdehyde content, and contributed to severe pulmonary dysfunction as evidenced by a significant decline in Pao2/Fio2 ratio and increase in pulmonary shunt fraction. Nebulization of epinephrine significantly reduced tracheal and main bronchial blood flows, ventilatory pressures, and lung malondialdehyde content. The treatment was further associated with significant improvements of Pao2/Fio2 ratio and pulmonary shunting.
Conclusions
Nebulization of epinephrine reduces airway blood flow and attenuates pulmonary dysfunction in sheep subjected to severe smoke inhalation injury. Future studies will have to improve the understanding of the underlying pathomechanisms and identify the optimal dosing for the treatment of patients with this injury.
Keywords: acute lung injury, adrenaline, aerosolization, airway blood flow, bronchodilatation, sheep
Approximately 7%–20% of burn patients admitted to burn centers suffer from concomitant inhalation injury (1, 2). The presence of inhalation trauma has major impact on severity and survival of these patients (1–3). The mortality rates of these patients range from 20% to 60% (2, 3), accentuating the significance of new effective treatment strategies for patients with this injury.
Acute lung injury represents a serious complication that mainly contributes to smoke inhalation-related mortality. Among the involved pathophysiologic alterations, airway dysfunction plays a crucial role in acute lung injury-induced pulmonary failure. Key factors that impair normal airway function secondary to smoke inhalation include bronchospasm (4) and increased airway blood flow (5); the latter leading to airway wall edema, leakage of procoagulant factors containing exudates into the airways, and subsequent formation of obstructive casts (6, 7). The described changes altogether narrow the airway lumen and impair the alveolar ventilation. The resultant ventilation/perfusion mismatch deteriorates the pulmonary gas exchange and leads to systemic hypoxemia. In addition, increased airway blood flow empties into the lung, thereby mechanically increasing pulmonary transvascular fluid flux. Furthermore, airway hyperemia can augment parenchymal lung damage by flushing the pulmonary microcirculation with inflammatory mediators (8, 9).
Epinephrine is a catecholamine with dose-dependent α-adrenergic and strong β receptor-mediated vasoconstrictive properties. Intravenously administered epinephrine is mainly used for cardiovascular resuscitation because of its strong hemodynamic effects; however, its local β- and α-adrenergic properties are also exploited for the treatment of acute bronchospasm in patients with asthma (10, 11) or decreasing mucosal edema in children with laryngotracheitis (12), respectively.
We hypothesized that nebulized epinephrine will ameliorate pulmonary dysfunction secondary to smoke inhalation by reducing airway hyperemia and edema formation and mediating bronchodilatation. We further assumed that nebulized epinephrine will attain local airway efficacy without undesirable systemic effects, especially in the presence of hypermetabolic and hemodynamic alterations in thermally injured patients (13, 14). We tested this hypothesis in an established, large animal model of smoke inhalation injury (15–17).
MATERIALS AND METHODS
This study was approved by the Animal Care and Use Committee of the University of Texas Medical Branch and conducted in compliance with the guidelines of the National Institutes of Health and the American Physiologic Society for the care and use of laboratory animals.
Surgical Preparation and Injury
Twenty-four healthy adult female sheep weighing 30–40 kg were included in this study. Following induction of anesthesia with ketamine (500 mg intramuscular, 300 mg intravenously), endotracheal intubation was performed. Anesthesia was maintained using an isoflurane (1.4–1.8 vol%)-oxygen mixture. The right femoral artery was cannulated with a polyvinylchloride catheter (Intracath, 16-G, 24 inches, Becton Dickinson Vascular Access, Sandy, UT) for continuous measurement of systemic arterial pressure and intermittent sampling of arterial blood. A thermodilution catheter (model 93A-131-7F, Edwards Critical Care Division, Irvine, CA) was inserted into the right external jugular vein through an introducer sheath (Edwards Lifescience, Irvine, CA) and advanced into the common pulmonary artery. Through the left fifth intercostal space, a Silastic catheter (0.062-inch inner diameter and 0.125-inch outer diameter, Dow Corning, Midland, MI) was positioned in the left atrium for continuous measurement of left arterial pressure. After a recovery period of 5–7 days, a baseline measurement was performed in spontaneously breathing sheep. Thereafter, the animals were anesthetized using intravenous ketamine (5 mg/kg). A tracheostomy was performed and anesthesia was maintained with a halothane (1.1–2.0 vol%)-oxygen mixture. A Foley urinary retention catheter was inserted. Under deep anesthesia, the animals received smoke inhalation injury according to an established protocol, which has previously been described in detail (15–17). In brief, the sheep were insufflated with a total of 48 breaths (four sets of 12 breaths each) of cotton smoke. The smoke was applied using a modified bee smoker filled with 40 g of burning cotton toweling and connected to the tracheostomy tube via a modified endotracheal tube containing an indwelling thermistor from a pulmonary artery catheter. During the insufflation procedure, the temperature of the smoke was monitored carefully and not allowed to exceed 40°C. Arterial carboxyhemoglobin concentrations were determined immediately after each set of smoke inhalation. Anesthesia was then discontinued and the sheep were allowed to awaken.
Experimental Protocol
After injury, the sheep were randomly allocated to the following three study groups: 1) sham-injured, non-treated animals (Sham; n = 5); 2) injured animals that received 10 mL of nebulized saline (Saline; n = 6); and 3) injured animals treated with 4 mg of nebulized epinephrine (Epi 4 mg; n = 6). The nebulization procedure was started 1 hr after the injury and repeated every 4 hrs using an ultrasonic nebulizer (Aeroneb Pro, Aerogen, Mountain View, CA). The respective dose of epinephrine was dissolved in 10 mL of saline and nebulized over 30 mins. All sheep were mechanically ventilated (Servo Ventilator 900C, Siemens, Elema, Sweden) with a tidal volume of 12–15 mL/kg and a positive end-expiratory pressure of 5 cmH2O. The Fio2 was set at 1.0 for the first 3 hrs post-injury and was then adjusted to maintain sufficient oxygenation (Sao2 >90%, Pao2 80–100 mm Hg) whenever possible. The respiratory rate was initially set at 20 breaths per minute and was then adjusted according to blood gas analyses to maintain the Paco2 within 5 mm Hg of the baseline value. All animals were fluid resuscitated with lactated Ringer’s solution to keep hematocrit and cardiac filling pressures close to baseline values (±3%). The sheep had free access to dry food, but not water, to control the fluid balance. At the end of the 48-hr study period, the animals were deeply anesthetized with ketamine (15 mg/kg) and euthanized by intravenous injection of 60 mL saturated potassium chloride.
Dose Finding
To identify an effective and safe dose, we performed dose response studies in an additional seven sheep. At first, we tested the effects of 2, 4, and 6 mg of nebulized epinephrine in uninjured and instrumented sheep and monitored systemic hemodynamic variables, arterial lactate and glucose concentrations, and serum levels of catecholamines. After each nebulization procedure, systemic hemodynamic variables were monitored and documented after 0, 1, 2, 3, 4, 5, 10, 15, and 60 mins, and blood was drawn at 0, 15, and 60 mins (Table 1). Afterward, we tested the effects of 2 and 4 mg nebulized epinephrine on the degree of lung injury over 48 hrs in sheep subjected to the above described smoke inhalation procedure (Table 2). Nebulization of 2 mg of epinephrine had no significant effects on systemic hemodynamic variables or metabolism in the healthy state and did not attenuate the development of pulmonary dysfunction after inhalation injury. Nebulization of 4 mg of epinephrine had moderate but less metabolic effects than 6 mg, but effectively attenuated the deteriorations of Pao2/Fio2 ratio, pulmonary shunt fraction, and ventilatory pressures. Accordingly, 4 mg nebulized epinephrine was considered as the dose that exerts minimal systemic effects on hemodynamics and metabolism, but beneficially influences the degree of lung injury.
Table 1.
Short-term effect of different doses of nebulized epinephrine on hemodynamic variables, arterial lactate, and glucose concentrations in healthy sheep
| Time After Epinephrine Nebulization (mins) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | 0 | 1 | 2 | 3 | 4 | 5 | 10 | 15 | 60 |
| Mean arterial pressure, mm Hg | |||||||||
| Epi 2 mg | 107 ± 6 | 107 ± 6 | 107 ± 6 | 106 ± 7 | 104 ± 7 | 106 ± 8 | 104 ± 7 | 107 ± 6 | 106 ± 5 |
| Epi 4 mg | 103 ± 5 | 104 ± 7 | 105 ± 7 | 104 ± 7 | 104 ± 6 | 104 ± 6 | 102 ± 6 | 102 ± 7 | 102 ± 5 |
| Epi 6 mg | 100 ± 6 | 105 ± 8 | 102 ± 7 | 103 ± 6 | 102 ± 7 | 102 ± 7 | 102 ± 8 | 105 ± 7 | 101 ± 4 |
| Heart rate, bpm | |||||||||
| Epi 2 mg | 98 ± 12 | 101 ± 15 | 101 ± 15 | 99 ± 14 | 101 ± 14 | 100 ± 14 | 102 ± 14 | 98 ± 16 | 99 ± 15 |
| Epi 4 mg | 94 ± 14 | 90 ± 12 | 91 ± 12 | 95 ± 13 | 96 ± 15 | 96 ± 15 | 97 ± 15 | 96 ± 15 | 95 ± 15 |
| Epi 6 mg | 90 ± 12 | 88 ± 12 | 89 ± 12 | 89 ± 11 | 89 ± 12 | 91 ± 12 | 93 ± 14 | 92 ± 13 | 89 ± 13 |
| Lactate, mmol/L | |||||||||
| Epi 2 mg | .9 ± .2 | — | — | — | — | — | — | .8 ± .2 | .7 ± .3 |
| Epi 4 mg | .8 ± .2 | — | — | — | — | — | — | 1.5 ± .5 | 1.5 ± .6 |
| Epi 6 mg | .7 ± .2 | — | — | — | — | — | — | 1.9 ± .7a | 2.2 ± .6a |
| Glucose, mg/dL | |||||||||
| Epi 2 mg | 75 ± 8 | — | — | — | — | — | — | 78 ± 5 | 76 ± 5 |
| Epi 4 mg | 72 ± 6 | — | — | — | — | — | — | 94 ± 11a | 111 ± 10a |
| Epi 6 mg | 76 ±3 | — | — | — | — | — | — | 98 ± 17a | 137 ± 11a |
Epi, epinephrine.
p < .05 vs. baseline (0 min).
Table 2.
Effects of different doses of nebulized epinephrine on the degree of lung injury after smoke inhalation
| Time After Injury (hrs) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Degree of Lung Injury | 0 | 3 | 6 | 12 | 18 | 24 | 36 | 48 |
| Pao2/Fio2, mm Hg | ||||||||
| Saline | 497 ± 13 | 407 ± 61 | 431 ± 55 | 439 ± 49 | 438 ± 42 | 393 ± 66 | 275 ± 65 | 192 ± 43 |
| Epi 2 mg | 475 ± 13 | 503 ±7 | 568 ± 21 | 556 ± 10 | 517 ± 30 | 454 ± 127 | 314 ± 120 | 222 ± 79 |
| Epi 4 mg | 528 ± 21 | 517 ± 24 | 574 ± 28 | 517 ± 51 | 485 ± 64 | 470 ± 64 | 442 ± 92a | 405 ± 65a |
| Shunt fraction, Qs/Qt | ||||||||
| Saline | .16 ± .01 | .21 ± .02 | .18 ± .02 | .18 ±.01 | .19 ± .03 | .23 ± .05 | .35 ± .07 | .34 ± .06 |
| Epi 2 mg | .17 ± .02 | .18 ± .01 | .15 ±.01 | .15 ± .02 | .15 ± .02 | .23 ± .10 | .30 ±.11 | .28 ± .10 |
| Epi 4 mg | .17 ± .02 | .19 ± .02 | .15 ±.01 | .17 ± .03 | .17 ± .03 | .16 ± .02 | .19 ± .04a | .19 ± .02a |
| Peak airway pressure, cmH2O | ||||||||
| Saline | 20 ± 1 | 19 ± 1 | 19 ± 1 | 20 ± 1 | 21 ± 1 | 21 ± 1 | 29 ± 3 | 31 ± 4 |
| Epi 2 mg | 19 ± 1 | 18 ± 1 | 19 ± 2 | 20 ± 2 | 21 ± 3 | 24 ± 5 | 27 ± 5 | 27 ± 7 |
| Epi 4 mg | 20 ± 1 | 19 ±2 | 19 ± 1 | 19 ± 1 | 20 ± 2 | 19 ±2 | 23 ± 2a | 22 ± 2a |
| Pause airway pressure, cmH2O | ||||||||
| Saline | 19 ± 0 | 18 ± 1 | 17 ± 1 | 18 ± 1 | 18 ± 1 | 19 ± 1 | 25 ±3 | 28 ± 4 |
| Epi 2 mg | 18 ± 1 | 17 ± 0 | 17 ± 2 | 18 ± 2 | 19 ± 2 | 23 ± 6 | 25 ± 4 | 26 ± 6 |
| Epi 4 mg | 17 ± 2 | 17 ± 2 | 17 ± 1 | 16 ± 1 | 17 ± 1 | 17 ± 2 | 20 ± 2 | 20 ± 2a |
Epi, epinephrine.
p < .05 vs. saline.
Hemodynamic Measurements
Systemic and pulmonary hemodynamic variables were determined from the femoral and pulmonary artery catheters using pressure transducers (Baxter-Edwards Critical Care, Irvine, CA) and recorded on a hemodynamic monitor (monitor V24C, Philips Medizin Systeme Böblingen, Böblingen, Germany). Cardiac output was measured in triplicate with the thermodilution technique (Monitor 9530, Baxter-Edwards Critical Care). Cardiac index was calculated using a standard equation.
Blood Analysis
Blood gases were measured using a blood gas analyzer (Synthesis 15, Instrumentation Laboratories, Lexington, MA). In addition, blood was centrifuged and plasma and serum samples were frozen at −80°C for the determination of serum aspartate aminotransferase, alanine aminotransferases, bilirubin, and creatinine (Vitros 5,1 FS, Ortho Clinical Diagnostics, Rochester, NY, USA). Plasma colloid oncotic pressure (πP) was determined with a colloid osmometer (model 4420, Wescor, Logan, UT). Plasma protein concentration (CP) was measured with a refractometer (National Instrument; Baltimore, MD). Catecholamine plasma levels were measured by high performance liquid chromatography using a commercially available assay kit (Bio-Rad Laboratories, Hercules, CA).
Pulmonary Function
The Pao2/Fio2 ratio was calculated as an index of pulmonary oxygenation. As an estimate of ventilation/perfusion mismatch, the pulmonary shunt fraction (Qs/Qt) was calculated using the following standard equation (18): Qs/Qt = (Cco2 – Cao2)/(Cco2 – CCvo2), where Cco2 is the end capillary oxygen content, Cao2 is the arterial oxygen content, and Cvo2 is the arterial oxygen content. It was assumed that the hemoglobin of the alveolar end capillaries was completely saturated with oxygen.
Tissue Analysis
After completion of the 48-hr experiment, the right lung was removed, and a 1-cm thick section was taken from the lower lobe and inflated with 10% formalin for histologic examination. Fixed samples were embedded in paraffin, sectioned into 4-µm pieces, and stained with hematoxylin-eosin. A pathologist who was unaware of the group assignments analyzed the samples. Airway obstruction was evaluated by estimating the degree of luminal obstruction (0% to 100%). Each airway was classified as a bronchus, a bronchiole, or a terminal bronchiole as previously described (6). From the remaining part of the right lower lobe, bloodless lung wet/dry weight ratio was calculated as an index of lung water content (19). Malondialdehyde formation was utilized to quantify the lipid peroxidation in the lung and measured as thiobarbituric acid-reactive material. Lung malondialdehyde levels were quantified with a commercially available assay (Northwest Life Science Specialties, Vancouver, WA, Canada). The level of lipid peroxides was expressed as malondialdehyde/protein.
Statistical Analysis
All values are expressed as means ± sem. Results were compared by a two-way analysis of variance (ANOVA) for repeated measures with appropriate Student-Newman-Keuls post hoc comparisons to compare differences within and between groups. One-way ANOVA was used to compare groups when measurements were made at only one time point, and the New-man-Keuls procedure was used for post hoc pairwise comparisons. A value of p < .05 was regarded as statistically significant.
RESULTS
Airway Microvascular Blood Flow and Edema Formation
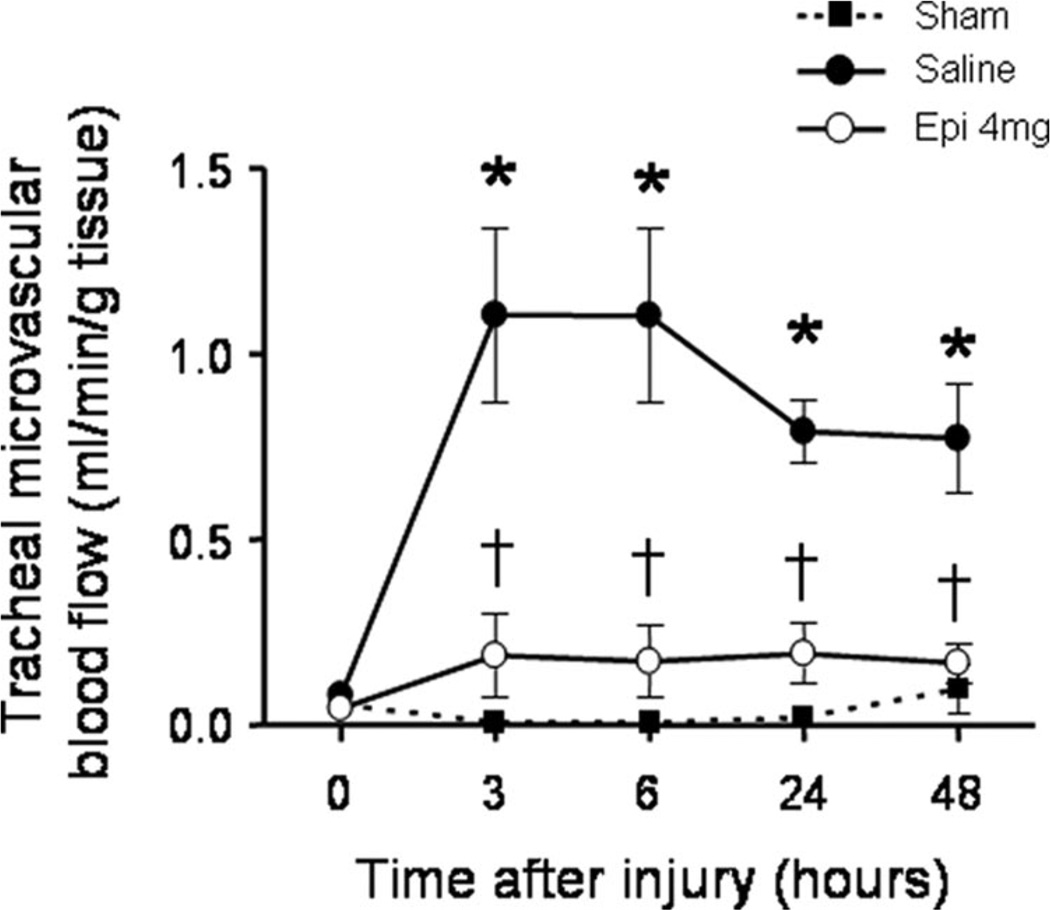
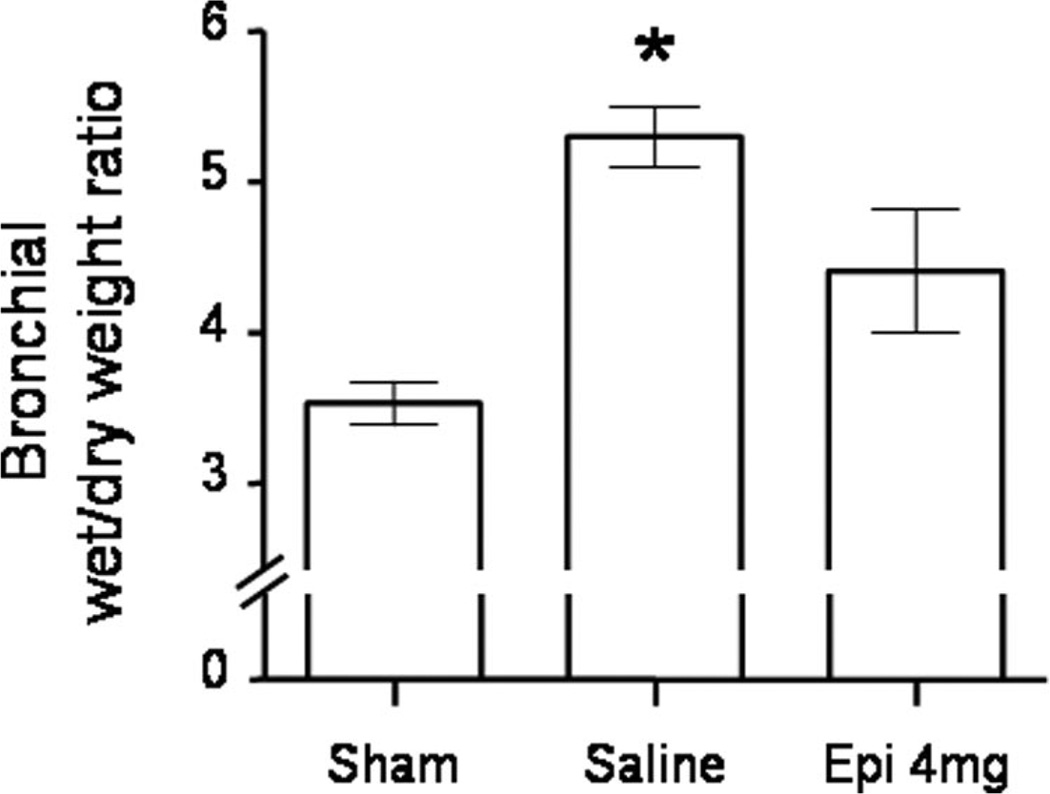
Tracheal and bronchial microvascular blood flow markedly increased in saline-treated injured sheep vs. sham animals. This increase was significantly attenuated by nebulization of 4 mg of epinephrine in the trachea and in the right and left main bronchi (Fig. 1, Table 3). The injury also induced a significant increase in bronchial wet/dry weight ratio in saline-treated, but not in epinephrine-treated, animals (Fig. 2). Total lung wet/dry weight ratio was not significantly different between groups.
Figure 1.
Impact of epinephrine (Epi) nebulization on tracheal blood flow as assessed by microsphere technique in sheep with combined burn and smoke inhalation injury. *p < .05 vs. Sham; †p < .05 epinephrine 4 mg vs. saline.
Table 3.
Changes in main and distal bronchial blood flow
| Time After Injury (hrs) | |||||
|---|---|---|---|---|---|
| Bronchial Blood Flow | 0 | 3 | 6 | 24 | 48 |
| Left main bronchial microvascular blood flow (mL/min/mg tissue) | |||||
| Sham | .09 ± .05 | .07 ± .02 | .04 ± .01 | .11 ±.01 | .14 ± .06 |
| Saline | .08 ± .01 | 1.07 ± .21a | .93 ± .22a | .75 ± .11a | .71 ± .05a |
| Epi 4 mg | .14 ± .04 | .67 ± .20a,b | .79 ± .23a | .64 ± .16a | .53 ± .16 |
| Right main bronchial microvascular blood flow (mL/min/mg tissue) | |||||
| Sham | .07 ± .01 | .08 ± .04 | .04 ± .02 | .10 ± .05 | .10 ± .07 |
| Saline | .11 ±.02 | 1.65 ± .38a | 1.49 ± .37a | 1.19 ± .19a | .79 ± .11a |
| Epi 4 mg | .08 ± .04 | .28 ± .10b | .39 ± .08b | .41 ± .09b | .53 ± .09 |
| Left distal bronchial microvascular blood flow (mL/min/mg tissue) | |||||
| Sham | .86 ± .09 | .74 ± .25 | .55 ± .13 | 1.29 ± .36 | .40 ± .44 |
| Saline | .53 ± .13 | 3.13 ± .55a | 2.57 ± .66a | 2.02 ± .40 | 2.25 ± .32 |
| Epi 4 mg | .42 ± .13 | 2.11 ± .55a | 2.91 ± .69a | 1.33 ± .33 | 1.57 ± .42 |
| Right distal bronchial microvascular blood flow (mL/min/mg tissue) | |||||
| Sham | .89 ± .15 | .72 ± .16 | .72 ± .18 | 1.31 ± .24 | 1.61 ± .42 |
| Saline | .31 ± .07 | 4.18 ± .90a | 4.01 ± .85a | 2.71 ± .36 | 1.43 ± .22 |
| Epi 4 mg | .48 ± .10 | 3.66 ± 47a | 3.14 ± .72a | 1.90 ± .32 | 2.12 ± .52 |
Epi, epinephrine.
p < .05 vs. sham
p < .05 Epi 4 mg vs. saline.
Figure 2.
Impact of epinephrine (Epi) nebulization on bronchial wet/dry weight ratio in sheep with combined burn and smoke inhalation injury. *p < .05 vs. sham.
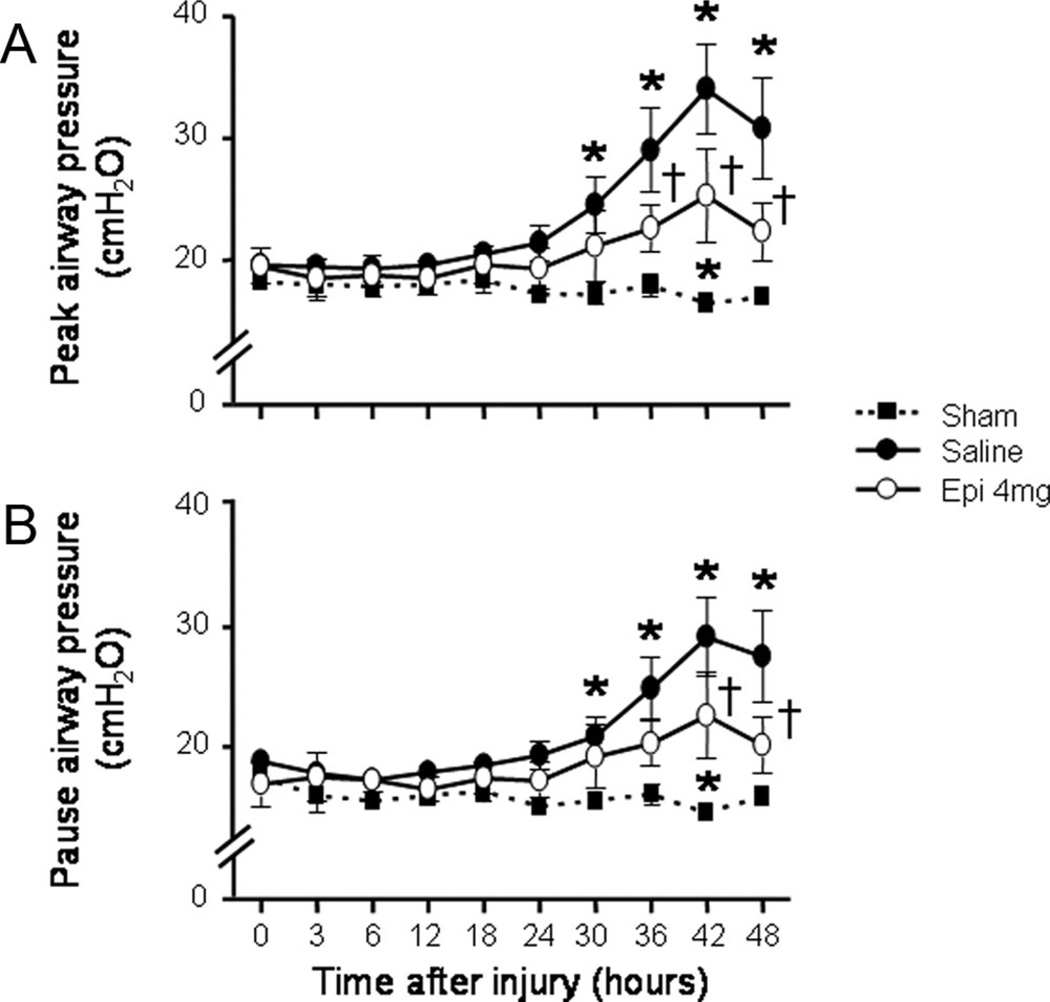
Ventilatory Pressures and Airway Obstruction
The injury-related elevations in both peak and pause airway pressures were significantly attenuated in sheep that received epinephrine nebulization (Fig. 3). Histologically determined bronchial obstruction score was significantly increased in injured, saline-treated vs. sham animals (24% ± 7% vs. 2% ± 1%; p = .02). This increase was slightly decreased by epinephrine nebulization (21% ± 8%), but no significant differences were found between saline- and epinephrine-treated groups (p > .05).
Figure 3.
Impact of epinephrine (Epi) nebulization on ventilatory pressures (A, peak airway pressure and B, pause airway in sheep with combined burn and smoke inhalation injury. *p < .05 vs. Sham; †p < .05 epinephrine 4 mg vs. saline.
Organ Functions
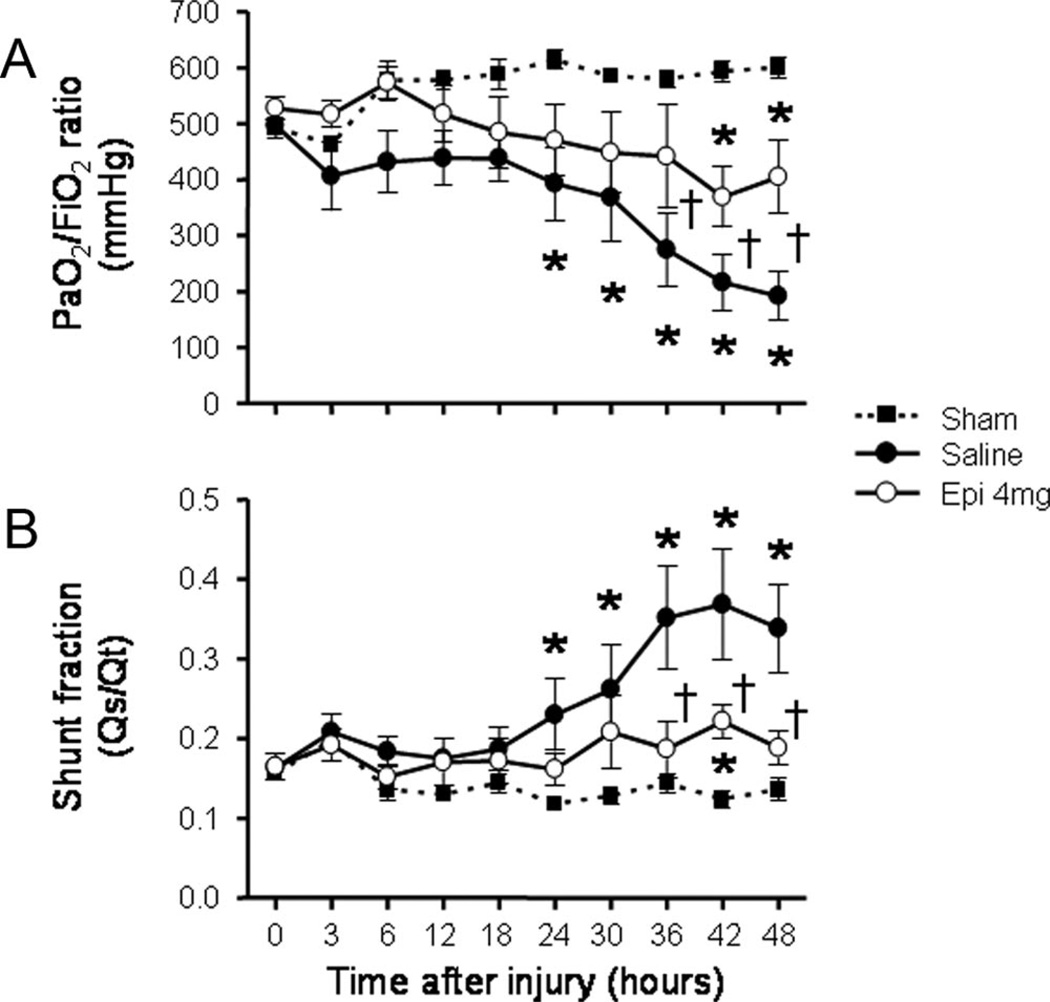
The injury was associated with a severe impairment in respiratory gas exchange as indicated by a decline in Pao2/Fio2 ratio below 200 mm Hg and a concurrent increase in pulmonary shunt fraction in saline-treated animals. In the epinephrine group, the Pao2/Fio2 ratio remained above 300 mm Hg during the entire study period, and was significantly higher than in the saline group from 36 to 48 hrs (Fig. 4A). Pulmonary shunt fraction was also significantly lower in epinephrine-treated than in saline-treated animals from 36 to 48 hrs post-injury (Fig. 4B). Surrogate parameters of liver function (aspartate aminotransferases, alanine aminotransferases, bilirubin), kidney function (creatinine, creatinine clearance, urine output), and systemic vascular permeability (plasma protein concentration, colloid oncotic pressure, fluid balance) were not affected by epinephrine nebulization (data not shown).
Figure 4.
Impact of epinephrine (Epi) nebulization on Pao2/Fio2 ratio (A) and pulmonary shunt fraction (B) of sheep with combined burn and smoke inhalation injury. *p < .05 vs. sham; †p < .05 epinephrine 4 mg vs. saline.
Formation of Malondialdehyde
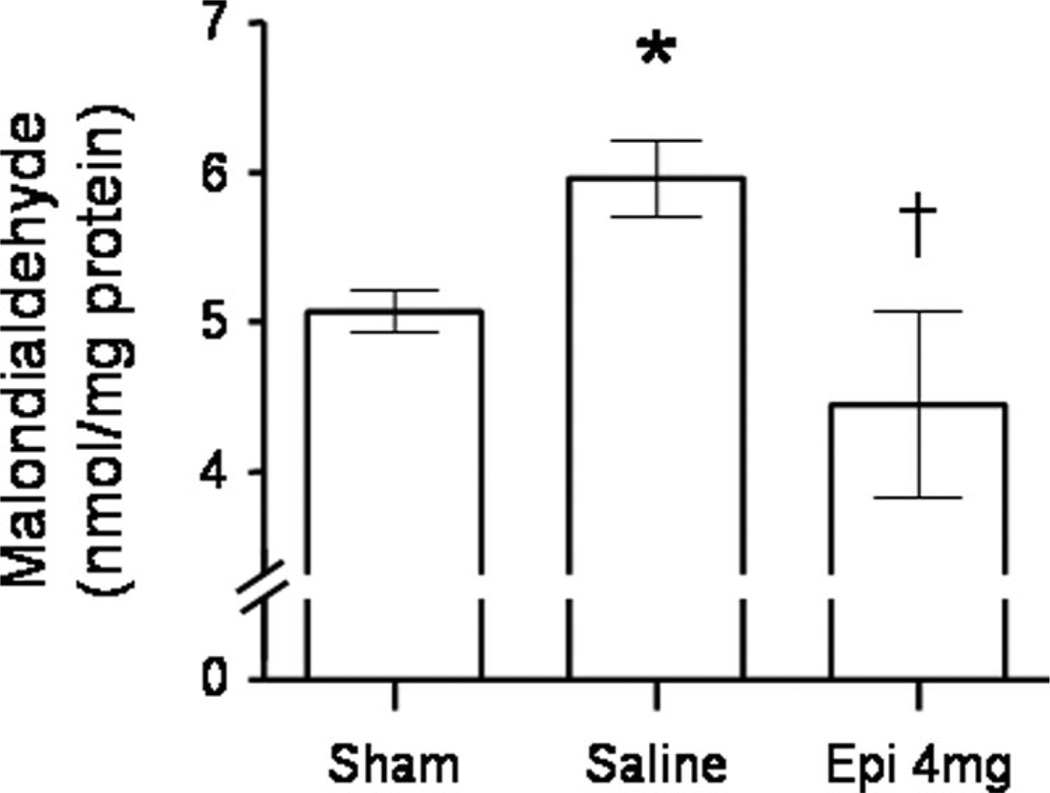
Malondialdehyde contents in whole lung homogenates were significantly increased post-injury in saline-treated vs. sham animals. In injured sheep treated with nebulization of 4 mg of epinephrine, the increase in malondialdehyde contents was abolished (Fig. 5).
Figure 5.
Impact of epinephrine (Epi) nebulization on lung homogenate malondialdehyde content, and index of lipid peroxidation, of sheep with combined burn and smoke inhalation injury. *p < .05 vs. sham; †p < .05 epinephrine 4 mg vs. saline.
Hemodynamic Variables, Metabolism, and Catecholamine Plasma Levels
Except for a transient elevation in cardiac index at 6 hrs, and a mild increase in arterial lactate concentration at 6 and 18 hrs post-injury, systemic and pulmonary hemodynamic variables and metabolism were not significantly affected by epinephrine nebulization (Table 4). Plasma catecholamine concentrations were similar in saline- and epinephrine-treated groups (data not shown).
Table 4.
Changes in cardiopulmonary hemodynamics and metabolism
| Hemodynamic and Metabolic Changes |
Time After Injury (hrs) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 12 | 18 | 24 | 36 | 48 | |
| Mean arterial pressure, mm Hg | ||||||||
| Sham | 94 ± 2 | 112 ± 5 | 106 ± 4 | 108 ± 4 | 111 ± 5 | 97 ± 5 | 93 ± 6 | 98 ± 5 |
| Saline | 93 ± 5 | 107 ± 7 | 106 ± 6 | 102 ± 5 | 98 ± 6 | 97 ± 6 | 99 ± 8 | 94 ± 6 |
| Epi 4 mg | 92 ± 4 | 96 ± 4 | 98 ± 5 | 97 ± 3 | 97 ± 3 | 92 ± 3 | 92 ± 3 | 90 ± 3 |
| Heart Rate, bpm | ||||||||
| Sham | 83 ± 6 | 97 ± 7 | 104 ± 2 | 101 ± 6 | 100 ± 7 | 103 ± 7 | 90 ± 7 | 84 ± 8 |
| Saline | 79 ± 3 | 93 ± 7 | 89 ± 8 | 88 ± 3 | 84 ± 4 | 96 ± 8 | 111 ± 10 | 101 ± 10 |
| Epi 4 mg | 83 ± 5 | 108 ± 4 | 100 ± 3 | 106 ± 4 | 96 ± 5 | 89 ± 4 | 103 ± 4 | 85 ± 2 |
| Cardiac index, L·min−1·m−2 | ||||||||
| Sham | 5.4 ± .3 | 6.1 ± .4 | 5.9 ± .5 | 5.6 ± .6 | 5.7 ± .3 | 5.4 ± .5 | 5.1 ± .7 | 5.1 ± .6 |
| Saline | 5.7 ± .4 | 5.8 ± .4 | 5.8 ± .5 | 5.0 ± .2 | 5.0 ± .2 | 5.3 ± .3 | 5.9 ± .5 | 5.7 ± .4 |
| Epi 4 mg | 6.1 ± .3 | 7.0 ± .5 | 7.4 ± 3a,b | 6.1 ± .1 | 6.0 ± .4 | 5.5 ± .3 | 5.4 ± .3 | 5.3 ± .3 |
| Mean pulmonary arterial pressure, mm Hg | ||||||||
| Sham | 20 ± 1 | 25 ± 1 | 25 ± 1 | 25 ± 1 | 25 ± 2 | 25 ± 1 | 25 ± 1 | 24 ± 2 |
| Saline | 20 ± 0 | 27 ± 2 | 28 ± 2 | 27 ± 2 | 25 ± 2 | 30 ± 2 | 31 ± 2a | 31 ± 3a |
| Epi 4 mg | 21 ± 0 | 26 ± 1 | 27 ± 1 | 26 ± 1 | 27 ± 1 | 26 ± 1 | 27 ± 1b | 26 ± 1b |
| Lactate, mmol/L | ||||||||
| Sham | .4 ± .1 | .8 ± .2 | .8 ± .1 | .7 ± .1 | .7 ± .1 | .4 ± .1 | .5 ± .1 | .4 ± 0 |
| Saline | .6 ± .1 | .9 ± .2 | 1.0 ± .2 | .8 ± .1 | .6 ± .1 | .6 ± .1 | .7 ± .2 | .8 ± .2 |
| Epi 4 mg | .4 ± .1 | 1.0 ± .1 | 1.5 ± 2a,b | 1.1 ± .2 | 1.3 ± .5a,b | .4 ± .1 | .5 ± .1 | .5 ± .1 |
| Glucose, mg/dL | ||||||||
| Sham | 87 ± 18 | 127 ± 13 | 134 ± 17 | 98 ± 10 | 92 ± 9 | 88 ±8 | 91 ± 16 | 100 ± 6 |
| Saline | 69 ± 5 | 91 ± 8a | 99 ± 6a | 94 ± 9 | 94 ± 4 | 77 ± 4 | 80 ± 6 | 71 ± 6 |
| Epi 4 mg | 74 ± 5 | 136 ± 13b | 138 ± 17b | 100 ± 19 | 120 ± 12 | 77 ± 7 | 84 ± 10 | 98 ± 1 |
| Oxygen delivery index, mL·min−1·m−2 | ||||||||
| Sham | 792 ± 61 | 908 ± 66 | 757 ± 56 | 767 ± 68 | 788 ± 43 | 696 ± 69 | 670 ± 88 | 637 ± 79 |
| Saline | 753 ± 36 | 826 ± 76 | 765 ± 63 | 619 ± 36 | 653 ± 39 | 698 ± 64 | 663 ± 45 | 681 ± 65 |
| Epi 4 mg | 796 ± 55 | 985 ± 74 | 968 ± 35 | 746 ± 39 | 767 ± 67 | 718 ± 50 | 666 ± 63 | 654 ± 41 |
| Oxygen consumption index, mL·min−1·m−2 | ||||||||
| Sham | 269 ± 12 | 255 ± 14 | 252 ± 15 | 281 ± 36 | 261 ± 12 | 253 ± 23 | 229 ± 20 | 211 ± 18 |
| Saline | 294 ± 15 | 262 ± 15 | 251 ± 26 | 218 ± 8 | 231 ± 10 | 269 ± 20 | 287 ± 43 | 250 ± 21 |
| Epi 4 mg | 283 ± 28 | 270 ± 27 | 298 ± 20 | 265 ± 20 | 268 ± 28 | 247 ± 10 | 244 ± 19 | 243 ± 16 |
Epi, epinephrine.
p < .05 vs. sham
p < .05 Epi 4 mg vs. saline.
DISCUSSION
The key findings of the current investigation are that nebulization of 4 mg of epinephrine every 4 hrs effectively attenuated the smoke inhalation-induced elevations in tracheal and main bronchial blood flow, increases in ventilatory pressures, and deteriorations in pulmonary shunting and oxygenation in sheep. Importantly, the beneficial effects of epinephrine nebulization on pulmonary function were not associated with detrimental effects attributed to epinephrine when administered systemically, such as increases in plasma catecholamine levels, and arterial lactate or glucose concentrations.
The pathophysiologic alterations following smoke inhalation injury are multifaceted and include the release of neuropeptides, such as calcitonin gene-related peptide (5), induction of nitric oxide synthases (15, 20), the release of reactive nitrogen and oxygen species (20), and activation of poly(adenosine diphosphate) polymerase (21) and nuclear factor-κB (22). Altogether these abnormalities contribute to an early 20- to 30-fold pathologic increase in airway blood flow, leading to airway wall edema formation and impairment of physiologic mechanisms, including hypoxic pulmonary vasoconstriction (23). Consequently, the blood flow to nonventilated areas of the lung increases which results in a ventilation/perfusion mismatch as reflected by an elevation in pulmonary shunt fraction. Furthermore, increased blood flow and the development of microvascular hyperpermeability in response to smoke inhalation injury lead to the transvascular leakage of plasma into the airways. This exudate contains procoagulant factors, which are activated by tissue factors present in alveolar macrophages or expressed from pulmonary epithelial cells and result in the formation of fibrin clots in the airways. Fibrin solidifies and forms a solid mass which obstructs 18%–26% of the airway lumen (6, 7). In addition, the inhalation of smoke induces airway hyperresponsiveness and bronchospasm, which further disrupts the normal air flow (4). All of these pathologic changes (airway wall edema, airway obstruction, and bronchospasm) lead to airway narrowing, impaired alveolar ventilation, and a significant ventilation/perfusion mismatch, which causes systemic hypoxemia. Additionally, increased airway blood flow can augment lung injury because it flushes the pulmonary microcirculation with various mediators, including accumulated neutrophils, cytokines, and reactive nitrogen and oxygen species (8, 9). This, in turn, may induce a severe inflammatory response of the lung parenchyma.
We have previously demonstrated that the above described chain of events can be interrupted experimentally at various levels by administration of specific pharmacologic antagonists (e.g., neuropeptide antagonists or nitric oxide synthase inhibitors [5, 15, 20]) or by surgical ablation of airway blood flow (8, 9). However, these innovative treatment strategies could not yet be translated into clinical practice because the compounds are not approved for human application and fear of uncontrollable side effects, respectively. In contrast, the proposed nebulization of epinephrine in the current study represents an effective treatment of inhalation injury that has already been established in the management of other airway diseases (10–12).
The current study is the first to provide evidence that epinephrine nebulization reduces the smoke inhalation-related ventilation/perfusion mismatch, as indicated by decreased pulmonary shunt fraction, and improves pulmonary oxygenation, as evidenced by increased Pao2/Fio2 ratio. With the applied methods, important mechanistic aspects of the amelioration of pulmonary function could be identified. The hypothesis that the topical vasoconstrictive α-adrenergic effects of nebulized epinephrine reduce airway hyperemia could be confirmed by the significantly lower tracheal and main bronchial blood flow in the treatment group. In addition, there was a trend toward lower bronchial wet/dry weight ratio, indicating less airway wall edema through epinephrine nebulization. Although the effects of nebulized epinephrine on bronchoconstriction were not directly measured, the significantly reduced ventilatory pressures in the treatment group give indirect evidence of wider airway lumens, probably due to the topical bronchodilator, β2-adrenergic effects. Furthermore, the reduction of airway blood flow by epinephrine nebulization in the present study was associated with significantly less malondialdehyde formation in lung tissue. Malondialdehyde is a marker of lipid peroxidation and is increasingly formed in response to inflammation. Thus, the current results suggest that reduced airway blood flow by epinephrine nebulization positively influences the inflammatory response of the lung parenchyma after inhalation of smoke. It is a limitation of the current study, however, that only the effects of epinephrine, a mixed α- and β-agonist, were tested. Future experiments are warranted to define the role of pharmacologic modulation of each receptor separately in the setting of severe inhalation injury.
Smoke inhalation also triggers mucus secretion by airway glands. We have previously shown that excessive mucus secretion plays an essential role in the formation of airway obstructive casts (24). Epinephrine is generally assumed to exert no significant effects on airway glands, but may even slightly increase mucus gland secretion (25, 26). Notably, epinephrine nebulization in the current study did not significantly alter histologically determined airway obstruction scores, indicating that the treatment had no major influence on mucus secretion and airway cast formation in this experimental model of severe smoke inhalation. The fact that ventilatory pressures decreased in the treatment group although bronchial obstruction scores were unaffected, indicates that nebulized epinephrine directly exerted bronchodilatory effects.
The dose of 4 mg nebulized epinephrine in sheep did not exert significant adverse hemodynamic effects, probably because this dose was just high enough to exert local effects on the airways. Nonetheless, it has to be kept in mind that the β-adrenergic effects of higher doses of epinephrine may cause potential harm by increasing heart rate, and the α-adrenergic effects may induce uncontrolled elevation of blood pressure by systemic vasoconstriction. These drug-induced hemodynamic alterations may become problematic in patients with significant comorbidities such as coronary heart disease or cerebral aneurysms. Importantly, we observed dose-dependent increases in arterial lactate and glucose concentrations within 1 hr after epinephrine nebulization. Even though these effects were only transiently detectable, metabolic alterations may represent another potential side effect of this treatment. Notably, inhalation injury is frequently associated with cutaneous burns, and endogenous catecholamines are known to be the primary mediators of the hypermetabolic response in burn patients (13, 14). Additional administration of exogenous epinephrine may support the burn-induced hypermetabolic reaction and increase the likelihood of complications in this patient population. Future studies are warranted to test the effects of nebulized epinephrine in an ovine model of combined smoke inhalation and large skin burn.
In the current study, we were seeking the most effective dose of epinephrine with minimum systemic effects. While 2 mg of nebulized epinephrine did not ameliorate pulmonary oxygenation, nebulization of 6 mg epinephrine was associated with the highest increase in arterial lactate concentrations. Thus, we selected a dose of 4 mg, which significantly improved the pulmonary gas exchange with less metabolic effects than the higher dose. However, it is a limitation of the present study that we did not test the effects of higher doses on pulmonary function. We refrained from conducting more extensive range-finding studies because the costs of large animal studies make such experiments prohibitively expensive. In addition, caution should be exercised when extrapolating the findings from the sheep model to humans with inhalation injury because of potential differences in species. Finally, the effects of epinephrine on survival have not been determined. The amelioration of physiologic parameters by the treatment may not necessarily be associated with improved long-term outcome.
CONCLUSIONS
Nebulization of 4 mg epinephrine every 4 hrs significantly attenuated the development of pulmonary dysfunction in sheep subjected to severe smoke inhalation injury, probably through both local vasoconstrictive α-adrenergic effects that reduced airway blood flow and β-adrenergic effects that caused bronchodilatation. Epinephrine has an advantage toward pure α- and β-agonists in this regard because it exerts both vasoconstrictive and bronchodilatory effects concurrently. Therefore, nebulization of epinephrine may represent a useful treatment adjunct for patients with severe inhalation injury. Future studies will have to further evaluate the understanding of the underlying pathomechanisms and to identify the optimal dosing for the treatment of patients with this injury.
Acknowledgments
Supported, in part, by National Institutes of Health grants P012 GM066312, R01 GM060688; Shriners Burns Institute 85041, 84050, 84080, and GM60915.
Footnotes
Dr. Lange, Dr. Hamahata, Dr. Kulp, and Dr. Nakano received funding from Shriners of North America. The remaining authors have not disclosed any potential conflicts of interest.
REFERENCES
- 1.American Burn Association. National burn repository: 2010 report. Dataset 6.0. [Accessed October 12, 2010]; Available at: http://www.ameriburn.org/2010NBRAnnualReport.pdf.
- 2.Saffle JR, Davis B, Williams P. Recent outcomes in the treatment of burn injury in the United States: A report from the American Burn Association Patient Registry. J Burn Care Rehabil. 1995;16:219–232. doi: 10.1097/00004630-199505000-00002. discussion 288–289. [DOI] [PubMed] [Google Scholar]
- 3.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205:82–87. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu TH, Kou YR. Airway hyperresponsiveness to bronchoconstrictor challenge after wood smoke exposure in guinea pigs. Life Sci. 2001;68:2945–2956. doi: 10.1016/s0024-3205(01)01088-8. [DOI] [PubMed] [Google Scholar]
- 5.Lange M, Enkhbaatar P, Traber DL, et al. Role of calcitonin gene-related peptide (CGRP) in ovine burn and smoke inhalation injury. J Appl Physiol. 2009;107:176–184. doi: 10.1152/japplphysiol.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox RA, Burke AS, Soejima K, et al. Airway obstruction in sheep with burn and smoke inhalation injuries. Am J Respir Cell Mol Biol. 2003;29:295–302. doi: 10.1165/rcmb.4860. [DOI] [PubMed] [Google Scholar]
- 7.Enkhbaatar P, Murakami K, Cox R, et al. Aerosolized tissue plasminogen inhibitor improves pulmonary function in sheep with burn and smoke inhalation. Shock. 2004;22:70–75. doi: 10.1097/01.shk.0000129201.38588.85. [DOI] [PubMed] [Google Scholar]
- 8.Hamahata A, Enkhbaatar P, Sakurai H, et al. Effect of ablated bronchial blood flow on survival rate and pulmonary function after burn and smoke inhalation in sheep. Burns. 2009;35:802–810. doi: 10.1016/j.burns.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakurai H, Soejima K, Nozaki M, et al. Effect of ablated airway blood flow on systemic and pulmonary microvascular permeability after smoke inhalation in sheep. Burns. 2007;33:885–891. doi: 10.1016/j.burns.2006.10.394. [DOI] [PubMed] [Google Scholar]
- 10.Abroug F, Nouira S, Bchir A, et al. A controlled trial of nebulized salbutamol and adrenaline in acute severe asthma. Intensive Care Med. 1995;21:18–23. doi: 10.1007/BF02425149. [DOI] [PubMed] [Google Scholar]
- 11.Adoun M, Frat JP, Doré P, et al. Comparison of nebulized epinephrine and terbutaline in patients with acute severe asthma: A controlled trial. J Crit Care. 2004;19:99–102. doi: 10.1016/j.jcrc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Kelley PB, Simon JE. Racemic epinephrine use in croup and disposition. Am J Emerg Med. 1992;10:181–183. doi: 10.1016/0735-6757(92)90204-B. [DOI] [PubMed] [Google Scholar]
- 13.Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 14.Jeschke MG, Mlcak RP, Finnerty CC, et al. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007;11:R90. doi: 10.1186/cc6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enkhbaatar P, Murakami K, Shimoda K, et al. The inducible nitric oxide synthase inhibitor BBS-2 prevents acute lung injury in sheep after burn and smoke inhalation injury. Am J Respir Crit Care Med. 2003;167:1021–1026. doi: 10.1164/rccm.200209-1031PP. [DOI] [PubMed] [Google Scholar]
- 16.Isago T, Fujioka K, Traber LD, et al. Derived pulmonary capillary pressure changes after smoke inhalation in sheep. Crit Care Med. 1991;19:1407–1413. doi: 10.1097/00003246-199111000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Westphal M, Noshima S, Isago T, et al. Selective thromboxane A2 synthase inhibition by OKY-046 prevents cardiopulmonary dysfunction after ovine smoke inhalation injury. Anesthesiology. 2005;102:954–961. doi: 10.1097/00000542-200505000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Suter PM, Fairley HB, Schlobohm RM. Shunt, lung volume and perfusion during short periods of ventilation with oxygen. Anesthesiology. 1975;43:617–627. doi: 10.1097/00000542-197512000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Pearce ML, Yamashita J, Beazell J. Measurement of pulmonary edema. Circ Res. 1965;16:482–488. doi: 10.1161/01.res.16.5.482. [DOI] [PubMed] [Google Scholar]
- 20.Saunders FD, Westphal M, Enkhbaatar P, et al. Molecular biological effects of selective neuronal nitric oxide synthase inhibition in ovine lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;298:L427–L436. doi: 10.1152/ajplung.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimoda K, Murakami K, Enkhbaatar P, et al. Effect of poly(ADP ribose) synthetase inhibition on burn and smoke inhalation injury in sheep. Am J Physiol Lung Cell Mol Physiol. 2003;285:L240–L249. doi: 10.1152/ajplung.00319.2002. [DOI] [PubMed] [Google Scholar]
- 22.Hassa PO, Hottiger MO. The functional role of poly(ADP-ribose)polymerase 1 as novel co-activator of NF-kappaB in inflammatory disorders. Cell Mol Life Sci. 2002;59:1534–1553. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westphal M, Cox RA, Traber LD, et al. Combined burn and smoke inhalation injury impairs ovine hypoxic pulmonary vasoconstriction. Crit Care Med. 2006;34:1428–1436. doi: 10.1097/01.CCM.0000215828.00289.B9. [DOI] [PubMed] [Google Scholar]
- 24.Cox RA, Mlcak RP, Chinkes DL, et al. Upper airway mucus deposition in lung tissue of burn trauma victims. Shock. 2008;29:356–361. doi: 10.1097/shk.0b013e31814541dd. [DOI] [PubMed] [Google Scholar]
- 25.Peatfield AC, Richardson PS. The control of mucin secretion into the lumen of the cat trachea by alpha- and beta-adrenoceptors, and their relative involvement during sympathetic nerve stimulation. Eur J Pharmacol. 1982;81:617–626. doi: 10.1016/0014-2999(82)90351-x. [DOI] [PubMed] [Google Scholar]
- 26.Rogers DF. Pharmacological regulation of the neuronal control of airway mucus secretion. Curr Opin Pharmacol. 2002;2:249–255. doi: 10.1016/s1471-4892(02)00146-7. [DOI] [PubMed] [Google Scholar]