Significance
Previous studies showed that the unfolded protein response (UPR) in plants is elicited by environmental stress, but not that it protects plants from stress. This paper demonstrates by blocking both arms of the UPR signaling pathway that the UPR protects plants from stress and supports growth and development. IRE1 is a key component of the UPR signaling pathway and has dual protein kinase (PK) and RNase activities. We showed that both the PK and RNase activities, but not its normal splicing target, bZIP60 mRNA, are required for root growth and male gametophyte development, while both RNase activity and bZIP60 are required for endoplasmic reticulum stress tolerance.
Abstract
The unfolded protein response (UPR) endows plants with the capacity to perceive, respond, and protect themselves from adverse environmental conditions. The UPR signaling pathway in Arabidopsis has two “arms,” one arm involving the bifunctional protein kinase (PK)/ribonuclease, IRE1, a RNA splicing enzyme, and another involving membrane-associated transcription factors, such as basic leucine zipper transcription factor 28 (bZIP28). Because of functional redundancies, single gene mutations in the plant UPR signaling pathway generally have not resulted in prominent phenotypes. In this study we generated multiple mutations in the UPR signaling pathway, such as an ire1a ire1b double mutant, which showed defects in stress tolerance and vegetative growth and development. Complementation of ire1a ire1b with constructs containing site-specific mutations in the PK or RNase domains of IRE1b demonstrated that a functional RNase domain is required for endoplasmic reticulum stress tolerance, and that both the PK and RNase domains are required for normal vegetative growth under unstressed conditions. Root growth under stress conditions was dependent on the splicing target of IRE1b, bZIP60 mRNA, and on regulated IRE1-dependent decay of target genes. However, root and shoot growth in the absence of stress was independent of bZIP60. Blocking both arms of the UPR signaling pathway in a triple ire1a ire1b bzip28 mutant was lethal, impacting pollen viability under unstressed conditions. Complementation with IRE1b constructs showed that both the PK and RNase domains are required for normal gametophyte development, but bZIP60 is not. Hence, the UPR plays a critical role in stress tolerance, and in normal vegetative growth and reproductive development in plants.
The plant unfolded protein response (UPR) is important in protecting plants from environmental stress. Adverse environmental conditions can interfere with sensitive biosynthetic processes in plants such as protein folding. The UPR has been subject of many recent reviews (for example, see ref. 1) and results from the accumulation of misfolded proteins in the endoplasmic reticulum (ER). The UPR signaling pathway in plants consists of two “arms,” one involving membrane associated transcription factors such as basic leucine zipper transcription factor 17 and 28 (bZIP17 and bZIP28) and another arm involving Inositol requiring enzyme1 (IRE1) (2). IRE1 is a dual PK/ribonuclease that is conserved from yeast to man. In response to stress, IRE1 is activated and splices a specific target mRNA in the cytoplasm that encodes a stress-response transcription factor.
IRE1 is a single-pass transmembrane protein in the ER membrane with its N terminus facing the ER lumen, serving as a sensor domain. The C terminus of the protein faces the cytosol and contains the PK and ribonuclease domains. Upon activation, IRE1 dimerizes or oligomerizes and undergoes transphosphorylation and activation of its ribonuclease domain (3, 4). Splicing involves cleavage in each of two loops of the substrate RNA, removal of the intervening segment or intron, and rejoining by tRNA ligase. In plants, the principal substrate is bZIP60 mRNA, and splicing removes a small intron (20 or 23 b), which causes a frame shift leading to the production of a transcription factor that no longer has a transmembrane domain (5, 6). Under certain circumstances, such as severe stress conditions, IRE1 becomes more promiscuous and degrades many mRNAs on the ER membrane encoding secreted proteins in a process termed regulated IRE1-dependent decay (RIDD) (7–9).
Arabidopsis encodes two full-length IRE1 isoforms, IRE1a and -b (10). In their study of autophagy in Arabidopsis, Liu et al. (11) found that ER stress induces autophagy in seedlings and that the major link between ER stress and autophagy was IRE1b. However, the surprising finding was that the reported target of IRE1b’s RNA splicing activity, bZIP60 mRNA, did not appear to be involved in the induction of autophagy. This observation suggested either that the PK activity of Arabidopsis IRE1 might link ER stress to autophagy or that IRE1 has RNA targets other than bZIP60 mRNA.
In this study, we used multiple mutations in the UPR signaling pathway to uncover the role of ER stress responses in plant development and stress tolerance. Up to this time, the analysis of single mutants in the pathway had not revealed functions for the UPR in plant growth and stress tolerance, although an ire1a ire1b double mutant was found to show a modest short root phenotype and increased sensitivity to ER stress agents (12). The lack of distinctive phenotypes led us to question whether the UPR really plays an important role in plant growth or stress tolerance. Therefore, in this study, we examined whether gene redundancies between the two arms of the UPR signaling pathway or redundancies within the arms obscured stress or normal growth phenotypes. In addition to revealing phenotypes, this study ascribes roles to the two IRE1 cytosolic domains, the PK and RNase domains, in producing these phenotypes.
Results
UPR in Vegetative Development.
Seedling growth is a demanding process requiring seed and seedling resources for active protein synthesis and secreted protein production. Therefore, we monitored the effects of UPR mutants on seedling root and shoot growth under unstressed and stressed conditions. Single gene mutants (bzip17, bzip28, bzip60, ire1a, or ire1b) had little effect on primary root growth under either condition (Fig. 1A). Arabidopsis encodes two full-length IRE1s (IRE1a and -b), and the double mutant knocking out both genes results in seedlings with less elongated primary roots under both conditions. bZIP60 mRNA is the principal target of and is on the same pathway as IRE1; hence, the inclusion of bzip60 in the triple ire1a ire1b bzip60 mutant had no further effect on root elongation.
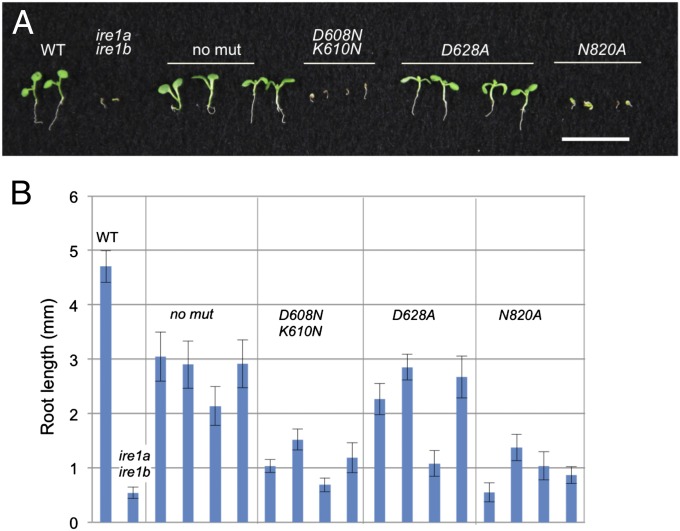
Fig. 1.
Multiple UPR mutations affect root and shoot growth under unstressed and stressed conditions. (A) Arabidopsis seedlings with the mutant genotypes as indicated were grown under unstressed (−DTT) and stressed (+1 mM DTT) conditions. Primary root lengths were measured in 7-d-old seedlings. Error bars indicate SE, n > 20. (B) Shoot growth of UPR mutants under unstressed and stressed conditions. Seedlings were grown for 7 d on LS plates in absence or presence of 1.5 mM DTT. (C) Growth of seedlings under unstressed conditions from a self cross of ire1a/ire1a ire1b/ire1b bzip28/+ bearing the transgene 35S:IRE1b. Seedlings were genotyped and 35S:IRE1b ire1a ire1b and 35S:IRE1b ire1a ire1b bzip28 homozygous seedlings are shown.
bZIP17 and bZIP28 are the major components in the membrane-associated transcription factor arm of the UPR pathway, and mutants in neither bZIP17 nor bZIP28 alone affected root elongation. Nonetheless, bZIP28 interacts cooperatively with IRE1 and bZIP60 in ER stress responses (2, 13). Therefore, to avoid compensatory gene activity from the transcription factor arm, we examined the effects of ire1 mutants in the background of bzip28 mutants. The double ire1b bzip28 mutant retarded primary root growth and root cell elongation comparable to the ire1a ire1b double mutant (Fig. 1A and Fig. S1). The double bzip28 bzip60 mutant does not interfere with normal root elongation under unstressed conditions, which was quite surprising because, as stated above, bZIP60 mRNA is the principal target of and on the same pathway as IRE1. Nonetheless, we interpret this result to mean that, under unstressed conditions, the growth-promoting effects of IRE1b in roots are independent of bZIP60.
There was very little effect of the single or multiple mutations on shoot growth under unstressed conditions, even for the double mutants ire1a ire1b and ire1b bzip28 and the triple ire1a ire1b bzip60 mutant, which affected root growth (Fig. 1B). We were not able to test the triple ire1a ire1b bzip28 mutant directly for its effects because it is lethal. However, we were able to partially rescue the triple ire1a ire1b bzip28 mutant by expressing IRE1b driven by the 35S promoter. This rescue was done by selfing a line bearing the 35S:IRE1b transgene that was homozygous for ire1a ire1b and heterozygous for bzip28. The progeny were genotyped and ire1a ire1b bzip28 progeny bearing the 35S:IRE1b transgene were identified (Fig. 1C). The rescued mutants were severely dwarfed with respect to both shoot and root development indicating that a functional UPR is vital for both shoot and root development under normal conditions.
It was unexpected to find that mutants in the UPR signaling pathway affect growth under unstressed conditions, because the UPR is a stress response. However, others have reported the presence of the spliced form of bZIP60 in Arabidopsis flowers, suggesting that UPR is active in these tissues under unstressed conditions (14). We investigated whether the UPR might be activated in young seedlings and found low levels of spliced bZIP60 mRNA in WT seedlings, but not ire1a ire1b seedlings (Fig. S2 A and B). The presence of spliced bZIP60 mRNA is indicative of some level of IRE1 activity, which may account for the UPR having a role in growth in seedlings in the absence of applied stress.
UPR in Vegetative Development Under Stress Conditions.
The role of UPR genes in root growth was different under ER stress conditions elicited by DTT, an ER stress agent. Increasing DTT concentrations led to a linear decline in root elongation (Fig. S3), with the most significant differences among the mutants under study occurring in 1 mM DTT. Under these conditions, the single mutants again showed little difference in root elongation compared with WT. Root elongation was significantly inhibited in the double ire1a ire1b mutant much like the triple mutant ire1a ire1b bzip60, indicating that these two mutants are sensitive to ER stress. However, the major difference between the stressed and unstressed conditions occurred in the double bzip28 bzip60 and ire1b bzip28 mutants (Fig. 1A). Root elongation in bzip28 bzip60 double mutant was highly impacted by ER stress treatment compared with the double ire1b bzip28 mutant (Fig. S3). Contrary to unstressed conditions, the root elongation effects of IRE1a and -b under ER stress conditions clearly involve bZIP60.
Shoot growth in seedlings was also evaluated under stress conditions. In contrast to unstressed conditions, when mutant seedlings were grown under stress conditions (in the presence of 1.5 mM DTT), the double ire1a ire1b mutant and the triple ire1a ire1b bzip60 mutant were severely growth inhibited (Fig. 1B). Again, because the double bzip28 bzip60 mutant was as growth inhibited on DTT as the double mutant ire1a ire1b, and the double bzip28 ire1b mutant was not, we concluded that bZIP60 also contributes to the tolerance of shoots to ER stress.
Another way to assess the effects of mutations in the UPR signaling pathway is to monitor BiP3 up-regulation, a reliable biomarker for UPR activity (6, 15). BiP3 up-regulation by DTT treatment was reduced slightly by the bzip28 mutation and more so by the bzip60 single mutation or by the double ire1a ire1b mutation (Fig. S4). However, BiP3 up-regulation was not observed in the bzip28 bzip60 double mutant. Thus, the effects of bZIP60 mRNA splicing on BiP3 up-regulation in response to DTT correspond to its impact on root elongation under the same ER stress conditions.
Effect of the Different IRE1 Domains.
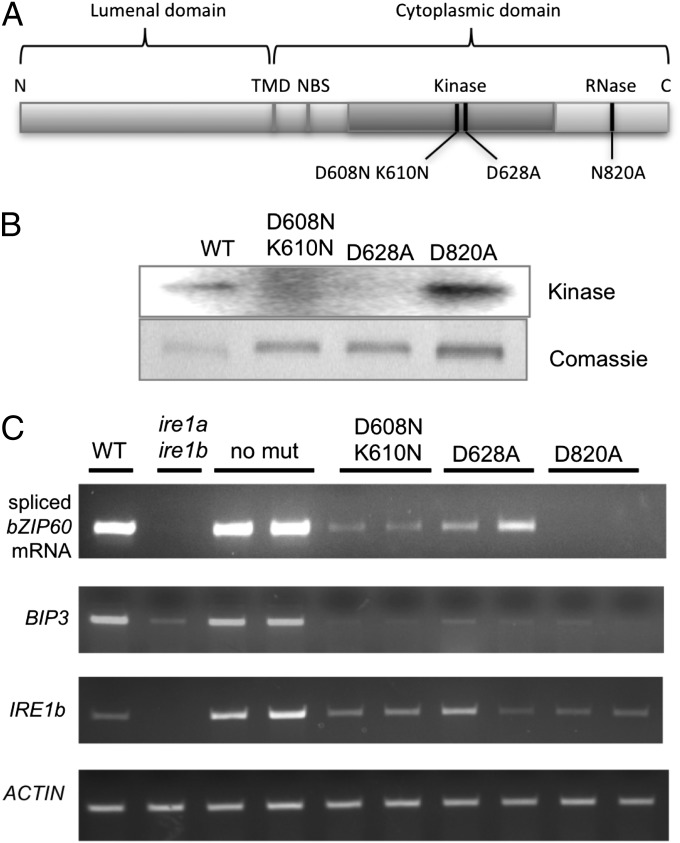
Because primary root elongation under unstressed conditions is affected by IRE1 in a manner that is independent of bZIP60, we were interested in determining which domains of IRE1 are required for normal root growth. To this end, we produced site-specific mutations in IRE1b that are predicted to differentially affect the PK and/or the ribonuclease activity of the RNA splicing enzyme (Fig. 2A).
Fig. 2.
Domain map of IRE1b and the effect of site–specific mutations in the domains. (A) The mutation D608N K610N in the protein kinase (PK) domain is predicted to block nucleotide binding to IRE1, D628 is expected to interfere with PK catalysis, and N820A is predicted to inhibit the RNase activity of IRE1. (B) Autophosphorylation assay was performed with 32P-γATP and IRE1b cytoplasmic domain constructs synthesized in E. coli bearing the mutations as listed. Coomassie-blue-stained IRE1b cytoplasmic domain was used as a loading control. WT (nonmutant) construct lane was underloaded because this protein was only produced at very low levels in E. coli. (C) bZIP60 mRNA splicing in vivo mediated by IRE1b full-length constructs bearing various mutations as indicated and expressed in ire1a ire1b mutant seedlings. Transgenic seedlings were subjected to ER stress (2 mM DTT) treatment for 2 h. bZIP60 splicing was measured in an RT-PCR assay along with IRE1b expression levels. Actin was used as a loading control. Number of PCR thermocycles used in amplifying the different RNAs: spliced bZIP60, 35; BiP3, 24; IRE1b, 26; ACTIN, 24.
We generated D608N K610N mutations in Arabidopsis IRE1b, corresponding to the yeast D797N K799N (called 1KR32), in the nucleotide-binding pocket. In yeast, 1KR32 incapacitates autophosphorylation and transautophosphorylation in in vitro kinase assays; however, these mutations retain their RNase activity in in vitro RNase assays and their ability to splice Hac1 mRNA in vivo (16). We produced a D628A mutation in Arabidopsis IRE1b within the conserved DFG kinase motif. The equivalent yeast mutation (D828A) is unable to undergo autophosphorylation in in vitro kinase assays, although it retains its ability to bind ATP (17). In yeast, D828 splices Hac1 mRNA following treatment with ER stress agents; however, the splicing reaction does not attenuate with time as it does with WT IRE1. We also generated a N820A mutation in Arabidopsis IRE1b corresponding to yeast N1057A. N1057A disables IRE1’s RNase activity in yeast, but not its PK activity (18).
Given the expected enzymatic properties for site-specific mutations in IRE1, we set out to determine whether the predictions applied to Arabidopsis IRE1b. To determine the effect of the mutations on the PK activity of IRE1b, the cytosolic domain was tagged and synthesized in Escherichia coli and tested for autophosphorylation in an in vitro PK system (Fig. 2B). We found, as predicted, that the D608N K610N and the D628A mutations knocked out the autophosphorylation activity of IRE1b, whereas the D820A mutation did not.
The RNase activities of these mutated forms of IRE1 were tested in vivo. The mutations were produced in constructs driven by the 35S promoter, introduced as transgenes into ire1a ire1b plants and assayed for bZIP60 splicing under unstressed and stressed conditions. As expected, under unstressed conditions, none of the constructs supported bZIP60 splicing in vivo. Under stressed conditions, no bZIP60 splicing was detected in D820A, which bears a mutation in the RNase domain, but has normal PK activity in vitro (Fig. 2C). Only very low levels of bZIP60 mRNA splicing were observed in D608N K610N, which bears a mutation in the nucleotide-binding site of IRE1, and in D628A, which has a mutation in the catalytic site of the PK domain. D628A supported bZIP60 splicing somewhat better than D608N K610N, which suggested that D628A restored more RNase activity compared with D608N K610N. However, the levels of splicing were so low in the two mutants that little, if any, stress induction of BiP3 was observed. Thus, nucleotide binding and/or PK activity appears to be required for efficient bZIP60 mRNA splicing in vivo.
Complementation of ire1a ire1b Mutants.
To determine the role of PK and RNase domains in conditioning the various phenotypes, we attempted to complement root growth under unstressed conditions in the double ire1a ire1b mutant with the constructs described above. We observed partial to full complementation with various lines expressing nonmutant constructs, but we did not observe consistent complementation with any of the mutant constructs (Fig. S5), suggesting that both the PK and RNase functions are required for promoting normal root elongation under unstressed conditions.
We were also successful in partial complementation of ER stress tolerance in the roots of ire1a ire1b mutant by expressing the nonmutant IRE1b construct (Fig. 3 A and B). With respect to the mutant constructs, we failed to detect complementation by expressing D608N K610N or by expressing N820A. However, we did observe some complementation with D628A. This result indicated that the nucleotide binding activity but not the PK catalytic activity of IRE1 is required for complementation. Nucleotide binding is probably required to activate IRE1b’s splicing activity.
Fig. 3.
Complementation by various IRE1b constructs of root growth in ire1a ire1b seedlings. (A and B) Transgenic lines bearing the IRE1b constructs as indicated were grown under stress conditions (1.5 mM DTT), and root lengths were measured in 7-d-old seedlings. Error bars indicate SE, n > 20.
With regard to shoot growth in the double ire1a ire1b mutant under stress conditions, neither D608N K610N nor N820A were able to complement the stress-tolerance defect (Fig. S6). However, D628A was able to do so, although not as well as the nonmutant construct. We again reasoned that because N820A failed to complement the stress-tolerance defect, the RNase activity, and not the PK activity of IRE1, is responsible for promoting shoot growth under ER stress conditions.
Thus, we conclude that the RNase activity of IRE1b is responsible for promoting root elongation and shoot growth under stress conditions and that both the PK and the RNase activities are involved in promoting root elongation under unstressed conditions. These findings raise the question about the identity of the RNA target under these circumstances. We had shown that root and shoot growth in the double bzip28 bzip60 mutant is highly sensitive to the DTT, suggesting that the principal target of IRE1b, bZIP60, is involved in promoting root elongation and shoot growth under stress conditions.
On the other hand, D628A, which is defective in bZIP60 mRNA splicing, can partially complement shoot and root growth in ire1a ire1b under stress conditions. On the face of it, this appears to be a dilemma in that bZIP60 is involved in shoot- and root-growth stress tolerance; however, D628A, which is defective in bZIP60 splicing, can partially complement shoot and root growth. One possibility is that other RNA targets may also be involved in shoot- and root-growth tolerance and that D628A, which cannot splice bZIP60 mRNA, may be active against these targets.
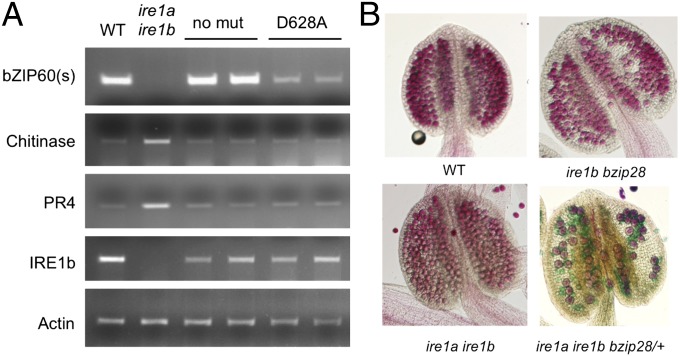
Mishiba et al. (12) recently showed that IRE1 is also involved in RIDD, a process by which mRNAs encoding secreted proteins on membrane–bound ribosomes are degraded in response to stress. In addition, they identified several RIDD target mRNAs in Arabidopsis. Therefore, we asked whether D628A degrades selected RIDD target mRNAs under stress conditions, even though it is only modestly able to splice bZIP60 mRNA. We observed that D628A does, indeed, degrade RIDD target mRNAs, such as PR4 mRNA (At3G04720) and a mRNA encoding a chitinase family protein (AT2G43620) (Fig. 4A and Fig. S7). The accumulation of these mRNAs is enhanced under stress conditions in the ire1a ire1b mutant. This finding indicates that Arabidopsis IRE1b PK activity is required for efficient bZIP60 splicing, but not for the degradation of RIDD target mRNAs in response to stress.
Fig. 4.
Effects of the double ire1a ire1b and the triple ire1a ire1b bzip28 mutants on stress tolerance and pollen viability. (A) bZIP60 RNA splicing and RIDD target RNA degradation in ire1a ire1b transgenic seedlings expressing the IRE1b non mutant or D628A mutant construct and treated with 2 mM DTT for 5 h. RIDD targets were RNAs encoding a chitinase family protein (AT2G43620) and PR4 (At3G04720). Actin was used as a loading control. (B) Pollen viability is reduced in the triple ire1a ire1b bzip28 mutant. Plants with the genotypes as indicated were selfed and stamens were stained with Alexander’s stain, a vital stain. Red-stained pollen is viable, blue- or green-stained pollen is not viable.
Thus, both bZIP60 and RIDD mRNAs are the targets of the RNase activity of IRE1b during root and shoot growth under stress, but not root and shoot growth under nonstressed conditions. Under these conditions, the bzip28 bzip60 double mutant does not interfere with growth, whereas bzip28 ire1b and 35S:IRE1b ire1a ire1b bzip28 do, indicating that targets other than bZIP60 mRNA are required for optimal root growth under nonstressed conditions.
The Effect of UPR Mutants on Reproductive Development.
In testing various single and multiple UPR mutants, we did not observe significant effects on reproductive development. However, as described above, the triple ire1a ire1b bzip28 mutant, which blocked both arms of the UPR signaling pathway, was lethal, as evidenced by the fact that we were unable to recover homozygous progeny. To determine whether the defects in the triple ire1a ire1b bzip28 mutant are manifest during reproductive stages, we evaluated the effects of the triple mutant in a hemizygous state during reproductive development. To do so, we selfed the mutant homozygous for ire1a and ire1b but heterozygous for bzip28 (ire1a ire1b bzip28/+) and scored for the transmission of the bzip28 allele to the next generation by genotyping progeny. If the gametes bearing the triple mutation are viable, then they should be capable of transmitting the bzip28 allele, and as such we would expect a 3:1 ratio of progeny bearing the bzip28 allele (in heterozygous or homozygous state). Instead, we recovered fewer progeny bearing bzip28 alleles, suggesting a loss of about half of the gametes bearing the bzip28 allele through the triple mutant (41 progeny with bzip28 alleles, 53 without. Goodness of fit to a 1:1 ratio, χ2 = 1.53, P = 0.22).
To confirm the observation that pollen viability was at stake in the triple mutant and to determine whether female gametophyte production was similarly affected, we performed a reciprocal cross between the ire1a ire1b bzip28/+ mutant and WT and scored for the transmission of the bzip28 allele (Table 1). It is clear that, when ire1a ire1b bzip28/+ was the female parent, the bzip28 allele was transmitted about on par with the WT allele. However, when the ire1a ire1b bzip28/+ mutant was the male parent, there was no transmission whatsoever of the bzip28 allele. Thus, the operation of the UPR signaling pathway is absolutely required for male gametophyte function in Arabidopsis. To verify this finding, we stained the anthers of the ire1a ire1b bzip28/+ mutant with a vital stain (Alexander’s stain), and found that about half of the pollen grains were viable, and half were not (Fig. 4B and Fig. S8) (308 were viable, 292 were not. Goodness of fit to a 1:1 ratio, χ2 = 0.427, P = 0.51).
Table 1.
Reciprocal crosses of ire1a ire1b bzip28/+ × WT
| Crosses | WT ♀ × ire1a ire1b bzip28/+ ♂ |
ire1a ire1b bzip28/+ ♀ × WT ♂ |
||
| Genotype of progeny at bZIP28 locus | bzip28/+ | +/+ | bzip28/+ | +/+ |
| No. of progeny with genotypes as indicated | 0 | 80 | 59* | 64* |
| Segregation ratio | 0 | 1 | 0.9 | 1 |
♀, female; ♂, male.
χ2 test for expected 1:1 ratio, χ2 = 0.2, P = 0.67, df = 1.
This finding, however, raises the question as to how UPR genes can play a role in reproductive development under normal conditions. We observed that, indeed, spliced forms of bZIP60 mRNA are found in flowers, albeit at fairly low levels, indicative of the UPR and IRE1 action, and that the appearance of the spliced forms is dependent on IRE1a and -b (Fig. S2).
It should be pointed out that, whereas the homozygous ire1a ire1b bzip28 triple mutant is lethal, the double bzip60 bzip28 mutant is not. Because bZIP60 mRNA is the principal target of IRE1’s splicing activity (5, 6), one might expect that the homozygous bzip60 bzip28 double mutant might also be lethal, but it is not. This result indicates that although bZIP60 mRNA is spliced in flowers, the viability conferred by IRE1a and IRE1b during reproductive development is independent of bZIP60.
Role of IRE1 Domains in Reproductive Development.
Because the action of IRE1 in conferring viability in the triple ire1a ire1b bzip28 mutant was independent of bZIP60, one might expect the RNase activity of IRE1 to be dispensable for these functions. To test which IRE1b domains were responsible for male gametophyte function, we performed complementation analysis in the ire1a ire1b bzip28/+ mutant. Expression of the nonmutant IRE1b construct was successful in restoring bzip28 allele transmission in that we were able to recover some bzip28 homozygotes and that the ratio of bzip28 heterozygotes to WT homozygotes was elevated in most of the lines (Table S1). However, we observed no complementation with any of the mutants even though the expression of the IRE1b construct could be demonstrated in these lines (Fig. S9). This result was similar to the outcome of efforts to complement root growth under unstressed conditions, and we conclude that, like root growth, both the protein kinase and the RNase activity of IREb are required for male gametophyte function, although it is independent of bZIP60.
Discussion
The UPR signaling pathway in Arabidopsis plays important roles in normal vegetative growth and reproductive development as well as in plant stress responses. The signaling pathway has two arms and because of redundant functions between and within the arms, single gene mutations generally have no discernable or only modest phenotypes. However, in this study, we show that multiple mutations incapacitating one or both arms can have profound effects on development and stress responses.
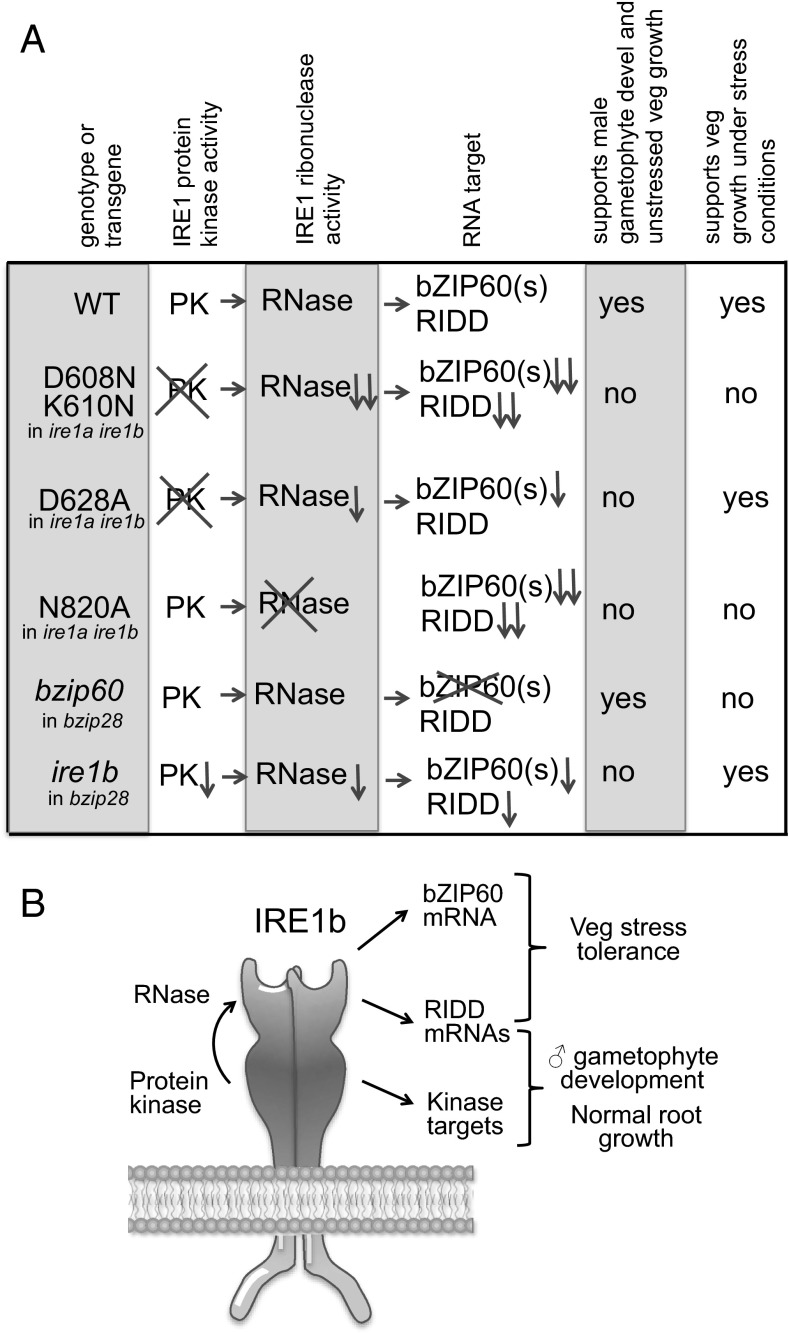
IRE1 is a dual-functioning stress transducer with both PK and RNase activities. We found that the N820A mutation in the RNase domain of IRE1b disabled its RNA splicing activity, but did not affect its PK activity (as ascertained in an autophosphorylation assay). However, mutations in the PK domain (D608N K610N and D628A) disabled IRE1b’s PK activity and sharply reduced its bZIP60 splicing activity, nonetheless, D628A did retain its activity against other RNA substrates. The effects of these IRE1b mutations are summarized in Fig. 5. In general, knocking out either the PK or RNase activity of IRE1b failed to complement the short root phenotype of the ire1a ire1b mutant, demonstrating that both functions are required to support growth under unstressed conditions.
Fig. 5.
Enzymatic activities and phenotypes of mutants and IRE1b constructs in various backgrounds. (A) Gene or domain knockouts in the various IRE1b constructs are indicated by crossouts; activity reductions are indicated by downward arrows. (B) Diagram of functions attributable to the PK and ribonuclease domains of IRE1b.
Mutations in both the PK and RNase domains of D628A and N820A, respectively, also demonstrate that RNase domain, but not PK domain, is required for stress tolerance. However, D628A, which retains some bZIP60 splicing activity and RNase activity against other RNAs (RIDD targets), was most informative. This mutant construct partially complements the ire1a ire1b mutant for growth under stress conditions, although still not complementing as well as the nonmutant construct, probably due to the inefficient support of bZIP60 splicing. In addition, a knock out of bZIP60 in a bzip28 background fails to support growth under stress conditions, likely meaning that both RIDD and bZIP60 mRNAs can serve as IRE1b targets under stress conditions. However, in a bzip28 background bZIP60 is required for stress tolerance, but otherwise, RIDD targets alone are enough to serve in conferring stress tolerance.
Mishiba et al. (12) recently reported that RIDD is required for the ER stress tolerance in Arabidopsis. They showed that the mRNAs for a set of secreted proteins were degraded in response to ER stress. Degradation of these mRNAs is presumably a cell-sparing process by lightening the load of protein secretion in plants under stress. Cell survival attributed to RIDD was demonstrated by the fact that knockouts of IRE1a and IRE1b were more susceptible to programmed cell death. Our studies show that both of the RIDD and bZIP60 splicing are required for the ER stress tolerance. This requirement was demonstrated by the fact that bzip28 bzip60 double mutants are much more sensitive to DTT compared with bzip28 ire1b double mutants and that the D628A mutant, which can more fully support RIDD target mRNA degradation than bZIP60 mRNA splicing, can only partially complement the stress tolerance phenotype.
The role of UPR signaling in ER stress responses is well documented, but the effect of UPR signaling on plant development is less well known and presents some dilemmas. For example, the PK and the RNase activity of IRE1 are required to promote optimal root growth under unstressed conditions. If the PK and RNA splicing activity of IRE1 are only induced under stress conditions, then how can IRE1 promote vegetative growth under normal conditions? There are several possible answers. One possible explanation is that, although the RNA-splicing activity of IRE1 is induced by ER stress, the RNase activity of IRE1 against other substrates might not be as stringent in its requirement for activation. We have shown in previous studies that purified Arabidopsis IRE1 has RNase activity in vitro under conditions that do not require any special activation (6). This observation is consistent with the finding in this study that the function of IRE1 during normal development is independent of bZIP60.
A second possible explanation for the function of IRE1 under unstressed conditions is that IRE1 is activated in certain tissues or in certain developmental stages in the absence of applied stress. Iwata et al. (14) found processed forms of bZIP60 protein in stamens of Arabidopsis plants that had not been subjected to stress treatment. In this study, we found low levels of the spliced form of bZIP60 mRNA in young seedlings and flowers under unstressed conditions, indicating some modest level of the UPR in the absence of applied stress. The short-root phenotype of ire1b bzip28 under unstressed conditions and the diminutive stature of 35S:IRE1b ire1a ire1b bzip28 in the absence of applied stress signifies that both arms of the UPR signaling pathway are activated and play overlapping roles during normal vegetative development.
We also show that genes encoding UPR components play essential roles in plant reproductive development. The effects of UPR signaling mutations are most profound during male gametophyte development. It is possible that the UPR is required to meet heavy secretion demands during male gametophyte development and that the UPR occurs in the absence of exogenously applied stress. Considering that the ire1a ire1b bzip28 triple mutant but not the ire1a ire1b double mutant showed reproductive defects, we conclude that both arms of UPR, the RNA splicing arm and the arm involving membrane associated transcription factors, play roles in reproductive development.
The finding that male gametophyte development and stress tolerance are bZIP60 independent seemingly contradicts the observation that the function of the male gametophyte depends on the RNase activity of IRE1b. bZIP60 is the principal target of IRE1’s splicing activity; therefore, if ER stress tolerance requires the RNase activity of IRE1b, then it would seem that it should be dependent on bZIP60, but it is not. A similar observation was made with respect to the link between ER stress and autophagy in Arabidopsis. Liu et al. (11) found that IRE1b was required to link ER stress to autophagy; however, the link was not dependent on bZIP60.
We show in this study that multiple mutations incapacitating both arms have profound effects on reproductive development and stress responses. Understanding how the ER stress response is interconnected to these developmental processes is an important challenge for the future.
Materials and Methods
Lines and Growth Conditions.
Arabidopsis thaliana ecotype Columbia-0 (Col-0) was used in this study, and the mutants bzip17(Col-0; SALK_104326), bzip28-2 (Col-0; SALK_132285), bzip60-1 (Col-0; SALK_050203), ire1a (Col-0; SALK_018112), and ire1b (Col-0; SAIL_238_F07) were obtained from Arabidopsis Biological Resource Center. Seeds were stratified at 4 °C for 3 d before germination. Unless indicated otherwise, plants were grown under continuous white light at 23–25 °C in soil or on Linsmaier Skoog (LS) medium (1× LS salts, 1% sucrose, 0.8% Agar). Agrobacterium-mediated transformation of Arabidopsis plants was carried out by the floral dip method. Agrobacterium strain GV3101 was used in all transformation experiments.
Stress Assays.
Analysis of gene expression by RT-PCR was performed as described (11). PCR primers used in this study are listed in Table S2.
In Vitro Autophosphorylation Assay.
Maltose-binding protein (MBP)-IRE1b (∼100 ng), MBP-D608N K610N, MBP-D628A, and MBP-N820A (500 ng each) were incubated in 20 μL of kinase buffer [20 mM Tris (pH 7.5), 100 mM NaCl, and 12 mM MgCl2] and 10 μCi 32P-γATP. After incubation at 37 °C for 40 min, the reactions were stopped by adding 7 μL of 4× SDS buffer and heated at 94 °C for 5 min. Proteins were resolved by a PAGE, and phosphorylation was detected by exposing the dried gel to storage phosphor screen.
Pollen Staining.
Analysis of pollen viability was performed using Alexander’s staining as described by Peterson et al. (19). Analysis of pollen tube elongation in pistils was performed using aniline blue staining as described by Jiang et al. (20).
Supplementary Material
Acknowledgments
We thank Dawei Zhang and Yanhai Yin for guidance for the in vitro autophosphorylation assay. This work was supported by the Iowa State University Plant Sciences Institute and by National Science Foundation Grant IOS90917 (to S.H.H.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 19189.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314749110/-/DCSupplemental.
References
- 1.Walter P, Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 2.Howell SH. ER stress responses in plants. Annu Rev Plant Biol. 2013;64:477–499. doi: 10.1146/annurev-arplant-050312-120053. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Korennykh AV, Behrman SL, Walter P. Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc Natl Acad Sci USA. 2010;107(37):16113–16118. doi: 10.1073/pnas.1010580107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shamu CE, Walter P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 1996;15(12):3028–3039. [PMC free article] [PubMed] [Google Scholar]
- 5.Nagashima Y, et al. Arabidopsis IRE1 catalyses unconventional splicing of bZIP60 mRNA to produce the active transcription factor. Sci Reports. 2011;1:29. doi: 10.1038/srep00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng Y, et al. Heat induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis. Proc Natl Acad Sci USA. 2011;108(17):7247–7252. doi: 10.1073/pnas.1102117108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollien J, et al. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186(3):323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han D, et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138(3):562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313(5783):104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 10.Koizumi N, et al. Molecular characterization of two Arabidopsis Ire1 homologs, endoplasmic reticulum-located transmembrane protein kinases. Plant Physiol. 2001;127(3):949–962. [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, et al. Degradation of the endoplasmic reticulum by autophagy during endoplasmic reticulum stress in Arabidopsis. Plant Cell. 2012;24(11):4635–4651. doi: 10.1105/tpc.112.101535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishiba K, et al. Defects in IRE1 enhance cell death and fail to degrade mRNAs encoding secretory pathway proteins in the Arabidopsis unfolded protein response. Proc Natl Acad Sci USA. 2013;110(14):5713–5718. doi: 10.1073/pnas.1219047110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu JX, Howell SH. bZIP28 and NF-Y transcription factors are activated by ER stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis. Plant Cell. 2010;22(3):782–796. doi: 10.1105/tpc.109.072173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwata Y, Fedoroff NV, Koizumi N. Arabidopsis bZIP60 is a proteolysis-activated transcription factor involved in the endoplasmic reticulum stress response. Plant Cell. 2008;20(11):3107–3121. doi: 10.1105/tpc.108.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez IM, Chrispeels MJ. Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular processes. Plant Cell. 2003;15(2):561–576. doi: 10.1105/tpc.007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubio C, et al. Homeostatic adaptation to endoplasmic reticulum stress depends on Ire1 kinase activity. J Cell Biol. 2011;193(1):171–184. doi: 10.1083/jcb.201007077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chawla A, Chakrabarti S, Ghosh G, Niwa M. Attenuation of yeast UPR is essential for survival and is mediated by IRE1 kinase. J Cell Biol. 2011;193(1):41–50. doi: 10.1083/jcb.201008071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korennykh AV, et al. Structural and functional basis for RNA cleavage by Ire1. BMC Biol. 2011;9:47. doi: 10.1186/1741-7007-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson R, Slovin JP, Chen C. A simplified method for differential staining of aborted and non-aborted pollen grains. Int J Plant Biol. 2010;1(2):66–69. [Google Scholar]
- 20.Jiang L, et al. VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell. 2005;17(2):584–596. doi: 10.1105/tpc.104.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.