Significance
Current efforts in malaria therapeutics and vaccine development include strategies aimed at deterring infection at the liver stage, preventing subsequent clinical complications and malaria transmission. To elucidate response mechanisms operating during liver stage infection, we searched for host genetic factors that control parasite expansion in a mouse model of malaria liver stage resistance. Here we report that the cell surface receptor TREM2 is expressed in innate immune cell types residing in the liver and takes part in a mechanism enabling such cells to control the yield of hepatocyte infection. This work highlights the relevance of innate immunity mechanisms in controlling expansion of the malaria parasite in the liver.
Abstract
Plasmodium liver stage infection is a target of interest for the treatment of and vaccination against malaria. Here we used forward genetics to search for mechanisms underlying natural host resistance to infection and identified triggering receptor expressed on myeloid cells 2 (TREM2) and MHC class II molecules as determinants of Plasmodium berghei liver stage infection in mice. Locus belr1 confers resistance to malaria liver stage infection. The use of newly derived subcongenic mouse lines allowed to map belr1 to a 4-Mb interval on mouse chromosome 17 that contains the Trem2 gene. We show that Trem2 expression in the nonparenchymal liver cells closely correlates with resistance to liver stage infection, implicating TREM2 as a mediator of the belr1 genetic effect. Trem2-deficient mice are more susceptible to liver stage infection than their WT counterparts. We found that Kupffer cells are the principle cells expressing TREM2 in the liver, and that Trem2−/− Kupffer cells display altered functional activation on exposure to P. berghei sporozoites. TREM2 expression in Kupffer cells contributes to the limitation of parasite expansion in isolated hepatocytes in vitro, potentially explaining the increased susceptibility of Trem2−/− mice to liver stage infection. The MHC locus was also found to control liver parasite burden, possibly owing to the expression of MHC class II molecules in hepatocytes. Our findings implicate unexpected Kupffer–hepatocyte cross-talk in the control Plasmodium liver stage infection and demonstrate that TREM2 is involved in host responses against the malaria parasite.
Malaria liver stage infection is asymptomatic but is absolutely required in the progression of Plasmodium infection in the vertebrate host, preceding propagation of parasites in the blood and clinical manifestations of malaria (1, 2). Current efforts in therapy and vaccine development include strategies aimed at deterring infection at the liver stage, preventing subsequent clinical complications and malaria transmission (3, 4). During liver stage infection, one Plasmodium sporozoite develops into thousands of merozoites inside each infected hepatocyte (5). Identification of host genetic factors that control liver parasite expansion may help elucidate response mechanisms operating during liver stage infection.
Gene deficiency models and gene expression studies focusing on hepatocyte infection have highlighted genes that control hepatocyte invasion and intrahepatocyte parasite expansion [e.g., CD81 (6), SR-B1 (7, 8)], but the mechanisms of host response to liver stage infection remain elusive. It has been proposed that sporozoites’ ability to traverse liver macrophages (9) and/or hepatocytes (10) in the course of liver stage infection may favor the release of proinflammatory factors at liver sites of sporozoite expansion (11, 12). Innate immune mechanisms might be involved in sensing Plasmodium sporozoites and in controlling liver stage infection (13).
Mouse models of liver stage infection suggest that sporozoites induce a innate inflammatory response associated with liver macrophage activation (14) and formation of inflammatory foci in the liver (15, 16). In mice, the severity of the inflammatory response depends on the genetic background, with, for example, BALB/c mice showing reduced sporozoite expansion in the liver and enhanced local inflammatory responses during malaria liver stage infection compared with C57BL/6 mice (17, 18). Little is known about host genetic factors and cell mechanisms that control the natural resistance to liver stage Plasmodium infection, however.
In the present study, we aimed to dissect the resistance to liver stage P. berghei ANKA infection in mice. We previously reported that partial resistance to liver stage P. berghei infection in the BALB/c strain can be attributed to intrahepatic factors. Genetic crosses with susceptible C57BL/6 strain mice revealed the contribution of mouse chromosome 17 and mapped the belr1 resistance locus distally to the H2 locus (19). Here we report the analysis of a newly generated panel of chromosome 17 subcongenic mouse strains that allowed us to unravel mechanisms of resistance to malaria liver stage infection mediated by the surface receptor TREM2 and MHC class II molecules.
Results
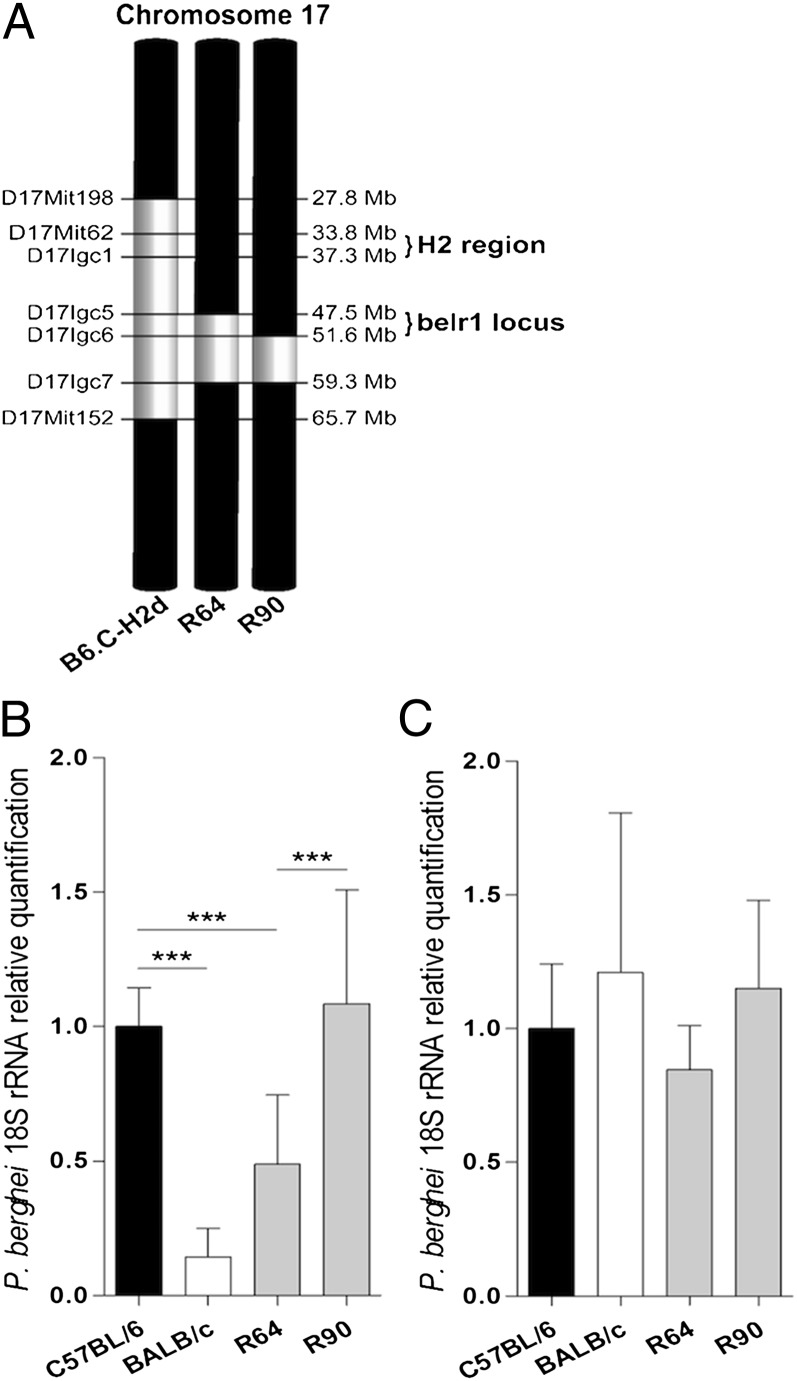
We used a subcongenic fine-mapping strategy to reveal the genetic and cellular basis of relative resistance to malaria liver stage infection observed in B6.C-H2d mice, a C57BL/6 congenic strain that carries both the belr1 locus and the H2 region within a 37.9-Mb chromosome 17 segment of BALB/c origin (19). Ten subcongenic mouse lines were generated by backcrossing B6.C-H2d mice on the C57BL/6 mouse strain and then selected for recombination using a high-density set of region-specific genetic markers (Fig. S1). Quantification of P. berghei ANKA rRNA at the end of the liver stage infection [at 40 h postinfection (p.i.)] served as a proxy of resistance against malaria liver stage infection in the subcongenic lines and allowed the dissection of two distinct controlling regions.
Trem2 Expression Correlates with the belr1 Resistance Phenotype.
Two double-recombinant subcongenic mouse strains that carried the MHC locus of C57BL/6 origin (R64 and R90) were instrumental in fine-mapping malaria liver stage resistance conferred by the belr1 locus. This narrowed down to belr1 within a 4.04-Mb region delimited by the upper recombination boundary of R64 (D17Igc5) and the upper boundary of R90 (D17Igc6) (Fig. 1 A and B). Analysis of parasite expansion in hepatocyte primary cultures indicated that the resistance phenotype observed in the R64 subcongenic line was not attributable to hepatocyte factors (Fig. 1C), strongly suggesting that other cell types in the liver mediated the belr1 effect.
Fig. 1.
belr1 maps within a 4.04-Mb region of mouse chromosome 17. (A) Diagram of BALB/c-derived congenic regions (in white) in R64 and R90 double-recombinant subcongenic lines with C57BL/6 background (in black). (B) Liver parasite burden in subcongenic lines at 40 h p.i. measured by relative quantification of P. berghei 18S rRNA using a parental C57BL/6 strain as the calibrator. Data are mean ± SD; n = 10. (C) In vitro parasite yield in infected primary hepatocyte cultures from C57BL/6, BALB/c, R64, and R90 mice. Data are mean ± SD of triplicate cultures. ***P < 0.0001.
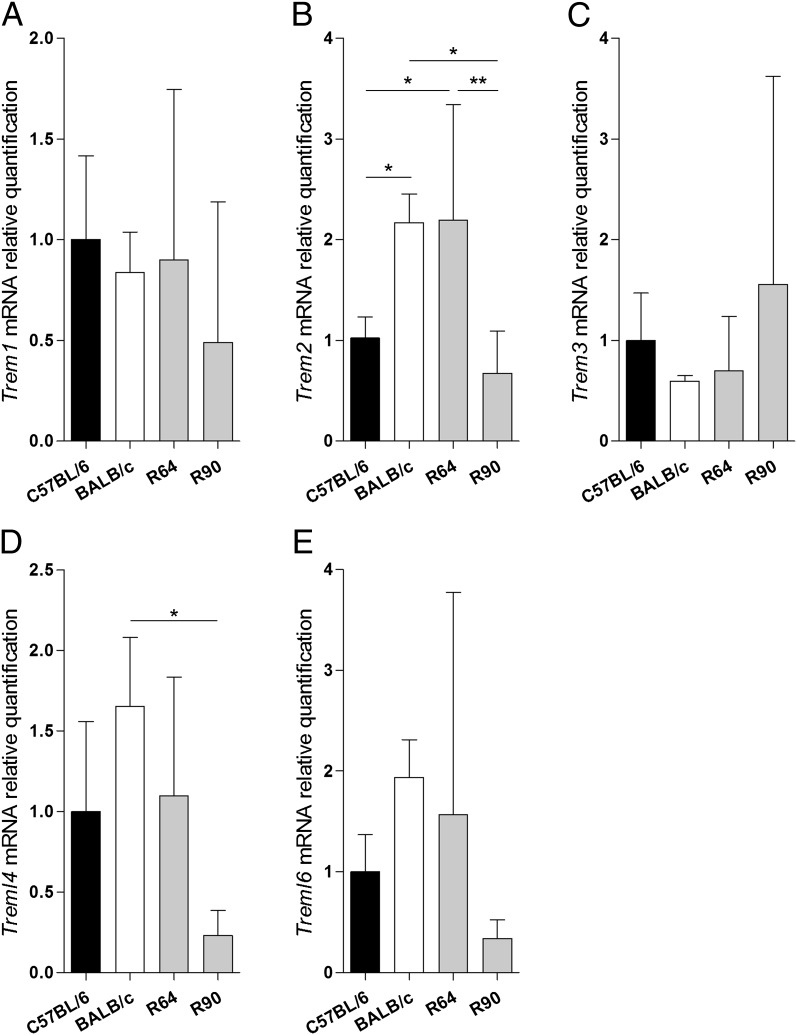
Thirty-four protein-coding genes map within the belr1 interval (D17Igc5–D17Igc6), including nine genes belonging to the Triggering Receptor Expressed on Myeloid cells (TREM) gene family (Table S1), orthologous to the TREM gene cluster on human chromosome 6 (20). To correlate the expression of genes in this interval with the liver stage resistance phenotype, we analyzed mRNA expression of all 34 protein-coding genes in isolated nonparenchymal liver cells (NPCs) from infected and noninfected parental strains and subcongenic lines R64 and R90. Nineteen genes, including five TREM genes, were expressed in NPCs (Fig. 2 and Fig. S2). Only Trem2 mRNA up-regulation from noninfected NPCs correlated closely with the liver stage resistance phenotype in the subcongenic lines that define the belr1 locus (Fig. 2B). Given that TREM genes are expressed mainly in cells of monocyte/macrophage lineage (21), these results suggest Trem2 as a belr1 positional and functional candidate gene.
Fig. 2.
Trem2 mRNA expression correlates with resistance to liver stage infection. mRNA quantification of Trem1 (A), Trem2 (B), Trem3 (C), Treml4 (D), and Treml6 (E) in NPCs isolated from noninfected R64, R90, and BALB/c mice, relative to the C57BL/6 strain. Data are mean ± SD; n > 6. *P < 0.05; **P ≤ 0.001.
Trem2 Controls Liver Stage Infection and Polarization of Kupffer Cells.
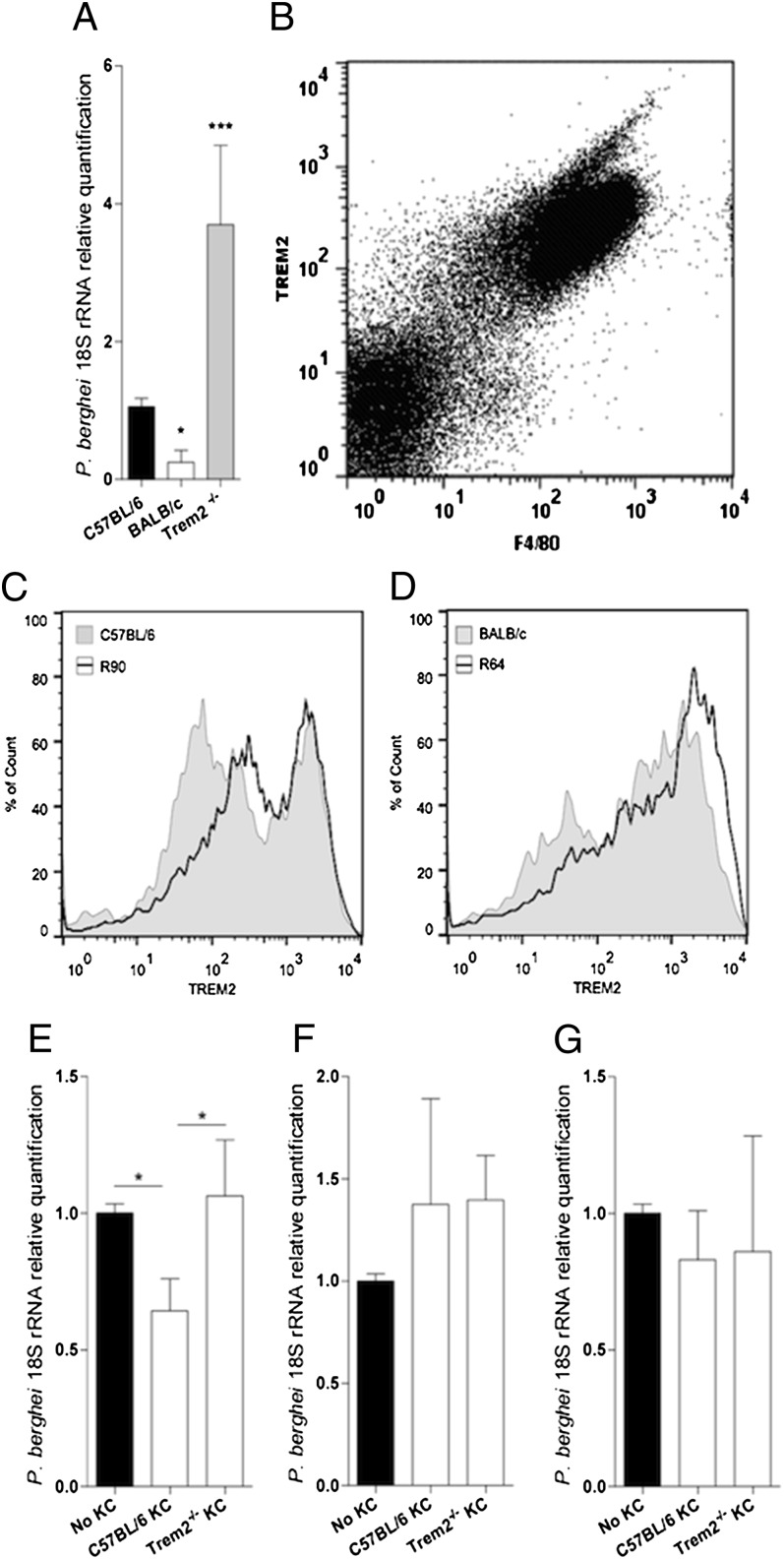
To investigate whether TREM2 is involved in resistance to malaria liver stage infection, we analyzed P. berghei-infected Trem2−/− mice. We found that in the absence of TREM2 expression, parasite expansion in the liver was increased (Fig. 3A). We further analyzed TREM2 expression in NPCs from C57B/6 mice by FACS analysis and identified Kupffer cells (KCs) as the principal NPC type expressing TREM2 (Fig. 3B). Flow cytometry analysis of TREM2 protein expression in KCs from subcongenic and parental strains in C57BL/6 and R90 susceptible mice showed that a sizeable fraction of F4/80+ cells expressed low to intermediate levels of TREM2 (Fig. 3C). A large majority of KCs from the resistant strains BALB/c and R64 expressed high levels of TREM2, indicating that surface expression of TREM2 in KCs correlates with the liver resistance phenotype conferred by the belr1 locus (Fig. 3D).
Fig. 3.
Trem2 deficiency dictates enhanced susceptibility to liver stage infection and reveals KCs’ control of hepatocyte infection. (A) Liver parasite burden in C57BL/6, BALB/c, and Trem2−/− mice at 40 h p.i. was quantified as described in Fig. 1. Data are mean ± SD; n = 10. (B) FACS plot of TREM2 and F4/80 surface expression in NPC from C57BL/6 mice. (C and D) FACS histograms of TREM2 expression in F4/80+ NPCs were obtained from C57BL/6 and R90 mice (C) or BALB/c and R64 mice (D). (E) Parasite yield at 40 h p.i. in isolated C57BL/6 hepatocytes or direct contact cocultures with sorted-purified F4/80+ NPCs from C57BL/6 or Trem2−/− mice. Data are mean ± SD of triplicate cultures. (F and G) Parasite yield at 40 h p.i. in C57BL/6 hepatocytes cultured in the lower chamber of a Transwell system with or without sort-purified F4/80+ liver cells from C57BL/6 or Trem2−/− mice in the upper chamber (mean ± SD of triplicate cultures) using a 4-μM Transwell filter and infection in the upper chamber only (F) or using a 0.3-μM Transwell filter and infection in both the upper and lower chambers (G). *P < 0.05; ***P < 0.0001.
Because liver stage parasite expansion occurs inside hepatocytes, we investigated whether KCs are able to control hepatocyte infection, using in vitro infection in coculture assays. We found a reduced parasite burden when primary hepatocytes were cocultured in direct contact with sort-purified WT KCs, but not with Trem2−/− KCs (Fig. 3E). This finding explains the increased susceptibility of Trem2−/− mice and indicates that TREM2 takes part in a mechanism enabling KCs to control the yield of hepatocyte infection.
We next used a Transwell culture system to examine whether direct KC–hepatocyte contact is required for the control of hepatocyte infection. We found no TREM2-dependent effects on parasite burden when hepatocytes were infected in the presence of soluble mediators derived from WT or Trem2−/− KCs exposed to P. berghei sporozoites (Fig. 3G), or any differences when sporozoites were exposed to WT or Trem2−/− KCs before migrating to infect hepatocytes in the lower Transwell chamber (Fig. 3F). These results suggest that KC exposure to sporozoites does not induce production of soluble macrophage-derived factors that influence the efficacy of liver stage infection, or affect sporozoite infectivity. Taken together, these in vitro results suggest that Trem2 expression in KCs plays a key role in sporozoite-induced KC activation, which on direct KC–hepatocyte contact leads to decreased efficacy of liver stage infection.
Trem2 Conditions the Polarization of Kupffer Cells by P. berghei.
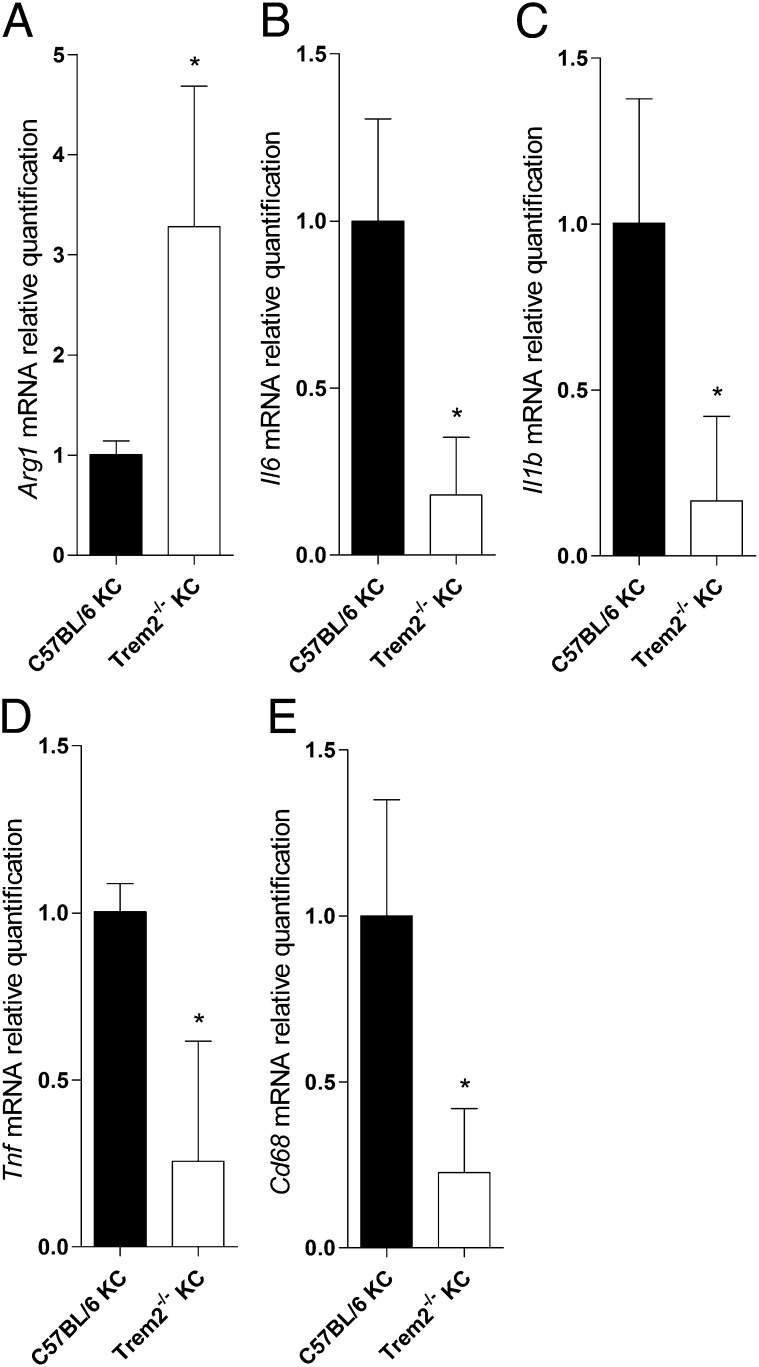
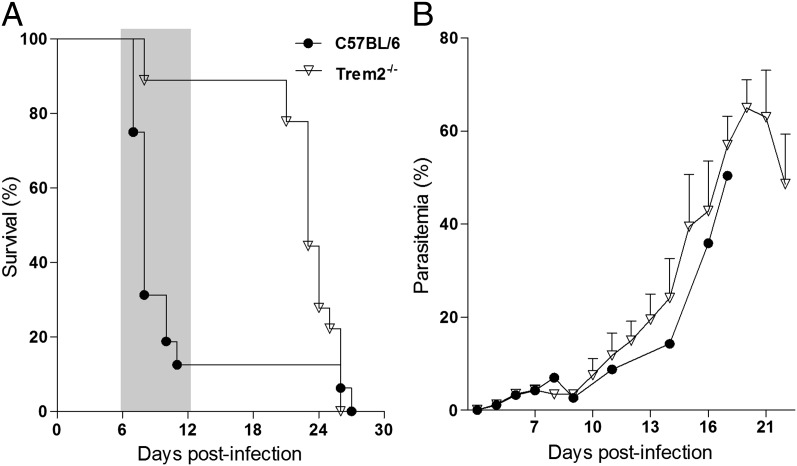
To test whether TREM2 expression has an impact on functional activation of KCs by P. berghei sporozoites, we profiled parasite-exposed cells measuring gene expression of activation markers associated to inflammatory effectors (M1 profile) or to anti-inflammatory properties (M2 profile) (22). Sort-purified Trem2−/− KCs exposed to P. berghei sporozoites for 40 h expressed high levels of Arg1 mRNA and low levels of Il6, Il1b, Tnf, and CD68 (Fig. 4). In the absence of TREM2, exposure to P. berghei sporozoites determines KC anti-inflammatory activation, indicating that TREM2 is involved in functional polarization of liver macrophages. Although the TREM2 ligands remain unknown, these findings suggest that contact with parasite components impinge on TREM2 signaling, raising the possibility that the effect of TREM2 on inflammation during malaria infection is also exerted in subsequent phases of malaria pathogenesis. We found that Trem2−/− mice infected with P. berghei sporozoites developed blood parasite levels comparable to those seen in WT mice, but were highly resistant to cerebral malaria, a neuroinflammatory syndrome to which C75BL/6 mice are highly susceptible (Fig. 5).
Fig. 4.
Trem2 determines KC functional polarization. mRNA quantification of Arg1 (A), Il6 (B), Ilb1 (C), Tnf (D), and Cd68 (E) in cultured, sort-purified KCs of Trem2−/− mice relative to C57BL/6, after a 40-h exposure to P. berghei ANKA sporozoites. Data are mean ± SD of triplicate cultures. *P < 0.05.
Fig. 5.
Trem2 deficiency confers resistance to cerebral malaria. (A) Kaplan–Meyer survival curves after infection with P. berghei sporozoites of Trem2−/− and C57BL/6 mice (n = 10 per group). The time window of fatal cerebral malaria development in C57BL/6 mice is shaded while death at a later stage is due to hyperparasitemia (P = 0.010, log-rank test). (B) Parasitemia was followed from day 4 to day 18 p.i.. Data are mean ± SD for each group.
Role for MHC Class II Molecules in Hepatocyte Resistance to Infection.
We further analyzed eight B6.C-H2d single-recombinant subcongenic lines and identified a 5.9-Mb region proximal to the belr1 locus that contributes to resistance to malaria liver stage infection (Fig. S3). Subcongenic lines R13 and R7 delimit this region within the D17Mit228–D17Igc1 interval, encompassing the H2 locus. This finding implies a role of the H2 locus, along with belr1, in controlling the resistance to liver stage infection in the B6.C-H-2d strain (19). Mice deficient in MHC class II genes had a reduced liver parasite burden compared with WT mice. Lack of expression of MHC class II molecules in purified hepatocytes decreased the in vitro expansion of liver stage parasites (Fig. S3), suggesting that the effect of H2 is independent of antigen presentation functions.
belr1 and H2 did not demonstrate an additive effect on the malaria liver resistance phenotype. Mouse lines containing both regions of BALB/c origin (i.e., R108 and R41) did not differ from lines carrying only one of the two controlling loci (i.e., R13 or R64). These results indicate that natural resistance to liver stage infection, previously ascribed to mouse chromosome 17 (19), can occur via one of two routes, dependent on either MHC class II expression in hepatocytes or on TREM2 expression in KCs.
Discussion
The study of malaria liver stage infection has focused primarily on Plasmodium–hepatocyte interactions during invasion, intracellular parasite growth, and parasite egress (2, 23, 24). Plasmodium sporozoites have been found to directly contact liver macrophages in the liver (9), modulating their cytokine profile (12). This work identifies TREM2 as underlying the belr1 genetic effect and implicates the functional activation of KCs by Plasmodium sporozoites in governing the resistance to liver stage infection.
Our findings imply that the C57B/6 and BALB/c parental strains carry different TREM2 genetic variants. Comparison of the published TREM2 genomic sequence in BALB/c and C57BL/6 mouse strains revealed only three single nucleotide differences: rs107941689 upstream of TREM2, rs49686564 in intron 2, and rs479921182 downstream of TREM2. rs107941689 maps 4.5 Kb upstream of the TREM2 protein coding sequence within a putative sequence regulatory element, suggesting a possible role in TREM2 transcriptional regulation (Ensembl, release GRC m38). This is in line with the observation that the level of TREM2 surface expression in KCs correlates with the liver resistance phenotype in the congenic strains that define the belr1 locus (Fig. 3). Although our data suggest a role for Trem2 in malaria liver stage infection, we cannot completely exclude the possibility that other genetic factors in the belr1 region could contribute to the liver stage resistance phenotype. mRNA expression analysis of protein-coding genes mapping within the belr1 region did not yield any other candidates.
Our data suggest that KCs play a role in liver stage infection at two stages: first, through activation by Plasmodium sporozoites in the course of their migration from sinusoidal vessels to invade the hepatocytes, and second, in the cross-talk with infected hepatocytes, leading to reduced intrahepatocytic parasite expansion. The idea that KC activation by Plasmodium sporozoites decreases the success of hepatocyte infection is corroborated by previous reports of activation on contact with sporozoites (12) and involvement in parasite clearance (25). The precise mechanism of KC cross-talk with infected hepatocytes requires further study to identify molecular pathways involved in this natural control mechanism of liver stage infection.
Expression of TREM2 in liver macrophages is in line with reports of its expression in resident macrophages in other tissues (21). TREM2 signaling was initially associated with negative regulation of macrophage activation (26), but multiple independent observations of TREM2 proinflammatory effects on macrophage activation in bacterial infections have been reported (27, 28). This suggests that cellular functions of TREM2 may be context-dependent. Our findings indicate that TREM2 is involved in determining proinflammatory macrophage activation on contact with the malaria parasite, similar to the profile recently described in microglia cells (29). Indirect evidence suggests expression of TREM2 ligands by both yeast and bacteria (30), supporting a role for TREM2 as a pathogen sensor (31). Our results indicate that P. berghei sporozoites activate macrophages in a TREM2-dependent fashion, suggesting that Plasmodium expresses as-yet unidentified TREM2 ligands.
TREM2 has been shown to contribute to both inflammatory and phagocytic responses to infectious agents (31, 32). Our in vitro experiments suggest that the triggering of TREM2 in macrophages is part of a decisive effector signal that enables a degree of control of parasite expansion inside hepatocytes, possibly by promoting killing/phagocytosis of infected hepatocytes and decreasing the yield of mature parasites. Histological examination of sporozoite-infected livers revealed no significant difference in the number of inflammatory foci between Trem2−/− and WT mice. Analysis of P. berghei infection over time in Trem2−/− mice led us to conclude that TREM2 plays a dual role in disease progression, favoring resistance against malaria liver stage infection while also promoting tissue-damaging responses elicited by infected erythrocytes (e.g., in the brain), further supporting a proinflammatory role for TREM2 in malaria infection. TREM1 has been implicated as a biomarker of macrophage activation in human malaria patients (33). TREM family genes are expressed mainly in the monocytic/macrophage lineage (34); our results support the idea that TREM genes control macrophage functional activation and thus intervene in innate immune responses, raising the possibility that TREM genes control effector functions that ultimately have impact on disease outcome in infections such as malaria.
The genetic dissection of two malaria liver stage resistance loci in mouse chromosome 17 (belr1 and H2) underlies the polygenic nature of the phenotypic difference between the BALB/c and C57BL/6 mouse strains. Although our observations pertain to P. berghei, it is possible that these genetic factors may control liver stage resistance against other Plasmodium species as well.
Our findings in this study demonstrate that identification of genetic factors underlying natural resistance to malaria liver stage infection provides insights into the host response to Plasmodium infection. Although multiple parasite–hepatocyte interactions are likely involved in successful infection of hepatocytes by the malaria parasite, we propose that KC functional activation is an effective mechanism for controlling the host response to malaria liver stage infection.
Materials and Methods
Mice.
All procedures involving laboratory mice were performed in accordance with national (Portaria 1005/92) and European regulations (European Directive 86/609/CEE) on animal experimentation and were approved by the Instituto Gulbenkian de Ciência’s Ethics Committee and the Direcção-Geral de Veterinária. Mice were bred and maintained in conventional housing facilities at the Instituto Gulbenkian de Ciência. H2-Ab1−/− and B6.C-H2d/bBy mice were obtained from the Jackson Laboratory. B6.C-H2d/bBy is a congenic mouse strain that carries a BALB/c-derived congenic segment of 37.9 Mb on chromosome 17 in a C57BL/6 genetic background, and is referred to herein as B6.C-H2d. Trem2-deficient mice (35) were kindly provided by Marco Colonna, Washington University School of Medicine, St. Louis, MO. To generate subcongenic lines (36), B6.C-H2d male mice were backcrossed to C57BL/6 females. In the second backcross generation, chromosome 17 recombinants were selected from among 187 progeny by individual genotyping with a high-density set of microsatellite markers covering the B6.C-H2d congenic region (Fig. S1). Markers were selected from the Whitehead/MIT Center for Genome Research collection (now at http://www.informatics.jax.org) or identified as polymorphic between C57BL/6 and BALB/c and termed Igc markers (Table S2). Ten recombinants (eight single and two double) were selected and expanded in a third backcross generation and bred to homozygosity by brother–sister mating in the fourth generation. All experiments were conducted using male mice age 8–15 wk.
Genotyping.
Tail genomic DNA was isolated, and genotyping was performed, applying conventional PCR and electrophoresis protocols for amplification and detection of polymorphic microsatellite markers (Fig S1). Primers for the markers identified in this study are listed in Table S2.
Parasites and Infection.
GFP-expressing P. berghei ANKA sporozoites (37) were obtained by dissection of infected salivary glands from Anopheles stephensi mosquitoes bred in the insectarium at the Instituto de Medicina Molecular, Lisbon, Portugal. Sporozoite suspensions in RPMI medium were injected i.v. in 100 μL of inocula containing104 sporozoites per mouse. Livers were collected at 40 h p.i. or survival, and parasitemia was followed for 28 d. For experimental cerebral malaria scoring, neurologic symptoms were monitored from day 5 p.i.. Hepatocyte primary cultures were infected with 4 × 104 P. berghei ANKA sporozoites, and noninfected controls were mock-infected with salivary glands from noninfected mosquitoes.
Liver Cell Preparation.
Primary mouse hepatocyte cultures were prepared as described previously (38). In brief, liver lobes were perfused, and hepatocytes were dissociated and separated using 1.12 g/mL, 1.08 g/mL, and 1.06 g/mL Percoll gradients (GE Healthcare). Hepatocytes were harvested from the gradient and cultured in Gibco Williams’ E complete medium (Life Sciences). NPCs were obtained as described previously with modifications (39). In brief, liver lobes were removed and perfused with liver perfusion medium (Life Sciences) supplemented with 750 mg/L of Collagenase H (Roche) at 37 °C. The resulting suspension was filtered through a 100-µm cell strainer (BD Falcon; BD Biosciences), and cells were suspended in RPMI complete medium (Life Sciences) and then mixed with Percoll solution (GE Healthcare) to a final concentration of 30% Percoll, followed by centrifugation at 850 × g for 10 min. The cell pellet was resuspended in RPMI and carefully layered on 30% Percoll solution, then centrifuged at 850 × g for 10 min. The cell pellet was washed and resuspended in ACK (NH4Cl 0.15 M, KHCO3 10 mM, Na2EDTA 2H2O 0.1 mM; pH 7.2) for 3 min to lyse remaining erythrocytes. Cells were washed and centrifuged at 153 × g for 20 s to discard the remaining hepatocytes. The supernatant was recovered, and NPCs were collected at 478 × g for 5 min.
Flow Cytometry and KC Sorting.
NPC preparations were stained with F4/80 APC (A3-1; Serotec) and anti-TREM2 PE antibodies (LifeSpan Bioscience) at 4 °C. A FACScalibur flow cytometer (BD Biosciences) and FlowJo software (Tree Star) were used for FACS analysis. Purified KCs were obtained by high-speed cell sorting using a FACSAria cell sorter (BD Biosciences) after staining of NPC preparations with F4/80 APC (Serotec).
Hepatocyte-KC Cultures.
Two culture systems were used. Sorted-purified KCs (from WT or Trem2−/− mice) were added to WT primary hepatocyte cultures that had been plated 24 h previously. The culture setups allowed modulation of the degree of cross-talk between cell types: in direct contact or separated by filters of different pore size. KCs in direct contact with hepatocytes were seeded at a 3:1 ratio and infected with 4 × 104 P. berghei ANKA sporozoites after 12 h. In filter-separated culture, KCs were seeded in the upper chamber of a Transwell culture system (Millipore). Two different filter systems were used. The first system consisted of a 0.4-µm filter that allows the exchange of soluble factors, but not parasites, between KCs (upper chamber) and hepatocytes; both chambers were infected with sporozoites. In the second system, to test changes in sporozoite infectivity on exposure to KCs, we used a 3-µm filters and infected only the upper chamber, allowing sporozoites to contact KCs and migrate through the filter to invade hepatocytes in the lower chamber. Hepatocytes were collected for parasite quantification at 40 h p.i. For KC activation studies, we allowed FACS-sorted KC cells to contact P. berghei sporozoites (1:1) for 40 h before collection for RNA analysis.
Parasite and Gene Expression Quantification.
Livers were collected at 40 h p.i. and immediately homogenized, and total RNA was extracted using the RNeasy Mini Kit (Qiagen). Then 1 μg of total RNA was converted to cDNA (Transcriptor First-Strand cDNA Synthesis Kit; Roche). Cultured cells were collected at 40 h p.i.. Cell lysis and reverse-transcriptase reactions were performed using the TaqMan Gene Expression Cell-to-CT kit (Ambion). cDNA specific to P. berghei 18S rRNA was amplified with TaqMan-specific primers: forward, 5′-CCG ATA ACG AAC GAG ATC TTA ACC T-3′; reverse, 5′-CGT CAA AAC CAA TCT CCC AAT AAA GG-3′; probe, 5′-ACT CGC CGC TAA TTA G-3′ (FAM/MGB). Trem1, Trem2, Trem3, Treml4, Treml6, Arg1, Il6, Il1b, Tnf, and Cd68 expression was quantified using best-coverage TaqMan Gene Expression Assays (Applied Biosystems). Endogenous control GAPDH (Mouse GAPD Endogenous Control; Applied Biosystems) was used in multiplex PCR reactions (Prism 7900HT; Applied Biosystems). Relative quantities were calculated by the ΔΔCt method.
Sample Size and Statistical Analysis.
All cell experiments were performed on a minimum of triplicate samples. Reported data are representative of at least three independent experiments. Two groups of samples were compared using the Mann–Whitney U test. Comparisons of more than two groups were performed by multiple comparisons using the Tukey–Kramer test. Survival curves were compared using the log-rank test. Between-group differences were considered statistically significant at a P value < 0.05.
Supplementary Material
Acknowledgments
We thank Marco Colonna and Susan Gilfillan for making Trem2−/− mice available, and Maria Mota for providing access to mosquitoes and insightful discussions. This work was supported by the Fundação para a Ciência e Tecnologia (FCT) through grants POCI/SAU-IMI/61057/2004 and HMSP-CT/SAU-ICT/0068/2009. L.A.G., L.R.D., J.R. and L.V.M. were supported by FCT fellowships (BD/44208/2008, BD/33566/2008, BD/29862/2006, and BPD/44486/2008, respectively). C.P.G. is an Affiliated Member of the European Virtual Institute of Malaria Research (EVIMaLaR).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306873110/-/DCSupplemental.
References
- 1.Borrmann S, Matuschewski K. Targeting Plasmodium liver stages: Better late than never. Trends Mol Med. 2011;17(9):527–536. doi: 10.1016/j.molmed.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Lindner SE, Miller JL, Kappe SH. Malaria parasite pre-erythrocytic infection: Preparation meets opportunity. Cell Microbiol. 2012;14(3):316–324. doi: 10.1111/j.1462-5822.2011.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derbyshire ER, Prudêncio M, Mota MM, Clardy J. Liver-stage malaria parasites vulnerable to diverse chemical scaffolds. Proc Natl Acad Sci USA. 2012;109(22):8511–8516. doi: 10.1073/pnas.1118370109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaughan AM, Kappe SH. Vaccination using radiation- or genetically attenuated live sporozoites. Methods Mol Biol. 2013;923:549–566. doi: 10.1007/978-1-62703-026-7_38. [DOI] [PubMed] [Google Scholar]
- 5.Prudêncio M, Rodriguez A, Mota MM. The silent path to thousands of merozoites: The Plasmodium liver stage. Nat Rev Microbiol. 2006;4(11):849–856. doi: 10.1038/nrmicro1529. [DOI] [PubMed] [Google Scholar]
- 6.Silvie O, et al. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med. 2003;9(1):93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues CD, et al. Host scavenger receptor SR-BI plays a dual role in the establishment of malaria parasite liver infection. Cell Host Microbe. 2008;4(3):271–282. doi: 10.1016/j.chom.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Yalaoui S, et al. Scavenger receptor BI boosts hepatocyte permissiveness to Plasmodium infection. Cell Host Microbe. 2008;4(3):283–292. doi: 10.1016/j.chom.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Pradel G, Frevert U. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology. 2001;33(5):1154–1165. doi: 10.1053/jhep.2001.24237. [DOI] [PubMed] [Google Scholar]
- 10.Mota MM, et al. Migration of Plasmodium sporozoites through cells before infection. Science. 2001;291(5501):141–144. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- 11.Torgler R, et al. Sporozoite-mediated hepatocyte wounding limits Plasmodium parasite development via MyD88-mediated NF-kappa B activation and inducible NO synthase expression. J Immunol. 2008;180(6):3990–3999. doi: 10.4049/jimmunol.180.6.3990. [DOI] [PubMed] [Google Scholar]
- 12.Klotz C, Frevert U. Plasmodium yoelii sporozoites modulate cytokine profile and induce apoptosis in murine Kupffer cells. Int J Parasitol. 2008;38(14):1639–1650. doi: 10.1016/j.ijpara.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liehl P, Mota MM. Innate recognition of malarial parasites by mammalian hosts. Int J Parasitol. 2012;42(6):557–566. doi: 10.1016/j.ijpara.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Epiphanio S, et al. Heme oxygenase-1 is an anti-inflammatory host factor that promotes murine plasmodium liver infection. Cell Host Microbe. 2008;3(5):331–338. doi: 10.1016/j.chom.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Khan ZM, Ng C, Vanderberg JP. Early hepatic stages of Plasmodium berghei: Release of circumsporozoite protein and host cellular inflammatory response. Infect Immun. 1992;60(1):264–270. doi: 10.1128/iai.60.1.264-270.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van de Sand C, et al. The liver stage of Plasmodium berghei inhibits host cell apoptosis. Mol Microbiol. 2005;58(3):731–742. doi: 10.1111/j.1365-2958.2005.04888.x. [DOI] [PubMed] [Google Scholar]
- 17.Khan ZM, Vanderberg JP. Role of host cellular response in differential susceptibility of nonimmunized BALB/c mice to Plasmodium berghei and Plasmodium yoelii sporozoites. Infect Immun. 1991;59(8):2529–2534. doi: 10.1128/iai.59.8.2529-2534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheller LF, Wirtz RA, Azad AF. Susceptibility of different strains of mice to hepatic infection with Plasmodium berghei. Infect Immun. 1994;62(11):4844–4847. doi: 10.1128/iai.62.11.4844-4847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonçalves LA, Almeida P, Mota MM, Penha-Gonçalves C. Malaria liver stage susceptibility locus identified on mouse chromosome 17 by congenic mapping. PLoS ONE. 2008;3(3):e1874. doi: 10.1371/journal.pone.0001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allcock RJ, Barrow AD, Forbes S, Beck S, Trowsdale J. The human TREM gene cluster at 6p21.1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur J Immunol. 2003;33(2):567–577. doi: 10.1002/immu.200310033. [DOI] [PubMed] [Google Scholar]
- 21.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009;21(1):38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ejigiri I, et al. Shedding of TRAP by a rhomboid protease from the malaria sporozoite surface is essential for gliding motility and sporozoite infectivity. PLoS Pathog. 2012;8(7):e1002725. doi: 10.1371/journal.ppat.1002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heussler V, Rennenberg A, Stanway R. Host cell death induced by the egress of intracellular Plasmodium parasites. Apoptosis. 2010;15(3):376–385. doi: 10.1007/s10495-009-0435-6. [DOI] [PubMed] [Google Scholar]
- 25.Vreden SG. The role of Kupffer cells in the clearance of malaria sporozoites from the circulation. Parasitol Today. 1994;10(8):304–308. doi: 10.1016/0169-4758(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 26.Sharif O, Knapp S. From expression to signaling: Roles of TREM-1 and TREM-2 in innate immunity and bacterial infection. Immunobiology. 2008;213(9-10):701–713. doi: 10.1016/j.imbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Sun M, et al. TREM-2 promotes host resistance against Pseudomonas aeruginosa infection by suppressing corneal inflammation via a PI3K/Akt signaling pathway. Invest Ophthalmol Vis Sci. 2013;54(5):3451–3462. doi: 10.1167/iovs.12-10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Q, et al. Triggering receptor expressed on myeloid cells-2 protects against polymicrobial sepsis by enhancing bacterial clearance. Am J Respir Crit Care Med. 2013;188(2):201–212. doi: 10.1164/rccm.201211-1967OC. [DOI] [PubMed] [Google Scholar]
- 29.Sieber MW, et al. Attenuated inflammatory response in triggering receptor expressed on myeloid cells 2 (TREM2) knock-out mice following stroke. PLoS ONE. 2013;8(1):e52982. doi: 10.1371/journal.pone.0052982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daws MR, et al. Pattern recognition by TREM-2: binding of anionic ligands. J Immunol. 2003;171(2):594–599. doi: 10.4049/jimmunol.171.2.594. [DOI] [PubMed] [Google Scholar]
- 31.Correale C, et al. Bacterial sensor triggering receptor expressed on myeloid cells-2 regulates the mucosal inflammatory response. Gastroenterology. 2013;144(2):346–356, e3. doi: 10.1053/j.gastro.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 32.N’Diaye EN, et al. TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. J Cell Biol. 2009;184(2):215–223. doi: 10.1083/jcb.200808080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chimma P, et al. A distinct peripheral blood monocyte phenotype is associated with parasite inhibitory activity in acute uncomplicated Plasmodium falciparum malaria. PLoS Pathog. 2009;5(10):e1000631. doi: 10.1371/journal.ppat.1000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erdman LK, et al. Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: A retrospective case-control study. PLoS ONE. 2011;6(2):e17440. doi: 10.1371/journal.pone.0017440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turnbull IR, et al. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol. 2006;177(6):3520–3524. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 36.Markel P, et al. Theoretical and empirical issues for marker-assisted breeding of congenic mouse strains. Nat Genet. 1997;17(3):280–284. doi: 10.1038/ng1197-280. [DOI] [PubMed] [Google Scholar]
- 37.Franke-Fayard B, et al. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol. 2004;137(1):23–33. doi: 10.1016/j.molbiopara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Gonçalves LA, Vigário AM, Penha-Gonçalves C. Improved isolation of murine hepatocytes for in vitro malaria liver stage studies. Malar J. 2007;6:169. doi: 10.1186/1475-2875-6-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goossens PL, Jouin H, Marchal G, Milon G. Isolation and flow cytometric analysis of the free lymphomyeloid cells present in murine liver. J Immunol Methods. 1990;132(1):137–144. doi: 10.1016/0022-1759(90)90407-m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.