Significance
We identified a human-specific endogenous retroviral insert (hsERV) that acts as an enhancer for human PRODH, hsERV_PRODH. PRODH encodes proline dehydrogenase, which is involved in neuromediator synthesis in the CNS. We show that the hsERV_PRODH enhancer acts synergistically with the CpG island of PRODH and is regulated by methylation. We detected high PRODH expression in the hippocampus, which was correlated with the undermethylated state of this enhancer. PRODH regulatory elements provide neuron-specific transcription in hippocampal cells, and the mechanism of hsERV_PRODH enhancer activity involves the binding of transcriptional factor SOX2. Because PRODH is associated with several neurological disorders, we hypothesize that the human-specific regulation of PRODH by hsERV_PRODH may have played a role in human evolution by upregulating the expression of this important CNS-specific gene.
Keywords: human-specific endogenous retrovirus, DNA methylation, central nervous system, human speciation, retroelement
Abstract
Using a systematic, whole-genome analysis of enhancer activity of human-specific endogenous retroviral inserts (hsERVs), we identified an element, hsERVPRODH, that acts as a tissue-specific enhancer for the PRODH gene, which is required for proper CNS functioning. PRODH is one of the candidate genes for susceptibility to schizophrenia and other neurological disorders. It codes for a proline dehydrogenase enzyme, which catalyses the first step of proline catabolism and most likely is involved in neuromediator synthesis in the CNS. We investigated the mechanisms that regulate hsERVPRODH enhancer activity. We showed that the hsERVPRODH enhancer and the internal CpG island of PRODH synergistically activate its promoter. The enhancer activity of hsERVPRODH is regulated by methylation, and in an undermethylated state it can up-regulate PRODH expression in the hippocampus. The mechanism of hsERVPRODH enhancer activity involves the binding of the transcription factor SOX2, whch is preferentially expressed in hippocampus. We propose that the interaction of hsERVPRODH and PRODH may have contributed to human CNS evolution.
Understanding the molecular basis of phenotypic differences between humans and chimpanzees can provide important clues to human-specific behavioral peculiarities and neurological disorders. For this purpose we conducted a genome-wide analysis of human-specific endogenous retroviral (hsERV) inserts that may induce new regulatory pathways by acting as promoters and enhancers (1, 2). HsERVs of the HERV-K(HML-2) group are one of the four families of transposable elements that were able to transpose at the time of the radiation of human lineage from the lineage of its most closely related species, chimpanzee (3). At least 50% of all hsERV elements exhibit promoter activity in human tissues (4). We found only six hsERV inserts in the upstream regions of known human genes, close to transcription start sites. Three of them displayed strong enhancer activity in transient transfection experiments; of these three, only one—near the PRODH gene—matched the transcriptional activity pattern of its endogenous genomic copy. This copy of hsERV is a full-length, almost intact betaretrovirus belonging to the HERV-K(HML-2) group. PRODH encodes a mitochondrial enzyme proline, dehydrogenase (oxidase), that converts proline to d-1-pyrroline-5-carboxylate (5). PRODH regulates proline catabolism, which is vital for normal CNS functioning. Several PRODH mutations are associated with neuropsychiatric disorders, such as schizophrenia (6). Gene knockouts in mice cause severe changes in the executive functioning of the brain (7). Given the potential importance of PRODH in brain functioning and disease, we attempted to characterize its newly recognized hsERVPRODH enhancer. We showed that hsERVPRODH enhancer activity is regulated by methylation and that the hsERVPRODH enhancer and PRODH internal CpG island act synergistically to activate its promoter. PRODH transcription analyses demonstrated the highest expression level in the hippocampus, where hsERVPRODH is hypomethylated. Moreover, the hsERVPRODH enhancer, together with the PRODH promoter and CpG island, caused neuron-specific expression. We also found that hsERVPRODH contains two functional sites for binding the transcription factor SOX2 that activates its enhancer activity and is expressed predominantly in hippocampus. Our data shed light on the transcriptional regulation of PRODH and identify a human-specific enhancer that is activated in hippocampus.
Results
Enhancer Activity Tests of Individual hsERVs.
Bioinformatic screening of all 133 hsERVs previously identified by us and other investigators (8) resulted in the identification of six elements inserted in the close vicinity (<5 kb of the transcription start sites) of known human genes. These hsERVs mapped upstream of SOCS4, PRODH, NDUFV1, ZFP3, KIAA1919, and c3orf17 (Fig. S1A).
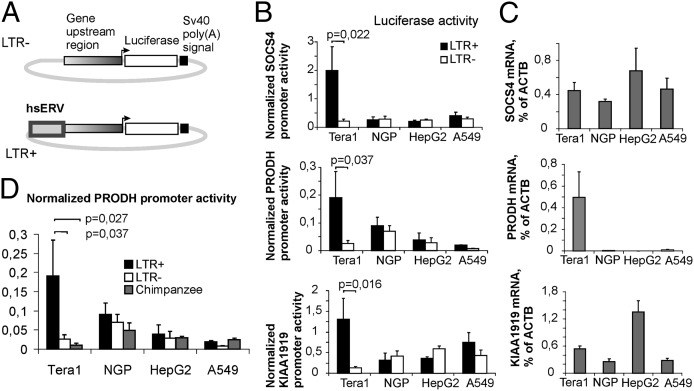
We next attempted to demonstrate the enhancer activity of these elements using transient transfection experiments. The genomic sequence upstream of the transcription start site including the hsERV element (LTR+ constructs) or excluding the hsERV element (LTR− constructs) was cloned into a luciferase-reporter construct for each of the six genes (Fig. 1A) and was used in a luciferase assay (Fig. 1B and Fig. S1B). For better accuracy, we used a Dual-Luciferase Reporter Assay (Promega). For the upstream regions of SOCS4, PRODH, and KIAA1919, we detected strong and statistically significant enhancer activity, with a normalized luciferase activity LTR+/LTR− ratio of seven- to 10-fold (Fig. 1B). For c3orf17, there was only a twofold difference in the luciferase activities of the LTR+ and LTR− constructs (Fig. S1B). The enhancer effects of the hsERVs were restricted to one of the four cell lines tried, Tera-1, suggesting that it is tissue specific.
Fig. 1.
Comparison of luciferase reporter assay data with transcriptional activities of the endogenous gene copies. (A) Scheme of luciferase reporter constructs. (B) Promoter activities of LTR+ and LTR− constructs in Tera-1, NGP127, HepG2, and A549 cell lines (normalized to the SV40 promoter activity). (C) mRNA levels of SOCS4, PRODH, and KIAA1919 genes in cell lines measured by qRT-PCR relative to the endogenous β-actin (ACTB) gene expression. (D) Promoter activities of human and chimpanzee PRODH upstream regions. LTR−, human PRODH promoter region; LTR+, human PRODH promoter region including hsERV LTR; Chimpanzee, orthologous chimpanzee PRODH promoter (lacking hsERV). Data show means ± SD of three independent experiments.
A number of studies suggest that transient transfection data may differ dramatically from the expression patterns seen for endogenous copies of the same genes when located in their native, genomic environment (9). Thus, examination of endogenous gene copy expression is needed to refine transient transfection data. We used quantitative RT-PCR (qRT-PCR) to evaluate transcriptional activities of all genes in our study in the same four cell lines used for the transient transfection experiments (Fig. 1C and Fig. S1C). In five of the six genes there was no correlation between the transcriptional activities of the endogenous gene copies and their transfected upstream regulatory regions for either the LTR+ and LTR− constructs. However, for PRODH (Fig. 1 B and C) we found a correlation between the promoter activities of the LTR+ construct and the endogenous copy of this gene, with clearly enhanced activities for both in Tera-1 cells, but there was no correlation between the transcription of the endogenous copy and the LTR− construct. Thus, only three elements displayed strong enhancer activity (by increasing the expression of neighboring genes from the authentic promoters by seven- to 10-fold) when tested in four human cell lines of the different etiologies. However, we cannot exclude the possibility that the other hsERVs tested may be able to show rather distinct enhancer activities in the other human cell types. Of these three hsERVs, only one element, hsERVPRODH, displayed enhancer activity in the luciferase tests that matched the transcriptional pattern of its endogenous copy.
We further attempted to compare the promoter activities of the human hsERVPRODH -lacking (LTR−) and hsERVPRODH -containing (LTR+) PRODH upstream regions (Fig. 1D) with the orthologous copy of the chimpanzee PRODH upstream region that lacks this retroviral insertion. For this purpose we amplified the chimpanzee 2.4-kb-long PRODH upstream region and cloned it into a luciferase-reporter vector. We found that the activity of the chimpanzee upstream sequence was comparable to that of the human LTR− construct and was weaker than that of the LTR+ construct. These data demonstrate that, in some cell types, the human PRODH upstream region harboring the hsERVPRODH insert is significantly more transcriptionally active than the orthologous chimpanzee sequence.
The hsERVPRODH enhancer therefore could represent a human-specific regulation of gene expression that arose after the separation of human and chimpanzee ancestors.
HsERVPRODH, a Human-Specific Enhancer That Acts Synergistically with a CpG Island to Activate PRODH Transcription.
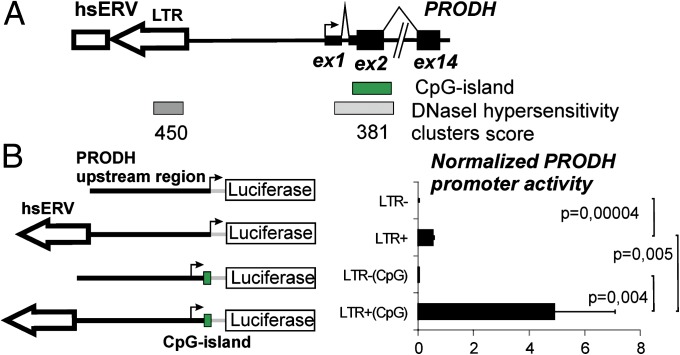
Little is known about the regulation of PRODH transcription. Sequence analysis indicates that this gene includes an internal CpG island (CpGPRODH) that partly overlaps with its second exon (Fig. 2A). To investigate whether the inclusion of CpGPRODH affects the activity of the hsERVPRODH enhancer, we added a 230-bp-long fragment overlapping with the CpGPRODH sequence to the luciferase reporter construct (Fig. 2B). This fragment included the whole first PRODH noncoding exon, the short first intron (62 bp), and a portion of the second exon up to the translation initiation codon. For these experiments, we used Tera-1 cells. We found that presence of the CpGPRODH sequence strongly reinforced the activity of the hsERVPRODH enhancer (Fig. 2B). In contrast, CpGPRODH had no effect on the promoter activity of the PRODH upstream sequence that lacked hsERVPRODH (Fig. 2B). These data suggest that hsERVPRODH and the CpGPRODH may act synergistically in PRODH transcriptional regulation. We also identified a DNase hypersensitivity cluster inside hsERVPRODH, which appeared to be even more important than that in the beginning of the PRODH gene itself, as reflected by its cluster score values (Fig. 2A). This finding clearly indicates that hsERVPRODH and CpGPRODH have functional activity in vivo.
Fig. 2.
Effect of the PRODH CpG island on hsERVPRODH-enhancer activity. (A) The PRODH gene upstream region. Black bars indicate PRODH exons; black arrow, PRODH transcription start site; open arrow, hsERV LTR; open bar, hsERV internal region; green bar, CpG island; gray bar, DNase I hypersensitive clusters. Numbers under gray bars indicate cluster scores. Data were taken from the University of California Santa Cruz Genome Browser, http://genome.ucsc.edu. (B) (Left) Schematic representation of luciferase reporter constructs. (Right) Relative PRODH promoter activity, normalized to SV40 promoter activity. Data show means ± SD of three independent experiments.
The Methylation Status of hsERVPRODH Regulates PRODH Transcription.
DNA methylation is thought to be involved in silencing HERV-K (HML 2) transcriptional regulatory elements (10, 11). To test this possibility, we transfected Tera-1 cells with in vitro-methylated LTR−, LTR+, or control constructs (the control included an SV40 promoter upstream of the luciferase reporter). The degree of methylation varied depending on the length of treatment with CpG-DNA methylase (0–30 min) (Fig. S2A). Results show that methylation dramatically reduces both hsERVPRODH and SV40 reporter transcription activities. This result is consistent with the idea that DNA methylation is vital for the regulation of HERV-K (HML 2) transcriptional activities.
These transient transfection results showed that hsERVPRODH hypermethylation may inhibit enhancer activity, and hypomethylation may increase enhancer activity. To uncover the methylation status of hsERVPRODH in the four human cell lines used thus far in this study, we performed bisulfite sequencing covering the whole length of the hsERVPRODH LTR. We found that the hsERVPRODH was extensively methylated in cell lines HepG2, A549, and NGP127 but was hypomethylated in Tera-1 cells (Fig. S2B). These low levels of methylation and increased expression of PRODH in Tera-1 cells suggest that the hsERVPRODH may be an active, tissue-specific enhancer in Tera-1 cells. A similar analysis of methylation of CpGPRODH revealed that, unlike hsERVPRODH, CpGPRODH was hypomethylated in all of the cells tested (Fig. S2B).
PRODH encodes an isoform of proline dehydrogenase that, according to previous reports (7, 12) (human http://genome.ucsc.edu/cgi-bin/hgGene?hgg_gene=uc002zoj.4&hgg_prot=E7EQL6&hgg_chrom=chr22&hgg_start=18900286&hgg_end=18923806&hgg_type=knownGene&db=hg19&hgsid=294069969), is predominantly transcribed in the heart, lung, liver, and CNS. We confirmed these reports with qRT-PCR data on samples of human testicular, bladder, heart, lung, and mixed brain tissues (Fig. S2C). The highest PRODH transcription was seen in brain samples, congruent with a putative role for PRODH in CNS functioning.
We examined hsERVPRODH and CpGPRODH methylation in the same brain samples using bisulfite sequencing. As in our previous data, CpGPRODH was equally undermethylated in all tissues (Fig. S2D). In contrast, hsERVPRODH was heavily methylated in most molecules; however, in several molecules hsERVPRODH was almost completely free of methylation (arrows in Fig. S2D). Methylated alleles may represent cells in which the hsERVPRODH-enhancer element is suppressed, whereas nonmethylated alleles may correspond to cells with active enhancer.
Overall, our data suggest that the activity of the hsERVPRODH enhancer is regulated differentially through its methylation and that the expression of PRODH is regulated by mechanism(s) other than promoter and CpGPRODH methylation. Moreover, the unmethylated state of the CpGPRODH that we observed in all tissues under investigation could not by itself be sufficient for providing high transcriptional activity for that gene.
hsERVPRODH–CpGPRODH Interplay in Human Brain.
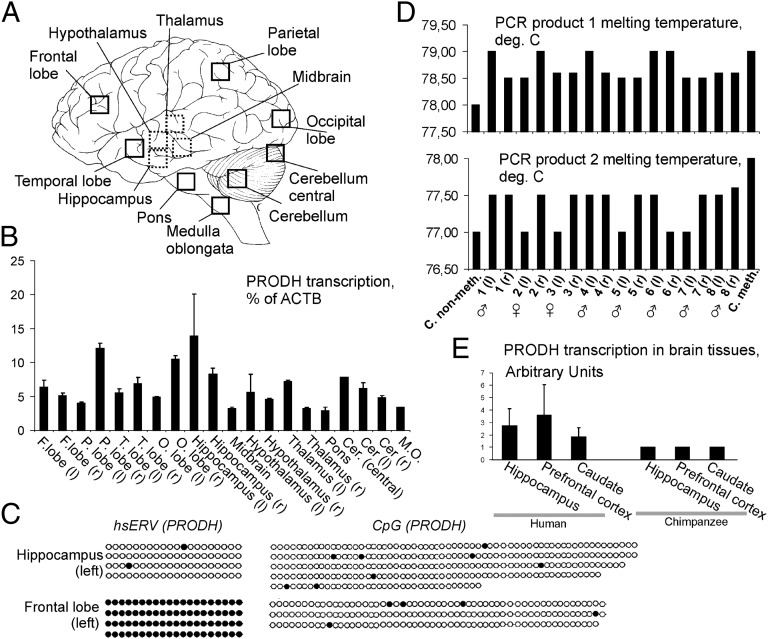
Because PRODH is brain specific, and because the brain contains a fraction of cells with unmethylated hsERVPRODH, we next examined PRODH expression and CpGPRODH in different brain tissue types using qRT-PCR. We performed qRT-PCR on 20 brain tissue types taken from a single donor (Fig. 3A) and found that PRODH is highly expressed in the hippocampus, parietal lobe, and occipital lobe (Fig. 3B). We also detected differences between the left and right hemispheres of the parietal lobe, occipital lobe, and thalamus (Fig. 3B), but further studies using a greater sampling are required to validate the significance of this effect.
Fig. 3.
Functional characterization of the PRODH locus in the brain tissues. (A) Schematic view of the brain sections investigated. (B) Expression of PRODH in human brain tissues measured using qRT-PCR relative to ACTB. Data show means ± SD of three independent experiments. (C) Representative methylation patterns of the hsERVPRODH (Left) and CpGPRODH (Right) in the left hippocampus and left hemisphere of the frontal lobe. Black circle, methylated CG dinucleotide; white circle, unmethylated CG dinucleotide. (D) High-resolution melting profiling of bisulfite-treated DNA from the left (l) and right (r) hemisphere of human hippocampi. Data are shown for two PCR-amplified bisulfite-treated CG-rich fragments of the hsERVPRODH. C.meth, methylated sequence control; C non-meth, unmethylated sequence control. (E) Transcriptional activity of PRODH in human and chimpanzee brain tissues. The data were extracted from the NCBI GEO database, and the fold-change differences in gene-expression levels in individual human tissue samples and in the average chimpanzee tissue were calculated. Arbitrary units represent the fold-change difference between the PRODH expression in human samples and the chimpanzee median PRODH expression.
We next used a bisulfite sequencing assay to assess the methylation status of hsERVPRODH and CpGPRODH in these tissues. CpGPRODH was hypomethylated in all tissues. In contrast, hsERVPRODH was strongly methylated in all tissues except the left hemisphere of the hippocampus (Fig. 3C). This region also contained the highest transcriptional activity of PRODH among the investigated tissues (Fig. 3B). Subsequent analyses using a methylation-sensitive high-resolution melting assay on eight additional, independent left and right hippocampus samples obtained from the healthy human donors revealed that undermethylation of the hsERVPRODH in the hippocampus is generally characteristic of the human population (Fig. 3D).
Finally, we compared PRODH gene transcription in human and chimpanzee using available microarray gene-expression data extracted from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo/). We interrogated 18 samples of human brain tissue and 18 samples of chimpanzee brain tissue, representing hippocampus, prefrontal cortex, and caudate (Dataset S1). These samples included seven human and six chimpanzee hippocampal samples. In all the brain sections tested we observed clear up-regulation of PRODH in human as compared with the chimpanzee samples (Fig. 3E).
Cell-Type Specificity of PRODH Transcriptional Activity.
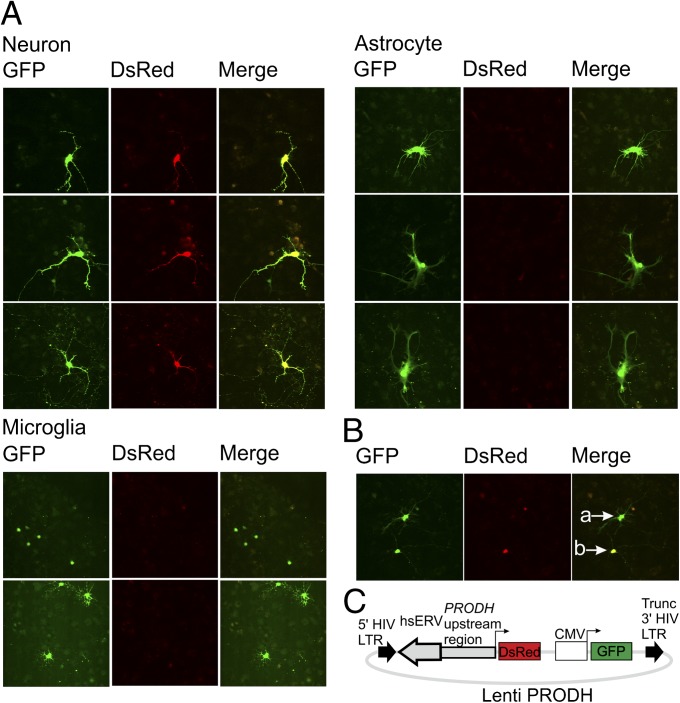
We next examined whether the transcriptional activity of PRODH is restricted to specific hippocampal cell types. We transformed cultured rat hippocampal cells with a lentiviral construct (Fig. 4A) containing GFP under the control of a constitutive CMV promoter and a reporter gene for the red fluorescent protein DsRed under the control of the PRODH promoter. We detected three clearly distinct morphological cell types in hippocampal tissue: neurons, astrocytes, and microglial cells. At least 50 fluorescent cells were observed for each cell type. In all cases, DsRed fluorescence was restricted to neurons, but GFP fluorescence was shown in all three cell types (Fig. 4 B and C), suggesting that PRODH expression in the hippocampus is specific to neurons.
Fig. 4.
Transformation of rat hippocampal cells with a lentiviral construct carrying the upstream human PRODH region. (A) Schematized structure of the lentiviral construct. Red fluorescent protein (DsRed) was placed under the transcriptional control of the PRODH promoter; the GFP gene was placed under the control of the constitutive CMV promoter (+ control). (B) PRODH promoter activity in different hippocampal cell types. (C) Comparison of DsRed vs. GFP fluorescence in astrocytes (a) and neurons (b).
Mechanism of hsERVPRODH Enhancer Activity.
To identify which transcription factors are responsible for hsERVPRODH enhancer activity, we bioinformatically screened the hsERVPRODH LTR for transcription factor-binding sites (TFBSs) using the MatIspector (13) and Alibaba2.1 (www.gene-regulation.com/pub/programs/alibaba2/index.html) programs. We identified ∼200 putative TFBSs. To identify TFBSs that may contribute functionally to hsERVPRODH enhancer activity, we compared transcriptomes of Tera-1 (hsERVPRODH enhancer–positive) cells with those of the cells that do not display hsERVPRODH enhancer activity, i.e., A549, NGP127, HepG2, and NT2/D1 (hsERVPRODH-enhancer–negative) (Fig. S3) and with the sample of human left hemisphere hippocampal tissue. We identified a set of transcription factor genes that were up-regulated in enhancer-positive cells (Datasets S2 and S3). Only two genes, SOX2 and NFKB1, exhibited TFBSs that had been predicted previously using bioinformatics. Subsequent qRT-PCR analyses showed that both genes were up-regulated simultaneously in Tera-1 cells and in the hippocampus and were down-regulated in all four enhancer-negative cell types (Datasets S2 and S3). A subsequent dendrogram analysis of gene-expression profiles showed that Tera-1 and hippocampus samples cluster together (Fig. S4).
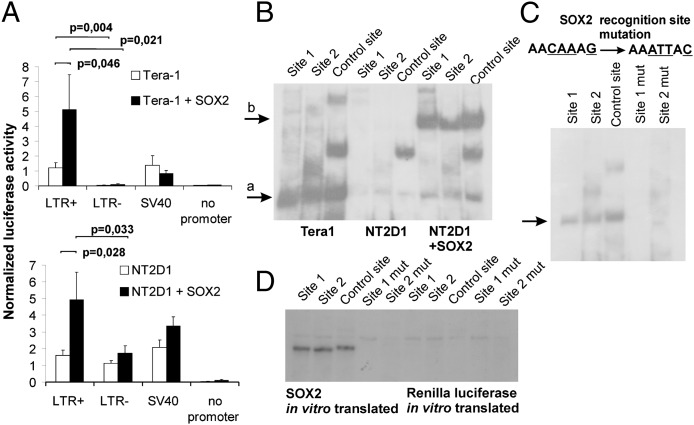
To determine if SOX2 and NFKB1 functionally affect hsERVPRODH activity, we overexpressed these genes in Tera-1 cells and in the enhancer-negative cell line NT2/D1. Overexpression of NFKB1 had no effect on enhancer activity of hsERVPRODH in either cell line (Fig. S5A), but overexpression of SOX2 in NT2/D1 cells resulted in a strong enhancer effect for hsERVPRODH (Fig. 5A, Lower). This effect also was seen in Tera-1 cells, despite their higher background levels of SOX2 (Fig. 5A, Upper).
Fig. 5.
Regulation of hsERVPRODH enhancer activity by the SOX2 transcription factor. (A) Effect of SOX2 overexpression on hsERVPRODH enhancer activity in Tera-1 (Upper) and NT2/D1 (Lower) cells. Data show means ± SD of three independent experiments. (B) EMSA for SOX2-binding sites with nuclear extracts of Tera-1 cells, NT2/D1 cells, and NT2/D1 cells overexpressing SOX2. (C) EMSAwith mutated SOX2-binding sites (mutated nucleotide positions are underlined). (D) EMSA for the SOX2-binding sites with in vitro-produced SOX2 protein. Renilla Luciferase protein was used as a negative control. Sites 1 and 2 SOX2, recognition sites 1 and 2 within hsERVPRODH; sites 1 and 2 mut, respective mutated sites; control site, control recognition sequence.
We next conducted an EMSA with radiolabeled double-stranded oligonucleotides corresponding to predicted hsERVPRODH TFBSs for NFKB1 and SOX2 gene products (Fig. S5B). We found no bands for predicted hsERVPRODH-binding sites for NFKB1 (Fig. S5 B and D), suggesting that the NFKB1 is not involved in mediating the enhancer activity of hsERVPRODH. In a similar experiment with SOX2, two binding sites within the hsERVPRODH sequence showed a strong positive signal (Fig. S5 B and C). Experiments with the competitor oligonucleotides confirmed that SOX2–TFBS binding was sequence specific for both the investigated recognition sites (Fig. S5B). Both these sites contained the predicted recognition motif AACAAAG. Subsequent experiments comparing TFBS binding of nuclear extracts from cell types confirmed binding in enhancer-positive cell types (Tera-1 and SOX2-overexpressed NTD/D1) and no binding in enhancer-negative cells (Fig. 5B). Our assays also found that four-nucleotide mutations in the SOX2 recognition motif disabled binding of SOX2 to the enhancer (Fig. 5C).
We next produced SOX2 protein in vitro to determine if TFBS recognition is mediated by SOX2 binding. SOX2-coding mRNA was synthesized and used for translation with the cytoplasmic extract of rabbit reticulocytes. Renilla luciferase was used as a negative control. Radiolabeled probes contained either wild-type or mutant SOX2 TFBSs (Fig. 5D). We detected binding with both wild-type TFBSs and with the positive control sequence, whereas in the SOX2 translation mix there was no binding with either mutant TFBS (Fig. 5D). These data provide strong evidence that the enhancer activity of hsERVPRODH is caused by SOX2 binding. Interestingly, our bioinformatic analysis revealed that both these SOX2 TFBSs are highly conserved among the LTRs of the endogenous retroviral family HERV-K (HML-2) and occur in 84% and 51% of them (i.e., in 557 and 366 genomic copies), respectively (Fig. S6). Thus, HERV-K (HML-2)–mediated SOX2 binding in vivo may not be limited to the regulation of the gene PRODH transcription but also may be used elsewhere in the human genome.
Discussion
The importance of PRODH for CNS functioning is clear, but its precise role is not. A deletion in the genomic locus 22q11, which contains PRODH, been implicated in DiGeorge syndrome, a condition associated with several neurological disorders, including schizophrenia (14). PRODH was identified as a schizophrenia-associated gene in some studies (15, 16) but not in another (17). PRODH is a mitochondrial enzyme catalyzing the first step of proline catabolism, and PRODH malfunctions cause hyperprolinemia type I. This condition is known to cause neurological abnormalities (18, 19). PRODH also may play a role in the synthesis of neuromediators. Proline can be converted to glutamate in two intermediate steps (20). Glutamate, in turn, may act as a neurotransmitter itself or may be further converted to GABA (21). Because proline may be used for glutamate synthesis in neurons (22), PRODH malfunction may cause a neuromediator misbalance (7).
Here, we show that PRODH is positively regulated by an hsERV insert. We hypothesize that, at some point in human evolution, the hsERVPRODH insertion may have influenced PRODH transcriptional activity significantly and increased its expression in the CNS. We show that the hsERVPRODH, together with the PRODH promoter, drives neuron-specific expression in cultured hippocampal cells. Remarkably, hsERVPRODH was hypomethylated in human hippocampal samples, where PRODH showed the highest transcriptional activity. We also uncovered a mechanism for the regulation of hsERVPRODH involving the binding of SOX2. In the brain, SOX2 is expressed preferentially in the hippocampus, which is known to be one of the brain structures most affected in schizophrenia (23). Our findings suggest a potential role of hsERVPRODH in CNS functioning. Discovery of this CNS-specific PRODH enhancer encourages further studies to discover the molecular features affecting its functional activity and its connection with neurological disorders. Our results can stimulate further studies of how methylation of PRODH regulatory regions interplays with human brain functioning in vivo. hsERVPRODH itself is a repetitive element and is flanked at both ends by other low-diverged genomic repeats (http://genome-euro.ucsc.edu/cgi-bin/hgTracks?position=chr22:18920392-18928317&hgsid=193710100&rmsk=full), making it a difficult target for precise DNA manipulation. However, further development of transgenesis technologies probably will make it possible in the future to explore in full the regulatory potential of hsERVPRODH and of other human-specific inserts of transposable elements.
Methods
Additional methods and detailed protocols are available in SI Methods.
Cell Lines.
The cell lines Tera-1 (testicular embryonal germ cell tumor), NT2/D1 (partly differentiated testicular germ cells with CNS-precursor cell characteristics), NGP127 (neuroblastoma), HepG2 (hepatocarcinoma), and A549 (lung carcinoma) were used in this study. Cells were grown on DMEM/F12 medium containing 10% (vol/vol) fetal calf serum (Invitrogen) at 37 °C and 5% CO2.
Tissue Samples.
Tissue samples were taken from human heart, lung, bladder, testicles, brain cortex, hippocampus, midbrain, pons, and cerebellum (left, central, right) and from the left and right frontal lobe, parietal lobe, temporal lobe, occipital lobe, hippocampus, hypothalamus, thalamus, and medulla oblongata,. All samples were taken within 24 h after death from adult (19- to 63- y-old) donors killed in road accidents. Informed consent was obtained from donor representatives, and the experiments were approved by the Institutional Review Board of the Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry.
Primary Neuronal Cell Culture.
Primary neuronal culture was prepared from newborn Wistar rat pup hippocampi.
DNA and RNA Extraction, cDNA Synthesis, PCR Amplification, and Cloning.
Genomic DNA was isolated using the Wizard Genomic DNA Purification Kit (Promega), RNA was extracted using the SV Total RNA Isolation System (Promega), and first-strand cDNA synthesis was conducted using the Mint reverse transcription kit (Evrogen) according to the manufacturers’ recommendations. PCR amplification of upstream gene regions and bisulfite-converted DNA was conducted with the Encyclo PCR Kit (Evrogen). For qRT-PCR, we used the qPCRmix-HS SYBR (Evrogen). qRT-PCR was performed using a MxPro3000 thermocycler (Stratagene). Primer sequences are listed in Table S1. For amplification of promoter regions we used genomic DNA isolated from human placenta and chimpanzee mixed brain tissues (∼40 ng per reaction). The pGEM-T Easy vector (Promega) was used for cloning PCR products, followed by subcloning into a pGL3-basic vector (Promega) and pDsRed-Express-N1 vector (Clontech).
Lentiviral Vector Construction.
A 42-bp linker containing Bsp120I and MluI restriction sites was cloned by AvaI and HpaI into a lentiviral vector p156RRLsinPPTCMVGFPPRE (a gift from Alon Chen, the Weizmann Institute of Science, Rehovot, Israel). An expression cassette containing DsRed fluorescent protein under the control of the PRODH upstream regulatory sequence was subcloned into the resulting vector using NotI and MluI restriction sites.
Cell Transfections and Luciferase Assays.
Cell transfections were performed in 24-well plates using Unifectin-56 transfection reagent according to the manufacturer’s recommendations; 0.5μg DNA was used per well. pGL3-based reporter constructs carrying the upstream region of the tested gene and the firefly luciferase gene were mixed in a ratio of 10/1 with pRL-TK vector used as an internal control. Firefly luciferase values were normalized to those of Renilla luciferase. For the overexpression of human transcriptional factors SOX2 and NFKB1, we used pMXs-hSOX2 (Addgene; vector was kindly provided by Maria Lagarkova, Vavilov Institute of General Genetics, Moscow) and phNFKB1 (vector was kindly provided by Alexander Belyavsky, Engelhardt Institute of Molecular Biology, Moscow). Here, SOX2 and NFKB1 genes are under the control of the standard CMV promoter. Luciferase activity was measured in quadruplicate 48 h after transfection using the Dual Luciferase Reporter Assay (Promega) and a GENios Pro luminometer (Tecan).
Microscopy.
Mounting was conducted with ProLong Gold antifade reagent (Life Technologies). Images were taken on an LSM5Life Confocal microscope and were pseudocolored using ImageJ software (24).
Microarray Hybridization.
Microarray hybridization was performed with the HumanHT-12 v4 Expression BeadChip Kit (Illumina) according to the manufacturer’s protocol. Microarray hybridization data are available on GEO (NCBI), accession no. GSE43773.
In Vitro DNA Methylation.
pGL3-based reporter constructs were methylated in vitro using M.Sss I CpG-methyltransferase (SibEnzyme) according to the manufacturer’s protocol.
Bisulfite Sequencing.
Bisulfite conversion of genomic DNA was performed using the EpiTect Bisulfite Kit (Qiagen) followed by amplification with the EpiTect Whole Bisulfitome Kit (Qiagen). DNA then was amplified by nested-PCR (primers are given in Table S1).
In Vitro Translation.
To obtain mRNA coding for SOX2, PCR products were synthesized using the pMXs-hSOX2 plasmid as the template, with the forward primer SOX2T7, which contained an introduced T7 priming site. The reverse primer, T50, contained a 50-nt-long 3′ poly(T) repeat as described in ref. 24. The RiboMAX kit (Promega) was used for in vitro transcription, which was followed by LiCl precipitation and an m7G capping step (ScriptCap; Epicentre Biotechnologies). This step was followed by a second LiCl precipitation. A capped and polyadenylated mRNA encoding Renilla luciferase was obtained in a similar way (25). For in vitro translation, nuclease-treated rabbit reticulocyte lysate (RRL; Promega) was used, and the manufacturer’s protocol was followed (26).
Nuclear Extract Preparation.
Nuclear extracts were performed as described in ref. 27 and were aliquoted and stored at −70 °C.
EMSA.
Annealed oligonucleotides were radiolabeled with an α32P-dATP using Klenow polymerase and then were purified in a polyacrylamide gel and stored at −20 °C. Nuclear extract (5 μg of total protein per reaction) was preincubated with 750 ng of noneukaryotic DNA. Labeled oligonucleotides (30,000 cpm) were added to nuclear extract and incubated for 30 min at 20 °C. After incubation, samples were loaded onto a polyacrylamide gel for electrophoresis. Gels were dried and exposed to X-ray film for 48–120 h.
Statistics and Dataset Analysis.
For statistical analysis we used STATISTICA software (www.statsoft.com/) and a t test. To compare gene expression in human and in chimpanzee, we chose the GSE33010 dataset from the GEO database (www.ncbi.nlm.nih.gov/geo/). We processed the samples analyzed on the platform Affymetrix Human Genome U133 Plus 2.0 Array. Raw microarray data from 36 samples were normalized with the GCRMA algorithm (28) and were summarized using redefined probe set definitions. For pairwise comparison of brain gene-expression profiles, we defined six groups of samples: Homo sapiens hippocampus (seven samples), Pan troglodytes hippocampus (six samples), Homo sapiens prefrontal cortex (four samples), Pan troglodytes prefrontal cortex (six samples), Homo sapiens caudate (seven samples), and Pan troglodytes caudate (six samples). Samples from Pan troglodytes were used as a reference.
Supplementary Material
Acknowledgments
We thank Dr. S. B. Akopov (Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry) for providing cell lines, Drs. S. A. Lukyanov and D. A. Shagin (Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry) for assistance with qRT-PCR, and the UMA Foundation for support in manuscript preparation. This work was supported by Russian Foundation for Basic Research Grants 12-04-33094 (to A.B.), 12-04-31028 (to M.S.), 12-04-33196 (to S.E.D.), and 11-04-00682 (to N.M.) and by the Presidium of the Russian Academy of Sciences Program “Dynamics and Conservation of Genomes.”
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318172110/-/DCSupplemental.
References
- 1.Bannert N, Kurth R. Retroelements and the human genome: New perspectives on an old relation. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14572–14579. doi: 10.1073/pnas.0404838101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gogvadze E, Stukacheva E, Buzdin A, Sverdlov E. Human-specific modulation of transcriptional activity provided by endogenous retroviral insertions. J Virol. 2009;83(12):6098–6105. doi: 10.1128/JVI.00123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills RE, et al. Recently mobilized transposons in the human and chimpanzee genomes. Am J Hum Genet. 2006;78(4):671–679. doi: 10.1086/501028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzdin A, Kovalskaya-Alexandrova E, Gogvadze E, Sverdlov E. At least 50% of human-specific HERV-K (HML-2) long terminal repeats serve in vivo as active promoters for host nonrepetitive DNA transcription. J Virol. 2006;80(21):10752–10762. doi: 10.1128/JVI.00871-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowaloff EM, Phang JM, Granger AS, Downing SJ. Regulation of proline oxidase activity by lactate. Proc Natl Acad Sci USA. 1977;74(12):5368–5371. doi: 10.1073/pnas.74.12.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kempf L, et al. Functional polymorphisms in PRODH are associated with risk and protection for schizophrenia and fronto-striatal structure and function. PLoS Genet. 2008;4(11):e1000252. doi: 10.1371/journal.pgen.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gogos JA, et al. The gene encoding proline dehydrogenase modulates sensorimotor gating in mice. Nat Genet. 1999;21(4):434–439. doi: 10.1038/7777. [DOI] [PubMed] [Google Scholar]
- 8.Buzdin A, et al. Human-specific subfamilies of HERV-K (HML-2) long terminal repeats: Three master genes were active simultaneously during branching of hominoid lineages. Genomics. 2003;81(2):149–156. doi: 10.1016/s0888-7543(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 9.Sestáková B, Vachtenheim J. Distinct co-regulation of endogenous versus transfected MITF-dependent tyrosinase promoter. Folia Biol (Praha) 2006;52(5):161–166. [PubMed] [Google Scholar]
- 10.Lavie L, Kitova M, Maldener E, Meese E, Mayer J. CpG methylation directly regulates transcriptional activity of the human endogenous retrovirus family HERV-K(HML-2) J Virol. 2005;79(2):876–883. doi: 10.1128/JVI.79.2.876-883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stengel S, Fiebig U, Kurth R, Denner J. Regulation of human endogenous retrovirus-K expression in melanomas by CpG methylation. Genes Chromosomes Cancer. 2010;49(5):401–411. doi: 10.1002/gcc.20751. [DOI] [PubMed] [Google Scholar]
- 12.Campbell HD, Webb GC, Young IG. A human homologue of the Drosophila melanogaster sluggish-A (proline oxidase) gene maps to 22q11.2, and is a candidate gene for type-I hyperprolinaemia. Hum Genet. 1997;101(1):69–74. doi: 10.1007/s004390050589. [DOI] [PubMed] [Google Scholar]
- 13.Cartharius K, et al. MatInspector and beyond: Promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21(13):2933–2942. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- 14.Bassett AS, et al. Clinical features of 78 adults with 22q11 Deletion Syndrome. Am J Med Genet A. 2005;138(4):307–313. doi: 10.1002/ajmg.a.30984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, et al. Genetic variation at the 22q11 PRODH2/DGCR6 locus presents an unusual pattern and increases susceptibility to schizophrenia. Proc Natl Acad Sci USA. 2002;99(6):3717–3722. doi: 10.1073/pnas.042700699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bender HU, et al. Functional consequences of PRODH missense mutations. Am J Hum Genet. 2005;76(3):409–420. doi: 10.1086/428142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ripke S, et al.; Multicenter Genetic Studies of Schizophrenia Consortium; Psychosis Endophenotypes International Consortium; Wellcome Trust Case Control Consortium 2; Management Committee; Data and Analysis Group; DNA, Genotyping, Data QC and Informatics Group; Publications Committee (2013) Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet 45(10):1150–1159. [DOI] [PMC free article] [PubMed]
- 18.Bavaresco CS, Streck EL, Netto CA, Wyse AT. Chronic hyperprolinemia provokes a memory deficit in the Morris water maze task. Metab Brain Dis. 2005;20(1):73–80. doi: 10.1007/s11011-005-2478-x. [DOI] [PubMed] [Google Scholar]
- 19.Clelland CL, et al. Evidence for association of hyperprolinemia with schizophrenia and a measure of clinical outcome. Schizophr Res. 2011;131(1-3):139–145. doi: 10.1016/j.schres.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu CA, Lin WW, Valle D. Cloning, characterization, and expression of cDNAs encoding human delta 1-pyrroline-5-carboxylate dehydrogenase. J Biol Chem. 1996;271(16):9795–9800. doi: 10.1074/jbc.271.16.9795. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JL, Roberts E. Proline, glutamate and glutamine metabolism in mouse brain synaptosomes. Brain Res. 1984;323(2):247–256. doi: 10.1016/0006-8993(84)90295-6. [DOI] [PubMed] [Google Scholar]
- 22.Renick SE, et al. The mammalian brain high-affinity L-proline transporter is enriched preferentially in synaptic vesicles in a subpopulation of excitatory nerve terminals in rat forebrain. J Neurosci. 1999;19(1):21–33. doi: 10.1523/JNEUROSCI.19-01-00021.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarley RW, et al. MRI anatomy of schizophrenia. Biol Psychiatry. 1999;45(9):1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dmitriev SE, et al. Efficient translation initiation directed by the 900-nucleotide-long and GC-rich 5′ untranslated region of the human retrotransposon LINE-1 mRNA is strictly cap dependent rather than internal ribosome entry site mediated. Mol Cell Biol. 2007;27(13):4685–4697. doi: 10.1128/MCB.02138-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dmitriev SE, Terenin IM, Rubtsova MP, Shatskiĭ IN. [Minor secondary-structure variation in the 5′-untranslated region of the beta-globin mRNA changes the concentration requirements for eIF2] Mol Biol (Mosk) 2003;37(3):494–503. [PubMed] [Google Scholar]
- 27.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z, Irizarry R, Gentleman R, Murillo FM, Spencer F. A model based background adjustment for oligonucleotide expression arrays. J Am Stat Assoc. 2004;99:909–917. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.